The promyelocytic leukemia gene, PML, is a growth and transformation suppressor. An additional role forPML as a regulator of major histocompatibility complex (MHC) class I antigen presentation has been proposed in a murine model, which would account for evasion from host immunity of tumors bearing malfunctioning PML, such as acute promyelocytic leukemia. Here we investigated a possible role ofPML for the control MHC class I expression in human cells. PML function was perturbed in human cell lines either byPML/RARα transfection or by PML- specific RNA interference. Impairment of wild-type PML function was proved by a microspeckled disassembly of nuclear bodies (NBs), where the protein is normally localized, or by their complete disappearance. However, no MHC class I down-regulation was observed in both instances. We next constructed a PML mutant, PML mut ex3,that is a human homolog of the murine PML mutant, truncated in exon 3, that was shown to down-regulate murine MHC class I. PML mut ex3 transfected in human cell lines exerted a dominant-negative effect since no PML molecules were detected in NBs but, instead, in perinuclear and cytoplasmic larger dotlike structures. Nevertheless, no down-regulation of MHC class I expression was evident. Moreover, neither transfection with PML mut ex3 nor PML-specific RNA interference affected the ability of γ-interferon to up-regulate MHC class I expression. We conclude that, in human cell lines, PML is not involved directly in the regulation of MHC class I expression.
Introduction
The promyelocytic leukemia (PML) gene has been identified in acute promyelocytic leukemia (APL) patients, in whom it is fused to the retinoic acid receptor α (RARα) gene as a result of the t(15;17) chromosomal translocation1 (reviewed in Salomoni and Pandolfi2). In normal cells, the PML gene comprises 9 exons that generate at least 7 spliced transcripts differing at their 3′ ends (PML I-VII).3 All PML isoforms, with the sole exception of the cytoplasmic PML isoform VII, are localized mainly within discrete nuclear structures termed nuclear bodies (NBs) (Jensen et al,3 and references cited therein). When PML is aberrant, such as in APL, NBs are disrupted.4-6 Overexpression of PML induces either growth arrest or apoptosis depending on cell type, level of expression, and PML isoforms7 (reviewed in Salomoni and Pandolfi2 and Pearson and Pelicci8). Conversely, PML gene inactivation results in increased cell proliferation and tumorigenesis,9 and expression of PML-RARα that exerts a dominant-negative action on PML10,11 induces leukemia with APL features.12 The tumor suppressor function of PML is regulated by its interaction with cell cycle- and apoptosis-regulatory proteins.13-17
A novel mechanism mediated by PML has been proposed recently, based on the observation that murine PML (mPML) regulates the expression of major histocompatibility complex (MHC) class I molecules.18 An mPML mutant was isolated that produced a truncated form of mPML protein in an MHC class I–negative tumor cell line. This mutant acted as a dominant-negative regulator and was considered responsible for an antigen-processing defect that caused a decreased MHC class I expression.18 In addition, a mPML isoform, called F12 (exons 1-4, 6, and 7 and part of exon 9 of themPML gene), was shown to up-regulate MHC class I expression in an MHC class I–negative tumor cell line and in untransformed murine fibroblasts.18 This mechanism would allow tumors bearing malfunctioning PML to escape the host immune surveillance. Thus, APL cells that harbor mutated PML should display a decreased expression of MHC class I molecules. However, expression of HLA A-B-C has been found in APLs19 at the same extent as in other acute myeloid leukemias that do not harbor PML alterations.20
Following these controversial results, we studied the relationship between PML and MHC class I in human cell lines. To this end, we affected the function of PML in 2 ways, that is, by inducing expression of the PML/RARα gene that acts as a dominant-negative regulator of PML, or by gene silencing through RNA interference.21 We also transfected, in human cells, a human homolog of the mPML mutant that down-regulates MHC class I.18 In no case was down-regulated MHC class I expression observed.
Alternatively, we asked whether PML could be involved in the up-regulation of MHC class I above its constitutive level. To this end, we studied whether an impaired or aberrant function of PML (induced by RNA interference or by the PML mutant) would affect the ability of γ-interferon to up-regulate the expression of MHC class I. No effect was observed on the increased level of MHC class I.
We conclude that, in human cell lines, PML is not directly involved in the regulation of MHC class I expression.
Materials and methods
PML mutants and expression vectors
Green fluorescent protein (GFP)–tagged PML(PML3/PMLIV/TRIM19lambda, Genbank accession no.AF230411)3,22,23 cDNA was subcloned either in the retroviral vector pBABE24 or in the plasmid pcDNA3.1 (Invitrogen, Milan, Italy). GFP-taggedPML/RARα25 cDNA was subcloned in the retroviral vector pBABE.
