Abstract
Natural killer (NK) cells are innate lymphocytes that provide cytokines critical for early host defense against pathogens. One subset of human NK cells (CD56bright) constitutively expresses the high-affinity interleukin 2 (IL-2) receptor and produces immunoregulatory cytokines. Here, we demonstrate that CD56bright NK cells are present in human lymph nodes and that endogenous T cell–derived IL-2, acting through the NK high-affinity IL-2 receptor, costimulates CD56bright NK cells to secrete IFN-γ. Thus, adaptive immunoregulators influence innate cytokine production, which in turn may influence the developing antigen-specific immune response. These data show a dynamic interaction between innate and adaptive human lymphocytes and emphasize the importance of studying interactions between immune components to understand the immune response as a whole.
Introduction
Immunity has long been classified as innate (early, nonspecific, and without memory) or adaptive (late, antigen-specific, and with memory) in nature.1 The study of these responses has been largely reductionist through focusing experiments on individual cell types or processes that contribute to immunity at the cellular or molecular level. Recently, interest has been shown in understanding the complex integration producing an effective adaptive immune response2 and novel interactions at the cellular level.3-6 A paradigm that the earlier innate immune response sets the stage for the subsequent adaptive response is well established7; however, experimental data in support of this theory in human systems is limited. Furthermore, while considerable study has been focused on the innate shaping of the adaptive immune response, only modest attention has been paid to the adaptive influencing the innate immune system beyond well-characterized antibody–Fc receptor interactions. Can adaptive immune cells affect the innate immunoregulatory response?
Natural killer (NK) cells are innate immune lymphocytes that mediate 2 major functions: recognition and lysis of tumor and virus-infected cells and production of immunoregulatory cytokines.8,9 The activation of an NK cell to kill a target cell is controlled by a complex interaction between activating and inhibitory receptor signals and can be modulated by cytokines.10-12 The production of cytokines such as interferon (IFN)-γ by NK cells is critical to early host defense against a variety of viral, bacterial, and parasitic pathogens.13,14 Innate immune signals that stimulate NK cell cytokine production are well established and include monokines such as interleukin 12 (IL-12), IL-15, IL-18, and IL-1β and NK receptor ligation.9,15 16 However, stimulatory signals for NK cell cytokine secretion from adaptive immune cells (eg, T cells) and a physiologic framework for such interactions have not been described.
IL-2 is produced primarily by antigen-specific CD4+and CD8+ T cells following activation and mediates an autocrine/paracrine proliferative program through the IL-2 receptor (IL-2R).17 Two signaling forms of the IL-2R complex have been identified: the intermediate affinity (IA) IL-2Rβγ (Kd = nanomolar, nM), and the high affinity (HA) heterotrimeric IL-2Rαβγ (Kd = picomolar, pM). Antigen-activated T cells express the HA IL-2Rαβγ and use low concentrations of IL-2 for proliferation and survival. In order to compete for limited amounts of T cell–derived IL-2 during an ongoing adaptive immune response, other cell types likely must be located within the T-cell microenvironment and express the HA IL-2Rαβγ.
Human NK cells comprise approximately 10% of peripheral blood lymphocytes and are characterized phenotypically by the presence of CD56 and the lack of CD3. The majority (approximately 90%) of human NK cells are CD56dim and express high levels of FcγRIII (CD16), whereas a minority (approximately 10%) are CD56bright and CD16dim/neg.9CD56bright NK cells constitutively express the HA IL-2Rαβγ and IA IL-2Rβγ and expand in vitro and in vivo in response to low (picomolar) doses of IL-2 but do not themselves make IL-2.18,19 CD56bright NK cells also have a distinct NK receptor repertoire with high expression of the C-type lectin CD94/NKG2 family and little expression of killer cell immunoglobulin (Ig)–like receptors (KIRs).20 In addition, CD56bright NK cells were recently identified as the major NK cytokine producer, consistent with a functional role as an innate immunoregulator. Moreover, this subset of NK cells expresses high levels of L-selectin and CCR7, both of which are involved in trafficking of immune cells to lymph nodes.21 22 Thus, in theory, CD56bright NK cells appear uniquely equipped to traffic to the site of developing adaptive immune responses (eg, lymph node) and have a dynamic interaction with both T cells and antigen-presenting cells (APCs) through cytokine secretion.
