Abstract
Megakaryocytes and functional platelets were generated in vitro from murine embryonic stem (ES) cells with the use of a coculture system with stromal cells. Two morphologically distinctive megakaryocytes were observed sequentially. Small megakaryocytes rapidly produced proplatelets on day 8 of the differentiation, and large hyperploid megakaryocytes developed after day 12, suggesting primitive and definitive megakaryopoiesis. Two waves of platelet production were consistently observed in the culture medium. A larger number of platelets was produced in the second wave; 104 ES cells produced up to 108 platelets. By transmission electron microscopy, platelets from the first wave were relatively rounder with a limited number of granules, but platelets from the second wave were discoid shaped with well-developed granules that were indistinguishable from peripheral blood platelets. ES-derived platelets were functional since they bound fibrinogen, formed aggregates, expressed P-selectin upon stimulation, and fully spread on immobilized fibrinogen. These results show the potential utility of ES-derived platelets for clinical applications. Furthermore, production of gene-transferred platelets was achieved by differentiating ES cells that were transfected with genes of interest. Overexpression of the cytoplasmic domain of integrin β3 in the ES-derived platelets prevented the activation of αIIbβ3, demonstrating that this system will facilitate functional platelet studies. (Blood. 2003;102:4044-4051)
Introduction
Various culture systems demonstrating megakaryocyte maturation and proplatelet formation from hematopoietic progenitor cells have been described. CD34+ stem cells from various sources, including bone marrow, peripheral blood, and cord blood cells, have been successfully differentiated into megakaryocyte lineages in vitro.1-4 Such culture systems include liquid culture or coculture systems with stromal cells, and most require the addition of a cytokine, thrombopoietin (TPO).5,6 A few reports have also demonstrated platelet release into a culture medium.4 Such methods have been used for studies of developmental biology of megakaryocytes or lineage-specific gene expression. Potential gains from these studies would be therapeutic applications, such as in transfusions or cell transplantation. However, the number of CD34 stem cells obtained and the difficulties in expansion of these cells in vitro are factors that limit such strategies from being able to generate sufficient amounts of megakaryocytes or platelets for clinical application or basic research.
Embryonic stem (ES) cells are another good source, as these cells can rapidly proliferate and are able to differentiate to a variety of cell types.7 Several techniques have been established to promote in vitro differentiation of murine ES cells to hematopoietic cell lineages, including megakaryocytes. In vitro differentiation has been performed by either formation of embryoid bodies,8 coculture with stromal cell lines,9,10 or culture on matrix-coated plates.11 Differentiation of human ES cells is also reported.12 However, there have been no reports focusing on the production of ES cell-derived functional platelets as a terminal differentiation of megakaryocytes.
The role of several gene products in platelet function has also been investigated by transfection into heterologous or megakaryocytic cell lines that could not undergo terminal differentiation or produce platelets. There are recent reports that primary megakaryocytes or megakaryocytes derived from ES cells can be used for such an approach.13,14 However, platelets expressing extrinsic gene products have not been generated in vitro. The forced expression of genes of interest in functional platelets could be a useful method for research in the field of platelet biology.
In this study, we used a coculture system with the stromal cell line OP9 to generate mature megakaryocytes from ES cells and identified the functional platelets produced in the culture supernatants. Further, we demonstrated that expression of extrinsic gene products could be achieved in platelets derived from ES cells. Our data suggest the potential utility of ES cell-derived platelets as a substitute for platelet transfusion. Combined with the genetic manipulability of ES cells, this system should facilitate functional studies using gene-transferred platelets and be a future approach for treatment of platelet dysfunction.
Materials and methods
Culture and differentiation of ES cells
A murine ES cell line, TT2,15 established from an F1 embryo of a C57B2/6 female and a CBA male mouse, was maintained as undifferentiated cells by coculture with mitomycin C-treated mouse embryonic fibroblast cells or STO cells in Dulbecco modified essential medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 15% Knockout SR serum replacer (Invitrogen) and 1000 U/mL leukemia inhibitory factor (Invitrogen).
Differentiation induction to hematopoietic progenitors was performed according to the method described by Nakano et al.9,16 ES cells were dissociated with 0.25% trypsin/EDTA (ethylenediaminetetraacetic acid) (Sigma, St Louis, MO), seeded onto confluent OP9 stromal cells derived from macrophage colony-stimulating factor (M-CSF)-deficient mice, and cultured in alpha-MEM medium (Invitrogen) supplemented with 20% fetal bovine serum (FBS). Differentiation was started with 1.0 × 104 ES cells in a well of a 6-well plate or 1.0 × 105 ES cells in a 10-cm culture dish. In 5 days, the ES cells were differentiated into hematopoietic progenitors without formation of embryonic bodies. For differentiation into megakaryocytes, the cells were trypsinized on day 5 and passed over the fresh mitomycin C-treated OP9 cells in the same culture medium containing 10 ng/mL TPO (Kirin Brewery, Tokyo, Japan). Then, 1.0 × 104 cells were seeded in a well of a 6-well plate for the experiments of megakaryocyte colony formation or for determination of platelet numbers. To obtain the large number of platelets needed for functional assay, 1.0 × 105 cells were seeded in a well of a 6-well plate or 1.0 × 106 cells were seeded in a 10-cm culture dish. Starting 2 days later (that is, starting on day 7 of differentiation), the culture medium was changed every day.
Characterization of megakaryocytes differentiated from ES cells
Megakaryocytes derived from ES cells were determined by morphology, immunocytochemistry, and Wright-Giemsa and acetylcholinesterase (AChE) staining.
