Abstract
Fibrinogen (FBG) assembles into matrix fibrils of fibroblasts, lung and mammary epithelial cells, but not endothelial cells. Furthermore, cryptic β15-21 residues are exposed in FBG fibrils with no evidence of thrombin or plasmin proteolysis. Herein, the effects of FBG on migration and proliferation of wounded dermal fibroblasts were investigated. FBG preassembled into matrix prior to scrape-wounding induced 3H-thymidine incorporation 8-fold and shortened the time to wound closure 1.6-fold ± 0.1-fold. FBG added immediately after wounding did not enhance either response. Fibroblast growth factor-2/platelet-derived growth factor (FGF-2/PDGF) stimulated cell proliferation 2.2-fold for FGF-2 and 3.2-fold for PDGF and wound closure 1.5-fold ± 0.1-fold in the absence of matrix-FBG. Surprisingly, exogenous growth factors had negligible effect on wound closure and cell proliferation already enhanced by matrix-FBG. Matrix-FBG-enhanced wound closure required active assembly of an FBG-fibronectin matrix, engagement of αvβ3, and FBG Aα-RGDS572-575 integrin recognition sites; Aα-RGDF95-98 sites were not sufficient for matrix-FBG assembly, enhanced wound closure, or cell proliferation. Although Bβ1-42 was not necessary for matrix assembly, it was required for matrix-FBG-enhanced cell migration. These data indicate that FBG serves as an important matrix constituent in the absence of fibrin formation to enhance wound repair and implicate Bβ1-42 as a physiologic inducer of signal transduction to promote an intermediate state of cell adhesion and a migratory cell phenotype. (Blood. 2003;102:4035-4043)
Introduction
Immediately following disturbances in homeostasis, such as infection, tissue injury, or immunologic disorders, the host responds by activation of a conserved set of nonadaptive defense mechanisms that constitute the innate immune system. These nonadaptive defenses include cellular immunity via activated neutrophils and macrophages, activation of complement, and induction of the acute phase response (APR).1 The APR is characterized by a series of local and systemic reactions that result in activation of various cell types to produce cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α). The cytokines in turn act on distant tissues and cells, resulting in fever, production of glucocorticoids, proliferation of cells of the immune system, and changes in synthesis of plasma proteins produced by the liver. Fibrinogen (FBG) is a well-characterized acute phase protein that is up-regulated as part of the innate immune response to inflammation.1 During coagulation, adhesive glycoproteins from plasma become incorporated into the fibrin clot by covalent cross-linking providing, in addition to the hemostatic plug, a scaffold for cell migration and proliferation; a reservoir for growth factors, proteases, and protease inhibitors; and a substrate for induction and modulation of cell function.2
Although FBG is regarded primarily for its hemostatic role in platelet aggregation and fibrin clot formation, we have shown that expression of the FBG genes and production of the intact protein occurs in pulmonary alveolar epithelial cells in response to inflammation.3,4 Furthermore, extrahepatic synthesis of intact FBG occurs in cervical epithelium5 and intestinal epithelial cells in response to inflammatory mediators.6 In addition to FBG, the acute phase proteins haptoglobin,7 serum amyloid A,8 α1-antitrypsin,9 and annexin I10 are up-regulated in lung during the APR to inflammation. Importantly, production of FBG, fibrin, and degradation products during inflammation and tissue injury, in excess of what is required to promote normal healing, modulates local immune function and interferes with wound repair (reviewed by Bini et al11 ).
Wound repair is an ordered process in which the production of proinflammatory cytokines by activated neutrophils and macrophages during the initial immune response induces both spatial and temporal changes in target gene expression at the site of injury. The local production of FBG by lung epithelium is regulated by IL-1β, IL-6, and glucocorticoids.3,12 Furthermore, the regulation of FBG expression in lung differs from that observed in the liver.12 The basolateral secretion of FBG by lung epithelial cells in response to glucocorticoids and IL-63,13 or during Pneumocystis carinii infection4 suggests that FBG synthesized locally at the site of injury, or derived from plasma due to increased vascular permeability, may incorporate into the provisional matrix. Previous studies have identified fibrillar strands within the provisional matrix of cutaneous and vascular wounds as fibrin.2 We have shown that both lung epithelial cell-derived and plasma FBG assemble into a pre-established, mature extracellular matrix (ECM) independently of conversion to fibrin, and matrix-FBG fibrils colocalize with other fibrillar proteins, including fibronectin (FN), laminin, and collagen type IV.14 FBG assembles into the ECM of fibroblasts, lung and mammary epithelial cells,14-17 and mouse embryonic cells18 but not quiescent endothelial cells (ECs) (P.J.S.-H. and S.O.L., unpublished observation, October 2002). Furthermore, fibroblast growth factor-2 (FGF-2) and vascular endothelial cell growth factor (VEGF) bind specifically and saturably to FBG and fibrin with high affinity,19,20 implicating FBG as a molecule essential to the maintenance of not only hemostasis, but homeostasis as well, by exerting biologic effects locally at sites of tissue injury. Taken together, these data suggest that FBG assembled into the ECM at sites of tissue damage contributes to cell type-specific mechanisms of wound repair. While extensive studies have elucidated the role of fibrin in the provisional matrix, little is known about the role of FBG independent of its conversion to fibrin in wound repair. Therefore, in this report we sought to determine how matrix-FBG affects cell migration and proliferation using an in vitro model of wound repair.