Therefore, we hypothesize that the CD56bright NK cell, with its constitutive HA IL-2Rαβγ expression and other unique attributes, is in a unique position to interact with T cells during the antigen-specific immune response. Here, we demonstrate that this subset of NK cells is present in the T-cell areas of human lymph nodes and can use low amounts of endogenous IL-2 acting through the HA IL-2Rαβγ to costimulate IFN-γ production. To our knowledge, this is the first evidence for immunoregulation of innate immune lymphocytes by adaptive lymphocytes, and it implies that dynamic bidirectional interactions occur between these branches of immunity.
Materials and methods
Reagents
Cells were cultured in RPMI 1640 with 10% human serum (C6 Diagnostics, Mequon, WI) and antibiotics. Recombinant (r) human (h) IL-12 (specific activity of 4.5 × 106 U/mg) was from Genetics Institute (Cambridge, MA), and rhIL-2 (specific activity of 1.53 × 107 U/mg) was from Hoffmann LaRoche (Nutley, NJ). PE/PC5-conjugated anti-CD56 monoclonal antibody (mAb), anti-CD94 mAb, and anti-CD16 mAb were from Coulter (Miami, FL). Mouse antihuman CD25 mAb (anti–IL-2Rα) and a nonreactive isotype control mAb were from Biosource (Camarillo, CA). Anti–IL-2 neutralizing antibody and control, antihuman CD3–fluorescein isothiocyanate (FITC), and antihuman CD16 were from BD Pharmingen (San Diego, CA).
Immunohistochemistry and RT in situ PCR
For the CD56, CD94 (clone 39B10), CD16, CD158b (clone GL183), and NKp46 immunohistochemistry (IHC) experiments, normal reactive axillary human lymph nodes were obtained from the pathology archives of the Istituto di Anatomia Patologica and IHC was performed as described.23 For reverse transcriptase (RT) in situ polymerase chain reaction (PCR) and CD3 colabeling experiments, tissues were acquired from the Department of Pathology at The Ohio State University. These tissues included 4 lymph nodes from patients with nonmalignant diagnoses that showed no pathologic alterations. Lymph nodes were tested for CD56 RNA expression using previously published RT in situ PCR techniques with the following CD56 primers: F′-TTGTTTTTCCTGGGAACTGC, R′-CCGGATCTGCAGGTAGTTGT, or anti-CD3 mAb as described.24 25
Flow cytometry
Fresh normal donor lymph nodes were obtained from the Human Tissue Network at The Ohio State University under an institutional review board (IRB)–approved protocol and were passed through sterile nylon mesh to create a single-cell suspension, red blood cells (RBCs) were lysed, and the suspension was stained with indicated mAbs as described.15 Forward and side scatter and fluorescence data were collected on a Coulter XL flow cytometer and at least 100 000 events were analyzed using the WinMDI software program (Joseph Trotter, Scripps, La Jolla, CA). Nonreactive isotype controls were used to set the quadrant gates with 99% or more of cells located in negative quadrants.
Isolation of human NK cell subsets
Purified NK cells were isolated from fresh healthy donor leukopacks (American Red Cross, Columbus, OH) as described.16 NK cells were stained with anti–CD56–phycoerythrin (PE) or control-PE and subsets were purified based on CD56 surface density by fluorescence-activated cell sorter (FACS) as described.16 Cell populations were routinely more than 98% pure by post-FACS analysis.
NK cell stimulation
Purified CD56bright or CD56dim NK cells (2 × 104) were cultured for 72 hours in the presence or absence of cytokines in 96-well plates in a total volume of 200 μL, after which cell-free supernatants were harvested and assayed by enzyme-linked immunosorbent assay (ELISA) for interferon-γ (IFN-γ; Gibco BRL, Gaithersburg, MD, or Endogen, Woburn, MA). Results represent the means of replicate wells ± SEMs. In some experiments, NK cells were preincubated with anti-CD25 or control mAb for 1 hour prior to the addition of cytokines. In time-course experiments, cells were harvested and cell-free supernatants collected at the indicated time points.
Real-time RT-PCR for IFN-γ gene expression
Coculture experiments
Purified NK cell subsets (5 × 104) were cocultured in 96-well plates with an established CD4+ T cell clone (1.5 × 104) reactive to tetanus toxoid (TT) antigen (Ag) and autologous APCs (B-LCL, 1 × 104) pulsed with TT (Wyeth Labs, Marietta, PA) plus rhIL-12 (10 U/mL). After 48 hours cell-free culture supernatants were harvested and assayed for IFN-γ protein by ELISA (Endogen). Control cocultures with T cells, Ag-pulsed APCs and rIL-12 (no NK cells) produced no IFN-γ protein (data not shown). T cells from those control cultures analyzed by intracellular flow cytometry produced IL-2 and IL-4 but not IFN-γ (data not shown). In some experiments, neutralizing anti–IL-2 or control antibodies (Abs) were added to the cocultures (5 μg/mL). Specificity of the anti–IL-2 antibody was shown in parallel cultures of NK cells with 10 pM IL-2 replacing the T cell/APC (Figure 4).