For immunostaining, a cytospin preparation or cultured cells in a 6-well plate were fixed with 4% formaldehyde-acetone solution and stained with antimouse CD41 (GPIIb/αIIb) monoclonal antibody MWReg30 (Pharmingen, San Diego, CA) followed by a polymeric alkaline phosphatase-conjugated secondary antibody (ENVISION/AP polymer; DAKO, Glostrup, Denmark).17 Visualization was performed with the use of a substrate mixture of naphthol AS-BI phosphate sodium salt (Sigma) and new fuchsin solution (Merck, Darmstadt, Germany) according to the manufacturer's instructions.
AChE staining was performed as described previously.18 Unfixed cells were incubated in 0.1 M phosphate-buffered saline (PBS) (pH 6.0) containing 0.05% acetylthiocholine iodide, 0.1 M sodium citrate, 30 mM copper sulfate, and 5 mM potassium ferricyanide at room temperature for 3 hours.
Megakaryocyte diameters were measured by comparing CD41+ megakaryocytes in the immunostained preparations and normal human erythrocytes from peripheral blood samples. Microscope photographs were taken at the same magnification (× 600), and the mean erythrocyte diameter was calculated as 7 μm.
DNA content of the differentiated cells was analyzed by flow cytometry as described previously.19 Cells were collected from the culture plate by mild pipetting, and the detached cells were labeled with MWReg30 followed by fluorescein isothiocyanate (FITC)-conjugated goat antirat immunoglobulin G (IgG) (Caltag, South San Francisco, CA). The cells were then washed, resuspended in hypotonic propidium iodide (50 μg/mL in 0.1% sodium citrate) containing 20 μg/mL RNAase (Sigma), and incubated for 30 minutes in the dark. The ploidy of the CD41+ cells was analyzed by a flow cytometer, Epics XL (Coulter, Fullerton, CA).
Flow cytometry and determination of the number of platelets derived from ES cells
Platelets released into the culture supernatant were determined by flow cytometer. Culture medium was gently collected and centrifuged at 150g for 20 minutes to remove the nucleated large cells. The supernatant was fixed with 1% paraformaldehyde for 1 hour and centrifuged at 900g for 10 minutes. The cells in the pellet were washed with Hanks balanced salt solution with Ca2+ (HBSS) containing 1% FBS and incubated with 10 μg/mL MWReg30 or antimouse glycoprotein (GP) V monoclonal antibody 1C2 (Seikagaku, Tokyo, Japan),20 followed by FITC-goat antirat IgG; each incubation was performed on ice for 1 hour. Finally, the cells were washed again and then analyzed by a flow cytometer. A single platelet gate was created by analyzing adult mouse peripheral platelets in the same manner.
The number of platelets produced from ES-derived megakaryocytes was counted by flow cytometer. Culture supernatants were collected from the same well of a 6-well plate every day from day 7 to day 16 of differentiation, and each day the cells in the supernatant were stained as described. The cells were finally suspended in 500 μL HBSS. By flow cytometer, data from 50-μL aliquots were collected several times during a continuous flow, and the numbers of platelet-sized and CD41+ cells were determined.
Electron microscopy of platelets derived from ES cells
Culture medium containing ES-derived platelets was collected as described. Mouse control peripheral blood was obtained from the retro-orbital plexus. Blood was collected in a tube containing acid-citrated-dextrose (ACD) (2.5% trisodium citrate, 1.5% citric acid and 2% glucose) solution, and platelet-rich plasma (PRP) was obtained by centrifugation at 150g for 20 minutes at room temperature. Then, 0.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) was added to the medium or PRP and centrifuged at 1000g for 10 minutes. Platelet pellets were then fixed in 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 60 minutes at 4°C. The samples were washed, then fixed with 1% osmium tetroxide in 0.1 M phosphate buffer for 60 minutes at 4°C, dehydrated with a graded ethanol series, and embedded in Epon (TAAB Laboratories, Aldermaston, Berkshire, United Kingdom) as described previously.21 Ultrathin sections were prepared, stained with uranyl acetate and lead citrate, and then examined with a JEM1200EX transmission electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 80 kV.
Functional studies of platelets derived from ES cells
For functional studies of the ES-derived platelets, the culture medium was changed to the same medium containing 1 μM prostaglandin E1 (PGE1) (Ono Pharmaceutical, Osaka, Japan) 1 day before assaying. Culture medium was collected and centrifuged at 150g for 20 minutes. To the supernatant, 1 μM PGE1, 1 U/mL apyrase (Sigma), and a 1:9 volume of ACD solution were added and centrifuged at 900g for 10 minutes. The cells in the pellet were resuspended and washed twice in 85 mM sodium citrate, 111 mM dextrose, and 71 mM citric acid pH 7.0, containing PGE1 and apyrase, and then resuspended in a modified Tyrode-HEPES (Tyrode-N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (138 mM NaCl, 0.36 mM NaH2PO4, 2.9 mM KCl, 12 mM NaHCO3, 10 mM HEPES, 5 mM glucose, 1 mM MgCl2, and 1 mM CaCl2, pH 7.4).
Fibrinogen binding was determined by flow cytometric analysis with the use of Alexa Fluor 488-labeled human fibrinogen (Molecular Probes, Eugene, OR). It is known that mouse platelets can bind human as well as mouse fibrinogen.13 ES-derived platelets in 50 μL of each aliquot were stimulated with 10 μM adenosine diphosphate (ADP) (Biopool, Ventura, CA) or 500 μM proteinase-activated receptor 4 (PAR4) thrombin receptor-activating peptide AYPGFK (BioSynthesis, Lewisville, TX)22 in the presence of 100 μg/mL Alexa Fluor 488-labeled human fibrinogen for 10 minutes at 37°C without stirring. The aliquot was then diluted with 500 μL HEPES buffer, and the mixture was directly analyzed by a flow cytometer. Single platelets were gated and analyzed. For controls, platelets were incubated with fluorescence-labeled fibrinogen without stimulation or were stimulated in the presence of 5 mM EDTA, instead of CaCl2, or 100 μg/mL MWReg30 in the same manner. Further, some samples were stimulated in the presence of unlabeled fibrinogen, and the aggregate formation was observed by microscopy.