A human homolog of the murine F12dG PML mutant18 was constructed. The murine F12dGmutant has a single base deletion in exon 3 (a deletion of a G at position 962 of the murine PML-F12 sequence)18that corresponds to position 875 relative to the first nucleotide of the start codon. F12 is an mPML isoform that comprises exons 1 to 4, 6, and 7 and part of exon 9.18 The point deletion in exon 3 causes a frameshift and early translational termination of the PML protein in exon 3. Because of the high homology between murine and human PML exons, the G to be deleted was easily identified in the human PML cDNA sequence and was found at position 958 relative to the first nucleotide of the humanPML start codon. By site-directed mutagenesis, this G was deleted in the GFP-PML construct within the pcDNA3.1 vector. The QuickChange Site-Directed Mutagenesis Kit from Stratagene (La Jolla, CA) was used, and the following oligonucleotide primers containing the desired mutation were synthesized by Invitrogen: 5′-GGCTGGGCCGCCTGATGCTGTGCTGCAGCGC-3′ and its complementary 5′-GCGCTGCAGCACAGCATCAGGCGGCCCAGCC-3′. The new mutated construct was checked by direct nucleotide sequencing using the Dye Terminator Cycle Sequencing Kit (ABI PRISM; Perkin-Elmer Applied Biosystems, Norwalk, CT). The following sequencing primers were used: the upstream forward oligonucleotides 5′-GCGCTCCTTGACAGCAGC-3′ and 5′-TGCAGGAGCAGGATAGATC-3′, and the downstream reverse oligonucleotides 5′-CTGGGCTGTCGTTGTATTGG-3′ and 5′-GCGCACCTTGAACTCGTCG-3′. Sequences were resolved and analyzed using the ABI 373A Sequence Apparatus (Perkin-Elmer Applied Biosystems).
Short-interfering (si)–RNA preparation for RNA interference
The mRNA sequence to be targeted by 21-nucleotide siRNA duplexes was selected on exon 2 of PML and corresponded to the coding region 398 to 418 relative to the first nucleotide of the start codon: 5′-GAGUCGGCCGACUUCUGGU-3′. This region is transcribed in all PML isoforms. The 2 21-nucleotide sense and antisense strands containing symmetric 2 nucleotide 3′ overhangs of 2 (2′-deoxy) thymidines (5′-GAGUCGGCCGACUUCUGGUdTdT-3′ and 5′-ACCAGAAGUCGGCCGACUCdTdT-3′) were synthesized by Pharmacon Research (Dharmacon Research, Lafayette, CO). The siRNA sequence was submitted to a BLAST search against the human genome sequence to ensure that only thePML gene of the human genome was targeted. Annealing of the 2 strands was performed by incubating 20 μM single strands in annealing buffer (100 mM potassium acetate, 30 mM HEPES-KOH [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] at pH 7.4, and 2 mM magnesium acetate) for one minute at 90°C, followed by one hour at 37°C.21
Cell cultures and transfections
Cell lines used were as follows: HeLa and HEK293 from the American Type Culture Collection (ATCC, Rockville, MD), and the neuroblastoma cell lines SK-N-BE2(c) and ACN kindly provided by Dr V. Pistoia (Genoa, Italy). Cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM glutamine, and antibiotics.
Transfection of cells with different PML isoforms, cloned in either pBABE or in pcDNA3.1 vectors, was performed with Lipofectamine 2000 (Invitrogen) in 24-well plates. DNA (1 μg) was dispensed in each well. Transfection of siRNA duplexes for RNA interference was carried out with Oligofectamine (Invitrogen), following the guidelines of the manufacturer for 24-well plates and using 0.84 μg siRNA duplex per well. Oligofectamine was used in cotransfection experiments, when both PML isoforms and siRNA duplexes were transfected. No reduction in transfection efficiency for PML was observed in comparison with transfections carried out with Lipofectamine 2000.
Flow cytometric immunofluorescence
Cells were incubated with mouse monoclonal antibodies (mAbs) to MHC class I followed by phycoerythrin (PE)–labeled goat anti–mouse IgG mAb (GAM, Southern Biotechnology Associates, Birmingham, AL). Anti–MHC class I mAbs were W6.32 (γ 2a, ATCC), 3A3 (μ), and A2 (γ1). The last 2 mAbs were produced in our laboratory and are specific for a monomorphic epitope of MHC class I. Controls were provided by cells incubated with isotype-matched mAbs of irrelevant specificity followed by PE-labeled GAM mAb. Samples were analyzed using a FacsCalibur flow cytometer and CellQuest software (Becton Dickinson, Milan, Italy).