Materials and methods
Fibrinogen purification, fluorophore labeling, and immunodetection assays
Purified human FBG was purified further as previously described.21 Conjugation of FBG to Oregon Green Fluorophore was performed as described previously.22 FBG missing the C-terminal Aα-RGDS572-575 sites was chromatographically separated from intact FBG as described23 and designated herein as FBG-ΔAαC. Purified FBG missing the Bβ1-42 domain, designated FBG-325,24 was a kind gift from Dr. A. Budzynski (Temple University, Philadelphia, PA). Purified and matrix-incorporated FBG were characterized with the following FBG-specific monoclonal antibodies (MoAbs): anti-Bβ1-21 (18C6)25 and anti-β15-21 (T2G1)26 from Accurate Chemical & Scientific, Westbury, NY; anti-Bβ262-269 (D73H)27 and anti-FPA Aα1-16 (RDV3), a generous gift from Dr J. R. Shainoff, Cleveland State University, OH; and anti-Aα-RGDF95-98 (LJ155B16) and anti-Aα-RGDS572-575 (LJ134B29), generous gifts from Dr Z. Ruggeri, Scripps Research Institute, La Jolla, CA. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), enzyme-linked immunosorbent assay (ELISA), and Western blot analysis were performed as previously described.23,27
Wound repair model
Human foreskin fibroblast (HFF) primary cultures were isolated from foreskins obtained from a local hospital as previously described.28 All tissue collection was approved by the University of Rochester's internal review board for ethical use of discarded human tissues. The cells used in this study were between passage 18 and 24 from the original primary culture. HFFs were grown in McCoy 5A medium plus 10% fetal bovine serum on 12 mm diameter glass coverslips or tissue-culture 2-chamber slides precoated with 0.2% porcine gelatin. Fibroblasts were grown to confluence so that a mature, fibrillar endogenous ECM was deposited, typically 7 days after plating. To wound HFFs grown on coverslips, the conditioned medium was removed from the cell monolayers and saved and the cells were wounded by scraping down the center of each coverslip with a sterile P20-pipette tip. After wounding, the cells were washed twice in warm serum-free medium. The saved conditioned medium was supplemented with various combinations of FBG, growth factors, antibodies, or 3H-thymidine and then added back to its original culture and returned to the incubator. The size of the original wound space was measured on representative coverslips of cells fixed immediately after wounding by measuring the distance between wound edges in 9 places along the length of the wound using the IP Lab image analysis software (Scanalytics, Fairfax, VA); the initial wound space averaged 0.8 ± 0.02 mm wide. Three experimental conditions were examined: (1) CONTROL, cells not treated with FBG, growth factors, or antibodies and scrape-wounded on day 8 after seeding; (2) FBG-ATOW, cells scrape-wounded on day 8, saved conditioned medium supplemented with 30 or 40 μg/mL FBG, and immediately added back to original culture after time of wounding (ATOW); and (3) FBG-PRE, cells treated with 30 or 40 μg/mL FBG on day 7 and incubated for 24 hours, FBG-containing conditioned medium removed, cells wounded as described above, and then saved FBG-containing conditioned medium added back to original culture. To determine the time to close the wound, scrape-wounded CONTROL, FBG-ATOW, and FBG-PRE-treated HFFs were allowed to recover and repopulate the denuded space for 8, 16, and 24 hours after wounding. At time points after wounding, coverslips were removed, washed, fixed, and permeabilized.22 To visualize the cells, polymerized F-actin was stained with rhodamine-phalloidin (Molecular Probes, Eugene, OR) at a dilution of 1:40 in phosphate-buffered saline (PBS)/2% ovalbumin for 30 minutes at 37°C. FBG-Oregon Green and rhodamine-phalloidin were detected by direct epifluorescence microscopy. FBG or FN was detected by indirect immunofluorescence using purified rabbit antihuman FBG or antihuman FN immunoglobulin G (IgG), respectively, and fluorescein isothiocyanate (FITC)-conjugated goat antirabbit IgG as described.22 A Nikon Eclipse E800 microscope, Spot II CCD camera from Diagnostic Instruments (Sterling Heights, MI), and IP Lab software were used for image capture and analysis. The term “matrix-FBG” is defined as the result of soluble FBG assembled over 24 hours into mature matrix fibrils. In some experiments, either platelet-derived growth factor (PDGF) at 2 ng/mL or FGF-2 at 50 ng/mL (Life Technologies, Bethesda, MD) was added to cultures ATOW.
In situ 3H-thymidine autoradiography
HFFs were grown in tissue-culture chamber slides as described above. The cell monolayers were wounded from approximately the center of the chamber to the outer edge with one pass of a sterile Teflon cell scraper. The wounded cells were rinsed twice in warm serum-free medium to remove the lifted cells; the attached cells were incubated in complete medium containing 1 μCi/mL (0.037 MBq) 3H-thymidine as described.29 In some samples, FBG was added 24 hours prior to wounding (FBG-PRE) and was maintained in the culture during incubations with the 3H-thymidine; other samples were treated with FBG with or without growth factors or antibodies and 3H-thymidine ATOW. After 16 hours of incubation at 37°C, cultures were rinsed 3 times in PBS and fixed in 3.7% formaldehyde in PBS for 20 minutes at room temperature. The slides were rinsed in PBS, dehydrated in increasing concentrations of ethanol, and then air-dried. Nonwounded monolayers were also treated with FBG and 3H-thymidine as above to determine the background rate of incorporation in the absence of wounding. Dried slides were dipped in 37°C NTB-2 nuclear tract emulsion (Eastman Kodak, Rochester, NY) diluted 1:1 (vol/vol) in distilled water, dried in the dark at room temperature, placed in slide boxes with desiccant, and stored in a light-tight outer container at 4°C for 3 days. The slides were developed and counterstained in Meyers Hematoxylin as described.4 Cells were counted from triplicate samples per condition from 2 separate experiments. The data are presented as the percent of cells showing 3H-thymidine uptake per total number of cells derived from the same surface area, typically three 10 × fields for each condition.
Inhibition of de novo matrix assembly with blocking antibodies
HFFs treated as described above were further treated ATOW with blocking MoAb for 16 hours. To block engagement of integrin αvβ3, neutralizing MoAb LM609 was used at 10 μg/mL (Chemicon, Temecula, CA). To inhibit FN polymerization into matrix fibrils, MoAb 9D2, which binds to human FN type III1 repeat30 (a kind gift from Dr D. F. Mosher, University of Wisconsin, Madison), was used at 2 μg/mL. Nonimmune IgG1 at 10 μg/mL was used as a negative control.
Statistical analysis
The entire wound width was visible using the 10 × objective. The numbers of cells migrating into the wound space were counted in 3 low-power (10 ×) fields per coverslip, which essentially encompassed the entire length of the wound. Wound closure was expressed as the fold change above or below the CONTROL at 16 hours to which no FBG or growth factors were added for each experiment. The experiments were repeated 3 to 13 times, and 2-way analysis of variance (ANOVA) was performed using StatView software (Abacus Concepts, Beverly, CA). A P value less than .05 was considered statistically significant.
Results
Fibrinogen preassembled into the ECM of fibroblasts enhances wound closure
To determine the time to close the wound, CONTROL, FBG-ATOW, and FBG-PRE-treated cells were allowed to recover and repopulate the denuded space for 8, 16, and 24 hours after wounding; the wound was completely closed in all conditions by 36 hours (not shown). As soon as 8 hours after wounding, FBG-PRE showed statistically significant enhanced wound closure over CONTROL (P < .05), while the CONTROL and FBG-ATOW treatments showed a similar but slower rate of wound closure (Figure 1A). Matrix-FBG shortened the time to wound closure in a statistically significant manner at 16 hours (P < .0001) and 24 hours (P < .02) after wounding, again when FBG was preassembled into the ECM prior to wounding (Figure 1A and Table 1). A representative image of actin-phalloidin-stained cells moving into the wound space is shown for each condition at 16 hours after wounding (Figure 1B). Actin stress fibers in cells at or near the wound margin were oriented perpendicular to the wound edge and in lamellipodia at the leading edge of the cell, indicating polarized movement onto the wound space (not shown). Furthermore, FBG-PRE-increased wound closure was statistically significant over FBG-ATOW at all time points (8 hours and 24 hours, P < .05; 16 hours, P < .0001). The effects of different treatments on the rate of wound closure were performed at 16 hours after wounding in subsequent experiments.
