Abstract
Transferrin receptor (TfR) facilitates cellular iron uptake by mediating endocytosis of its ligand, iron-loaded transferrin. Although TfR is widely believed to be important for iron acquisition by all mammalian cells, direct experimental evidence is lacking. We have previously shown that mouse embryos homozygous for a disrupted transferrin receptor allele (TfR-/-) die of anemia before embryonic day 12.5, although most other embryonic tissues appear to be developing normally. Here, we have investigated the importance of TfR postnatally, by using TfR-/- embryonic stem cells to produce chimeric animals. We find that TfR-/- embryonic stem cells give rise to most tissues and organs, but do not contribute to hematopoietic tissues on a wild-type C57BL/6J background, indicating that both adult erythropoiesis and lymphopoiesis require TfR. On an immunodeficient RAG2-/- background, TfR-/- B-cell development proceeds at least to the IgM+ stage, although significantly fewer IgM+ cells are present in peripheral lymphoid organs. Conversely, T cells lacking TfR are arrested very early in their development, at the CD4-8-3- stage. These results indicate that TfR is necessary for the normal maturation of thymocytes, but that B-cell development is less severely affected by the absence of TfR. (Blood. 2003;102:3711-3718)
Introduction
Transferrin receptor 1 (TfR) is a widely expressed type II membrane protein that plays a central role in iron metabolism. Its ligand, transferrin (Tf), binds ferric iron with high affinity to solubilize it, render it nonreactive, and deliver it to cells. At the cell surface, holotransferrin (Fe2-Tf) binds to TfR and is internalized by receptor-mediated endocytosis. Iron dissociates from Tf within acidified endosomes and is transferred across the endosomal membrane by divalent metal transporter 1 (DMT1; for a review, see Andrews1 ). The apoprotein-Tf-TfR (apoTf-TfR) complex is then recycled back to the cell surface. This process, termed the Tf cycle, is reiterated many times by each TfR and Tf molecule, providing a highly efficient mechanism for cellular iron uptake.
The erythroid bone marrow requires an exceptionally large amount of iron to support the production of hemoglobin. However, other cells that are not actively dividing have minimal iron requirements. Accordingly, 2 mutant mouse strains, deficient in TfR or Tf, have revealed a critical role for the Tf cycle in erythropoiesis. Embryos lacking TfR appear to form nonhematopoietic tissues normally during early embryogenesis, but die from severe anemia at mid-gestation, indicating that they become dependent on the Tf cycle prior to the onset of fetal liver hematopoiesis.2 The fact that TfR-/- embryos can produce a substantial red cell mass until embryonic day 10.5 (E10.5) suggests that TfR-independent mechanisms of iron uptake are adequate for the earliest erythropoiesis as well as for the initial formation of most tissues. Similarly, hypotransferrinemic (Tfhpx/hpx) animals are profoundly anemic due to an inactivating mutation within a splice donor site in the Tf gene.3 They also appear to have normal development of most nonhematopoietic tissues. Tfhpx/hpx mice survive if they are given exogenous Tf or red blood cell transfusions soon after birth.4 Interestingly, these animals develop systemic iron overload similar to human patients with atransferrinemia,5 providing further evidence that the Tf cycle may be dispensable for the survival, proliferation, and differentiation of many cell types. However, TfR-/- mice and Tfhpx/hpx mice show subtle abnormalities in neuroepithelial precursors2 and central nervous system architecture,6 respectively, indicating that the Tf cycle may be important for neuronal development.
There are limitations to the conclusions that can be drawn from these studies of TfR-/- and Tfhpx/hpx mice. First, because TfR-/- embryos die between E10.5 and E12.5, it is impossible to evaluate whether cells and tissues that have not yet developed require TfR. In particular, we were unable to determine whether TfR was necessary for definitive, adult erythropoiesis, for the development of other hematopoietic lineages, or for the formation of tissues that arise later in gestation. Second, although severely deficient in serum Tf, Tfhpx/hpx mice have a small amount of circulating, abnormal Tf that is likely to be functional.3 Finally, it is possible that TfR has other roles, in addition to iron uptake, which do not require Tf and would not have been revealed in studies of Tfhpx/hpx mice. For these reasons, we sought an alternative approach to determining the importance of TfR in the formation of adult mouse tissues.
We generated chimeric mice by introducing TfR-/- embryonic stem (ES) cells into wild-type (TfR+/+) blastocysts. The blastocysts were allowed to develop, and chimeric mice were analyzed to determine which tissues contained descendants of the TfR-/- ES cells and which were exclusively derived from the TfR+/+ host cells. A substantial contribution of TfR-/- ES cells to an organ or tissue indicated that TfR was not essential for the development of most or all of the cells of that tissue. Conversely, cell types and tissues that showed no contribution from TfR-/- ES cells must require TfR for their normal development.
Similar to earlier studies of TfR-/- embryos and Tfhpx/hpx mice, we found that TfR-/- ES cells participated in the formation of most tissues. However, TfR-/- cells were notably missing from hematopoietic tissues including bone marrow, spleen, and thymus. This suggested that TfR was not only necessary for normal erythropoiesis, but also for the proliferation or maturation (or both) of other hematopoietic cell types. We extended our studies of the role of TfR in T and B lymphopoiesis by producing chimeric mice on a recombinase-activating gene-2-deficient (RAG2-/-) background, because RAG2-/- mice have an early block in the production of lymphocytes. We found that TfR-/- cells did not give rise to T cells beyond the triple-negative (CD4-8-3-) stage of development. However, B-lymphoid cells that had undergone heavy-chain gene rearrangement and matured to at least the immunoglobulin M+ (IgM+) stage of development were present in at least some of the TfR-/- chimeras. These results indicate that TfR is important in early lymphoid development, but differentially so. TfR may play a key role in the differentiation of T-lymphoid precursor cells, in the expansion of early B- and T-lymphoid cell populations, or in both processes.
