Abstract
Activation of the phosphatidylinositol 3- kinase/AKT pathway antagonizes apoptosis in diverse cellular systems. We previously showed that human plasma activated AKT and potently blocked the ability of chlorambucil or gamma radiation to induce apoptosis of B-chronic lymphocytic leukemia (CLL) cells. Here we report experiments that identify albumin as the major component of plasma that blocks CLL cell killing by chlorambucil or radiation. Intact plasma depleted of albumin by chromatography on Cibacron blue–Sepharose or plasma from a subject with analbuminemia failed either to activate AKT or to protect CLL cells from chlorambucil-induced apoptosis. Both functions were restored by re-addition of albumin. The protective action of albumin as well as AKT activation was compromised by the binding of lipids. Fluorescence-activated cell sorter (FACScan) analysis demonstrated the uptake of fluoresceinated albumin by CLL cells. Accumulation of albumin in intracellular vesicles was also shown by confocal microscopy. Indirect inhibition of AKT activation by the phosphatidylinositol 3-kinase inhibitor LY294002 reversed the blockade of chlorambucil-induced killing by plasma albumin. The data suggest that activation of AKT consequent to binding of albumin by CLL cells blocks chlorambucil- and radiation-induced apoptosis. Strategies designed to block albumin-induced antiapoptotic signaling may, therefore, be of value in enhancing cytotoxic drug action on CLL cells.
Introduction
B-chronic lymphocytic leukemia (CLL) is the most common leukemia in the Western world and is currently considered to be incurable.1,2 Although CLL cells survive for extended periods in vivo, they rapidly undergo apoptosis when cultured in vitro,3 suggesting that survival signals available in vivo are lacking under in vitro culture conditions. Although several cytokines, including interleukin 4,4,5 interferons α6,7 and γ,8 and stromal cell-derived factor-1,9 have been shown to promote CLL survival in vitro, the contribution of these ligands to extended survival in vivo is unclear. During studies designed to define antiapoptotic factors present in peripheral blood, we observed that plasma, but not serum, promoted CLL cell survival in vitro and also blocked the ability of the cytotoxic drug chlorambucil or of gamma radiation to induce apoptosis.10 There was no consistent difference in the antiapoptotic actions of plasma from healthy subjects or patients with CLL, suggesting that the cytoprotective component was a common constituent of plasma.
Significantly, we observed that addition of plasma to CLL cells rapidly activated the AKT protein kinase. AKT has been implicated as a major modulator of cell survival,11 via the phosphorylation and inactivation of proapoptotic proteins, including the BCL-2 family member BAD12 and Forkhead family transcription factors.13 Diverse survival factors trigger AKT via activation of phosphatidylinositol 3-kinase (PI3-K) and the consequent elevation of 3-phosphoinositides, which serve as allosteric modulators of AKT.14
The cytoprotective action of plasma was not abrogated by neutralizing antibodies against a variety of cytokines, including interleukin-4, interferons α and γ, and stromal cell–derived factor-1.10 In addition, the failure of plasma to activate Janus kinases (JAKs) of CLL cells suggested that a noncytokine signaling component of plasma was responsible for antiapoptotic signaling.
Here we report studies that implicate albumin as the major antiapoptotic signaling component present in plasma. We also show that the protective action of albumin is compromised by binding of lipids and that CLL cells can bind and internalize albumin. Some of these studies have been reported in a preliminary form.15
Materials and methods
Reagents
Delipidated human albumin (HA) was obtained from Sigma (Poole, United Kingdom) and recombinant HA (Recombumin) from Delta Biotechnology (Nottingham, United Kingdom). They were dissolved in RPMI 1640 (Invitrogen, Paisley, Scotland) and dialyzed exhaustively against the same medium. Albumins loaded with equimolar amounts of oleic and linoleic acids were prepared by addition of 50 mM ethanol stock solutions of the fatty acids, sonication for four 5-second bursts, and dialysis against RPMI 1640. For fluorescein isothiocyanate (FITC) labeling, HA was dissolved at 50 mg/mL in 250 mM carbonate buffer, pH 9, and dialyzed exhaustively against the same buffer. Fluorescein isothiocyanate (Sigma) was then added to 2.5 mg/mL, and the reaction was allowed to proceed for 8 hours at 4°C. The FITC-HA was then dialyzed against phosphate-buffered saline. Spectroscopic analysis16 showed that the final preparation contained 4 moles FITC per mole albumin.
Preparation of human serum and plasma
For the preparation of plasma, peripheral blood from patients with CLL or healthy subjects was collected in preservative-free heparin (10 U/mL). Following centrifugation at 700g for 10 minutes, the plasma was withdrawn, clarified by a further centrifugation step, and stored at −20°C. A plasma sample was also obtained from a subject with analbuminemia, a rare recessive trait resulting in the complete absence of plasma albumin.17
Fatty acids were depleted from human serum by a modification of a published procedure.18 Following adjustment of the pH to 4 by addition of 5 N HCl, 200 mg/mL acid-washed charcoal (Sigma) was added. The tube was rotated for 1 hour, and the charcoal was then removed by centrifugation. The pH of the serum was re-adjusted to 7.4 by addition of 5 N NaOH, followed by exhaustive dialysis against RPMI 1640. Free fatty acids were determined using the NEFA C enzymatic assay kit (WAKO GmbH, Neuss, Germany).