To determine the expression of P-selectin upon agonist stimulation, platelets were stimulated with 500 μM AYPGFK for 10 minutes. After fixation with 2% paraformaldehyde for 1 hour, the cells were washed, incubated with anti-P-selectin rabbit polyclonal serum23 followed by FITC-conjugated antirabbit secondary antibody, and then analyzed by flow cytometer. Control cells were fixed without stimulation, stained, and analyzed simultaneously.
Platelet spreading on the immobilized fibrinogen was analyzed. An 8-well chamber slide was coated with 100 μg/mL fibrinogen overnight. After blocking with 1 mg/mL bovine serum albumin (BSA) for 1 hour, platelets were added to each well with 20 μg/mL epinephrine (Biopool) and 1 U/mL apyrase and incubated at room temperature for 30 minutes. After fixation with 2% paraformaldehyde, platelets were treated with 0.1% Triton X-100 for 10 minutes, and the actin filament was stained with 50 U/mL FITC-conjugated phalloidin (Molecular Probes). Platelet spreading was observed by fluorescence microscopy.
Production of gene-transferred platelets
An expression vector pCX-EGFP (provided by Dr M. Okabe, Osaka University, Osaka, Japan)24 containing chicken beta-actin promoter and green fluorescence protein (GFP) cDNA was used for the expression of GFP. For lineage-specific expression of megakaryocytes and platelets, an expression vector (pBK-PF4-GFP) was constructed. A 1.6-kilobase pair (kbp) human platelet factor 4 (PF4) promoter25 was amplified by polymerase chain reaction (PCR) and cloned into pBluscript II (Stratagene, La Jolla, CA). GFP cDNA and a polyadenylation signal were excised from pEGFP-C1 (Clontech, Palo Alto, CA) and linked with the PF4 promoter. After linearization by enzyme digestion, the plasmids were cotransfected into TT2 cells with a vector, pKJ2 (GIBCO, Carlsbad, CA), containing phosphoglycerate kinase-1 promoter and a neomycin-resistance gene.26 Transfection was performed by electroporation by means of Gene Pulser Apparatus (Bio-Rad, Richmond, CA). Cells were cultured with G418 (Invitrogen) for 10 days and the colonies picked up. Positive clones were screened by PCR with the use of primer sets for amplification of GFP cDNA. Differentiation into megakaryocytes was started with the positive clones.
Mutant cDNA for a fusion protein of extracellular and transmembrane domains of interleukin 2 (IL2) receptor α-chain (Tac antigen/CD25) and cytoplasmic domain of integrin β3 was constructed by PCR according to a previously described strategy.27 Primer sets were prepared in which half of the sequence was identical to the end of the transmembrane domain of the IL2 receptor until Leu187, and the other half was identical to the beginning of the cytoplasmic domain of β3. Two separate PCRs were performed to amplify the extracellular and transmembrane domains of the IL2 receptor and the cytoplasmic domain of β3. The PCR products were mixed, and a final PCR was performed with the use of outside primers and then cloned into an expression vector pBK-EF that contained human elongation factor-1α promoter.28,29 The construct was verified by nucleotide sequencing by means of an automated DNA sequencer (ABI 310; Applied Biosystems, Foster City, CA). Platelets expressing the fusion protein (Tac-β3) were produced from ES cells as described above. The expression of the protein on cell surfaces was determined by anti-IL2 receptor antibody, Tac (provided by Dr T. Uchiyama, Kyoto University, Kyoto, Japan).30
Results
Formation of megakaryocyte colonies from ES cells in vitro
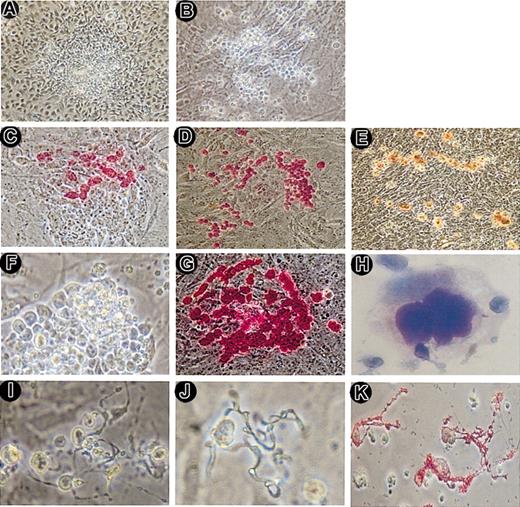
In our initial experiments, where we tried to differentiate several murine ES cell lines (eg, J1 and D3 cells), it was observed that TT2 cells were the ones most effectively differentiated to megakaryocytes. Therefore, all studies were performed with TT2 cells, which were cultured on OP9 layers that induced the differentiation to mesodermal-like hematopoietic progenitors. From day 5, a combination of several cytokines, including IL3, IL6, IL11, and TPO, was tried for differentiation. Although other cytokines enhanced the number of megakaryocytes, megakaryocyte purity was highest with a single cytokine, TPO. Furthermore, low concentration of TPO induced sufficient differentiation into megakaryocytes and production of platelets even if TPO was present at all times. Thus, cells were thereafter differentiated in the presence of just 10 ng/mL TPO. Initially, cells formed isolated colonies with unclear borders (Figure 1A), which showed a clear contrast with the distinct borders of colonies of undifferentiated ES cells. On day 8 of the differentiation, some colonies produced individually identifiable cells, with most of the colonies starting to produce such cells after day 12. Typically, large cells appeared at the periphery of the colonies and migrated outward thereafter (Figure 1B). The large cells were positive for immunostaining with anti-CD41 (GPIIb/αIIb) antibody and AChE staining (Figure 1C-E). Subsequently, almost all cells within the colonies became large and CD41+ (Figure 1F-G). Then, some of the cells spontaneously floated into the culture medium and were easily detached by gentle pipetting, suggesting only a loose adhesion between differentiated and OP9 cells. Wright-Giemsa staining showed that the cells recovered from the culture supernatant exhibited morphologic features of mature megakaryocytes (Figure 1H), indicating that mature megakaryocytes were produced from ES cells. These megakaryocytes were quite viable because, by flow cytometric analysis with propidium iodide (PI) staining, almost all CD41+ cells were PI-.