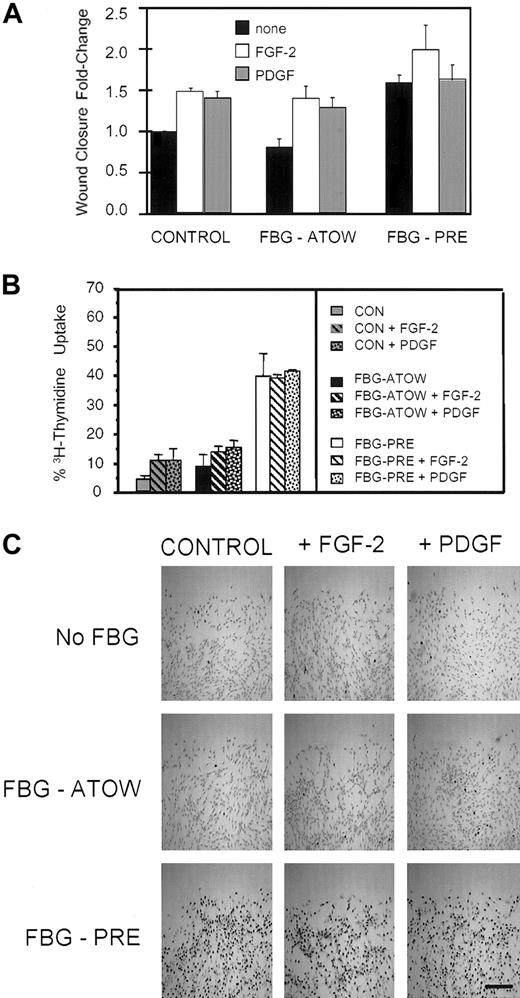
FBG preassembled into the ECM of fibroblasts enhances wound closure. Fibroblasts were left untreated (CONTROL), treated with 40 μg/mL FBG immediately after the time of wounding (FBG-ATOW), or treated with FBG 24 hours prior to wounding (FBG-PRE). (A) At 8, 16, and 24 hours after wounding, the numbers of cells migrating into the denuded space were quantified as described in “Materials and methods.” The data are presented as the mean ± SEM; n = 7 to 13 per condition. (B) The effect of FBG treatment on cell migration into the wound space was visualized by low-power microscopy in CONTROL cells (left), FBG-ATOW-treated (middle), and FBG-PRE-treated (right) cells 16 hours after wounding. The original wound margins are denoted by dotted lines. Scale bar represents 50 μm. (C) Fibroblasts were treated with or without FBG as described above. 3H-thymidine was added ATOW to the culture media. A representative low-power field of each treatment condition at 16 hours after wounding is shown. Positive DNA synthesis is denoted by the presence of black-appearing silver grains over the nuclei of actively proliferating cells. Scale bar represents 100 μm.
FBG preassembled into the ECM of fibroblasts enhances wound closure. Fibroblasts were left untreated (CONTROL), treated with 40 μg/mL FBG immediately after the time of wounding (FBG-ATOW), or treated with FBG 24 hours prior to wounding (FBG-PRE). (A) At 8, 16, and 24 hours after wounding, the numbers of cells migrating into the denuded space were quantified as described in “Materials and methods.” The data are presented as the mean ± SEM; n = 7 to 13 per condition. (B) The effect of FBG treatment on cell migration into the wound space was visualized by low-power microscopy in CONTROL cells (left), FBG-ATOW-treated (middle), and FBG-PRE-treated (right) cells 16 hours after wounding. The original wound margins are denoted by dotted lines. Scale bar represents 50 μm. (C) Fibroblasts were treated with or without FBG as described above. 3H-thymidine was added ATOW to the culture media. A representative low-power field of each treatment condition at 16 hours after wounding is shown. Positive DNA synthesis is denoted by the presence of black-appearing silver grains over the nuclei of actively proliferating cells. Scale bar represents 100 μm.
The results of in situ 3H-thymidine incorporation indicated that FBG enhanced the number of proliferating cells only when FBG was pre-established in the cell matrix 24 hours prior to wounding (Figure 1C and Table 1). Control experiments, in which cells were not wounded but FBG was added for 24 hours and then incubated an additional 16 hours to mimic the post-wounding time to repair, did not show enhanced DNA synthesis over CONTROL (not shown).
Exogenous growth factors do not affect matrix-FBG-enhanced wound closure
Both FGF-2 and PDGF are released from cells at sites of wound injury, are mitogenic for fibroblasts, and FGF-2 is necessary for appropriate wound healing.31 Therefore, to determine whether addition of exogenous growth factors could further enhance matrix-FBG stimulation of wound closure and proliferation, FGF-2 or PDGF was added ATOW to fibroblasts untreated, treated with FBG-ATOW, or treated with FBG for 24 hours prior to wounding. At 16 hours after wounding, FGF-2 and PDGF significantly stimulated wound closure (Figure 2A and Table 1) in CONTROL cells (FGF-2 and PDGF, P < .002) and fibroblasts treated with FBG-ATOW (FGF-2 and PDGF, P < .002). In the absence of either growth factor, FBG-PRE treatment increased wound closure by 1.6-fold ± 0.1-fold (P < .0001) and the mitotic index by 8-fold over CONTROL cells (Figure 2A and Table 1). Furthermore, the addition of either growth factor had negligible effect on further enhancement of either wound closure or cell proliferation as measured by 3H-thymidine uptake in the FBG-PRE condition. In contrast, growth factor treatment resulted in 2.2-fold to 3.2-fold increase in the number of cells showing 3H-thymidine uptake in CONTROL or FBG-ATOW conditions (Figure 2 and Table 1), but this increase in cell proliferation was significantly less than in the FBG-PRE-alone condition. The lack of mitogenic effect of FGF-2 and PDGF on matrix-FBG-PRE-treated cells is strikingly apparent, as demonstrated by the density of silver grain deposition over the nuclei in the FBG-PRE with or without growth factor treatment conditions (Figure 2C).