Materials and methods
Generation of TfR-/- ES cells
Previously derived TfR+/- ES cells2 were expanded on feeder layers of γ-irradiated embryonic fibroblasts in Dulbecco modified Eagle medium containing 4.5 g/L glucose supplemented with 15% fetal calf serum (FCS), 1 mM sodium pyruvate, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 5.8 mM NaOH, 0.087 mM β-mercaptoethanol, 200 U/mL penicillin G sodium, 200 μg/mL streptomycin sulfate, and 104 U/mL leukemia inhibitory factor (R&D Systems, Minneapolis, MN). To inactivate the remaining wild-type TfR allele, TfR+/- cells were treated with 1 to 2.5 mg/mL G418 (Gibco BRL, Grand Island, NY) as part of a neomycin step-up experiment7 to select for cells that carried 2 copies of the targeted chromosome and had lost the chromosome carrying the normal TfR allele. Clones were isolated after 10 or 17 days of selection. Nontransferrin-bound iron was provided for the selected cells by supplementation with 50 μM, 100 μM, or 150 μM ferric salicylaldehyde isonicotinoyl hydrazone (Fe-SIH, a gift from P. Ponka). Ninety clones were screened by polymerase chain reaction (PCR) amplification of a microsatellite polymorphism that distinguishes between wild-type and null TfR alleles as previously described.2 Clones that appeared to be TfR-/- in this assay were confirmed by Southern blot analysis.2 Two TfR-/- clones were obtained, both of which arose in cultures supplemented with 50 μM Fe-SIH. Karyotype analysis showed that both clones had a normal complement of 40 chromosomes. Because long-term maintenance in Fe-SIH appeared to be toxic to the cells, the clones were subsequently maintained with 20 μg/mL ferric ammonium citrate as the iron source.
Generation and analysis of C57BL/6J chimeras
Cells from each TfR-/- clone and control TfR+/- cells were microinjected into wild-type C57BL/6J blastocysts to produce chimeric mice. Chimeras and nonchimeric littermates were bled and tissues were harvested 4 to 6 weeks after birth. Blood was collected into heparinized capillary tubes. Hemoglobin electrophoresis was performed as described previously.8 Isoelectric focusing was performed using standard techniques. Tissue DNA was prepared using the Puregene DNA Isolation Kit (Gentra Systems, Minneapolis, MN) according to the manufacturer's instructions. Samples (10 μg) were digested with NheI and subjected to Southern blot analysis to distinguish wild-type and disrupted TfR alleles,2 or analyzed by PCR.
RAG2-/- blastocyst complementation and flow cytometry
For the first experiment, RAG2-/- mice9 were maintained in the barrier mouse facility at Children's Hospital (Boston, MA). TfR-/- and TfR+/- ES cell clones were microinjected into blastocysts to produce chimeric mice as described (see “Generation and analysis of C57BL/6J chimeras”). At 18 to 26 days of age, a distal portion of the tail was removed under anesthesia for preparation of DNA. DNA was purified from tails by incubation in proteinase K lysis buffer (100 mM Tris [tris(hydroxymethyl)aminomethane], pH 8.5, 5 mM EDTA [ethylenediaminetetraacetic acid], 0.2% sodium dodecyl sulfate [SDS], 200 mM NaCl, 200 μg/mL proteinase K) followed by phenol/chloroform extraction and ethanol precipitation. Southern analysis was performed on 10 μg tail DNA to identify chimeras (see “Generation and analysis of C57BL/6J chimeras”). In addition, 100 μL blood for lymphocyte analysis was obtained by retro-orbital bleeding into heparinized capillary tubes and transferred to 5-mL polystyrene tubes. Red blood cells were lysed with 1 mL red blood cell lysis buffer (8.3 g/L ammonium chloride in 10 mM Tris-Cl, pH 7.5, at 37°C) for 2 to 3 minutes. Ice-cold phosphate-buffered saline (PBS) supplemented with 2% FCS was added and the remaining cells were pelleted at 4°C. Cell pellets were resuspended in a small amount of the remaining liquid and 100 μL antibody solution, containing 1:100 dilutions of fluorescein isothiocyanate (FITC)-Ly9.1, phycoerythrin (PE)-Thy1.2, and cychrome-B220 (CD45R) antibody (PharMingen, San Diego, CA), was added. Samples were incubated on ice for 10 minutes, then washed in 2% FCS/PBS and centrifuged as before. The pellets were resuspended in a small amount of the remaining liquid and 10 000 to 50 000 cells were counted for flow cytometry. Lymphocytes were identified by forward and side light scatter characteristics.