Megakaryocyte colony and proplatelet formation by ES-derived hematopoietic cells. A hematopoietic colony with unclear borders was formed on day 6 of differentiation (A). On day 12, a megakaryocyte colony with individually identifiable large cells appeared (B). These cells were visualized by immunostaining with anti-CD41 (GPIIb) antibody (C-D) and AChE staining (E). CD41+ mature megakaryocytes appeared at the periphery of the colonies. On day 13, almost all cells within the colonies became large (F). These cells were immunostained with an anti-CD41 antibody and were CD41+ (G). Cytospin preparation of the culture medium on day 13 was stained by Wright-Giemsa staining (H). On day 14 of culture, cells attached on the layer (I), floated in the medium (J), and in cytospin preparation (K) displayed proplatelet formation from mature megakaryocytes. The cytospin preparation (K) was immunostained with anti-CD41 antibody. Panels T and J were not stained. The proplatelets were CD41+ up to the top of the projections. Original magnifications were × 100 for panels A-E, × 200 for panels F-G, × 600 for panel H, and × 400 for panels I-K.
Megakaryocyte colony and proplatelet formation by ES-derived hematopoietic cells. A hematopoietic colony with unclear borders was formed on day 6 of differentiation (A). On day 12, a megakaryocyte colony with individually identifiable large cells appeared (B). These cells were visualized by immunostaining with anti-CD41 (GPIIb) antibody (C-D) and AChE staining (E). CD41+ mature megakaryocytes appeared at the periphery of the colonies. On day 13, almost all cells within the colonies became large (F). These cells were immunostained with an anti-CD41 antibody and were CD41+ (G). Cytospin preparation of the culture medium on day 13 was stained by Wright-Giemsa staining (H). On day 14 of culture, cells attached on the layer (I), floated in the medium (J), and in cytospin preparation (K) displayed proplatelet formation from mature megakaryocytes. The cytospin preparation (K) was immunostained with anti-CD41 antibody. Panels T and J were not stained. The proplatelets were CD41+ up to the top of the projections. Original magnifications were × 100 for panels A-E, × 200 for panels F-G, × 600 for panel H, and × 400 for panels I-K.
After day 13 or 14, the cells showed a dramatic morphologic change (Figure 1I-K). Numerous cells exhibited long beaded projections, so-called proplatelets, from which platelets were produced.31 Proplatelets were observed in cells attached on the layer and those floating in the medium. By immunostaining, the proplatelets were strongly CD41+ up to the top of the projections.
Two-wave differentiation to megakaryocytes and platelets from ES cells
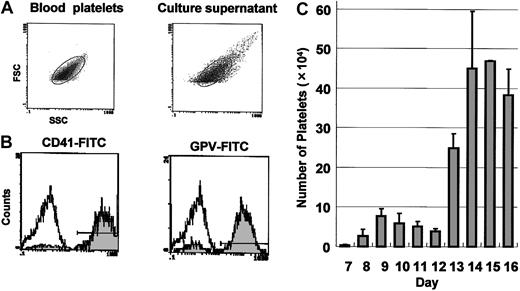
We determined whether platelets were produced from the proplatelet-bearing megakaryocytes. Culture medium was collected, and nucleated cells were removed by low-speed sedimentation. Cells in the supernatant were analyzed by flow cytometer. A gate was fixed in the forward- and side-scatter histogram with the use of peripheral blood platelets from adult mice. The size of the most cells was within the platelet gate (Figure 2A), and almost all cells were positive for CD41 and another platelet-specific antigen, GPV (Figure 2B). We concluded that platelets were released to the culture medium from mature megakaryocytes derived from ES cells. To determine the number produced, platelets were collected from medium of the same well of a 6-well culture plate every day from day 7 to day 16, and the number of platelet-sized and CD41+ cells were accurately counted by flow cytometer (Figure 2C). Two waves of platelet production were observed; the first wave appeared on day 8 of the induction and reached a peak on day 9, and thereafter the number of platelets decreased gradually. A second wave of platelet production appeared on day 13. These 2 waves were reproducibly observed in more than 5 independent experiments. Larger numbers of platelets were produced in the second wave than in the first. Approximately 1.8 × 106 platelets were produced from 1 × 104 cells on day 5 (3 × 105 platelets from days 8 to 12, and 1.5 × 106 platelets from days 13 to 16). Since an initial culture of 1 × 104 ES cells typically yielded 1 × 106 cells on day 5, 1 × 104 ES cells could eventually produce as many as 1.8 × 108 platelets.