Albumin was depleted by chromatography of 5 mL plasma on a 5-mL column of Cibacron blue–Sepharose 6B (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). The flow-through fraction was collected and reconcentrated to 5 mL, using a Centricon centrifugal filter unit (Millipore, Watford, United Kingdom).
Cell purification
CLL was diagnosed by established clinical criteria and confirmed by immunocytochemical analysis for the expression of CD5, CD19, and monoclonal immunobglobulin.1 Patients whose cells were known to be resistant to chlorambucil in vitro were excluded from the study. Peripheral blood was obtained from patients following informed consent. Mononuclear cells were isolated by sedimentation on Lymphoprep gradients (Nyegaard, Oslo, Norway). Monocytes were removed by adhesion to plastic. The proportion of contaminating T lymphocytes was determined following incubation with FITC-labeled anti-CD2 monoclonal antibody (Becton Dickinson, Cowley, United Kingdom) and analysis by fluorescence-activated cell sorter (FACScan; Becton Dickinson). Samples containing more than 5% contaminating T cells were repurified by rosetting with neuraminidase-treated sheep erythrocytes.5Cells were cultured in RPMI 1640 medium containing 100 U/mL penicillin, 100 μg/mL streptomycin (basal medium), and other supplements as indicated in individual figures.
Incubations
CLL cells were cultured in basal medium containing 10% fetal calf serum (FCS). They were then left untreated or exposed to chlorambucil for 1 hour at 37°C. The cells were recovered by centrifugation at 700g, washed once in Hanks saline, and resuspended in basal medium containing supplements as indicated in the figures. Serum and plasma preparations were added at 50% (vol/vol). Titration studies (not shown) have established that maximal protection was achieved at plasma concentrations between 20% and 30%. HA or rHA was added at 25 mg/mL. Following incubation at 37°C for 24 hours, viability and apoptosis were determined as described in “Determination of viability and apoptosis.” The cleavage of poly-adenosine-diphosphate-ribose polymerase (PARP) was determined following a 12-hour incubation.
Cells were exposed to gamma radiation using a calibrated137Cs source (Gammacell 3000; Nordion, Kanata, Canada).
Determination of viability and apoptosis
Cell viability was determined by quantifying the reduction of 3-4,5-dimethylthiazol-2,5-diphenyl tetrazolium bromide (MTT).10,19 Assays were carried out in triplicate, and the results are presented as mean ± standard error. The percentage of apoptotic cells was determined by counting of May-Grunwald Giemsa–stained cytospin slide preparations using an oil-immersion objective. Cells with condensed or fragmented nuclei were scored as apoptotic.20 Slides were coded prior to counting of a total of at least 1000 cells. Data are presented as the mean percentages of apoptotic cells (± standard error) in 4 randomly selected fields per slide.
Cell lysis and Western blotting
Cells (10 million) per experimental point were centrifuged at 700g for 5 minutes, washed in Hanks balanced saline, and lysed in 100 μL 20 mM, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–KOH (pH 7.4), 50 mM NaCl, 2% nonidet P40, 0.5% sodium deoxycholate, 0.2% sodium dodecyl sulfate (SDS), 1 mM EGTA (ethyleneglycoltetraacetic acid), 1 mM Na orthovanadate, 10 mM NaF, 2.5 mM Na pyrophosphate, 1 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, and 10 μL/mL protease inhibitor cocktail (Sigma). Following a 15-minute incubation on ice, samples were centrifuged for 15 minutes at 14 000g at 4°C. The supernatants were stored at −70°C.
Gel electrophoresis was carried out using 4% to 12% SDS-polyacrylamide gradient gels (Invitrogen) using the loading and run buffers provided by the manufacturer. Proteins were transferred electrophoretically to ECL Hybond membranes (Amersham Pharmacia Biotech). Membranes were blocked for 30 minutes in 20 mM Tris (tris(hydroxymethyl)aminomethane)-HCl, pH 7.4, 137 mM NaCl, 0.2% Tween 20 (TBS-Tween) containing 5% polyvinylpyrrolidone, 2% calf serum, and 0.1% sodium azide prior to addition of primary antibodies. Membranes were rinsed in 3 changes of TBS-Tween and exposed to appropriate horseradish peroxidase–conjugated secondary antibodies (DAKO, Glostrup, Denmark) in the above blocking solution minus azide. Following further rinsing, immunoreactive bands were detected by enhanced chemiluminescence (ECL; Amersham Biotech). Band intensities were quantified by laser densitometry using a GS-700 Imaging Densitometer and Quantity One software (Bio-Rad, Hemel Hempstead, United Kingdom).
The following primary antibodies were used: p85 PARP (Promega, Southampton, United Kingdom), p116 PARP (Pharmingen, Oxford, United Kingdom), β-actin (Sigma), and caspase 3 (Santa Cruz, CA).