Fluorescence microscopy
Control and transfected cells grown on glass coverslips in 24-well plates were processed for immunostaining with the following primary antibodies: PG-M3, a mouse mAb that recognizes all PML isoforms since its epitope lies in the amino terminal region of human PML (residues 37-51)26 and SP100 (Santa Cruz Biotechnology, Heidelberg, Germany). Fluorochrome-conjugated secondary antibodies included GAM–Alexa 594 (Molecular Probes, Eugene, OR), donkey antimouse fluorescein isothiocyanate (FITC), and donkey antigoat cytochrome 3 (Cy3) (Jackson ImmunoResearch Laboratories, West Grove, PA).
Cells were fixed with 3.7% paraformaldehyde for 10 minutes at room temperature followed by 0.1% Triton X-100 for 10 minutes at room temperature (for PML), or with methanol for 3 minutes at −20°C followed by rehydration in phosphate-buffered saline (PBS) for 20 minutes at room temperature (for SP100). The last fixation procedure was used for PML as well, in 2-color staining experiments, as no significant difference was observed in the PML staining pattern between paraformaldehyde and methanol fixation. After washing with PBS, primary antibodies were added for 30 minutes at room temperature, followed by fluorochrome-conjugated secondary antibodies for 30 minutes at room temperature. Primary antibodies were omitted in controls. Nuclear staining was revealed by 4′,6-diamidino-2-phenylindole (DAPI, 1:200; Sigma Chemical, St Louis, MO). Coverslips were mounted using Mowiol 488. Analyses were performed with an epifluorescence inverted microscope (Olympus Optical, Hamburg, Germany), and images were acquired using a chilled Hamamatsu CCD black-and-white camera and processed with IPLab (Scanalytics, Fairfax, VA) and Adobe Photoshop software (San Jose, CA).
Results
MHC class I expression is not affected by a dominant-negative mutant of PML
We studied whether an impairment of PML function would affect surface expression of MHC class I molecules. A first approach to inhibit PML was to transfect cells with the APL-associated fusion genePML/RARα. The chimeric PML/RARα protein acts as a dominant-negative oncogene by delocalizing PML molecules to aberrant microspeckled nuclear structures.11 In Figure1, results with HeLa cells are shown. Controls were provided by vector alone or PML-transfected cells. As shown by flow cytometric measurements of GFP fluorescence, 39% of the cells were transfected with PML and 34% withPML/RARα MHC class I expression of PML/RARα-expressing cells had a mean fluorescence value almost identical to that of untransfected and of PML-transfected cells. Detection of MHC class I molecules with antibodies different from W6.32, such as the 3A3 and A2 mAb, yielded the same results (not shown). Time-course measurements of MHC class I expression were also carried out, from 16 hours after transfection (when GFP reached a significant level of detection) up to the seventh day, and provided identical results (not shown). The microscopic examination of cells grown on glass slides and stained with anti-PML mAb demonstrated the dominant action of PML/RARα that disassembled PML molecules in NBs leading to a diffuse microspeckled pattern. At variance, PML-transfected cells displayed classical NBs that were larger than normal NBs of control cells (eg, compare the GFP-positive cell with the GFP-negative cell, in second row of Figure 1). Experiments carried out with HEK293 cells also indicated that the surface amount of MHC class I molecules is not affected by PML/RARα expression (not shown).
MHC class I expression is not affected by PML/RARα, a dominant-negative mutant of PML.
(A) HeLa cells were transfected with the retroviral vector pBABE alone (top row), with pBABE containing GFP-PML (middle row), or GFP-PML/RARα (bottom row), using the Lipofectamine 2000 procedure. At 3 days after transfection, cells were processed for fluorescence microscopy and flow cytometry. Cells growing on glass coverslips were immunostained with anti-PML followed by GAM-Alexa 594 antibodies (red fluorescence, middle column) and counterstained with DAPI (blue fluorescence, right column). GFP green fluorescence is shown in the left column. Cells processed for flow cytometry were immunostained with anti–MHC class I mAb (W6.32) followed by GAM-PE. (B) Bivariate dot plots are shown: x-axis, green fluorescence; y-axis, red fluorescence. Percentage of GFP-positive cells (ie, of transfected cells) is reported. The negative control for MHC class I expression, obtained using isotype-matched antibodies followed by GAM-PE, is shown in gray.
MHC class I expression is not affected by PML/RARα, a dominant-negative mutant of PML.