Two-wave platelet production from ES cells. (A) Cells released to the culture medium on day 15 were analyzed by flow cytometry. A platelet gate was fixed in the forward- and side-scatter profiles of peripheral blood platelets from adult mice (left). Most cells in the culture medium of ES cells were within the gate (right). (B) Cells in culture supernatant were labeled with megakaryocyte- and platelet-specific monoclonal antibodies. Almost all cells were positive for CD41 (left) and GPV (right), as shown by the gray histogram. The open histogram represents cells stained with control antibody. (C) Cells were collected from medium of the same well of a 6-well culture plate every day (days 7 to 16), and the numbers of platelets were counted as platelet-sized and CD41+ cells by flow cytometry. Two waves of platelet production were observed. The values shown are the mean ± SD from 5 independent experiments.
Two-wave platelet production from ES cells. (A) Cells released to the culture medium on day 15 were analyzed by flow cytometry. A platelet gate was fixed in the forward- and side-scatter profiles of peripheral blood platelets from adult mice (left). Most cells in the culture medium of ES cells were within the gate (right). (B) Cells in culture supernatant were labeled with megakaryocyte- and platelet-specific monoclonal antibodies. Almost all cells were positive for CD41 (left) and GPV (right), as shown by the gray histogram. The open histogram represents cells stained with control antibody. (C) Cells were collected from medium of the same well of a 6-well culture plate every day (days 7 to 16), and the numbers of platelets were counted as platelet-sized and CD41+ cells by flow cytometry. Two waves of platelet production were observed. The values shown are the mean ± SD from 5 independent experiments.
The phenomenon of the 2 waves of platelet production reminded us of primitive and definitive hematopoiesis.16 Therefore, the time course of megakaryocyte maturation was observed in greater detail. Consistent with the first wave of platelet production, small megakaryocytes were observed in some colonies around day 8. However, because these small cells rapidly produced proplatelets and disappeared by day 12, they were not likely to be precursors of the megakaryocytes produced after day 12. After this, large megakaryocytes were observed in other colonies, consistent with the second wave. The 2 megakaryocytes showed distinctive morphologies (Figure 3A). Megakaryocytes on days 9 and 13 were both positive by immunostaining with anti-CD41 antibody and by AChE staining, but the cells on day 9 showed an obviously weaker intensity in immunostaining and AChE staining than those on day 13. Furthermore, the size of the day-9 megakaryocytes was smaller than those of days 13 or 16 (Figure 3B) (means of diameters: day 9 = 13.1 μm, day 11 = 13.8 μm, day 13 = 20.2 μm, day 16 = 24.2 μm). On day 9, most cells were uniformly about 12 to 15 μm, whereas cells of day 13 and 16 were more than 20 μm, and some large megakaryocytes with diameters more than 30 μm were observed. In parallel with cell size, the DNA content was also different (Figure 3C). The ploidy of day-9 cells was predominantly 4n, whereas day-13 megakaryocytes contained hyperploid cells with 8n to 128n. These results indicated that the cells from the second wave were morphologically close to a mature adult type of megakaryocyte, and that the platelets in the first wave were released from qualitatively different small megakaryocytes. The results suggested primitive and definitive megakaryopoiesis from ES cells.
Primitive and definitive megakaryopoiesis from ES cells. (A) Megakaryocytes on days 9 (i,iii,v) and 13 (ii,iv,vi) are shown in pairwise fashion. Cells are observed under phase-contrast microscopy (i-ii). Consistent with the first-wave platelet production, small megakaryocytes on day 9 already display numerous proplatelets. These megakaryocytes are stained with anti-CD41 (iii-iv) and AChE (v-vi). Panels Ai and Aii were not stained. Original magnification in all photographs is × 200. (B) The diameter of the megakaryocytes on days 9, 11, 13, and 16 was measured as described in “Materials and methods.” The values shown are the mean ± SD in 50 cells. (C) The DNA content of the megakaryocytes on day 9 (top panels) and day 13 (bottom panels) was analyzed by flow cytometry. Wright-Giemsa staining of the typical analyzed cells is shown on the right (original magnification, × 400).
Primitive and definitive megakaryopoiesis from ES cells. (A) Megakaryocytes on days 9 (i,iii,v) and 13 (ii,iv,vi) are shown in pairwise fashion. Cells are observed under phase-contrast microscopy (i-ii). Consistent with the first-wave platelet production, small megakaryocytes on day 9 already display numerous proplatelets. These megakaryocytes are stained with anti-CD41 (iii-iv) and AChE (v-vi). Panels Ai and Aii were not stained. Original magnification in all photographs is × 200. (B) The diameter of the megakaryocytes on days 9, 11, 13, and 16 was measured as described in “Materials and methods.” The values shown are the mean ± SD in 50 cells. (C) The DNA content of the megakaryocytes on day 9 (top panels) and day 13 (bottom panels) was analyzed by flow cytometry. Wright-Giemsa staining of the typical analyzed cells is shown on the right (original magnification, × 400).
Transmission electron micrograph of ES-derived platelets
The morphology of platelets derived from ES cells was examined by transmission electron microscopy. Control normal platelets from mouse peripheral blood exhibited discoid forms with 2 major granules (alpha-granules, which predominated, and dense granules), an open canalicular system, and other organelles (Figure 4A). ES cell-derived platelets from the first wave (day 10) were mostly rounder than normal platelets. Granules and other organelles were present, but the numbers and sizes of the granules were smaller compared with normal peripheral platelets (Figure 4B). In contrast, platelets from the second wave (day 15) were relatively larger than the peripheral platelets, but exhibited well-developed granules and normal organelles (Figure 4C). Some platelets attached to each other, probably by spontaneous activation. The morphology of the platelets from the second wave was indistinguishable from mouse peripheral platelets.