Chimeras and control mice (nonchimeric littermates, wild-type 129 mice, and RAG2-/- mice) were humanely killed in the eighth week of life. The bone marrow (BM) cavity of the femur was flushed with PBS to obtain cells for DNA preparation. Spleen, thymus, and lymph nodes (inguinal, brachial, axial, and mesenteric) were dissected into 3 mL ice-cold 2% FCS/PBS. BM, heart, and kidney, as well as a portion of the dissected spleen and thymus, were harvested for DNA. The dissected spleen, thymus, and lymph nodes were placed between pieces of mesh and the tissue was gently scraped apart. Further disruption was achieved by gently pipetting the cell suspension through a glass pipette and red blood cells were lysed. The resulting single-cell suspensions were kept on ice and centrifuged at low speed for 6 to 7 minutes at 4°C. The pellets were resuspended in a small amount of residual supernatant liquid. Then, 100 μL resuspended cells plus 100 μL antibody solution were incubated on ice for 10 minutes. The cells were washed with 2% FCS/PBS and recentrifuged, and the pellet was resuspended in a small amount of residual supernatant liquid for flow cytometry. Cells (200 000-500 000) were analyzed for each sample from the chimeras. For the thymus, the antibody solution consisted of 1:100 dilutions of FITC-α mouse Ly9.1, PE-α mouse CD4, and cychrome-α mouse CD8a (Ly-2). For the spleen and lymph nodes, the antibody solution consisted of 1:100 dilutions of FITC-antimouse Ly9.1, PE-antimouse Thy1.2 (CD90.2), and cychrome-antimouse B220/CD45R. All antibodies were obtained from PharMingen. All flow cytometry was performed on a FACSCalibur machine (Becton Dickinson, Bedford, MA) using CellQuest software (BD Biosciences, Milan, Italy) and analyzed with FlowJo software (Tree Star, San Carlos, CA). DNA was prepared with the Puregene DNA Isolation Kit according to manufacturer's instructions (Gentra Systems).
A second set of chimeras was obtained from blastocyst injections performed by the Dana-Farber Cancer Institute DF/HCC Mouse Specialized Services Core Facility (Boston, MA). Both TfR+/- and TfR-/- chimeras were identified by Southern blot analysis of tail DNA. When the animals were 5 to 6 weeks old, lymphocytes were isolated and prepared from blood, spleen, and lymph nodes. BM, kidney, heart, and sections of the dissected spleen and thymus were also harvested for DNA. Peripheral blood lymphocytes were also stained with FITC-antimouse Ly9.1, PE-antimouse IgM, allophycocyanin (APC)-antimouse CD3ϵ, and then fixed in 0.5% formaldehyde/PBS for later analysis. Thymocytes, splenocytes, and lymph node cells were analyzed. In addition, additional aliquots of spleen and lymph node cells were also stained with FITC-antimouse Ly9.1, PE-antimouse IgM, and APC-antimouse CD3ϵ. At least 10 000 cells were counted from blood, and at least 100 000 cells were counted from thymus, spleen, and lymph nodes when enough material was present. All antibodies were obtained from PharMingen. All flow cytometry was performed on a FACSCalibur machine (Becton Dickinson) using CellQuest software (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Results
Generation of TfR-/- ES cells and analysis of C57BL/6J chimeras
We chose a chimera approach for this study, reasoning that adult tissues that were not populated with TfR-null cells must require TfR for their development. Two independent, homozygous TfR-/- ES cell clones (E12 and H5) were isolated using a neomycin step-up selection strategy on heterozygous parental TfR+/- ES cells (Figure 1A). These TfR-/- ES clones were used in all subsequent experiments. TfR-/- or parental TfR+/- ES cells were injected into wild-type C57BL/6J mouse blastocysts to produce chimeric animals. Chimeras were identified by patches of brown coat color, which indicated contribution from cells of ES (strain 129S4/SvJae) origin. Typically, mice showing chimerism in coat color also showed chimerism in other tissues. Eleven adult chimeric mice were analyzed from animals produced using TfR-/- ES cells, and 3 adult chimeric mice were analyzed from animals produced using TfR+/- cells. The degree of chimerism based on coat color was 10% to 85% in the TfR-/- chimeras, with 4 animals about 30% chimeric, and 5 animals having chimerism equal to or more than 50%.
TfR-/- ES cell clones and C57BL/6J chimera hemoglobin analysis. (A) Southern blot analysis of the parental TfR+/- ES cell line and the 2 TfR-/- ES cell clones obtained after neomycin step-up. (B) Isoelectric focusing of hemoglobins from peripheral blood of E12 TfR-/- chimeras (CM-1 to CM-3), nonchimeric wild-type littermates, C57BL/6J (BL6), and 129 wild-type controls. M indicates marker lanes. (C) Hemoglobin electrophoresis of peripheral blood samples from TfR+/- chimeras (het nos. 1-3), a 129 wild-type control, and 6 (of 8) H5 TfR-/- chimeras. H5 chimeras no. 1 and no. 3 are not shown here, but in all cases, TfR-/- chimeras show contribution only from the host C57BL/6J hemoglobin to circulating erythrocytes, indicating that the 129-derived TfR-/- cells are unable to produce mature erythrocytes.
TfR-/- ES cell clones and C57BL/6J chimera hemoglobin analysis. (A) Southern blot analysis of the parental TfR+/- ES cell line and the 2 TfR-/- ES cell clones obtained after neomycin step-up. (B) Isoelectric focusing of hemoglobins from peripheral blood of E12 TfR-/- chimeras (CM-1 to CM-3), nonchimeric wild-type littermates, C57BL/6J (BL6), and 129 wild-type controls. M indicates marker lanes. (C) Hemoglobin electrophoresis of peripheral blood samples from TfR+/- chimeras (het nos. 1-3), a 129 wild-type control, and 6 (of 8) H5 TfR-/- chimeras. H5 chimeras no. 1 and no. 3 are not shown here, but in all cases, TfR-/- chimeras show contribution only from the host C57BL/6J hemoglobin to circulating erythrocytes, indicating that the 129-derived TfR-/- cells are unable to produce mature erythrocytes.