(A) HeLa cells were transfected with the retroviral vector pBABE alone (top row), with pBABE containing GFP-PML (middle row), or GFP-PML/RARα (bottom row), using the Lipofectamine 2000 procedure. At 3 days after transfection, cells were processed for fluorescence microscopy and flow cytometry. Cells growing on glass coverslips were immunostained with anti-PML followed by GAM-Alexa 594 antibodies (red fluorescence, middle column) and counterstained with DAPI (blue fluorescence, right column). GFP green fluorescence is shown in the left column. Cells processed for flow cytometry were immunostained with anti–MHC class I mAb (W6.32) followed by GAM-PE. (B) Bivariate dot plots are shown: x-axis, green fluorescence; y-axis, red fluorescence. Percentage of GFP-positive cells (ie, of transfected cells) is reported. The negative control for MHC class I expression, obtained using isotype-matched antibodies followed by GAM-PE, is shown in gray.
MHC class I expression is not affected by PML-specific RNA interference
RNA interference is a process of sequence-specific gene silencing initiated by double-stranded RNA that is homologous to the silenced gene.27 The mediators of sequence-specific messenger RNA degradation are 21-nucleotide short-interfering RNAs (siRNA) generated by ribonuclease III cleavage from longer double-stranded (ds) RNAs.28 Based on this physiologic mechanism, an in vitro gene silencing technique that introduces duplexes of 21-nucleotide siRNA homologous to the down-regulated gene has been developed for mammalian cells.21 We used this technique for silencing PML.
In Figure 2, the effects of PML-specific RNA interference on surface MHC class I expression are shown, both in control cells and in GFP-PML–transfected HeLa cells. RNA interference was effective.
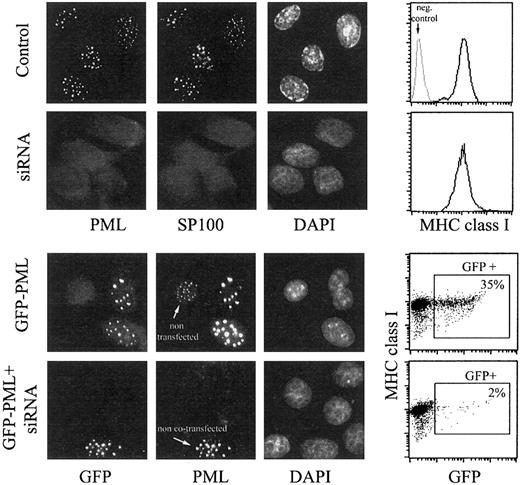
MHC class I expression is not affected by PML-specific RNA interference.
HeLa cells were transfected with PML-specific siRNA duplex (second row), with GFP-PML (third row), or cotransfected with siRNA duplex and GFP-PML (fourth row) using the Oligofectamine procedure. Control cells in the first row were treated with Oligofectamine alone. At 48 hours after transfection, cells were processed for fluorescence microscopy and flow cytometry. Cells growing on glass coverslips were immunostained with anti-PML mAb followed by donkey antimouse FITC and anti-SP100 mAb followed by donkey antigoat Cy3 (control and siRNA duplex-treated samples, first 2 rows), or with anti-PML mAb only, followed by GAM-Alexa 594 (samples transfected with GFP-PML, last 2 rows). The green fluorescence is shown in the first column, the red fluorescence in the second, and the blue fluorescence from DAPI in the third. In the fourth row, a microscopic field comprising one of the few GFP-positive cells was chosen, which presumably was not transfected with siRNA but only with GFP-PML, to emphasize the disappearance of PML-containing NBs induced by RNA interference. Cells processed for flow cytometry were immunostained with anti–MHC class I mAb (W6.32) followed by GAM-PE. Data are reported in the fourth column as histograms of MHC class I expression (top 2 rows, negative control provided by isotype-matched mAb indicated in gray) and bivariate dot plots (bottom 2 rows) of GFP fluorescence (x-axis) and MHC class I expression (y-axis).
MHC class I expression is not affected by PML-specific RNA interference.
HeLa cells were transfected with PML-specific siRNA duplex (second row), with GFP-PML (third row), or cotransfected with siRNA duplex and GFP-PML (fourth row) using the Oligofectamine procedure. Control cells in the first row were treated with Oligofectamine alone. At 48 hours after transfection, cells were processed for fluorescence microscopy and flow cytometry. Cells growing on glass coverslips were immunostained with anti-PML mAb followed by donkey antimouse FITC and anti-SP100 mAb followed by donkey antigoat Cy3 (control and siRNA duplex-treated samples, first 2 rows), or with anti-PML mAb only, followed by GAM-Alexa 594 (samples transfected with GFP-PML, last 2 rows). The green fluorescence is shown in the first column, the red fluorescence in the second, and the blue fluorescence from DAPI in the third. In the fourth row, a microscopic field comprising one of the few GFP-positive cells was chosen, which presumably was not transfected with siRNA but only with GFP-PML, to emphasize the disappearance of PML-containing NBs induced by RNA interference. Cells processed for flow cytometry were immunostained with anti–MHC class I mAb (W6.32) followed by GAM-PE. Data are reported in the fourth column as histograms of MHC class I expression (top 2 rows, negative control provided by isotype-matched mAb indicated in gray) and bivariate dot plots (bottom 2 rows) of GFP fluorescence (x-axis) and MHC class I expression (y-axis).