Transmission electron microscopy of platelets derived from ES cells.The morphology of platelets derived from ES cells was examined by transmission electron microscopy. Control normal platelets from mouse peripheral blood (A), ES cell-derived platelets from the first wave (day 10) (B), and platelets from the second wave (day 15) (C) are shown. Original magnification, × 10 000.
Transmission electron microscopy of platelets derived from ES cells.The morphology of platelets derived from ES cells was examined by transmission electron microscopy. Control normal platelets from mouse peripheral blood (A), ES cell-derived platelets from the first wave (day 10) (B), and platelets from the second wave (day 15) (C) are shown. Original magnification, × 10 000.
This result suggested that cells released into the culture medium were not a simple fragmentation of megakaryocytes, but were platelets produced from ES cell-derived megakaryocytes through a physiologic process.
Platelets derived from ES cells were functional
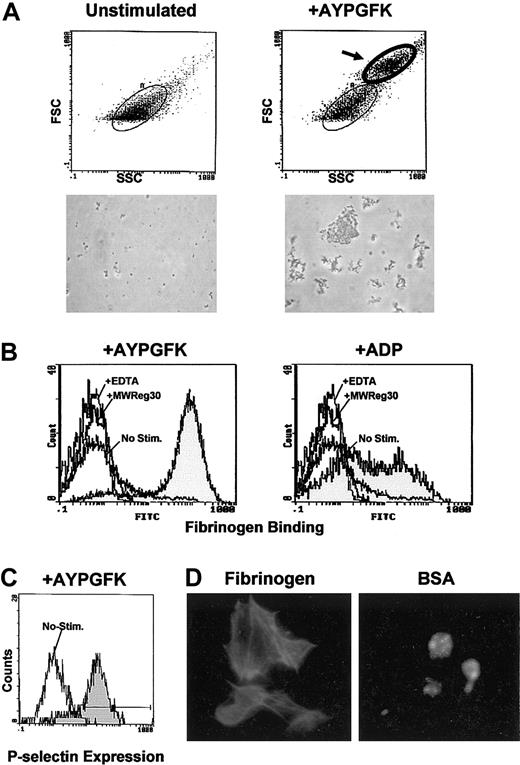
Platelets were obtained from the second wave of production (days 14 and 15), and a functional assay was performed. By flow cytometry, platelet particles were shifted to a higher level in the forward scatter after stimulation of PAR4 thrombin receptor-activating peptide AYPGFK without stirring, indicating the formation of aggregates (Figure 5A). When platelets were stimulated with vigorous stirring, most of the platelets were shifted to a higher level. Numerous platelet aggregates were observed under microscopy, whereas unstimulated platelets showed no aggregation. Aggregation was probably mediated by fibrinogen binding since the binding of Alexa Fluor 488-labeled fibrinogen to the platelets was detected in response to AYPGFK or ADP (Figure 5B). Binding of fibrinogen depended on αIIbβ3 because it was blocked by EDTA or the anti-CD41 (GPIIb/αIIb) inhibitory antibody MWReg30.17 Surface expression of P-selectin was also observed after the stimulation, but not in unstimulated platelets (Figure 5C), suggesting that release reaction occurred. These platelets spread fully and exhibited formation of actin stress fibers on the immobilized fibrinogen, whereas they kept a round morphology on the control BSA surface (Figure 5D). These results indicated that platelets derived from ES cells in vitro were as functional as the platelets from peripheral blood.
Functional assay of the platelets derived from ES cells. Platelets were obtained from the culture medium on days 14 to 15, and functional assays were performed. (A) Unstimulated platelets (left panels) and stimulated platelets by PAR4 thrombin receptor-activating peptide AYPGFK without stirring (right panels) were analyzed by flow cytometry. In the stimulated platelets, particles with higher forward scatter are observed (indicated by an arrow), indicating aggregate formation. A microscopic photograph (original magnification, × 600) of the analyzed platelets is shown below each histogram. (B) Platelets were stimulated by AYPGFK (left) or ADP (right) in the presence of Alexa Fluor 488-labeled fibrinogen, and the fibrinogen binding was determined by flow cytometry (gray histograms). Simultaneous binding to unstimulated platelets and control binding in the presence of EDTA or the anti-CD41 inhibitory antibody MWReg30 are shown as open histograms. (C) Platelets were stimulated by AYPGFK and stained with anti-P-selectin antibody followed by FITC secondary antibody. Surface expression of P-selectin was analyzed by flow cytometry (gray histogram). The open histogram represents the control experiment with unstimulated platelets. (D) Platelets were allowed to adhere and spread on the immobilized fibrinogen (left) and BSA surface (right), stained with FITC-conjugated phalloidin, and observed under fluorescence microscopy. Original magnification, × 1000.
Functional assay of the platelets derived from ES cells. Platelets were obtained from the culture medium on days 14 to 15, and functional assays were performed. (A) Unstimulated platelets (left panels) and stimulated platelets by PAR4 thrombin receptor-activating peptide AYPGFK without stirring (right panels) were analyzed by flow cytometry. In the stimulated platelets, particles with higher forward scatter are observed (indicated by an arrow), indicating aggregate formation. A microscopic photograph (original magnification, × 600) of the analyzed platelets is shown below each histogram. (B) Platelets were stimulated by AYPGFK (left) or ADP (right) in the presence of Alexa Fluor 488-labeled fibrinogen, and the fibrinogen binding was determined by flow cytometry (gray histograms). Simultaneous binding to unstimulated platelets and control binding in the presence of EDTA or the anti-CD41 inhibitory antibody MWReg30 are shown as open histograms. (C) Platelets were stimulated by AYPGFK and stained with anti-P-selectin antibody followed by FITC secondary antibody. Surface expression of P-selectin was analyzed by flow cytometry (gray histogram). The open histogram represents the control experiment with unstimulated platelets. (D) Platelets were allowed to adhere and spread on the immobilized fibrinogen (left) and BSA surface (right), stained with FITC-conjugated phalloidin, and observed under fluorescence microscopy. Original magnification, × 1000.