Statistical analysis
Statistical analysis was performed using the Student pairedt test; P < .05 was considered significant.
Results
Human NK cells are located in the parafollicular T-cell region of lymph nodes
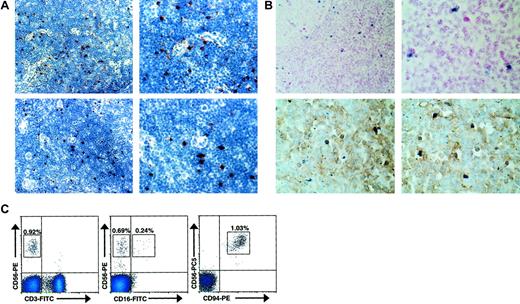
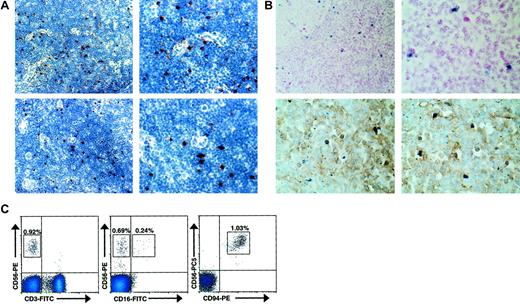
As we hypothesized that CD56bright NK cells may interact with IL-2–producing T cells during an ongoing adaptive immune response, we examined histologically normal human lymph nodes for their presence. Normal human lymph nodes were tested for expression of CD56, CD94, CD16, CD158b (GL183), and the natural cytotoxicity receptor (NCR) NKp46 by IHC. Distinct CD56+ and CD94+ lymphocytes were identified within the parafollicular T-cell areas of histologically normal lymph nodes (Figure1A). In contrast, no reactivity was observed with CD16-, CD158b-, or NKp46-specific antibodies (data not shown). Normal human lymph nodes were also studied using in situ RT-PCR to detect cells that express CD56 transcript (Figure 1B). In 4 of 4 lymph nodes tested, a discrete number of lymphocytes, again primarily located in the parafollicular T-cell areas of the lymph node, strongly expressed CD56 mRNA. Furthermore, upon colabeling CD3 these CD56+ lymphocytes lacked surface CD3 expression, consistent with an NK cell phenotype. To better characterize the immunophenotype of the observed CD56+CD3−lymphocytes, flow cytometric analysis of normal human lymph nodes was also performed (Figure 1C). In 4 of 4 lymph nodes analyzed, a discrete population of CD56brightCD3− cells was consistently detected (0.94% ± 0.12%). Similar to peripheral blood CD56bright NK cells,9 a subset seen in the lymph node (approximately 30%) express low density of CD16 and virtually all express high density of CD94, in agreement with the data presented above. While nearly all (> 95%) lymph node CD56+ NK cells were CD56bright, this differs from the peripheral blood, where the majority (approximately 90%) of NK cells are CD56dim and a minority (approximately 10%) are CD56bright.9 Thus, a distinct population of CD56bright NK cells is located at the anatomic site of ongoing adaptive immune responses.
CD56bright NK cells are present in human lymph nodes.
(A) CD56+ and CD94+ lymphocytes are present in the paracortical T-cell areas of normal human lymph nodes. Low-power (× 40, left) and high-power (× 100, right) photomicrographs of normal human lymph nodes stained for CD56 (top panels) and CD94 (bottom panels). Results are representative of 2 reactive lymph nodes. (B) CD56+CD3− lymphocytes were observed in the parafollicular T-cell areas of human lymph nodes. Low-power (× 40, left) and high-power (× 100, right) photomicrographs of normal human lymph nodes evaluated for CD56 expression using in situ RT-PCR (top panels). High-power photomicrographs of similar sections from lymph nodes colabeled with anti-CD3 mAb and CD56 via in situ PCR demonstrating that the CD56+ cells lack CD3 expression (bottom panels). Results are representative of at least 4 different experiments. (C) Distinct populations of CD56brightCD3− NK cells were observed via flow cytometry in normal human lymph nodes. Flow cytometric density plots are shown gated on lymphocytes stained with mAbs reactive to CD56 and CD3 (left), CD56 and CD16 (middle), and CD56 and CD94 (right). Percentage of lymphocytes in each boxed region is shown. Results are representative of 3 independent experiments on at least 3 normal human lymph nodes.