Activation of AKT
CLL cells were incubated for 2 hours at 37°C in basal medium containing 10% FCS. They were recovered by centrifugation and resuspended in prewarmed basal medium containing supplements as indicated in figure legends. Following 10 minutes of further incubation, cells were recovered by centrifugation, washed once in Hanks saline, and lysed as described in “Cell lysis and Western blotting.” In incubations containing 20 μM LY294002, the inhibitor was added 20 minutes prior to the end of the preincubation period and was also present during exposure to serum, plasma, or HA. Following electrophoresis of protein extracts and transfer to ECL Hybond membranes, AKT activation was determined by exposure to phosphoserine473 AKT antibody (New England Biolabs, Hitchin, United Kingdom). Blots were stripped by exposure to 150 mM Tris-HCl, pH 6.7, 2% sodium dodecyl sulfate, and 100 mM 2-mercaptoethanol at 55°C, reblocked, and normalized by exposure to a pan-reactive AKT antibody (New England Biolabs).
FACScan analysis of FITC-HA uptake
CLL cells (1 million) were incubated with 3 mg/mL FITC-HA in 100 μL phosphate-buffered saline (PBS). The sample was incubated for 30 minutes at 4°C or 37°C, recovered by centrifugation, washed in PBS, and analyzed by FACScan (Becton Dickinson, Oxford, United Kingdom). Dead cells were excluded from analyses by appropriate gating on forward-angle light scatter versus side-angle light scatter dot plots as described.21
Confocal microscopy
CLL cells were labeled for 30 minutes at 37°C with FITC-HA as described in “FACScan analysis of FITC-HA uptake.” Cells were washed and visualized by using an Ultraview confocal microscope (PE Biosystems, Warrington, United Kingdom).
Results
Depletion of plasma albumin compromises its cytoprotective action
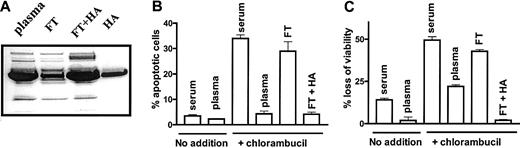
Chromatography of plasma on a Cibacron blue-Sepharose column decreased its albumin content to approximately 25% that of intact plasma, as determined by densitometric analysis of a Coomassie blue-stained SDS-polyacrylamide gel (Figure1A). A 1-hour pulse treatment of CLL cells with 80 μM chlorambucil followed by a 24-hour incubation in basal medium supplemented with 50% human serum resulted in the induction of apoptosis relative to untreated cells incubated in the same medium (Figure 1B). Replacement of serum with plasma dramatically decreased the induction of apoptosis by chlorambucil. However, the ability of plasma to protect CLL cells from chlorambucil-induced apoptosis was lost following depletion of albumin. Re-addition of HA to the depleted flow-through fraction restored its cytoprotective action (Figure 1B). An essentially similar pattern of cytoprotection by plasma, loss of protection following albumin depletion and its restoration by re-addition of HA, was observed when chlorambucil-induced loss of viability was quantified by the MTT dye reduction assay (Figure 1C). These observations suggest that albumin accounts for the antiapoptotic action of plasma.
Albumin depletion compromises the antiapoptotic action of plasma.
(A) SDS-polyacrylamide gel electrophoresis of plasma. FT indicates flow-through from Cibacron Blue-Sepharose column; and FT + HA, flow-through supplemented with 50 mg/mL HA. (B) Percentage of apoptotic cells and (C) loss of viability of untreated CLL cells or cells pretreated with 80 μM chlorambucil and incubated for 24 hours with the indicated supplements. The data are representative of 3 experiments.
Albumin depletion compromises the antiapoptotic action of plasma.
(A) SDS-polyacrylamide gel electrophoresis of plasma. FT indicates flow-through from Cibacron Blue-Sepharose column; and FT + HA, flow-through supplemented with 50 mg/mL HA. (B) Percentage of apoptotic cells and (C) loss of viability of untreated CLL cells or cells pretreated with 80 μM chlorambucil and incubated for 24 hours with the indicated supplements. The data are representative of 3 experiments.
The 116-kDa enzyme poly(ADP ribose) polymerase (p116 PARP) is cleaved by caspase 3, a key cysteine protease whose activation is a key event in the classical apoptotic pathway, resulting in the generation of an inactive fragment (p85 PARP).22 The ability of plasma, serum, or HA to protect CLL cells from chlorambucil-induced apoptosis was, therefore, studied by quantifying PARP cleavage. When CLL cells pulsed with 80 μM chlorambucil were subsequently cultured in the presence of FCS or human serum, there was a substantial increase in PARP cleavage compared with untreated controls (Figure 2A-B). In contrast, incubation of the drug-pulsed cells in medium supplemented with plasma or HA suppressed PARP cleavage. The albumin-depleted Cibacron blue–Sepharose flow-through fraction was less effective in suppressing chlorambucil-induced PARP cleavage compared with intact plasma (Figure 2A-B). Therefore, quantitation of PARP cleavage provides molecular evidence that confirms the conclusion that albumin protects CLL cells from chlorambucil-induced apoptosis.