Gene-transferred platelets derived from ES cells for functional assay
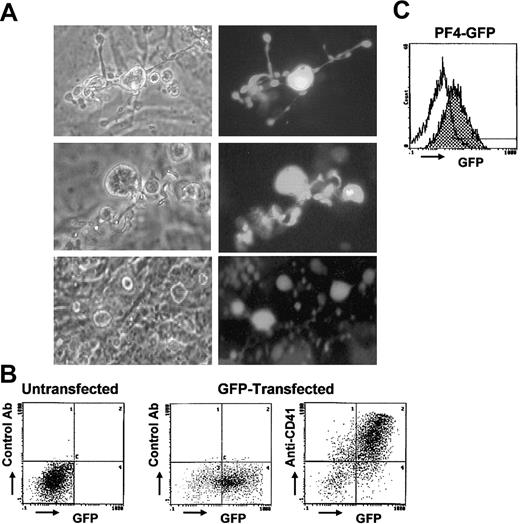
To generate platelets expressing extrinsic gene products, platelets expressing GFP were produced as a test case. We prepared constructs in which actin (pCX-EGFP) or megakaryocyte-specific PF4 (pBK-PF4-GFP) promoters were linked to GFP cDNA. ES cells were transfected with the constructs and positive clones selected. When differentiation was started with these cells, GFP+ megakaryocytes were detected. Differentiation was started with the stably transfected ES clones, and the percentage of the transfected cells was 100%, which was confirmed by the positivity of GFP. In the case of pCX-EGFP, strong green fluorescence was observed in the megakaryocytes and also in proplatelets under fluorescence microscopy (Figure 6A). GFP+ platelets were observed in the culture medium. However, numerous platelets were attached on the OP9 layer or were possibly present under the layer, although such platelets were hardly recognized under phase-contrast microscopy (Figure 6A, lower panel). By flow cytometry, platelets in the supernatant were found to be both GFP+ and anti-CD41+ (Figure 6B). With pBK-PF4-GFP, lineage-specific expression of GFP was observed because only mature megakaryocytes and platelets were GFP+ by flow cytometry, although the expression was not strong enough for microscopic observation (Figure 6C).
GFP-expressing megakaryocytes and platelets derived from ES cells. In panels A and B, megakaryocytes and platelets expressing GFP were produced from ES cells transfected with pCX-EGFP. (A) Paired photographs of phase-contrast microscopy (left column) and fluorescence microscopy (right column) are shown (original magnification, × 400). Proplatelet-bearing megakaryocytes are strongly GFP+ up to the top of the proplatelets (top and middle rows, day 14). GFP+ platelets are observed on or under the stromal layer, although platelets are hardly recognized under the phase-contrast microscopy (bottom row, day 15). (B) Platelets in the culture supernatants (day 15) were stained with anti-CD41 antibody followed by PE-conjugated secondary antibody, and 2-color flow cytometric analysis (PE and GFP) was performed. Platelets derived from GFP-transfected ES cells are both GFP+ and anti-CD41+ (right panel). Results of the platelets stained with a control antibody (middle), and of the platelets derived from untransfected ES cells (left) are shown. (C) Platelets on day 15 derived from ES cells transfected with GFP linked to megakaryocyte-specific PF4 promoter (pBK-PF4-GFP) are also GFP+ by flow cytometry (gray histogram). Open histogram represents the fluorescence of platelets derived from untransfected ES cells.
GFP-expressing megakaryocytes and platelets derived from ES cells. In panels A and B, megakaryocytes and platelets expressing GFP were produced from ES cells transfected with pCX-EGFP. (A) Paired photographs of phase-contrast microscopy (left column) and fluorescence microscopy (right column) are shown (original magnification, × 400). Proplatelet-bearing megakaryocytes are strongly GFP+ up to the top of the proplatelets (top and middle rows, day 14). GFP+ platelets are observed on or under the stromal layer, although platelets are hardly recognized under the phase-contrast microscopy (bottom row, day 15). (B) Platelets in the culture supernatants (day 15) were stained with anti-CD41 antibody followed by PE-conjugated secondary antibody, and 2-color flow cytometric analysis (PE and GFP) was performed. Platelets derived from GFP-transfected ES cells are both GFP+ and anti-CD41+ (right panel). Results of the platelets stained with a control antibody (middle), and of the platelets derived from untransfected ES cells (left) are shown. (C) Platelets on day 15 derived from ES cells transfected with GFP linked to megakaryocyte-specific PF4 promoter (pBK-PF4-GFP) are also GFP+ by flow cytometry (gray histogram). Open histogram represents the fluorescence of platelets derived from untransfected ES cells.
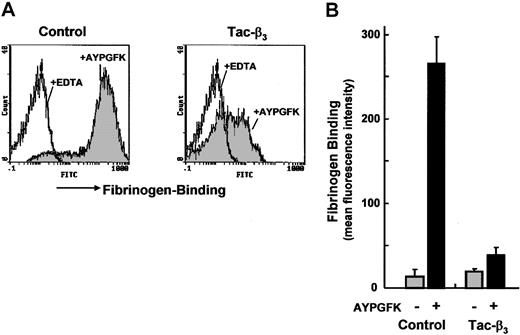
To verify the utility of the gene-transferred platelets in functional studies, a construct, which produced a fusion protein of IL2 receptor and the cytoplasmic domain of integrin β3 (Tac-β3), was tested. It has been reported, in Chinese hamster ovary (CHO) cells or megakaryocytes, that the overexpression of the cytoplasmic domain of β3 by the same construct prevented the conversion of αIIbβ3 to the active form, probably because of the interference of the interaction of intracellular associated factors to the cytoplasmic domains of αIIbβ3.13,32 However, such effects were not confirmed in platelets. Platelets from ES cells transfected with Tac-β3 cDNA were strongly positive for anti-IL2 receptor antibody Tac (data not shown). When these platelets were stimulated with AYPGFK, the binding of Alexa Fluor 488-labeled fibrinogen was dramatically reduced compared with control platelets (Figure 7). Therefore, it was demonstrated that in platelets too, the overexpression of the cytoplasmic domain of β3 prevented activation of integrin αIIbβ3.