Chimeric animals were similar to wild-type littermates in their hematocrit values, hemoglobin concentrations, and red blood cell parameters (data not shown). To determine whether the TfR-/- and TfR+/- cells could contribute to definitive adult erythropoiesis, we took advantage of the fact that C57BL/6 and 129 hemoglobins have different electrophoretic properties. C57BL/6 hemoglobin migrates as a single band (s type) and 129 hemoglobin migrates diffusely, producing multiple bands (d type). Hemoglobin electrophoresis and isoelectric-focusing experiments were performed on peripheral blood from TfR+/- and TfR-/- chimeras. Although one TfR+/- chimera (with 25% coat color chimerism) did not show a contribution of TfR+/- ES cells to circulating erythrocytes, the other 2 TfR+/- chimeras (50%-70% chimerism by coat color) did have 129 hemoglobin, indicating that the parental TfR+/- ES cells were able to contribute substantially to circulating erythrocytes (Figure 1C). In contrast, all chimeric animals generated from injection of TfR-/- ES cells showed no 129 hemoglobin, indicating that there was no contribution of TfR-/- cells to circulating erythrocytes (Figure 1B-C). We conclude from this experiment that TfR is also essential for adult erythropoiesis.
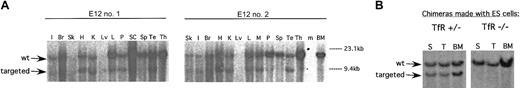
The likely role of TfR in erythropoiesis is obvious—erythroid precursors must incorporate large amounts of iron to produce hemoglobin, and, as observed previously in mouse embryos,2 this requires TfR. Immature erythroid precursors that fail to hemoglobinize properly are probably destroyed in the BM. However, other cell types need far less iron and may be able to rely on alternative iron uptake pathways. We investigated this by using Southern blot analysis to determine the contribution of TfR-/- and control TfR+/- ES cells to other tissues (Figure 2). The presence of the smaller (approximately 9 kb) band indicates contribution from ES cells carrying the null TfR allele.
TfR-/- cells do not contribute to hematopoietic tissues on a wild-type C57BL/6J background. (A) Representative Southern blots of tissues from 2 E12 chimeras show contribution of TfR-/- cells (targeted allele) to all tissues except spleen, thymus, and bone marrow. I indicates intestine; Br, brain; Sk, skin; H, heart; K, kidney; Lv, liver; L, lung; P, pancreas; SC, spinal cord; SP, spleen; Te, testis; Th, thymus; M, skeletal muscle; BM, bone marrow; m, marker lane. (B) TfR+/- and TfR-/- cell contribution to spleen (S), thymus (T), and bone marrow (BM) in chimeras was re-examined to confirm the absence of TfR-/- cells in these tissues.
TfR-/- cells do not contribute to hematopoietic tissues on a wild-type C57BL/6J background. (A) Representative Southern blots of tissues from 2 E12 chimeras show contribution of TfR-/- cells (targeted allele) to all tissues except spleen, thymus, and bone marrow. I indicates intestine; Br, brain; Sk, skin; H, heart; K, kidney; Lv, liver; L, lung; P, pancreas; SC, spinal cord; SP, spleen; Te, testis; Th, thymus; M, skeletal muscle; BM, bone marrow; m, marker lane. (B) TfR+/- and TfR-/- cell contribution to spleen (S), thymus (T), and bone marrow (BM) in chimeras was re-examined to confirm the absence of TfR-/- cells in these tissues.
Figure 2 shows representative results of Southern blot analyses for chimerism in multiple tissues. In these and other experiments, we found that both TfR+/- and TfR-/- ES cells could contribute substantially to brain, spinal cord, pancreas, intestine, heart, kidney, skeletal muscle, liver, lung, and testis, indicating that TfR is not essential for the normal development of cells in those tissues and organs. However, because these structures are made up of multiple types of cells, we cannot exclude the possibility that some individual cell types do require TfR. Although we had seen increased apoptosis of neuroepithelial precursor cells in mid-gestation TfR-/- mouse embryos, we have not yet been able to determine whether TfR is necessary for development of specific structures in the central or peripheral nervous system of adult animals. However, no gross abnormalities in brain architecture were noted on necropsy of the TfR-/- chimeras (data not shown).
Results from our analysis of hematopoietic tissues were different from those of nonhematopoietic tissues. As expected, we found that control TfR+/- cells could also contribute substantially to BM, spleen, and thymus (Figure 2B). However, with the exception of one highly chimeric animal (estimated 85% from coat color), none of the chimeras produced from TfR-/- ES cells showed any detectable contribution of TfR-/- cells to those hematopoietic tissues (Figure 2). The approximate 85% chimera did show a very small contribution of TfR-/- cells to spleen and thymus, but not BM (data not shown). This suggests that the spleen and thymus chimerism seen in this animal may be due to stromal or epithelial cells or both.
The fact that TfR-/- cells were not detected in the BM in any C57BL/6J chimeras suggested that most, if not all, hematopoietic cells (or a hematopoietic stem cell) were dependent on TfR for their proliferation or differentiation. This conclusion is further supported by the fact that thymus and spleen, both populated primarily by hematopoietic cells, also showed little or no contribution of TfR-/- cells. In the remainder of our analysis we focused on the role of TfR in lymphopoiesis.