CD56bright NK cells are present in human lymph nodes.
(A) CD56+ and CD94+ lymphocytes are present in the paracortical T-cell areas of normal human lymph nodes. Low-power (× 40, left) and high-power (× 100, right) photomicrographs of normal human lymph nodes stained for CD56 (top panels) and CD94 (bottom panels). Results are representative of 2 reactive lymph nodes. (B) CD56+CD3− lymphocytes were observed in the parafollicular T-cell areas of human lymph nodes. Low-power (× 40, left) and high-power (× 100, right) photomicrographs of normal human lymph nodes evaluated for CD56 expression using in situ RT-PCR (top panels). High-power photomicrographs of similar sections from lymph nodes colabeled with anti-CD3 mAb and CD56 via in situ PCR demonstrating that the CD56+ cells lack CD3 expression (bottom panels). Results are representative of at least 4 different experiments. (C) Distinct populations of CD56brightCD3− NK cells were observed via flow cytometry in normal human lymph nodes. Flow cytometric density plots are shown gated on lymphocytes stained with mAbs reactive to CD56 and CD3 (left), CD56 and CD16 (middle), and CD56 and CD94 (right). Percentage of lymphocytes in each boxed region is shown. Results are representative of 3 independent experiments on at least 3 normal human lymph nodes.
IL-2, acting through the HA IL-2Rαβγ, costimulates CD56bright NK cell IFN-γ production
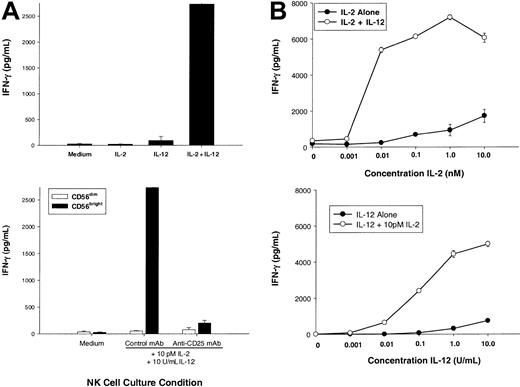
IL-12 appears to be the central regulator of NK cell–derived IFN-γ, whereas optimal IFN-γ production by an NK cell requires costimulation, often with another monokine or NK receptor ligation. CD56bright NK cells are unique among resting lymphocytes for their constitutive expression of a functional HA IL-2Rαβγ,18 and we postulated that this NK subset was uniquely able to use IL-2 during an ongoing T-cell response. First, we examined the ability of very low (pM) amounts of IL-2, which selectively saturate the HA IL-2Rαβγ, to potentiate IL-12–induced IFN-γ production (Figure 2A, top panel). While little or no IFN-γ was produced by CD56bright NK cells stimulated with medium, 10 pM IL-2, or IL-12, there was a synergistic increase induced by 10 pM IL-2 plus IL-12 (P < .05). 10 pM IL-2 (0.01 nM or 2.3 IU/mL) binds approximately 50% of HA IL-2Rαβγ and less than 1% of IA IL-2Rβγ.17 Further, when CD56bright NK cells were preincubated with a monoclonal antibody that selectively blocks the HA IL-2Rαβγ, IFN-γ production was abrogated (Figure 2A, bottom panel). As a control, CD56dim NK cells that lack the HA IL-2Rαβγ18 failed to produce IFN-γ in response to 10 pM IL-2 plus IL-12.
Low concentrations of IL-2 acting through the high-affinity IL-2 receptor costimulate CD56bright NK cell secretion of IFN-γ.
(A) Sorted CD56bright NK cells were cultured for 72 hours with medium alone, IL-2 (10 pM, 2.3 IU/mL), IL-12 (10 U/mL), or IL-2 plus IL-12, and cell culture supernatants were then assayed for human IFN-γ protein production (top panel). Prior to the addition of cytokines, cells were preincubated for one hour with saturating concentrations of an anti-CD25 mAb (selectively blocking the α subunit of the high-affinity IL-2R) or an isotype control mAb (bottom panel). As a negative control, CD56dim NK cells do not express the high-affinity IL-2R and fail to produce IFN-γ. (B) Sorted CD56bright NK cells were cultured with various concentrations of IL-2 with or without 10 U/mL IL-12 (top panel) or various concentrations of IL-12 with or without 10 pM IL-2 (bottom panel). Cell culture supernatants were harvested at 72 hours and assayed for IFN-γ protein by ELISA. Results represent the means ± SEMs of replicate wells, expressed in pg/mL, and are representative of at least 3 separate experiments.