Exogenous FGF-2 and PDGF have no effect on wound closure enhanced by matrix-FBG. After the time of wounding, no growth factor (none), FGF-2 (50 ng/mL), or PDGF (2 ng/mL) was added to fibroblasts left untreated (CONTROL), treated with FBG-ATOW, or treated with FBG 24 hours prior to wounding (FBG-PRE). (A) At 16 hours after wounding, the cells were fixed and stained with rhodamine-phalloidin to visualize actin filaments in order to count the cells that had migrated into the wound space. The data are presented as the mean ± SEM; n = 5 to 7 per condition. (B) The fibroblasts were treated with or without FBG and with or without growth factors as described above; however, ATOW 3H-thymidine was also added to the culture media. At 16 hours after wounding, the cells were fixed, dehydrated, and dipped into NTB-photographic silver emulsion to detect cells in S phase by the deposition of silver grains over the nuclei that have actively incorporated 3H-thymidine. The data are presented as the mean ± SEM; n = 5 to 6 per condition from 2 separate experiments. (C) A representative low-power field of each treatment condition is shown. Positive DNA synthesis is denoted by the presence of black-appearing silver grains over the nuclei of actively proliferating cells. Scale bar represents 125 μm.
Exogenous FGF-2 and PDGF have no effect on wound closure enhanced by matrix-FBG. After the time of wounding, no growth factor (none), FGF-2 (50 ng/mL), or PDGF (2 ng/mL) was added to fibroblasts left untreated (CONTROL), treated with FBG-ATOW, or treated with FBG 24 hours prior to wounding (FBG-PRE). (A) At 16 hours after wounding, the cells were fixed and stained with rhodamine-phalloidin to visualize actin filaments in order to count the cells that had migrated into the wound space. The data are presented as the mean ± SEM; n = 5 to 7 per condition. (B) The fibroblasts were treated with or without FBG and with or without growth factors as described above; however, ATOW 3H-thymidine was also added to the culture media. At 16 hours after wounding, the cells were fixed, dehydrated, and dipped into NTB-photographic silver emulsion to detect cells in S phase by the deposition of silver grains over the nuclei that have actively incorporated 3H-thymidine. The data are presented as the mean ± SEM; n = 5 to 6 per condition from 2 separate experiments. (C) A representative low-power field of each treatment condition is shown. Positive DNA synthesis is denoted by the presence of black-appearing silver grains over the nuclei of actively proliferating cells. Scale bar represents 125 μm.
Matrix-FBG-enhanced wound repair is dependent on active assembly of an ECM
Fibroblasts bind to the RGD sequences in the Aα chain of FBG via αvβ3 integrin receptors.32 To determine whether engagement of αvβ3 is required to support matrix-FBG-enhanced wound closure, we used MoAb LM609 to block RGD-dependent ligand binding to the αvβ3 receptor complex. The results show that neutralization of αvβ3 with LM609 inhibited deposition of both FN and FBG into mature matrix fibrils (Figure 3Aii,Av). This, in turn, inhibited wound closure by 53% in CONTROL (P < .0001), 32% in FBG-ATOW (P < .003), and 20% in FBG-PRE (not significant) treatment conditions (Figure 3B,Cii,Cv) compared with CONTROL plus nonimmune IgG1. However, compared with FBG-PRE plus nonimmune IgG1, FBG-PRE plus 9D2 treatment significantly inhibited wound closure by 60% (P < .015). We have shown previously that FBG coaligns with FN fibrils in the ECM of fibroblasts and epithelial cells14 and that assembly of FBG into mature matrix fibrils is dependent on the active assembly of an FN matrix.18 To determine whether enhanced wound closure is dependent upon continued formation of an FN-FBG matrix, we used MoAb 9D2, which recognizes a cryptic site on the FN type III1 domain necessary for homophilic self-polymerization of FN into matrix fibrils,30 to inhibit both FN and FBG matrix assembly. The results indicate that 9D2 inhibited FN and FBG assembly into matrix fibrils (Figure 3Aiii,Av). In addition, wound closure was inhibited by 27% in CONTROL (P < .01), 20% in FBG-ATOW (P < .003), and 15% in the FBG-PRE (Figure 3B,Ciii,Cv and Table 1) treatment conditions compared with CONTROL with no treatment (P < .05). Compared with FBG-PRE plus nonimmune IgG1, FBG-PRE plus LM609 treatment significantly inhibited wound closure by 68% (P < .05). These results indicate that the continued assembly of FBG and FN into matrix fibrils involving engagement of αvβ3 is required to support dermal fibroblast wound closure in all conditions but is most pronounced in the FBG-PRE condition.
Matrix-FBG-enhanced wound repair is dependent on integrin activation and active assembly of FN and FBG in the ECM. (A) HFFs were treated with 40 μg/mL FBG-Oregon Green plus 10 μg/mL either nonimmune IgG1 (i,iv), anti-αvβ3 (LM609; ii,v), or 2 μg/mL anti-FN-III1 (9D2; iii,vi) IgG1 for 24 hours. (A) The intensity and pattern of FN fibrils was detected by indirect immunofluorescence (i-iii), and FBG-Oregon Green assembled into matrix fibrils was visualized by direct epifluorescence (iv-vi). Scale bar represents 25 μm. (B) Fibroblasts treated with nonlabeled FBG and nonimmune IgG1, LM609 (anti-αvβ3), or 9D2 (anti-FN-III1) MoAb were wounded, and the number of cells migrating into the denuded space over 16 hours was quantified as described in “Materials and methods.” The data are presented as the mean ± SEM; n = 4 to 6 per condition from 3 separate experiments. (C) The effect of antibody treatment on wound closure was visualized by staining F-actin and low-power microscopy in CONTROL cells (i-iii) and FBG-PRE-treated cells (iv-vi). Scale bar represents 40 μm.
Matrix-FBG-enhanced wound repair is dependent on integrin activation and active assembly of FN and FBG in the ECM. (A) HFFs were treated with 40 μg/mL FBG-Oregon Green plus 10 μg/mL either nonimmune IgG1 (i,iv), anti-αvβ3 (LM609; ii,v), or 2 μg/mL anti-FN-III1 (9D2; iii,vi) IgG1 for 24 hours. (A) The intensity and pattern of FN fibrils was detected by indirect immunofluorescence (i-iii), and FBG-Oregon Green assembled into matrix fibrils was visualized by direct epifluorescence (iv-vi). Scale bar represents 25 μm. (B) Fibroblasts treated with nonlabeled FBG and nonimmune IgG1, LM609 (anti-αvβ3), or 9D2 (anti-FN-III1) MoAb were wounded, and the number of cells migrating into the denuded space over 16 hours was quantified as described in “Materials and methods.” The data are presented as the mean ± SEM; n = 4 to 6 per condition from 3 separate experiments. (C) The effect of antibody treatment on wound closure was visualized by staining F-actin and low-power microscopy in CONTROL cells (i-iii) and FBG-PRE-treated cells (iv-vi). Scale bar represents 40 μm.