TfR-/- lymphocyte development on a RAG2-/- background is differentially affected
We further tested the ability of TfR-/- cells to give rise to lymphocytes in vivo by generating TfR+/- and TfR-/- ES cell chimeras on a RAG2-/- background. The RAG2-/- complementation assay was developed to address the problem that homozygous disruption of some genes results in early embryonic lethality, precluding study of their roles in lymphopoiesis.10 RAG2-/- mice cannot produce mature lymphocytes because they cannot undergo somatic recombination of T-cell receptor and immunoglobulin chain loci. In these mice, T-cell development is interrupted at the CD4-8-3-44-25+ (early pre-T) stage, and B-cell development is arrested at the B220+ CD43+ early pro-B-cell stage.9 Therefore, all mature lymphocytes in RAG2-/- chimeric animals must be derived from the injected ES cells.10 Consequently, this system allows for a more sensitive analysis of lymphocyte development from modified ES cells than can be achieved on a wild-type genetic background.
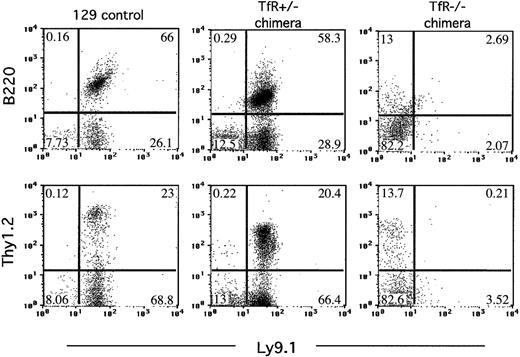
Chimeras were identified by Southern blot analysis of tail DNA taken at 18 to 26 days of age. Chimerism assessed in this way ranged from about 10% to 50% (data not shown). Flow cytometry was performed on blood samples taken at the same time, to detect T and B lymphocytes in the circulation. Anti-Ly9.1 antibody recognizes an allele of a surface glycoprotein antigen present on strain 129 lymphocytes but absent from our RAG2-/- cells, which display the C57BL/6 strain Ly9.2 specificity.11-13 This difference permits the identification of lymphocytes derived from the injected ES cells. Two chimeras produced by blastocyst injection of parental TfR+/- ES cells showed full reconstitution of the mature, circulating lymphocyte pool, rescuing the defect in lymphocyte development seen in RAG2-/- mice (Figure 3). In contrast, although 10 000 to 50 000 nucleated blood cells were analyzed, very few, if any, Ly9.1+Thy1.2+ or Ly9.1+B220+ cells were present in the circulation of chimeras generated from injection of TfR-/- ES cells. This indicated that TfR-/- cells, in contrast to parental TfR+/- cells, were severely limited in their ability to develop into mature T or B lymphocytes.
TfR-/- chimeras fail to produce mature B or T cells in the circulation. Flow cytometry was performed on blood from TfR chimeras generated on a RAG2-/- background. Nucleated blood cells were stained with Ly9.1 (an antibody recognizing cells of 129 strain origin), B220 (pan B-cell marker), and Thy1.2 (pan T-cell marker). Cells (10 000-50 000) were counted for each sample. The plots shown for the TfR-/- chimera are representative of all 6 chimeras obtained. Percentages of cells in each quadrant are indicated. Fewer total cells were counted for the 129 control, therefore fewer cells appear on the dot plot.
TfR-/- chimeras fail to produce mature B or T cells in the circulation. Flow cytometry was performed on blood from TfR chimeras generated on a RAG2-/- background. Nucleated blood cells were stained with Ly9.1 (an antibody recognizing cells of 129 strain origin), B220 (pan B-cell marker), and Thy1.2 (pan T-cell marker). Cells (10 000-50 000) were counted for each sample. The plots shown for the TfR-/- chimera are representative of all 6 chimeras obtained. Percentages of cells in each quadrant are indicated. Fewer total cells were counted for the 129 control, therefore fewer cells appear on the dot plot.
TfR-/- chimeras were killed in the eighth week of life to analyze the lymphocyte content of the spleen, thymus, and lymph nodes by flow cytometry. For each sample, 200 000 to 500 000 cells were examined to increase the sensitivity of the analysis and to allow detection of very small populations of cells. Spleen and lymph node cells were stained with Ly9.1, Thy1.2, and B220 antibodies. Thymus cells were stained with Ly9.1, CD4, and CD8 antibodies.
Thymus-derived cell populations from all of the TfR-/- chimeras were indistinguishable from the cell populations seen in RAG2-/- control mice, indicating that TfR-/- cells cannot develop beyond the triple-negative (CD4-8-3-) developmental stage, in which RAG2-/- cells are also arrested (Figure 4). The few dots appearing in the CD4+ and CD8+ quadrants probably result from nonspecific background staining, because RAG2-/- mice have no CD4+ or CD8+ cells in the thymus.9
TfR-/- chimeras, like RAG2-/- animals, fail to produce thymic cells that express CD4 or CD8. Flow cytometry was performed on thymocytes from controls and 7-week-old TfR-/- chimeras generated on a RAG2-/- background. Thymus cells were stained with Ly9.1, CD4, and CD8 antibodies. Cells (200 000-500 000) were counted in these analyses. The plot shown for TfR-/- chimera is representative of the 6 chimeras obtained. Percentages of cells in each quadrant are indicated.
TfR-/- chimeras, like RAG2-/- animals, fail to produce thymic cells that express CD4 or CD8. Flow cytometry was performed on thymocytes from controls and 7-week-old TfR-/- chimeras generated on a RAG2-/- background. Thymus cells were stained with Ly9.1, CD4, and CD8 antibodies. Cells (200 000-500 000) were counted in these analyses. The plot shown for TfR-/- chimera is representative of the 6 chimeras obtained. Percentages of cells in each quadrant are indicated.