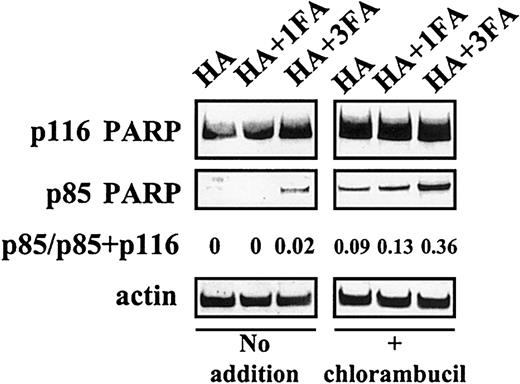
Because plasma and serum contain similar levels of albumin, we sought an explanation for the observation that the cytoprotective action of serum was substantially less than that of plasma. Heating of plasma to 56°C for 30 minutes did not abrogate its antiapoptotic actions (not shown). Therefore, the different antiapoptotic properties of FCS and of plasma are not accounted for by the routine heating of commercial FCS. However, delipidation of serum by charcoal extraction dramatically augmented its ability to block chlorambucil-induced PARP cleavage (Figure 2A-B), suggesting that a lipid component of serum masks the cytoprotective action of albumin. At a 1:1 molar ratio, fatty acids only marginally impaired the ability of HA to block chlorambucil-induced apoptosis. However, at a 3:1 ratio of fatty acid to HA, the antiapoptotic action of HA was decreased, as evidenced by a 4-fold increase in PARP cleavage in chlorambucil-pulsed CLL cells (Figure 3).
PARP cleavage in CLL cells.
(A) Generation of p85 PARP in CLL cells preincubated for 1 hour with no addition or with 80 μM chlorambucil and subsequently incubated for 12 hours with the indicated supplements. P indicates plasma; HA, human albumin; SER, human serum; dSER, delipidated human serum; FT, Cibacron blue flow-through. (B) Densitometric quantitation of PARP cleavage data of panel A. Blots were probed with an actin antibody as a control for loading. Data are representative of 4 experiments.
PARP cleavage in CLL cells.
(A) Generation of p85 PARP in CLL cells preincubated for 1 hour with no addition or with 80 μM chlorambucil and subsequently incubated for 12 hours with the indicated supplements. P indicates plasma; HA, human albumin; SER, human serum; dSER, delipidated human serum; FT, Cibacron blue flow-through. (B) Densitometric quantitation of PARP cleavage data of panel A. Blots were probed with an actin antibody as a control for loading. Data are representative of 4 experiments.
Fatty acid binding compromises the ability of albumin to block PARP cleavage.
CLL cells preincubated with no additions or with 80 μM chlorambucil were subsequently incubated with the indicated supplements. FA indicates fatty acid.
Fatty acid binding compromises the ability of albumin to block PARP cleavage.
CLL cells preincubated with no additions or with 80 μM chlorambucil were subsequently incubated with the indicated supplements. FA indicates fatty acid.
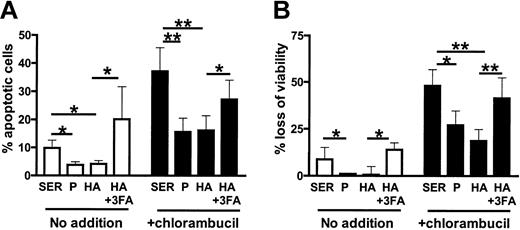
CLL cells from 9 patients cultured in either plasma or albumin were protected from spontaneous and chlorambucil-induced apoptosis relative to cells cultured in serum (Figure 4A-B). The cytoprotective action of albumin was inhibited by fatty acids (Figure 4A-B).
HA triggers rapid activation of AKT
HA activated AKT as effectively as did plasma (Figure5A). Augmentation of AKT activity by both plasma and HA was abrogated by the PI-3K inhibitor LY294002 (LY), suggesting that AKT activation was PI3-K dependent. Human serum failed to activate AKT. Serum delipidated by charcoal extraction, however, showed an increased ability to activate this pathway (Figure 5B).
Fatty acid binding compromises the anticytotoxic actions of albumin.
(A) Percentage of apoptotic cells and (B) loss of viability of CLL cells from 9 patients preincubated with no additions or with 80 μM chlorambucil and subsequently incubated for 24 hours with the indicated supplements. Mean values ± standard errors are shown. The significance of the differences between individual values was assessed using Student t test for paired samples. *.02 < P < .05; **P < .01.
Fatty acid binding compromises the anticytotoxic actions of albumin.
(A) Percentage of apoptotic cells and (B) loss of viability of CLL cells from 9 patients preincubated with no additions or with 80 μM chlorambucil and subsequently incubated for 24 hours with the indicated supplements. Mean values ± standard errors are shown. The significance of the differences between individual values was assessed using Student t test for paired samples. *.02 < P < .05; **P < .01.
Activation of AKT kinase by plasma or HA.
Activation of AKT was determined as described in “Materials and methods.” Cells from 2 different patients were used in A and B. Serum, delipidated serum (dSerum), plasma, and FT were added as 50% mixtures with basal medium except where indicated. HA was at 25 mg/mL. Data are representative of 8 experiments.
Activation of AKT kinase by plasma or HA.
Activation of AKT was determined as described in “Materials and methods.” Cells from 2 different patients were used in A and B. Serum, delipidated serum (dSerum), plasma, and FT were added as 50% mixtures with basal medium except where indicated. HA was at 25 mg/mL. Data are representative of 8 experiments.