To determine the effects of these neutralizing antibodies on DNA synthesis, the percentage of FBG-PRE cells showing 3H-thymidine uptake in the presence of nonimmune IgG1, LM609, or 9D2 was compared with the percentage of CONTROL cells similarly treated. The mitotic index was calculated by dividing the percentage of cells showing 3H-thymidine uptake due to the specified treatment by the percentage of CONTROL cells showing 3H-thymidine uptake (Table 1). Neutralization of αvβ3 engagement by LM609 reduced the mitotic index from 8.0 to 0.75 in FBG-PRE (P < .0001) and from 1.0 to 0.5 in the CONTROL (P < .0001) cells (Table 1). Compared with CONTROL plus nonimmune IgG1, inhibition of FN and FBG assembly into the matrix reduced the mitotic index in both CONTROL plus 9D2 (0.75) (P = .003) and FBG-PRE plus 9D2 (0.64) (P = .0056) -treated cells (Table 1).
The role of FBG cell-binding domains in mediating enhanced wound closure
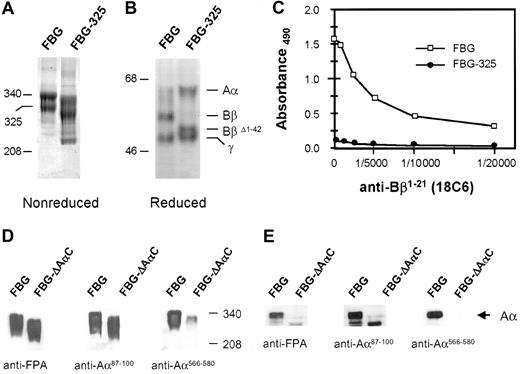
The dimeric structure of FBG is important in its role as a bridging molecule between cells and adhesion of cells to the ECM.11 Among the many cell-binding domains identified on the FBG Aα, Bβ, and γ chains,21 the Aα chain RGD integrin-binding domains and the fibrin(ogen) heparin-binding domain (HBD) contained within Bβ1-42 have been shown to mediate cell-matrix binding interactions (reviewed by Bini et al11 ). Furthermore, we have shown that the deposition of FBG into the ECM occurs in a heparan sulfate proteoglycan (HSPG)-dependent manner.15 These findings suggested that FBG residues Bβ1-42 and Aα RGD sequences play a role in matrix-FBG-mediated enhancement of wound closure. Thus, we used FBG-325 to examine the functional significance of Bβ1-42 in the assembly of FBG into mature matrix fibrils and to support matrix-FBG-mediated enhancement of wound closure. FBG-325 was characterized by SDS-PAGE and Coomassie staining and by ELISA to detect the presence or absence of the FBG Bβ1-21 domain. The data show that FBG-325 migrates faster under nonreducing conditions by SDS-PAGE (Figure 4A) and that upon reduction only the Bβ chain migrates at a lower apparent Mr (Figure 4B). The fastest migrating band observed in nonreduced FBG-325 likely represents a small portion of the FBG lacking both the Bβ1-42 region and the protease-sensitive AαC domain. Prolonged digestion of FBG with Protease III from Crotalus atrox leads to cleavage of the AαC domain subsequent to cleavage of Bβ1-42 (not shown). ELISA indicated that Bβ1-21 was essentially undetectable in FBG-325 at all dilutions of MoAb 18C6 tested, whereas the Bβ1-21 epitope was still detectable in intact FBG at a 1:20 000 dilution of 18C6 (Figure 4C).
Characterization of FBG-325 and FBG-ΔAαC. FBG-325 was analyzed by SDS-PAGE under nonreducing (A) and reducing (B) conditions followed by Coomassie blue staining. The positions of migration of intact FBG (340 kDa), FBG-325 (325 kDa), and reduced FBG Aα, Bβ, BβΔ1-42 , and γ chains are marked. ELISA (C) was performed using serial dilutions of MoAb 18C6 to detect the presence (FBG, □) or absence (FBG-325, •) of Bβ1-21 . Western blot analysis using MoAbs against FPA, Aα87-100 containing the RGDF95-98 domain, and Aα566-580 containing the RGDS572-575 domain was performed on FBG and FBG-ΔAαC resolved under nonreducing (D) or reducing (E) conditions.
Characterization of FBG-325 and FBG-ΔAαC. FBG-325 was analyzed by SDS-PAGE under nonreducing (A) and reducing (B) conditions followed by Coomassie blue staining. The positions of migration of intact FBG (340 kDa), FBG-325 (325 kDa), and reduced FBG Aα, Bβ, BβΔ1-42 , and γ chains are marked. ELISA (C) was performed using serial dilutions of MoAb 18C6 to detect the presence (FBG, □) or absence (FBG-325, •) of Bβ1-21 . Western blot analysis using MoAbs against FPA, Aα87-100 containing the RGDF95-98 domain, and Aα566-580 containing the RGDS572-575 domain was performed on FBG and FBG-ΔAαC resolved under nonreducing (D) or reducing (E) conditions.
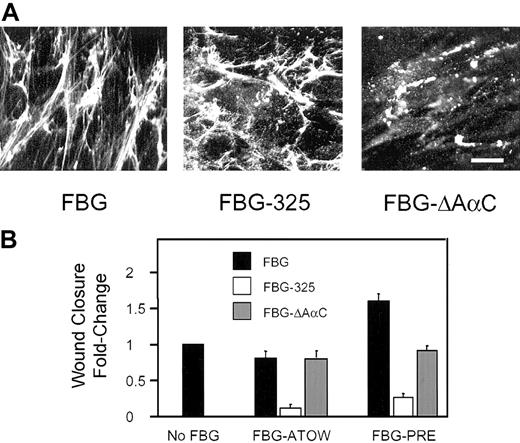
Although we showed that assembly of FBG into matrix fibrils requires cell surface-HSPG binding interactions,15 we did not determine whether the Bβ1-42 domain was involved. To determine the role of Bβ1-42 in FBG assembly into ECM, FBG-325 was added to confluent monolayers of fibroblasts and incubated for 24 hours. The results show that FBG-325 assembly into mature matrix fibrils occurred in the absence of Bβ1-42 (Figure 5A), suggesting that the HSPG-dependent assembly of FBG into matrix fibrils does not involve the HBD within the FBG Bβ1-42 sequence. This interpretation is further supported by the finding that the assembly of FN into the ECM is dependent on cell surface-HSPG interactions.33 Because FBG assembly into matrix fibrils is dependent on active assembly of an FN matrix,18 the HSPG-dependent assembly of FBG into fibrils is likely secondary to the role of HSPG in support of FN deposition. In contrast, the FBG Bβ1-42 region is required for matrix-FBG-enhanced wound closure as shown in the wound-scrape model of tissue injury (Figure 5B and Table 1). FBG-325-ATOW reduced wound closure 7-fold (P < .0001) compared with CONTROL and 5.6-fold compared with intact FBG-ATOW (P < .0001). When FBG-325 was added 24 hours prior to wounding, wound closure was reduced 4-fold compared with CONTROL (P < .0001) and 6.4-fold compared with intact FBG-PRE (P < .0001) (Figure 5B and Table 1). Furthermore, the FBG-PRE mitotic index fell from 8.0 to 0.9 for FBG-325-PRE (P < .0001) in the 3H-thymidine uptake assay (Table 1).