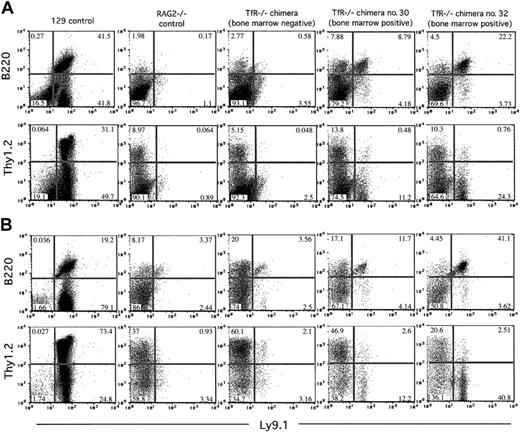
Furthermore, similar to RAG2-/- controls, few, if any, Ly9.1+Thy1.2+ cells were observed in samples from the spleen and lymph nodes of TfR-/-RAG2-/- chimeras, indicating that there were no TfR-/- T-lymphoid cells present in those sites (Figure 5). In contrast, Southern blot analysis showed substantial (30%-60%) TfR-/- ES cell contribution to other, nonhematopoietic organs as we had previously seen in the C57BL/6J chimeras (data not shown).
TfR-/- chimeras display a differential ability to produce T and B cells in secondary lymphoid organs on a RAG2-/- background. Nucleated cells from 7-week-old chimeras and control animals were stained with Ly9.1, B220, and Thy1.2 and analyzed by flow cytometry. Cells (200 000-500 000) were counted for these analyses. Percentages of cells in each quadrant are indicated. (A) Splenic cells. (B) Lymph node (axial, brachial, inguinal, and mesenteric) cells. All TfR-/- chimeras show few, if any, Ly9.1+Thy1.2+ cells. Only those TfR-/- chimeras with TfR-/- cells in the bone marrow (no. 30 and no. 32) display a small number of Ly9.1+B220+ cells. The TfR-/- BM-negative chimera shown is representative of the other 4 TfR-/- BM-negative chimeras obtained.
TfR-/- chimeras display a differential ability to produce T and B cells in secondary lymphoid organs on a RAG2-/- background. Nucleated cells from 7-week-old chimeras and control animals were stained with Ly9.1, B220, and Thy1.2 and analyzed by flow cytometry. Cells (200 000-500 000) were counted for these analyses. Percentages of cells in each quadrant are indicated. (A) Splenic cells. (B) Lymph node (axial, brachial, inguinal, and mesenteric) cells. All TfR-/- chimeras show few, if any, Ly9.1+Thy1.2+ cells. Only those TfR-/- chimeras with TfR-/- cells in the bone marrow (no. 30 and no. 32) display a small number of Ly9.1+B220+ cells. The TfR-/- BM-negative chimera shown is representative of the other 4 TfR-/- BM-negative chimeras obtained.
In our analysis of B lymphopoiesis, however, we did see a very small, but significant, contribution from TfR-/- cells to the B-cell compartment in RAG2-/- chimeras. Four of 6 chimeric animals examined had no detectable TfR-/- cells in the bone marrow (by Southern blot analysis; data not shown) and no Ly9.1+B220+ cells in the spleen or lymph nodes (by flow cytometry; Figure 5), indicating an absence of TfR-/- B cells early in development and in the periphery. However, 2 animals, chimeras no. 30 and no. 32, did have TfR-/- cells in the bone marrow by Southern blot analysis (approximately 20% and approximately 30%, respectively; data not shown). These animals also had a very small Ly9.1+B220+ population in the spleen and lymph nodes (by flow cytometry; Figure 5). Due to the paucity of RAG2-/-B220+ cells in these tissues, TfR-/- B cells account for up to 22% and 41% of the lymphocyte-gated cells in the spleen and lymph nodes, respectively, in chimera no. 32. The absolute numbers of Ly9.1+B220+ cells in the spleens of TfR-/- chimeras were 1740 of 306 600 total cells counted for chimera no. 30, 3143 cells of 283 500 total cells counted for chimera no. 32, and 24 648 of 190 950 total cells counted for the 129 control mouse. In the lymph nodes, chimera no. 30 had 594 Ly9.1+B220+ cells of 268 200 total cells counted, chimera no. 32 had 2770 of 312 000 total cells counted, and the 129 control mouse had 16 883 of 211 050 total cells counted. PCR analysis of total spleen DNA from TfR-/- chimeras no. 30 and no. 32 showed a low level of D-JH immunoglobulin heavy-chain rearrangement in these animals (data not shown), suggesting that the B220+ cell population included B-lymphoid cells that had matured at least as far as the early pre-B stage.