The strong activation of AKT by HA was dose dependently diminished by complexing with fatty acids (Figure 5B). Although plasma activated AKT in a dose-dependent fashion, the albumin-depleted flow-through fraction from a Cibacron blue-Sepharose column failed to do so (Figure 5B). Taken together, the data of Figure 5 suggest that the ability of plasma to activate AKT kinase of CLL cells is dependent on albumin. Furthermore, the inability of serum to activate AKT is attributable to charcoal-extractable material, because its removal unmasked a latent ability to trigger this signaling pathway.
Cytoprotective action of albumin is not attributable to a contaminating biomolecule
Both natural and recombinant human albumin (rHA) preparations activated AKT to a comparable level (Figure6A). Although intact plasma activated AKT, plasma from the analbuminemic subject failed to do so (Figure 6B). Figure 6C confirms that the albumin band present in intact plasma was completely absent in the analbuminemic plasma.
Actions of recombinant HA and analbuminemic plasma on AKT.
(A-B) Western blot analysis of AKT activation. SER indicates serum; HA, human albumin; rHA, recombinant human albumin; NP, intact donor plasma; AP, analbuminemic plasma. (C) Polyacrylamide gel electrophoresis of NP and AP. Data representative of 3 experiments.
Actions of recombinant HA and analbuminemic plasma on AKT.
(A-B) Western blot analysis of AKT activation. SER indicates serum; HA, human albumin; rHA, recombinant human albumin; NP, intact donor plasma; AP, analbuminemic plasma. (C) Polyacrylamide gel electrophoresis of NP and AP. Data representative of 3 experiments.
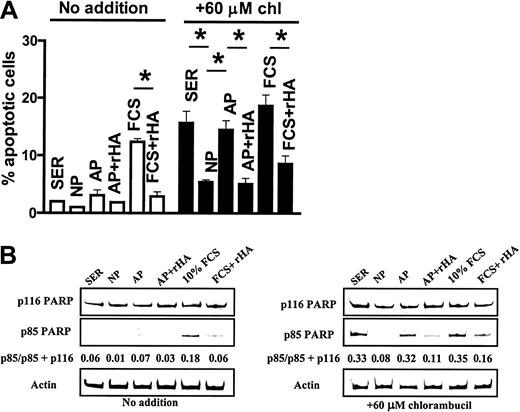
CLL cells cultured in 50% human serum, intact plasma, or analbuminemic plasma for 24 hours without prior exposure to chlorambucil showed low levels of spontaneous apoptosis (Figure7A). However, cells cultured in 10% FCS showed a higher level of spontaneous apoptosis that was significantly decreased by the addition of rHA. Chlorambucil pretreatment resulted in augmented apoptosis when the cells were cultured in human serum. This drug-induced cell death was diminished by culture in intact plasma, but not in analbuminemic plasma (Figure 7A). Addition of rHA to the analbuminemic plasma resulted in a level of cytoprotection comparable to that seen in intact plasma. The high level of apoptosis seen when the chlorambucil-pulsed cells were incubated in 10% FCS was also decreased by addition of rHA (Figure 7A).
Actions of recombinant HA and analbuminemic plasma on CLL apoptosis.
Apoptosis was quantified by morphologic criteria (A, *P < .01) or by Western blot analysis of PARP cleavage (B). Human serum or plasma was added at 50% (vol/vol). FCS was at 10% (vol/vol). chl indicates chlorambucil.
Actions of recombinant HA and analbuminemic plasma on CLL apoptosis.
Apoptosis was quantified by morphologic criteria (A, *P < .01) or by Western blot analysis of PARP cleavage (B). Human serum or plasma was added at 50% (vol/vol). FCS was at 10% (vol/vol). chl indicates chlorambucil.
Western blot analysis of PARP cleavage mirrored the results obtained by morphologic analysis. Spontaneous PARP cleavage detected in untreated cells cultured in 10% FCS was suppressed by the addition of recombinant albumin (Figure 7B). The chlorambucil-induced PARP cleavage evident in cells cultured in human serum was diminished by culture in intact but not analbuminemic plasma (Figure 7B). Addition of rHA to analbuminemic plasma increased its ability to block drug-induced PARP cleavage. rHA also protected against chlorambucil-induced PARP cleavage in cultures containing 10% fetal calf serum (Figure 7B). Therefore, the experiments shown in Figures 6 and 7 suggest that the signaling and antiapoptotic properties of albumin are functions of the protein itself and not of an associated biomolecule.
HA protects CLL cells from radiation-induced apoptosis
Exposure of CLL cells to gamma radiation followed by 24 hours of incubation in FCS-supplemented medium resulted in an apoptotic response that was partially blocked by incubation with rHA (Figure8A). The ability of rHA to retard radiation-induced apoptosis was also shown by analysis of PARP cleavage. A representative example is shown in Figure 8B. Similar results were obtained using 2 additional CLL isolates (not shown).
Albumin protects CLL cells from radiation-induced apoptosis.