The role of FBG Aα-RGD and Bβ1-42 cell-binding domains in mediating enhanced wound closure. Confluent monolayers of HFFs were treated with 30 μg/mL FBG, FBG-325, or FBG-ΔAαC either ATOW or 24 hours prior to wounding, FBG-PRE. (A) The intensity and pattern of FBG (left), FBG-325 (middle), and FBG-ΔAαC (right) assembled into matrix fibrils was visualized by indirect immunofluorescence. Scale bar represents 25 μm. (B) At 16 hours after wounding, wound closure was quantified as described in “Materials and methods.” The data are presented as the mean ± SEM; n = 3 to 5 per condition from 3 independent experiments.
The role of FBG Aα-RGD and Bβ1-42 cell-binding domains in mediating enhanced wound closure. Confluent monolayers of HFFs were treated with 30 μg/mL FBG, FBG-325, or FBG-ΔAαC either ATOW or 24 hours prior to wounding, FBG-PRE. (A) The intensity and pattern of FBG (left), FBG-325 (middle), and FBG-ΔAαC (right) assembled into matrix fibrils was visualized by indirect immunofluorescence. Scale bar represents 25 μm. (B) At 16 hours after wounding, wound closure was quantified as described in “Materials and methods.” The data are presented as the mean ± SEM; n = 3 to 5 per condition from 3 independent experiments.
To determine whether regions of the FBG Aα chain containing the RGD domains are required to support assembly of FBG into mature matrix fibrils and to enhance wound repair, FBG missing the protease sensitive C-terminal portion of the Aα chain (FBG-ΔAαC) was used in the wound repair assay. Western blot analysis of FBG and FBG-ΔAαC under nonreducing (Figure 4D) or reducing (Figure 4E) conditions revealed that more than 90% of the immunoreactive FBG was missing the C-terminal RGDS572-575 site while retaining the NH2-terminal FPA residues and the RGDF95-98 site. In contrast to FBG-325, FBG-ΔAαC was incapable of assembling into mature matrix fibrils; however, FBG-ΔAαC bound to cells in discrete patches, suggesting receptor binding and/or protein-protein interactions at the cell surface (Figure 5A). The addition of FBG-ΔAαC-ATOW had essentially no effect on the rate of wound closure. When FBG-ΔAαC was added 24 hours prior to wounding, the 1.6-fold ± 0.1-fold enhanced wound closure observed in the intact FBG-PRE condition was significantly reduced to near CONTROL values (P < .01) observed in the absence of added FBG (Figure 5B and Table 1). In the presence of FBG-ΔAαC-PRE, the mitotic index was reduced to 0.65 (Table 1). Together, these results suggest that matrix-FBG-enhanced cell proliferation requires both the Bβ1-42 and the AαC domain of FBG and that the presence of the AαC domain in FBG-325 is not sufficient for promoting either cell proliferation or cell migration.
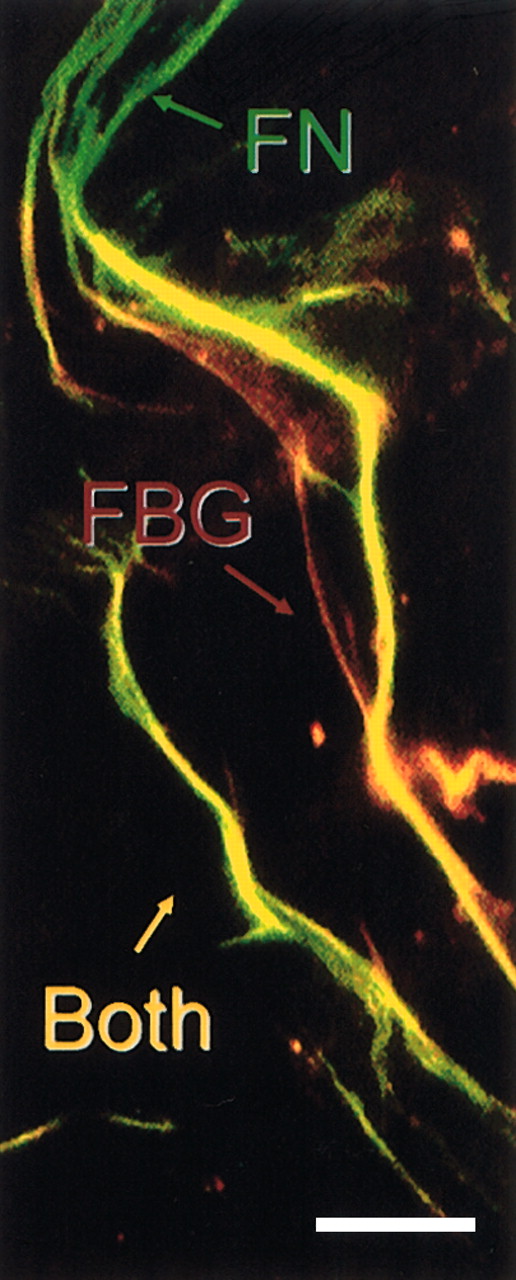
We have shown previously that both FBG-specific (fibrinopeptides A and B) and fibrin-specific epitopes (the T2G1 epitope at β15-21 ) are exposed in matrix-FBG, with no evidence of thrombin or plasmin proteolysis or covalent cross-linking into higher molecular weight multimers.14 To determine whether the β15-21 “fibrinlike” epitope is exposed along the length of FBG fibrils that colocalize with FN matrix fibrils, postconfluent fibroblasts were treated with plasma FBG at 40 μg/mL for 24 hours and immunostained with MoAb T2G126 and polyclonal antihuman FN. The results indicate that β15-21 is exposed in FBG matrix fibrils that coalign uniformly with FN fibrils (Figure 6). Thus, the functionally important FBG Bβ1-42 region is exposed and regularly distributed throughout the matrix.