Microscopic analyses of the distribution of PML and SP100, which colocalizes with PML in NBs, demonstrated the complete disappearance of NBs in 97% of the cells. To support further the efficiency of PML silencing by siRNA, we performed experiments in whichGFP-PML and siRNA duplexes were cotransfected. In these experimental conditions the percentage of GFP-positive cells decreased from 35% to 2%, and the negative cells (98%) did not display a reduced GFP fluorescence but, instead, a completely undetectable GFP. This indicates the complete absence of any exogenously expressed PML protein in these cells and, thus, the absence of endogenous PML is proved further. However, despite the absence of PML expression, surface expression of MHC class I molecules was unchanged, as observed from 48 hours after transfection (when the effect of RNA interference became significant; Figure 2) up to the fifth day (not shown).
MHC class I expression is not altered by PML mut ex3
In the work mentioned previously,18 a specific mPML mutant termed “F12dG” was shown to induce an antigen-processing defect by decreasing MHC class I expression in murine cells. This mutant has a point deletion in exon 3 of the mPML gene that causes a frameshift and translational termination of PML at exon 3. It has been proposed that the mechanism responsible for MHC class I down-regulation is a dominant-negative action of F12dG that impairs the physiologic activity of wild-type PML, possibly by dimerizing with wt PML and thus interfering with its function.18
We observed that impairment of PML function had no effect on the surface expression of MHC class I. Therefore, we reasoned thatF12dG could code for a protein that actively affects the pathway of MHC class I regulation, instead of negatively regulating the function of PML. F12dG is much shorter than wt mPML and could display a different tertiary structure (F12dG is composed of 308 amino acids [aa's], while the 2 mPML isoforms deposited on Genbank [accession nos. U33626 and NM_008884] consist of 808 aa's). In addition, F12dG contains a 16-aa string at its C-terminus that is not present in wt mPML; this could account for some specific activity. Thus, we constructed a GFP-tagged human homolog of F12dG,“PML mut ex3” (see details in “Materials and methods”), and transfected it into HeLa, HEK 293, ACN, and SK-N-BE2(c) cell lines. Sequences of the human PML mutant (PML mut ex3) and the mPML mutant (F12dG) are compared in Figure3A. Transfected cells exhibited a completely different pattern of PML localization, following immunofluorescence staining with anti-PML mAb, as shown in Figure 3. The localization of PML molecules in NBs was replaced by perinuclear or cytoplasmic dots, which were larger and in lower numbers (eg, HeLa and HEK293) or smaller but more numerous (eg, ACN and SK-N-BE2(c)). Whether the dots were fewer/larger or more/smaller did not depend on the cell type, but rather on the cell density of the monolayer. Cells not surrounded by neighboring elements and able to extend large cytoplasmic expansions displayed a thicker and more speckled PML distribution. The almost complete absence of other PML molecules within NBs suggests a dominant-negative action of PML mut ex3. Yet, PML mut ex3 did not affect the expression of MHC class I, because transfected cells did not exhibit a decreased expression of surface MHC class I molecules. This result is shown in Figure 3B by flow cytometric GFP/MHC class I bivariate plots of ACN cells, and it is superimposable to data obtained with all cell lines tested (not shown). Time-course measurements of MHC class I expression were also carried out, from 16 hours after transfection (when GFP reached a significant level of detection) up to the seventh day, and provided identical results (not shown).
PML mut ex3 induces cytoplasmic localization of PML molecules and does not affect MHC class I expression.
(A) Cells growing on glass coverslips were either untreated (top row) or transfected with PML mut ex3 (bottom row) by the Lipofectamine 2000 procedure. At 2 days after transfection, cells were stained with anti-PML mAb followed by GAM-Alexa 594 (red) and counterstained with DAPI (blue). Localization of PML molecules in relation with the cell nucleus was achieved by merging the 2 fluorescence pictures. The amino-acid sequences of the PML mut ex3 protein and of the murine PML mutant F12dG are compared. Both mutants arise from a point deletion in exon 3 of the PML gene that causes a frameshift and translational termination of PML proteins at exon 3. The 16-aa string underlined at the C-terminus of both proteins corresponds to the sequence that derives from the mutation-induced frameshift and that is not present in wt PML. (B) ACN cells were transfected with GFP-tagged PML mut ex3. At 2 days after transfection, cells were processed for microscopic and flow cytometric analyses. Cells growing on glass coverslips were immunostained with GAM-Alexa 594 (middle column) either alone (top row) or preceded by anti-PML mAb (bottom row) and counterstained with DAPI (right column). GFP fluorescence is shown in the left column. Of note is the presence of one untransfected cell that was negative for GFP and displayed a PML pattern typical of NBs after immunostaining with anti-PML mAb. Cells processed for flow cytometry were immunostained with anti–MHC class I mAb (W6.32) followed by GAM-PE. In the bivariate dot plot of GFP (x-axis, green fluorescence) versus MHC class I (y-axis, red fluorescence), the percentage of GFP-positive cells (ie, of transfected cells) is reported. The negative control for MHC class I expression, obtained using isotype-matched antibodies followed by GAM-PE, is shown in gray.