(A) CLL cells from 3 patients were exposed to the indicated doses of gamma radiation. They were then incubated in RPMI medium supplemented with FCS or rHA. The percentage of apoptotic cells was determined following 24-hour incubation. The mean values ± standard errors are shown. (B) Quantitation of PARP cleavage in irradiated CLL cells incubated for 12 hours with FCS or rHA.
Albumin protects CLL cells from radiation-induced apoptosis.
(A) CLL cells from 3 patients were exposed to the indicated doses of gamma radiation. They were then incubated in RPMI medium supplemented with FCS or rHA. The percentage of apoptotic cells was determined following 24-hour incubation. The mean values ± standard errors are shown. (B) Quantitation of PARP cleavage in irradiated CLL cells incubated for 12 hours with FCS or rHA.
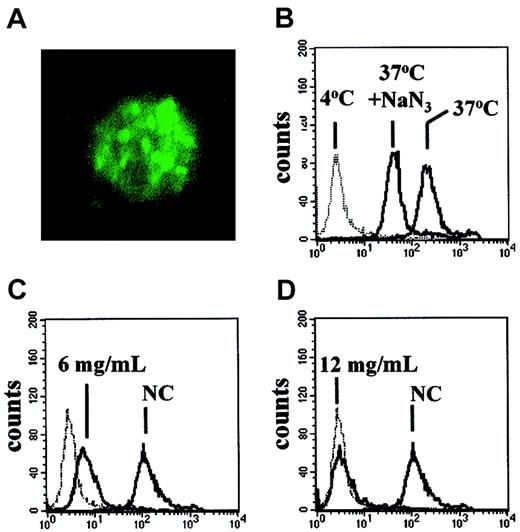
CLL cells bind and internalize HA
The observations that albumin could activate the AKT signaling pathway and modulate spontaneous and chlorambucil-induced apoptosis suggested that this protein may bind to CLL cells. Analysis by confocal microscopy showed that incubation of CLL cells with FITC-labeled albumin resulted in internalization of the labeled conjugate into structures resembling endocytic vesicles (Figure9A).
Uptake of FITC-rHA by CLL cells.
(A) Confocal micrograph of a CLL cell incubated with FITC-rHA for 30 minutes prior to fixation. Original magnification × 2500. (B-D) FACScan analysis of CLL cells exposed to FITC-rHA. (B) Effect of 30-minute preincubation with 1.5 mM NaN3 on uptake of FITC-rHA. (C-D) Competitive effect of unlabeled rHA on the uptake of FITC-rHA. Concentrations of added unlabeled rHA are indicated in individual panels. NC indicates no competing unlabeled rHA. The dotted lines (B-D) denote the fluorescence of CLL cells incubated with FITC-rHA at 4°C. All other incubations were at 37°C.
Uptake of FITC-rHA by CLL cells.
(A) Confocal micrograph of a CLL cell incubated with FITC-rHA for 30 minutes prior to fixation. Original magnification × 2500. (B-D) FACScan analysis of CLL cells exposed to FITC-rHA. (B) Effect of 30-minute preincubation with 1.5 mM NaN3 on uptake of FITC-rHA. (C-D) Competitive effect of unlabeled rHA on the uptake of FITC-rHA. Concentrations of added unlabeled rHA are indicated in individual panels. NC indicates no competing unlabeled rHA. The dotted lines (B-D) denote the fluorescence of CLL cells incubated with FITC-rHA at 4°C. All other incubations were at 37°C.
The uptake of FITC-labeled recombinant albumin was also quantified by FACScan analysis. Incubation of the cells with FITC-rHA at 4°C resulted in weak staining. An approximately 200-fold increase in cell-associated fluorescence was detected when the incubation was carried out at 37°C (Figure 9B). A 30-minute preincubation with azide decreased the cell-associated fluorescence. Taken together with the microscopic evidence (Figure 9A), our results suggest that CLL cells bind albumin weakly at 4°C, but that a temperature- and energy-dependent uptake results in accumulation of this protein within the cells. A decrease in uptake of FITC-rHA was seen when a 2-fold excess of unlabeled rHA was added to the incubation (Figure 9C). Uptake was almost completely eliminated by a 4-fold excess of rHA (Figure 9D). These competition experiments, together with the ability of HA to activate AKT, suggest that uptake of FITC-rHA by CLL cells was by a receptor-mediated mechanism. Similar results were obtained when FITC-HA was used in the FACScan study in place of FITC-rHA (not shown).
PI3-K inhibition augments sensitivity to chlorambucil
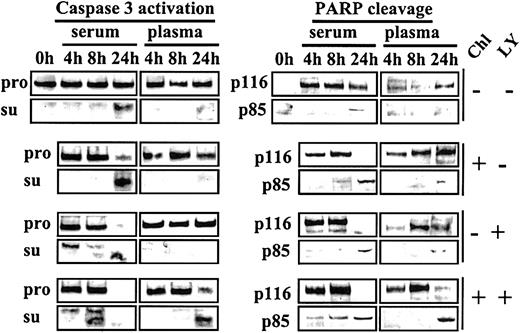
Because activation of AKT by plasma or HA is sensitive to the PI3-K inhibitor LY, we carried out experiments to determine whether LY could reverse the blockade of chlorambucil-induced apoptosis in plasma cultures. CLL cells were either left untreated or exposed to a 1-hour pulse of 60 μM chlorambucil. The drug was then removed, and the cells were cultured in media supplemented with either serum or plasma. Commitment to apoptosis was monitored by Western blot analysis for the processing of caspase 3 to its active subunits,23 as well as for the cleavage of PARP (Figure10).