The cryptic fibrinlike β15-21 epitope is exposed in matrix-FBG fibrils that colocalize with FN fibrils. Confluent monolayers of HFFs were treated with purified plasma FBG at 40 μg/mL for 24 hours. FBG matrix fibrils were detected with MoAb T2G1 (red fluorescence), which recognizes the neo-NH2-terminus (β15-21 ) of the fibrin β chain, and FN matrix fibrils (green fluorescence) were detected with polyclonal antihuman FN IgG. Very few isolated fibrils of FN (green) or FBG (red staining) are found in the matrix; most FBG colocalizes with FN fibrils (yellow fluorescence), indicating that the cryptic β15-21 epitope is exposed in FBG matrix fibrils distributed along the length of FN matrix fibrils. Scale bar represents 25 μm.
The cryptic fibrinlike β15-21 epitope is exposed in matrix-FBG fibrils that colocalize with FN fibrils. Confluent monolayers of HFFs were treated with purified plasma FBG at 40 μg/mL for 24 hours. FBG matrix fibrils were detected with MoAb T2G1 (red fluorescence), which recognizes the neo-NH2-terminus (β15-21 ) of the fibrin β chain, and FN matrix fibrils (green fluorescence) were detected with polyclonal antihuman FN IgG. Very few isolated fibrils of FN (green) or FBG (red staining) are found in the matrix; most FBG colocalizes with FN fibrils (yellow fluorescence), indicating that the cryptic β15-21 epitope is exposed in FBG matrix fibrils distributed along the length of FN matrix fibrils. Scale bar represents 25 μm.
Discussion
The β15-42 NH2-terminus exposed by thrombin cleavage of the FBG Bβ chain can be identified by reactivity with MoAb T2G1, which recognizes β15-21 in fibrin but not soluble or surface-immobilized FBG.26 The functional significance of exposure of the β15-21 epitope is underscored by data from Sporn et al and Bruce et al demonstrating that the exposure of β15-42 promotes cell adhesion, spreading and proliferation of ECs and fibroblasts,34,35 cell adhesion and spreading of platelets,36 and release of stored von Willebrand factor from Weibel-Palade bodies of ECs.37 In addition, we have shown that exposure of a cryptic HBD at β15-42 is enhanced by thrombin cleavage of the FBG Bβ chain.38 Furthermore, we show in this report that the β15-21 epitope is exposed in FBG that is assembled along with FN into matrix fibrils, leaving the β15-42 HBD on matrix-FBG accessible for receptor-mediated binding interactions. Taken together, these data indicate that the exposure of β15-42 at sites of injury serves as a physiologic inducer of cell activation.
Disruptions in homeostasis induce cells to respond to local stimuli that bring about alterations in cellular morphology, recruitment of inflammatory cells, the release of stored constituents, and modulation of target gene expression because of, and leading to, cell-cell and cell-ECM communication. Animal models of cutaneous wounds frequently serve as a paradigm for tissue organization and healing. Wound fibroblasts go through dynamic changes in gene expression of matrix constituents and altered expression of cell surface receptors leading to an intermediate state of cell adhesion, followed by reorganization of the actin cytoskeleton and enhanced matrix deposition leading to planar cell polarity, wound contraction, and a strong state of cell adhesion to bring about resolution of wound injury.39 The integrity of the barrier function of highly polarized cells is disrupted during various types of injury. Because exposure of the β15-42 domain is necessary for fibrin-mediated EC sprouting during angiogenesis,40 we reasoned that exposure of this domain in matrix-FBG would play a role in enhancing wound closure. Therefore, we used an in vitro model of tissue injury to elucidate the role of matrix-FBG in modulating the time to wound closure and cell proliferation during wound repair.
The data in this report show that matrix-FBG significantly shortens the time required to achieve wound closure. The enhanced wound closure induced by matrix-FBG was essentially not affected by the addition of exogenous growth factors, whereas both FGF-2 and PDGF significantly enhanced wound closure and proliferation of CONTROL cells. The addition of exogenous growth factors stimulated 3H-thymidime uptake to a greater extent than wound closure in all conditions, but the mitotic index of FGF-2 and PDGF on CONTROL cells or cells treated with FBG-ATOW was only 2.2 to 3.2, whereas the mitotic index for FBG-PRE in the absence of added growth factors was 8.0. No additional increase in DNA synthesis was induced by added growth factors in the FBG-PRE condition, suggesting that “preconditioning” matrix with FBG stimulates synthesis and/or release of endogenous growth factors leading to autocrine cell proliferation. These data indicate that cell adhesion to matrix-FBG plays a role in signal transduction leading to increased cell proliferation.
The FN integrin receptor α5β1 plays a crucial role in mediating assembly of FN into the ECM,41 presumably by regulating a series of sequential self-interactions that result in the polymerization of FN.42 Although some evidence suggests that FBG binds to Mn2+-activated α5β1 on ECs,43 fibroblasts bind preferentially to FBG via αvβ3.32 Moreover, engagement of αvβ3 promotes cell migration during metastasis, angiogenesis, and wound repair.32,44-46 Therefore, to determine whether engagement of αvβ3 plays a role in enhanced wound repair of dermal fibroblasts, MoAb LM609 was used to inhibit RGD-dependent ligand binding to activated αvβ3. The results show that engagement of αvβ3 was required to mediate wound closure in CONTROL cells, indicating that cell binding to RGD-dependent ligands of the endogenous matrix, such as FN, promotes normal wound closure. A requirement for FN in wound closure is further supported by the data showing that neutralization of αvβ3 ligation reduced assembly of endogenous FN into mature matrix fibrils in CONTROL cells; the immature matrix is composed of shorter, thinner fibrils and stitchlike fragments of FN.18 In mouse embryonic fibroblasts rendered null for expression of the a5 integrin subunit, αvβ3 provides an alternative pathway for the assembly of soluble FN into the ECM by a process independent of α5β1.47
LM609 inhibited assembly of FBG into mature matrix fibrils as well, which resulted in inhibition of matrix-FBG-enhanced wound closure. However, these data did not distinguish between the role of αvβ3 integrin activation and active assembly of FN into the ECM in the regulation of matrix-FBG-enhanced wound repair. Therefore, to determine whether active assembly of an FN-containing ECM was necessary for matrix-FBG enhancement of wound closure, we used MoAb 9D2, which blocks homophilic interactions of the FN-III1 module required to promote FN fibril formation. This MoAb does not inhibit binding of FN to cell surface matrix assembly sites or integrin receptors; nor does 9D2 bind to the RGD cell-binding domain of FN.30 We have shown previously that inhibiting FN fibrillogenesis with MoAb 9D2 also inhibits the assembly of FBG into matrix fibrils.18 The data indicate that by inhibiting active assembly of FN fibrils and subsequent FBG matrix assembly, wound closure is slightly reduced in CONTROL cells but is dramatically reduced in the FBG-PRE condition. Together, these data indicate that wound closure depends to a small extent on active assembly of an FN-containing ECM but that the enhanced wound closure induced by matrix-FBG depends on the assembly of FBG into matrix fibrils in an FN-dependent manner involving activated αvβ3.