Low concentrations of IL-2 acting through the high-affinity IL-2 receptor costimulate CD56bright NK cell secretion of IFN-γ.
(A) Sorted CD56bright NK cells were cultured for 72 hours with medium alone, IL-2 (10 pM, 2.3 IU/mL), IL-12 (10 U/mL), or IL-2 plus IL-12, and cell culture supernatants were then assayed for human IFN-γ protein production (top panel). Prior to the addition of cytokines, cells were preincubated for one hour with saturating concentrations of an anti-CD25 mAb (selectively blocking the α subunit of the high-affinity IL-2R) or an isotype control mAb (bottom panel). As a negative control, CD56dim NK cells do not express the high-affinity IL-2R and fail to produce IFN-γ. (B) Sorted CD56bright NK cells were cultured with various concentrations of IL-2 with or without 10 U/mL IL-12 (top panel) or various concentrations of IL-12 with or without 10 pM IL-2 (bottom panel). Cell culture supernatants were harvested at 72 hours and assayed for IFN-γ protein by ELISA. Results represent the means ± SEMs of replicate wells, expressed in pg/mL, and are representative of at least 3 separate experiments.
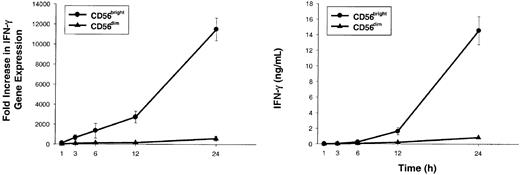
To better characterize the amount of IL-2 required to costimulate IL-12–induced IFN-γ production, dose-response experiments were performed with varying concentrations of IL-2 or IL-12 (Figure 2B). CD56bright NK cells produced near-maximal amounts of IFN-γ when stimulated with 10 pM (0.01 nM) IL-2 in combination with IL-12 (Figure 2B, top panel). Moreover, addition of 10 pM IL-2 reduced by 1000-fold the amount of IL-12 required to induce a given amount of IFN-γ (Figure 2B, bottom panel). Increase in IFN-γ gene expression was rapidly followed by IFN-γ protein secretion when CD56bright NK cells were costimulated with 10 pM IL-2 plus IL-12 (Figure 3). Again, as a control, CD56dim NK cells failed to produce IFN-γ under identical culture conditions. Thus, IFN-γ gene expression and protein secretion is rapidly induced in CD56bright NK cells when costimulated with pM concentrations of IL-2 acting through the HA IL-2Rαβγ in combination with IL-12. These in vitro experiments using highly purified human NK cells and recombinant cytokines suggest that a CD56bright NK cell, which itself does not produce IL-2, may be activated through IL-2 secretion by antigen-activated T cells in the lymph node. In the next experiment, we simulated an antigen-specific T-cell response in vitro to test this hypothesis under more physiologic conditions.
CD56bright NK cells stimulated with low concentrations of IL-2 and IL-12 rapidly accumulate IFN-γ transcript followed by IFN-γ protein secretion.
Sorted CD56bright (●) and CD56dim (▴) NK cells were cultured for 1, 3, 6, 12, and 24 hours with IL-2 (10 pM) plus IL-12 (10 U/mL). At each time point, cells and supernatants were collected and IFN-γ gene expression (left panel) and protein levels (right panel) were assessed. Results represent the means ± SEMs of replicate wells, expressed as fold increase in gene expression or ng/mL, and are representative of 4 separate experiments.
CD56bright NK cells stimulated with low concentrations of IL-2 and IL-12 rapidly accumulate IFN-γ transcript followed by IFN-γ protein secretion.
Sorted CD56bright (●) and CD56dim (▴) NK cells were cultured for 1, 3, 6, 12, and 24 hours with IL-2 (10 pM) plus IL-12 (10 U/mL). At each time point, cells and supernatants were collected and IFN-γ gene expression (left panel) and protein levels (right panel) were assessed. Results represent the means ± SEMs of replicate wells, expressed as fold increase in gene expression or ng/mL, and are representative of 4 separate experiments.