Acceleration of chlorambucil-induced caspase 3 activation and PARP cleavage by LY.
CLL cells cultured in basal medium containing 10% FCS were incubated for 1 hour at 37°C in the absence or presence of 60 μM chlorambucil. The cells were recovered by centrifugation, washed once in Hanks saline, and resuspended in basal medium containing either 50% serum or 50% plasma. Each culture was divided into 2 aliquots, one of which received no further additions, and 20 μM LY was added to the other. Cells were processed for Western blot analysis at the indicated times. Blots were probed with caspase 3, p85 PARP, and p116 PARP antibodies. Pro indicates caspase 3 proenzyme; and su, caspase 3 subunits. The 0-hour sample expressed no detectable p116 PARP. This finding is attributable to the storage of these cells at 4°C prior to commencement of the experiment.
Acceleration of chlorambucil-induced caspase 3 activation and PARP cleavage by LY.
CLL cells cultured in basal medium containing 10% FCS were incubated for 1 hour at 37°C in the absence or presence of 60 μM chlorambucil. The cells were recovered by centrifugation, washed once in Hanks saline, and resuspended in basal medium containing either 50% serum or 50% plasma. Each culture was divided into 2 aliquots, one of which received no further additions, and 20 μM LY was added to the other. Cells were processed for Western blot analysis at the indicated times. Blots were probed with caspase 3, p85 PARP, and p116 PARP antibodies. Pro indicates caspase 3 proenzyme; and su, caspase 3 subunits. The 0-hour sample expressed no detectable p116 PARP. This finding is attributable to the storage of these cells at 4°C prior to commencement of the experiment.
In serum-supplemented cultures, chlorambucil induced nearly complete processing of both the caspase 3 proenzyme and of PARP by 24 hours of culture. Both these events were dramatically suppressed in plasma cultures (Figure 10). LY alone also induced complete cleavage of caspase 3 and PARP following 24-hour culture with serum, suggesting that the low level of active AKT in these cultures (Figure 5A) was essential for the maintenance of viability. LY was unable to induce these cleavage reactions in cells cultured in plasma (Figure 10).
When chlorambucil-pulsed cells were cultured with serum plus LY, cleavage of caspase 3 and PARP was accelerated relative to comparable serum cultures in the absence of LY. Strikingly, addition of LY to plasma cultures of chlorambucil-pulsed cells reversed the blockade of caspase 3 processing and PARP cleavage that was observed when chlorambucil-treated cells were incubated in plasma cultures in the absence of LY (Figure 10). These observations suggest that activation of the PI3-K/AKT signaling pathway by plasma contributes to the induction of cytotoxic drug resistance and that inhibition of this pathway by LY restores the apoptotic response to DNA damage induction by chlorambucil. Similar results were obtained in studies using 6 additional CLL isolates (not shown).
Discussion
We have used viability assessment by the MTT assay, morphologic quantitation of apoptosis, and Western blot analysis of PARP cleavage and AKT activation to identify the plasma component responsible for the blockade of apoptosis in CLL cells exposed to chlorambucil. Taken together, the results suggest that stimulation of PI3-K/AKT signaling by plasma albumin inhibits cell killing by the cytotoxic drug. First, plasma chromatographically depleted of albumin or analbuminemic plasma were impaired, relative to intact plasma, in their ability to block chlorambucil-induced apoptosis. In each case, the protective action was restored by re-addition of albumin. Second, addition of albumin alone to chlorambucil-pulsed CLL cultures was cytoprotective. Third, confocal microscopic and FACScan analyses showed that albumin was bound and internalized by CLL cells. Fourth, albumin clearly activated the antiapoptotic protein kinase AKT. Although intact plasma also activated AKT, albumin-depleted plasma or analbuminemic plasma were impaired in AKT activation.
The pulse procedure used in the present experiments suggests that the ability of albumin to block chlorambucil-induced apoptosis cannot be explained by sequestration of the drug by extracellular proteins. This interpretation is strongly reinforced by the observation that albumin also blocked gamma radiation–induced cell death.
In contrast with the ability of albumin to block chlorambucil- or radiation-induced cell killing, preliminary experiments (not shown) suggest that this protein failed to protect CLL cells from apoptosis induction by the nucleoside analog 2-chlorodeoxyadenosine. Whereas both chlorambucil and radiation initiate cell death pathways consequent to damaging DNA, 2-chlorodeoxyadenosine may induce CLL cell killing by directly interfering with mitochondrial integrity, resulting in the release of proapoptotic proteins, including cytochrome c and apoptosis-inducing factor.24 It is, therefore, plausible that the failure of albumin to protect against death induced by 2-chlorodeoxyadenosine may be accounted for by the different mechanisms of apoptosis induction by DNA-damaging agents versus nucleoside analogs.