PML mut ex3 induces cytoplasmic localization of PML molecules and does not affect MHC class I expression.
(A) Cells growing on glass coverslips were either untreated (top row) or transfected with PML mut ex3 (bottom row) by the Lipofectamine 2000 procedure. At 2 days after transfection, cells were stained with anti-PML mAb followed by GAM-Alexa 594 (red) and counterstained with DAPI (blue). Localization of PML molecules in relation with the cell nucleus was achieved by merging the 2 fluorescence pictures. The amino-acid sequences of the PML mut ex3 protein and of the murine PML mutant F12dG are compared. Both mutants arise from a point deletion in exon 3 of the PML gene that causes a frameshift and translational termination of PML proteins at exon 3. The 16-aa string underlined at the C-terminus of both proteins corresponds to the sequence that derives from the mutation-induced frameshift and that is not present in wt PML. (B) ACN cells were transfected with GFP-tagged PML mut ex3. At 2 days after transfection, cells were processed for microscopic and flow cytometric analyses. Cells growing on glass coverslips were immunostained with GAM-Alexa 594 (middle column) either alone (top row) or preceded by anti-PML mAb (bottom row) and counterstained with DAPI (right column). GFP fluorescence is shown in the left column. Of note is the presence of one untransfected cell that was negative for GFP and displayed a PML pattern typical of NBs after immunostaining with anti-PML mAb. Cells processed for flow cytometry were immunostained with anti–MHC class I mAb (W6.32) followed by GAM-PE. In the bivariate dot plot of GFP (x-axis, green fluorescence) versus MHC class I (y-axis, red fluorescence), the percentage of GFP-positive cells (ie, of transfected cells) is reported. The negative control for MHC class I expression, obtained using isotype-matched antibodies followed by GAM-PE, is shown in gray.
γ-Interferon (γ-IFN)–induced increase of surface MHC class I expression is not affected by either PML-specific RNA interference or by expression of PML mut ex3
Data presented so far indicate that impairment of PML function does not affect the constitutive level of MHC class I expression. We then investigated a possible involvement of PML in the up-regulation of MHC class I above its basal level by exploring the effect of an impaired or aberrant function of PML on the ability of γ-IFN to up-regulate MHC class I expression.
HEK293 and SK-N-BE2(c), 2 cell lines that display low surface MHC class I expression, were treated with 1000 U/mL γ-IFN, and, after 6 hours, they were transfected with either PML-specific siRNA duplexes or GFP-PML mut ex3. Enlargement of NBs observed in Figure4 for HEK293 cells treated with γ-IFN confirms that PML is an IFN-inducible gene.29However, γ-IFN was not able to counterbalance the “negative” effects of siRNA and of PML mut ex3 on PML expression and distribution, though it was added 6 hours prior. RNA interference abolished almost completely PML expression (95%-98% negative cells) also in γ-IFN–treated cells (Figure 4). Moreover, in cells expressing PML mut ex3 (identified by GFP positivity), no PML molecules were detected in NBs, though molecules were detected in cytoplasmic dots, both in control and γ-IFN–treated cultures (Figure 4). However, the ability of γ-IFN to up-regulate MHC class I expression was unaltered, as all cells displayed the same increase (+ 290% compared with control cells), regardless of the PML status (Figure 4). Identical results were obtained with SK-N-BE2(c) cells (not shown). These data suggest that PML does not interfere with the regulatory pathway of MHC class I enhanced by γ-IFN.
γ-IFN–induced increase of surface MHC class I expression is not affected by PML-specific RNA interference or by expression of PML mut ex3.