CD56bright NK cells use endogenous IL-2 produced by antigen-activated T cells to costimulate IFN-γ production
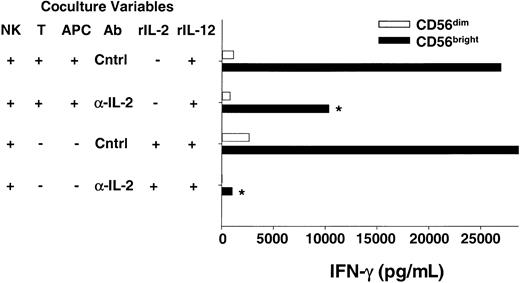
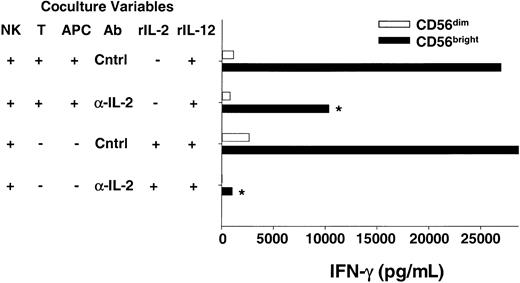
For a T cell to respond to an antigen, the peptide must be presented by a major histocompatibility complex (MHC) molecule on the surface of an APC (eg, dendritic cell, B cell, or macrophage). Recent studies have shed light on the importance of the interactions between APCs and T cells on the outcome of the subsequent adaptive immune response.23,26 27 To provide more physiologic evidence that CD56bright NK cells are able to use endogenous T cell–derived IL-2 to costimulate IFN-γ secretion, we developed an in vitro coculture system where an antigen-activated T cell clone was used as a source of IL-2. The CD4+ T-cell clone specific for tetanus toxoid (TT) used in these experiments produced IL-2 but not IFN-γ following stimulation with TT-pulsed autologous APCs (B-LCL, data not shown). Sorted CD56brightNK cells were cocultured with the T cell/Ag-pulsed APCs plus exogenous IL-12 and NK-derived IFN-γ secreted in the supernatant was measured (Figure 4). Abundant IFN-γ protein was detected when CD56bright NK cells were cocultured with T cell/Ag-pulsed APCs and IL-12, whereas control cocultures containing CD56dim NK cells yielded no IFN-γ. Addition of anti–IL-2 antibody to cocultures of CD56bright NK cells/T cells/Ag-pulsed APCs/IL-12 significantly abrogated IFN-γ production, demonstrating that endogenous IL-2 is an important costimulus in this system (47% ± 5% decrease; P < .03). Thus, data from experiments using T cells as a source of endogenous IL-2 in this coculture system are consistent with earlier data presented here with exogenous rIL-2 and highly purified CD56bright NK cells.
CD56bright NK cells can use T cell–derived IL-2 to costimulate IFN-γ production when cocultured in vitro.
Sorted NK cell subsets were cocultured with a tetanus toxoid–specific T-cell clone (T) and peptide-pulsed autologous APCs plus IL-12 in the presence of neutralizing anti–IL-2 (α-IL-2) or control (Cntrl) Abs. After 48 hours cell-free culture supernatants were harvested and assayed for IFN-γ production by ELISA. The T-cell clone used does not produce IFN-γ, and thus all IFN-γ is NK cell–derived (data not shown). As a positive control, NK cells were also cultured with rIL-2 (10 pM) plus IL-12 in the presence of α-IL-2 or Cntrl Abs. Results shown are the means ± SEMs of replicate wells and summarize 6 independent experiments. *P < .03.
CD56bright NK cells can use T cell–derived IL-2 to costimulate IFN-γ production when cocultured in vitro.
Sorted NK cell subsets were cocultured with a tetanus toxoid–specific T-cell clone (T) and peptide-pulsed autologous APCs plus IL-12 in the presence of neutralizing anti–IL-2 (α-IL-2) or control (Cntrl) Abs. After 48 hours cell-free culture supernatants were harvested and assayed for IFN-γ production by ELISA. The T-cell clone used does not produce IFN-γ, and thus all IFN-γ is NK cell–derived (data not shown). As a positive control, NK cells were also cultured with rIL-2 (10 pM) plus IL-12 in the presence of α-IL-2 or Cntrl Abs. Results shown are the means ± SEMs of replicate wells and summarize 6 independent experiments. *P < .03.