Because albumin binds numerous biomolecules, it was important to establish that the signaling and antiapoptotic actions were attributable to the protein itself and not to a bound species. We showed that recombinant HA expressed in yeast shared the ability of purified native albumin to block cytotoxic killing, to be internalized by CLL cells, and to activate AKT, suggesting that the anticytotoxic properties were functions of albumin per se.
Because serum and plasma contain very similar concentrations of albumin, we sought to explain the apparent paradox that plasma was able to activate AKT and to protect CLL cells from apoptosis more effectively than serum. Charcoal extraction of serum simultaneously increased its ability to activate AKT and to protect CLL cells from chlorambucil-induced apoptosis, suggesting that an extractable component present in serum at higher concentration than in plasma was responsible for masking antiapoptotic signaling by serum albumin. Although charcoal extraction decreased the free fatty acid content of serum by approximately 10-fold, we cannot formally eliminate the possibility that an extractable nonlipid molecule was responsible for impairing the cytoprotective potential of serum albumin. However, the ability of fatty acids to abrogate the AKT-activating and antiapoptotic functions of albumin suggests that lipids may potentially mask the cytoprotective actions of albumin. We are currently attempting to identify lipids present in higher concentration in serum than in plasma and that may account for their different antiapoptotic actions.
Indirect inhibition of AKT by PI-3K antagonists sensitizes malignant cells to cytotoxic drug-induced apoptosis, implying an antiapoptotic role for AKT.25,26 Plasma, delipidated serum, or albumin activated AKT and also protected CLL cells from cytotoxic killing. In contrast, albumin-depleted plasma, analbuminemic plasma, or serum or albumin complexed with fatty acids were relatively impaired in their ability to activate AKT and to confer protection, consistent with a role for AKT activation in mediating antiapoptotic signaling by albumin. The ability of the PI3-K inhibitor LY to impair AKT activation by albumin and also to reverse the cytoprotective action of plasma further implies that activation of the PI3-K/AKT pathway by albumin plays a key role in mediating the ability of plasma to protect CLL cells from killing by chlorambucil. In contrast, Moran et al21 reported that albumin blocked basal apoptosis of CLL cells by sequestering oxidants from the culture medium. The ability of albumin to protect renal tubular cells and macrophages from spontaneous apoptosis has also been attributed to oxidant scavenging.27 It is, therefore, likely that albumin inhibits apoptosis by multiple mechanisms. However, the effect of albumin on drug-induced apoptosis was not evaluated in either of these studies.21 27
Depending on the cellular context, AKT blocks apoptosis by diverse mechanisms that involve phosphorylation and inactivation of proapoptotic proteins, including BAD12 and the forkhead transcription factor FKHRL1.13 However, CLL cells do not express BAD.28 Although FKHRL1 is thought to induce apoptosis via triggering transcription of the apoptosis-inducing Fas ligand,13 induction of CLL cell apoptosis by chlorambucil does not involve the Fas/Fas ligand system.29 Therefore, the mechanism by which AKT activation blocks apoptosis in CLL cells is unclear. Ongoing studies are directed at pinpointing the specific step in the complex pathway of apoptosis induction that is blocked by AKT activation.
To the best of our knowledge, we have presented the first evidence that albumin activates the AKT pathway and also promotes resistance of CLL cells to DNA damage–induced apoptosis in vitro. Because the apoptotic blockade could be achieved by approximately half the concentration of albumin present in plasma in vivo, it is plausible that this mechanism plays a significant role in blocking the response of patients with CLL to cytotoxic therapy. It is currently unclear whether cytoprotection by albumin is relevant to cell types other than CLL. We failed, however, to observe a significant protective action of albumin against etoposide-induced killing of human vascular endothelial cells or the chlorambucil-induced apoptosis of intact T or B lymphocytes (S.Y.L., D.T.J., unpublished observation, May 2002). Although it is surprising that a protein as abundant and ubiquitous as albumin may possess signaling and antiapoptotic functions, receptors for this protein have previously been described. These include the large (> 400 000 kDa) proteins cubilin and megalin, which are involved in the reabsorption of albumin in kidney proximal tubules,30 and a 60-kDa sialoglycoprotein gp60/albondin, which mediates transcytosis of albumin across the vascular endothelium.31-33 Albumin binding has also been shown to initiate signal transduction events, including the activation of the transcription factor nuclear factor κB (NF-κB) in proximal tubule-derived cell lines34 and the gp60/albondin-mediated activation of the pp60 cSrc protein kinase in endothelial cells.33 A link between albumin receptors and cell signaling is also suggested by the dependence of albumin endocytosis on an active PI3-K pathway.35 Characterization of the receptor and signal transduction mechanism through which albumin mediates antiapoptotic signaling will extend understanding of the biology of CLL and may contribute to the design of novel therapeutic protocols.
We thank David Harry, Mark Lowdell, and Paul Travers for advice.
Prepublished online as Blood First Edition Paper, December 12, 2002; DOI 10.1182/blood-2002-07-2143.
Supported by the Leukaemia Research Fund, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
R. Gitendra Wickremasinghe, Department of Haematology, Royal Free and University College Medical School, Rowland Hill St, London NW3 2PF, United Kingdom; e-mail:r.wickremasinghe@rfc.ucl.ac.uk.