Inhibition of αvβ3 engagement in the FBG-PRE condition decreased the mitotic index 10-fold, but the relative rate of wound closure was decreased only 2.25-fold. In contrast, inhibition of αvβ3 engagement in CONTROL cells decreased the mitotic index by 2-fold, which corresponded with the 2.1-fold reduction in the rate of wound closure. However, the rate of wound closure in the FBG-PRE plus LM609-treated cells still showed a 1.6-fold enhancement over wound closure in the CONTROL plus LM609-treated cells, indicating that matrix-FBG enhances cell migration in the absence of enhanced cell proliferation to close the wound at a faster rate. Furthermore, inhibition of FN and FBG assembly in the ECM by MoAb 9D2 reduced the matrix-FBG-enhanced wound closure, indicating that the cell-mediated process of assembling FBG into the ECM is required to promote enhanced wound closure.
To examine the functional significance of Bβ1-42 in the ability of FBG to assemble into mature matrix fibrils and to support matrix-FBG-mediated enhancement of wound closure, we used FBG-325 in which the Bβ1-42 residues containing the HBD are deleted. Although FBG Bβ1-42 was not necessary for assembly of FBG into matrix fibrils, the lack of FBG Bβ1-42 significantly inhibited wound closure regardless of whether FBG was added after the time of wounding or preincubated with cells 24 hours prior to wounding. In FBG-325-PRE-treated cells, proliferation was equivalent to CONTROL cells but significantly reduced compared with intact FBG-PRE cells. These data suggest that FBG lacking Bβ1-42 exerts stronger cell adhesive forces to significantly reduce cell migration into the wound space (eg, RGD-mediated adhesion). Thus, both cell proliferation and migration require the presence of FBG Bβ1-42 sequences, consistent with the data showing that exposure of β15-42 induces proliferation of ECs and fibroblasts.34,35
Because the endogenous ECM of fibroblasts contains, in addition to FN, other RGD-containing ligands such as thrombospondin, vitronectin, tenascin (TN), and laminin,48 we could not conclude that Aα chain RGD domains in matrix-FBG were responsible for αvβ3-dependent enhanced wound closure. Therefore, we used FBG-ΔAαC lacking the AαC domain containing the Aα-RGDS572-575 site while retaining the Aα-RGDF95-98 domain to determine whether Aα chain sequences containing either of these RGD domains play a role in assembly of FBG into matrix fibrils and/or support of matrix-FBG-enhanced wound repair. The data indicate that the FBG Aα-RGDS572-575 site is required and that the Aα-RGDF95-98 domain is not sufficient to support assembly of FBG into the ECM. Although FBG-ΔAαC bound to cell surfaces, in the absence of FBG, fibrillogenesis wound closure was reduced to CONTROL levels and cell proliferation was reduced to below CONTROL levels. These data suggest that active assembly of FBG into the ECM requires Aα-RGDS572-575 ligation of αvβ3. In addition, enhanced wound closure requires the presence of Bβ1-42 NH2-termini and fibrillogenesis of FBG into mature matrix fibrils. These data suggest that β15-42 region containing the HBD, which is exposed in matrix-FBG, transduces signals to promote a less adhesive cell phenotype as shown for TN-C.49
In contrast to the severe or lethal phenotypes seen in mice with targeted deletions in genes encoding VEGF, αv, or FN, mice with targeted disruptions in the FBG Aα 50 or γ chain51 genes show no evidence of fetal loss and are born normal in appearance. Besides the lack of FBG-mediated platelet aggregation and fibrin formation, the most notable defect is the inability of homozygous null females to carry a pregnancy to term. Pregnancy in FBG-/- mice uniformly results in fatal uterine bleeding around the 10th day of gestation.50 Thus, heterozygous females are mated to produce the FBG-null animals. During cutaneous wound healing, the FBG-deficient mice show an abnormal pattern of tissue repair, including misguided and hypertrophied epithelium, enhanced collagen deposition, delayed wound closure, and reduced wound tensile strength.52 The most profound defect in tissue organization found in FBG-deficient mice is the inability of cells to efficiently organize and migrate into the wound space. Although wound repair ultimately proceeds in a relatively normal manner in the absence of fibrin(ogen) or its biologically active fragments derived from proteolysis, the data show that fibrin(ogen) is important for appropriately directed cellular migration in a temporal and spatial manner within the wound space and to establish wound strength and stability.52
We show in this report that matrix-FBG enhances both wound closure and cell proliferation to significantly shorten the time to wound closure in a dermal fibroblast model of tissue injury. Specific cell-binding domains on matrix-FBG are required to support accelerated wound closure. In this context, FBG demonstrates properties of matricellular proteins such as TN-C, thrombospondins, and soluble syndecans.53 These matricellular proteins play an important role in modulating the strength of cell adhesion, signal transduction, and changes in target gene expression by modifying the composition of the ECM to promote wound healing in a cell type- or tissue-specific manner.53,54 Altogether, the data in this report indicate that matrix-FBG functions in wound repair in a manner analogous to matricellular proteins, and we propose, henceforth, that matrix-FBG belongs to this specialized class of matrix constituents.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-03-0822.
Supported by grants HL50615 and HL30616 from the National Institutes of Health (P.J.S.-H.).
B.J.R. and S.O.L. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Ms LiHua Rong for expert technical assistance in culturing cells and, from the University of Rochester, Dr C. G. Haidaris for critical comments on the manuscript, Dr G. G. Vaday for help with immunofluorescent microscopy, and Drs M. Pereira, J. Sottile, and D. C. Hocking for helpful discussions on the mechanisms of matrix assembly. We are grateful for the kind gifts of the following monoclonal antibodies: RDV3 from Dr J. R. Shainoff, Cleveland State University, OH; LJ155B16 and LJ134B29 from Dr Z. Ruggeri, Scripps Research Institute, La Jolla, CA; and 9D2 from Dr D. F. Mosher, University of Wisconsin, Madison. FBG-325 was a generous gift from Dr A. Budzynski, Temple University, Philadelphia, PA.