Discussion
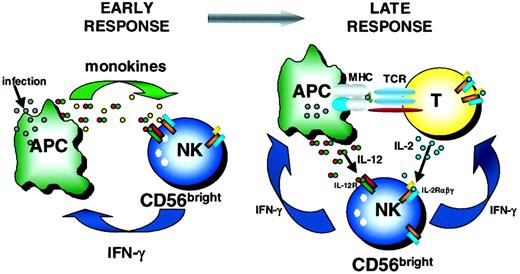
NK cells and T cells are thought to be the critical immunoregulatory lymphocytes of innate and adaptive immunity, respectively. T cells have been extensively studied as key sources of cytokines during the adaptive immune response.1 More recently, CD56bright NK cells have been identified as important innate immunoregulators, capable of producing abundant IFN-γ and other cytokines and chemokines in response to monokine stimulation and NK receptor ligation.9 Here, we have identified for the first time a distinct population of CD56bright NK cells in the T cell regions of normal human lymph nodes. Further, we show that endogenous T cell–derived IL-2, acting through the HA IL-2Rαβγ constitutively expressed on CD56bright NK cells, costimulates their production of IFN-γ. These data suggest that a more complex interaction between CD56bright NK cells and T cells exists in which not only NK cell–derived IFN-γ influences T cells, but also T cell–derived IL-2 provides a costimulatory signal for NK cytokine production. Therefore, this study provides novel data showing a dynamic 2-way dialogue between the innate and adaptive immune systems (Figure5).
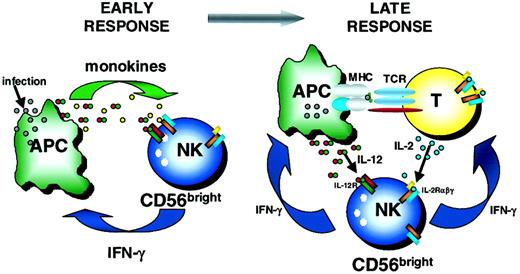
Schema of early and late immunoregulatory functions of the CD56bright NK cell subset.
Early during the innate immune response, CD56bright NK cells are costimulated with monokines (eg, IL-12 plus IL-18, IL-15, IL-1β) to produce IFN-γ. Later, CD56bright NK cells may use T cell–derived IL-2 and APC-derived IL-12 to synergistically induce IFN-γ, which may then influence the developing adaptive immune response. TCR indicates T-cell receptor.
Schema of early and late immunoregulatory functions of the CD56bright NK cell subset.
Early during the innate immune response, CD56bright NK cells are costimulated with monokines (eg, IL-12 plus IL-18, IL-15, IL-1β) to produce IFN-γ. Later, CD56bright NK cells may use T cell–derived IL-2 and APC-derived IL-12 to synergistically induce IFN-γ, which may then influence the developing adaptive immune response. TCR indicates T-cell receptor.
Previous studies have examined secondary lymphoid tissues for NK cells using immunohistochemistry (IHC) for CD56 and PEN5,28CD57,29 and granzymes A/B.30 Vivier and colleagues analyzed coexpression of PEN5, a posttranslational modification of P-selectin glycoprotein ligand-1 (PSGL-1),20 and CD56 on human lymphoid tissues.28 In this study, weak CD56 expression was noted only on very rare cells, whereas more numerous PEN5+ cells were identified within the parafollicular T-cell regions of lymph nodes. The lack of CD56 expression on the PEN5+population was attributed to technical difficulty in detecting CD56 on human cells using IHC. We used both IHC and in situ RT-PCR that clearly detected distinct CD56+CD3− NK cells, and flow cytometry that further characterized these cells as CD56bright NK cells that coexpress CD94. As PEN5 is expressed by a subset of CD56dim NK cells,20the functional significance of PEN5+CD56−cells in lymphoid tissue and their relationship to the CD56bright NK cells identified here is unclear.
There are 2 explanations for why CD56bright NK cells are the primary NK subset present in lymph nodes: trafficking or differentiation. Unlike CD56dim NK cells, peripheral blood CD56bright NK cells express adhesion molecules (eg, L-selectin) and chemokine receptors (eg, CCR7) required to traffic to peripheral lymph nodes through high endothelial venules. Thus, it is likely that CD56bright NK cells traffic to lymph nodes using these mechanisms, similar to other lymphocytes.31,32Alternatively, NK precursors may travel to lymph nodes from the bone marrow and differentiate into CD56bright NK cells in the periphery, perhaps under the direction of IL-15.33 The “snapshot” photomicrographs and flow cytometry identifying lymph node CD56bright NK cells presented here do not address the dynamic movements of immune cells in vivo or the response of immune cells to infection. Ultimately, to best understand the relationship between NK cell subsets present in various tissue compartments (eg, peripheral blood, bone marrow, spleen, liver, lymph nodes, lung) and their functional roles, experiments monitoring the movement of cells in vivo in humans will be necessary.