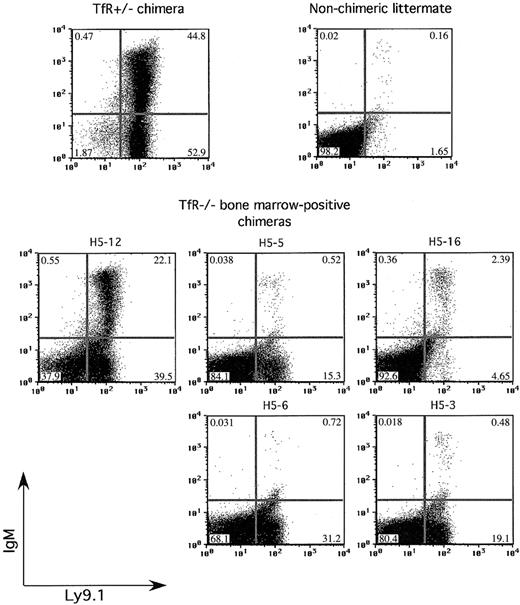
In a second group of chimeras, we further examined the ability of TfR-/- cells to produce mature lymphocytes. In accord with previous experiments, no TfR-/-RAG-/- chimeras had Ly9.1+CD4+ or Ly9.1+CD8+ cells in the thymus (data not shown). They also had no detectable Ly9.1+CD3ϵ+ cells above background in the spleen or blood (data not shown). Figure 6 shows flow cytometry on spleen samples taken from a control TfR+/- chimera, a nonchimeric littermate, and the TfR-/- chimeras with presence of TfR-/- cells in the BM. TfR-/- chimera H5-12 had TfR-/- cells in both the bone marrow (approximately 40% chimerism) and spleen (approximately 50% chimerism) by Southern blot analysis (data not shown). This chimera had approximately half the percentage of IgM+ cells in the spleen as the TfR+/- control chimera, and approximately 30% as many total IgM+ cells (8731 of 101 490 total cells counted versus 30 001 of 106 545 total cells counted for the TfR+/- chimera control). A second TfR-/- chimera, H5-16, showed fewer IgM+ cells in the spleen (1062 of 101 685 total cells counted). These 2 chimeras, which displayed Ly9.1+IgM+ cells in the spleen, also showed Ly9.1+IgM+ cells in the lymph nodes, whereas only chimera H5-12 had Ly9.1+IgM+ cells in the peripheral blood (data not shown). The presence of IgM+ cells correlates with the extent of BM chimerism—the most chimeric animal (H5-12) had the greatest number and percentage of IgM+ cells in the spleen, lymph nodes, and blood. A less chimeric animal (H5-16) displayed fewer IgM+ cells. Other TfR-/- chimeras, with no or very little TfR-/- contribution to the BM and spleen, had few if any IgM+ cells in the spleen, lymph nodes, or blood. These results indicate overall that the absence of TfR impedes T-cell differentiation beyond the earliest stage, whereas B lymphopoiesis is not as severely affected.
Some TfR-/- chimeras are able to produce IgM+ B cells. Splenocytes from control and TfR-/-RAG2-/- chimeras are stained with Ly9.1 and IgM. Two of the BM-positive TfR-/-RAG2-/- chimeras show IgM+ cells, one with a substantial amount, indicating some ability to produce B cells that have completed V(D)J recombination.
Some TfR-/- chimeras are able to produce IgM+ B cells. Splenocytes from control and TfR-/-RAG2-/- chimeras are stained with Ly9.1 and IgM. Two of the BM-positive TfR-/-RAG2-/- chimeras show IgM+ cells, one with a substantial amount, indicating some ability to produce B cells that have completed V(D)J recombination.
Discussion
We extended our previous analysis2 to ask whether TfR is necessary for adult erythropoiesis and for the development of other adult tissues. Adult TfR-/- chimeras produced on a wild-type C57BL/6J background showed the contribution of TfR-/- cells to all tissues tested except those of hematopoietic origin—marrow, spleen, and thymus. We conclude that most cell types are capable of assimilating iron through a mechanism that is not mediated by TfR.
At least 4 TfR-independent iron acquisition pathways have been characterized in mammalian systems. TfR-independent uptake of Tf-bound iron has been noted in TfR-deficient Chinese hamster ovary cells.14,15 Low level, receptor-mediated uptake of ferritin has been shown in human erythroid precursors.16-18 Non-Tf-bound iron has been shown to be taken up directly by a variety of cell types in vitro,19-23 in accord with the observation that atransferrinemic patients and Tfhpx/hpx mice develop systemic iron overload in nonhematopoietic tissues.4,5,24,25 Most recently, iron delivery and uptake mediated by 24p3/neutrophil gelatinase-associated lipocalin (NGAL) has been described. 59Fe-labeled 24p3/NGAL was shown to deliver 59Fe to developing kidney epithelial cells, with a pattern of receptor binding, trafficking, and topologic distribution that was distinct from that of Tf.26 The tissue distribution and developmental timing of 24p3/NGAL-mediated iron transport appear to be different from, but complementary to, those of Tf.26
24p3/NGAL-mediated iron delivery or the other TfR-independent pathways must function efficiently when the Tf cycle is not available for iron delivery. It is possible that these alternative routes are preferred in many tissues, and that the Tf cycle plays little, if any, normal role in iron uptake by most mammalian cells. However, it will be difficult to investigate that possibility further until the molecular mechanisms underlying these alternative uptake pathways have been fully elucidated.
The absence of TfR-/- cells in bone marrow and spleen of C57BL/6J chimeras, and the absence of strain 129 hemoglobin, revealed that TfR is required for adult erythropoiesis. The absence of TfR-/- cells in the spleen and thymus likewise showed, surprisingly, that lymphocyte development is also severely compromised in the absence of TfR.