HEK293 cells were either untreated (left panel) or treated with 1000 U/ml γ-IFN (right panel) (time [t] = 0), and subsequently transfected with PML-specific siRNA duplex (middle row) or with GFP-PML mut ex3 (bottom row) (t = 6 hours). After 2 additional days (t = 54 hours), cells were processed for fluorescence microscopy and flow cytometry. Cells growing on glass coverslips were immunostained with anti-PML followed by GAM-Alexa 594 antibodies (red fluorescence, first and fourth columns) and counterstained with DAPI (blue fluorescence, second and fifth columns). Cells processed for flow cytometry were immunostained with anti–MHC class I mAb (W6.32) followed by GAM-PE. Histograms of MHC class I expression are shown for control and siRNA-transfected cell cultures, while bivariate dot plots of GFP fluorescence (x-axis) and MHC class I expression (y-axis) are reported for PML mut ex3–transfected cell cultures. Percentage of GFP-positive cells (ie, of PML mut ex3–transfected cells) is reported. The negative control of MHC class I expression, obtained using isotype-matched antibodies followed by GAM-PE, is shown in gray.
γ-IFN–induced increase of surface MHC class I expression is not affected by PML-specific RNA interference or by expression of PML mut ex3.
HEK293 cells were either untreated (left panel) or treated with 1000 U/ml γ-IFN (right panel) (time [t] = 0), and subsequently transfected with PML-specific siRNA duplex (middle row) or with GFP-PML mut ex3 (bottom row) (t = 6 hours). After 2 additional days (t = 54 hours), cells were processed for fluorescence microscopy and flow cytometry. Cells growing on glass coverslips were immunostained with anti-PML followed by GAM-Alexa 594 antibodies (red fluorescence, first and fourth columns) and counterstained with DAPI (blue fluorescence, second and fifth columns). Cells processed for flow cytometry were immunostained with anti–MHC class I mAb (W6.32) followed by GAM-PE. Histograms of MHC class I expression are shown for control and siRNA-transfected cell cultures, while bivariate dot plots of GFP fluorescence (x-axis) and MHC class I expression (y-axis) are reported for PML mut ex3–transfected cell cultures. Percentage of GFP-positive cells (ie, of PML mut ex3–transfected cells) is reported. The negative control of MHC class I expression, obtained using isotype-matched antibodies followed by GAM-PE, is shown in gray.
Discussion
The purpose of our study was to clarify whether PML regulates MHC class I–mediated antigen presentation in human cells, as proposed previously in a murine model.18 According to this hypothesis, since expression of PML is altered in several solid tumors30-32 (though the gene mutation patterns are not established yet2), it could be envisaged that tumorigenesis of cells bearing aberrant PML may be caused by inactivation of PML-regulated tumor suppressive pathways related to cell-cycle control and apoptosis,13-17,33 but also by their invisibility to the immune system. On the other hand, APL cells, harboring a well-known PML inactivation, do not display reduced MHC class I expression.19 It is possible that in human cellsPML behaves differently from murine cells, although the protein itself bears a very high homology in the 2 systems.
In this study PML function in human cell lines was inhibited by transfecting cells with the fusion gene PML/RARα that acts as a dominant-negative oncogene,11 or by performing PML-specific RNA interference.21 In both cases MHC class I expression was not down-regulated.
Once shown that impairment of PML function did not affect surface expression of MHC class I molecules on human cells, we followed an alternative route suggested by the murine model.18 A specific mPML mutant called F12dG was shown to decrease MHC class I expression by a dominant-negative action.18 Since we had observed that impairment of PML function had no effect on surface expression of MHC class I, we asked whether F12dG could code for a protein that actively affects the pathway of MHC class I regulation, instead of negatively regulating the function of PML. F12dG has a point deletion in exon 3 of the mPML gene that causes a frameshift and early truncation of the protein. Thus, F12dG is much shorter than wt mPML and may display a different tertiary structure. In addition, F12dG contains a 16-aa string at its C-terminus that is not present in wt mPML; this could account for some specific activity. We constructed a human homolog of F12dG (“PML mut ex3”) and transfected it into human cell lines. At variance from the mutant F12dG in murine cells, the human mutant PML mut ex3 did not affect surface expression of MHC class I.
Transfected cells exhibit a completely different pattern of PML localization. The typical distribution of PML molecules in NBs is replaced by perinuclear or cytoplasmic large dots. The cytoplasmic localization pattern of PML mut ex3 could be due, at least in part, to a loss of the nuclear localization signal (NLS) domain in exon 6, caused by a truncation of the protein in exon 3. The almost complete absence of PML-NBs suggests a dominant-negative action of PML mut ex3 molecules. The mechanism of dominant-negative regulation is unclear. In murine cells the mutant F12dG protein was suggested to dimerize with wt PML,18 thus interfering with its function. This could be possible also in our case, as the PML mut ex3 protein retains the first 91 aa's of 95 aa's of the whole coiled-coil region, which is responsible for dimerization.