The quality and character of the innate immune response, especially the cytokines induced by various pathogens, influences the subsequent adaptive T-cell response.7,14,34,35 In vitro studies in both human and murine systems have demonstrated the importance of IFN-γ in the induction of a type 1 (Th1) immune response.36-39 IFN-γ mediates this effect through multiple mechanisms, including priming for IL-12 production by APCs and inducing the IL-12Rβ on CD4+ T cells.40-42In addition, murine NK cells were identified as a critical source of IFN-γ leading to a protective CD4+ Th1 response in vivo following Leishmania major infection.43 Thus, our finding that human CD56bright NK cells are present in the T-cell areas of lymph nodes and can respond to endogenous T cell–derived IL-2 to produce IFN-γ is consistent with their potential role as an innate immunoregulator of the primary adaptive immune response. However, our findings also identify a mechanism whereby adaptive T lymphocytes, through elaboration of IL-2, activate innate NK cell IFN-γ, thus providing novel data demonstrating bidirectional regulation between these immune system branches.
NK cells have also been shown to influence the differentiation of CD8+ cytotoxic T cells and their functional responses.44 Human NK cells were shown to be required for the differentiation of alloreactive CD8+ cytotoxic T lymphocytes (CTLs) in mixed cultures in vitro.45Furthermore, NK cells (NK1.1+) were required in vivo for the differentiation of protective B16-melanoma–specific CD8+ T cells, as well as for the differentiation of effector influenza-virus–specific CD8+ CTLs, using murine models.44,46 47 Thus, CD56bright NK cells, through cell surface interactions or the elaboration of cytokines, could also play a role in human CD8+ CTL differentiation and successful responses to viral infection and malignant transformation in peripheral tissues.
Murine NK cells, while similar to their human counterparts in many fundamental respects, fail to express a CD56 (neural cell adhesion molecule) homolog. Functionally distinct murine NK subsets, similar to those recognized in humans, have yet to be identified in the mouse.9 Moreover, there appear to be some fundamental differences between human and murine NK cell regulation, such as the use of KIR NK receptors in humans but not in mice. Such differences highlight the importance of understanding basic human NK cell biology and regulation as a basis for elucidating their role in disease and/or immunotherapy. It will be interesting to examine NK cell subsets present in human lymph nodes in the setting of infectious (eg, chronic viral infections) and malignant disease. Furthermore, therapies aimed at augmenting innate immunity may need to assess responses in multiple tissue compartments in addition to peripheral blood.
CD56bright and CD56dim NK cells exhibit a functional dichotomy, with CD56bright NK cells serving as immunoregulators and CD56dim NK cells being cytotoxic effectors.9 The findings presented here are consistent with an immunoregulatory role for CD56bright NK cells and identify this subset in lymphoid tissues where they may exert influences on the T-cell response through elaboration of cytokines (eg, IFN-γ). The finding that CD56bright NK cells are the primary NK subset in lymph nodes suggests that human NK cells exhibit different predilections for tissue compartments (eg, blood, spleen, lymph node, uterus, bone marrow, liver), and the tissue compartment in which they reside should be investigated and correlated with their biologic roles. In addition to NK subset distinction based upon CD56, several groups have postulated the division of NK cells based upon the type of cytokine production (NK1 and NK2), similar to CD4 helper T cells.48 49 While such studies to date present data on cloned or extensively cultured NK cells, the idea is interesting and should be further pursued in freshly isolated primary NK cells. In humans, such NK1 and NK2 subsets would likely be found within the CD56bright NK cell subset.
In conclusion, we demonstrate that CD56bright NK cells are present in human lymph nodes and that endogenous T cell–derived IL-2, acting through the high-affinity IL-2 receptor, costimulates CD56bright NK cells to secrete IFN-γ. This novel immunoregulatory link between adaptive and innate lymphocytes provides evidence for a more dynamic communication between these 2 arms of the immune system. As meaningful immune interactions are identified, it will be important to integrate these connections to better understand the immune response as a whole.
We thank A. Oberyszyn for cell sorting as well as Tamra Brooks and Donna Bucci for administrative assistance.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood-2002-09-2876.
Supported in part by National Institutes of Health grants CA68458, CA65670, and P30CA16058. T.A.F. is supported in part by the Resident Physician Scientist Training Pathway at the Washington University Department of Internal Medicine. M. A. Cooper is the recipient of Medical Scientist Program fellowships from The Ohio State University College of Medicine and Public Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael A. Caligiuri, The Ohio State University, 458A Starling Loving Hall, 320 W 10th Ave, Columbus, OH, 43210; e-mail: caligiuri-1@medctr.osu.edu.