There is a large body of literature on the roles of Tf and TfR in lymphocytes. Tf has long been known to be an essential factor for in vitro culture of lymphoid cells.27,28 Little, if any, TfR is detectable on the surface of resting, mature T and B lymphocytes, but it is markedly induced upon antigen and mitogen stimulation, presumably to support the iron needs of rapidly proliferating cells.29-36 Accordingly, anti-TfR antibodies have been shown to inhibit proliferation of stimulated peripheral lymphocytes.32,37,38 In mature T cells, TfR expression is induced in response to autocrine activity of interleukin-2 and modulated by cellular iron content.31,39-41
To further investigate the ability of TfR-/- cells to undergo normal lymphocyte differentiation, we took advantage of the sensitive RAG2-/- complementation assay. Under normal conditions, T-cell development proceeds through well-delineated stages, from thymic lymphoid progenitors to CD4-8-3- triple-negative substages distinguished by differing CD44 and CD25 expression, to CD4+CD8+ double-positive cells and then to mature CD4-8+ or CD4+CD8- single-positive T cells (for a review, see Zuniga-Pflucker and Lenardo42 ). TfR has been shown to be expressed only by proliferating thymocytes, including some subsets of CD4-8-3- (triple-negative) cells, and CD4+8+3- (double-positive) cells prior to expression of the T-cell receptor.43 In our TfR-/-RAG2-/- chimera analysis, we never detected TfR-/- T-lymphoid cells beyond the triple-negative stage, indicating a very early block in differentiation. This is consistent with experiments carried out with fetal thymus organ cultures, which have shown that anti-TfR antibodies arrest the development of αβ T cells at the CD4-8-3-44-25+ (early pre-T) stage, with very few cells developing to the double-positive CD4+8+3- stage.44
B-cell development also proceeds through well-defined stages, characterized by differential expression of distinct cell surface markers including CD43 and the μ heavy chain. Immature B cells have completed V(D)J recombination and express IgM, whereas fully mature B cells express both surface IgM and IgD (for reviews, see Rolink and Melchers45 and Osmond et al46 ). In contrast to T-cell development, however, there is little available information about the expression of TfR or its importance during B-cell development.
Although some of the B220+ cells seen in RAG2-/- chimeras may be natural killer cells and other non-B cells,9,47 detection of immunoglobulin heavy chain D-J rearrangements by PCR and identification of Ly9.1+IgM+ cells in the spleen and lymph nodes of 2 of the TfR-/- chimeras indicate that a considerable fraction of TfR-/-B220+ cells can pass the stage at which RAG2-/- lymphopoiesis is blocked. We interpret our observation that these cells were present in a substantial numbers in only one TfR-/-RAG-/- chimera, but absent in most other BM-positive TfR-/-RAG-/- chimeras, as an indication that absence of TfR impairs proliferation of early B-lymphoid precursors as well, but does not prevent their differentiation. The presence, but decreased number, of these B cells suggests that TfR is important during the stages in which B cells normally receive signals to proliferate (eg, after successful pairing of the VDJ-recombined Ig heavy chain with the surrogate light chain). It is likely that B-lymphoid precursors use TfR for iron acquisition to support proliferation. It has been estimated that maturation from the early pro-B stage (the point at which RAG2-/- mice are blocked) to the large pre-B stage (where VDJH recombination is complete and cells begin to express the pre-B-cell receptor) requires approximately 6 cell divisions.48-50 Perhaps B cells cannot effectively sequester storage iron51 or cannot otherwise obtain the iron necessary for these divisions in the absence of TfR.
Although we have observed that both T- and B-lymphoid development are impaired when cells are actively proliferating and might be expected to have increased iron needs, we cannot rule out the possibility that TfR plays some other role in lymphoid development. It is possible, for example, that TfR mediates an important signal transduction event. Such a role has previously been proposed for TfR in the activation of mature T cells,52 where it was shown that TfR interacts with the ζ chain of the T-cell receptor-CD3 complex and with ZAP70, a T-cell-specific protein tyrosine kinase. Stimulation with anti-CD3ϵ and with anti-TfR antibodies induces phosphorylation of both TfR and CD3ζ, presumably initiating a signaling cascade of unknown function. We cannot exclude the possibility that lymphocyte proliferation is required for proper differentiation, but there are examples of differentiation in the presence of moderately to severely affected proliferation.53-56
In summary, we have demonstrated that the TfR is not required for the development of most tissues and organs. However, TfR is required for adult erythropoiesis and has disparate roles in T- and B-lymphocyte development. B-cell maturation can proceed normally, although there appears to be a proliferation defect in the absence of TfR. In contrast, T lymphopoiesis is completely arrested at a very early stage of maturation. We speculate that TfR may play another role in T-cell development, in addition to iron delivery.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-04-1086.
Supported by National Health Institutes (NIH) grant R01 HL51057 (N.C.A.). R.M.N. was supported by a Ford Foundation predoctoral fellowship. N.C.A. is an Associate Investigator of the Howard Hughes Medical Institute. The Children's Hospital Gene Manipulation Facility is partially supported by NIH grant P30 HD18655.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We appreciate help from Fred Alt in supplying RAG2-/- mice, providing access to his RAG2-/- blastocyst injection services, and discussing the manuscript. RAG2-/- blastocyst injections were also performed at the Dana-Farber Cancer Institute DF/HCC Mouse Specialized Services Core Facility. We thank Prem Ponka for providing Fe-SIH and Joanne Levy for help and advice in the isolation of the TfR-/- ES cell clones. The wild-type C57BL/6 blastocyst injections were carried out by the staff at the Children's Hospital Gene Manipulation Facility. The Clinical Hematology Laboratory at Children's Hospital performed hemoglobin electrophoresis and isoelectric focusing. We appreciate discussions with, and advice from, other members of the Andrews group.