Since we did not observe a down-regulation of MHC class I expression in human cells either by affecting PML or by transfecting a human homolog of F12dG, we focused on another experimental approach described by Zheng et al.18 They found that a DNA sequence, called F12, was able to restore surface MHC class I expression in a tumor cell line that had lost it (ReB7), as well as in NIH3T3 cells derived from Stat-1–deficient mice that do not exhibit detectable surface MHC class I. DNA sequencing revealed thatF12 cDNA is an isoform of PML comprising exons 1-4, 6, and 7, and part of exon 9 of the mPML gene. Accordingly, we searched for a human homolog of F12 to determine whether transfection of “human F12” may up-regulate MHC class I expression. However, no human PML isoform expressing the same pattern of exon distribution has been found yet in human cells, as indicated by a recent review that summarizes all data on PML isoforms and splice variants.3 We also performed a BLAST search of the theoretical cDNA sequence of “human F12” (obtained by replacing the murine exons with the corresponding human exons) against the human expression sequence tags (ESTs) database (Genbank). No matches were found. The lack of expression of a human form of F12 in human cells ruled out any rationale for this issue. An additional consideration excludes the existence of a “human F12” protein that regulates MHC class I in human cells. Should such a molecule exist, it would have been knocked down in the RNA interference experiments, since the siRNA sequence chosen to silence all forms of PML was on exon 2 of the PML gene. Consequently, a reduction of MHC class I expression should have been observed, at variance from the results of this study.
Data from γ-IFN–treated cell cultures, as well, do not support the contention of a regulatory involvement of PML in the expression of MHC class I in human cells. Thus, the overall results indicate that impairment of PML neither decreases expression of MHC class I nor affects the mechanism of up-regulation of MHC class I triggered by the cytokine.
We conclude that, in human cell lines, PML does not regulate the expression of MHC class I molecules. This is in accordance with the observation of patients with APL, in whom alteration of PMLdue to the t(15;17) translocation does not abrogate MHC class I expression on the leukemic cells.19
Prepublished online as Blood First Edition Paper, December 27, 2002; DOI 10.1182/blood-2002-11-3335.
Supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC), Ministero per l'Istruzione l'Università e la Ricerca Scientifica (MIUR), Progetto Finalizzato Ministero della Salute (“Alterazioni Geniche nelle Leucemie Acute”), and Compagnia di S. Paolo (E.C. and C.E.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Silvia Bruno, Department of Experimental Medicine, Section of Human Anatomy, Via De Toni, 14; 16132 Genova, Italy; e-mail: silvia.bruno@unige.it.



![Fig. 4. γ-IFN–induced increase of surface MHC class I expression is not affected by PML-specific RNA interference or by expression of PML mut ex3. / HEK293 cells were either untreated (left panel) or treated with 1000 U/ml γ-IFN (right panel) (time [t] = 0), and subsequently transfected with PML-specific siRNA duplex (middle row) or with GFP-PML mut ex3 (bottom row) (t = 6 hours). After 2 additional days (t = 54 hours), cells were processed for fluorescence microscopy and flow cytometry. Cells growing on glass coverslips were immunostained with anti-PML followed by GAM-Alexa 594 antibodies (red fluorescence, first and fourth columns) and counterstained with DAPI (blue fluorescence, second and fifth columns). Cells processed for flow cytometry were immunostained with anti–MHC class I mAb (W6.32) followed by GAM-PE. Histograms of MHC class I expression are shown for control and siRNA-transfected cell cultures, while bivariate dot plots of GFP fluorescence (x-axis) and MHC class I expression (y-axis) are reported for PML mut ex3–transfected cell cultures. Percentage of GFP-positive cells (ie, of PML mut ex3–transfected cells) is reported. The negative control of MHC class I expression, obtained using isotype-matched antibodies followed by GAM-PE, is shown in gray.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/9/10.1182_blood-2002-11-3335/4/m_h80934202004.jpeg?Expires=1765028516&Signature=jR~0W-nZie88Wd272bGxGfoj9zVHupPLMJ7jZziE71fKgBvvPrsx4tRzCxUpauvFAy-uNmL8ug5DoAo-zSttfDdF2uyGjZgv7QGUz5WORKtHLFDBYDgyL7gIsf8mIeyETjjV2GrBvv3wyUAMNLiF-jTT7DgGmrG0cbWhim~e1H1F556B603NMxT1xnioGcyxsInNVRnl3Nd08orx4DeAbJSLEYl8fhWJ7ufdchyyaLl7ayRNJY23is-HTxB0heQTrdlK9ThftM8VuxCpwDPfej1veE2yDlOJUAMOQqx4T108fsjjSnXk4ayT8nFerlfTh0GkfyuWt5hmzAWv7cZrUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