Gene-transferred platelets derived from ES cells for functional assay. A cDNA construct that produced a fusion protein of IL2 receptor and cytoplasmic domain of integrin β3 (Tac-β3) was transfected into ES cells, and platelets were produced. (A) Platelets from the transfected ES cells (day 15) were stimulated with AYPGFK, and the binding of Alexa Fluor 488-labeled fibrinogen was analyzed by flow cytometry (gray histogram, right panel). Results obtained with control platelets produced from ES cells transfected with pKJ2 are shown in the left panel. Open histograms represent the fibrinogen binding in the presence of EDTA. (B) Fibrinogen binding is expressed as mean fluorescence intensity. Data represent the mean ± SEM from platelets produced by 3 independently transfected ES clones.
Gene-transferred platelets derived from ES cells for functional assay. A cDNA construct that produced a fusion protein of IL2 receptor and cytoplasmic domain of integrin β3 (Tac-β3) was transfected into ES cells, and platelets were produced. (A) Platelets from the transfected ES cells (day 15) were stimulated with AYPGFK, and the binding of Alexa Fluor 488-labeled fibrinogen was analyzed by flow cytometry (gray histogram, right panel). Results obtained with control platelets produced from ES cells transfected with pKJ2 are shown in the left panel. Open histograms represent the fibrinogen binding in the presence of EDTA. (B) Fibrinogen binding is expressed as mean fluorescence intensity. Data represent the mean ± SEM from platelets produced by 3 independently transfected ES clones.
These results demonstrated that the gene-transferred platelets derived from ES cells would be a powerful tool in the field of biologic research with platelets.
Discussion
In this study, megakaryocyte differentiation and functional platelet production from murine ES cells were demonstrated with the use of a coculture system with a stromal cell line, OP9, derived from M-CSF-deficient mice.9 We demonstrated for the first time that ES-derived platelets, especially those produced at a late phase of differentiation induction, were morphologically and functionally comparable to normal murine peripheral blood platelets.
In vitro platelet production was demonstrated from CD34+ progenitor cells from human peripheral blood. Choi et al4 demonstrated that such platelets were ultrastructurally identical to plasma-derived platelets and functional in several assays. However, CD34+ progenitor cells are not suitable as starting materials for clinical applications or functional studies of platelets because of the number of obtained cells and the difficulties of expansion in vitro. ES cells are a good source since they can rapidly proliferate and might enable an unlimited supply of platelets in vitro to be produced.
Nakano et al9 first reported that ES cells could give rise to hematopoietic cells when cultured with OP9 cells. They demonstrated erythroid, myeloid, B-lymphoid, and megakaryocyte lineages developed by this system (the OP9 system).16,33 Recently, Eto et al14 reported that megakaryocytes were generated in quantity from murine ES cells by the OP9 system, and that forced expression of genes and functional assay of αIIbβ3 can be achieved with these megakaryocytes. However, the authors in these 2 studies observed megakaryocytes maturation only until day 12, but did not give attention to platelets released into the culture medium. Our data clearly demonstrated that quantitative platelets were generated after day 12 and that therefore these would have been missed by the previous reports. We also consider that as compared with megakaryocytes, the ES-derived platelets have advantages for the study of platelet-specific gene products because upon stimulation they can undergo more dynamic changes, such as aggregate formation.
TT2 cells were the best starting cell lines for in vitro platelet production in ES cell lines used for the differentiation. Other cell lines (J1 and D3 cells) similarly differentiated to hematopoietic cells but, for reasons unknown, ultimately produced lower numbers of platelets (approximately 1 × 107 total platelets) than did TT2 cells (not shown). However, TT2 cells have different characteristics from other commonly used murine ES cells.15 For instance, when TT2 cells were injected into blastocysts, the colonization into tissues was very low, but when injected into 8-cell embryos, the cells efficiently colonized each tissue of pups. Furthermore, pups were disproportionately male, and TT2-derived cells were dominant, accounting for over half of the total cells. It remains to be determined whether such characteristics of ES cells could affect the potency of the platelet production in vitro.
Two morphologically distinctive megakaryocytes appeared sequentially over the time course of differentiation. Small megakaryocytes rapidly produced proplatelets on day 8, and large hyperploid megakaryocytes developed thereafter. Consistently, 2 waves of platelet production were observed, with the 2 waves producing 2 types of untrastructurally distinctive platelets. The data indicates there are 2 distinct megakaryopoiesis during differentiation from ES cells in vitro, possibly demonstrating primitive and definitive megakaryopoiesis. It is well known that erythropoiesis originates in the yolk sac, where primitive erythrocytes are produced, and then migrates to fetal liver, where definitive erythrocytes are produced.34 However, such transitions in other cell lineages, including myelocytic and megakaryocytic cells, are not well understood. Nakano et al16 demonstrated that ES cells gave rise to primitive and definitive erythropoiesis in an OP9 system, and 2 types of erythrocytes that differ in their morphology and hemoglobin type were sequentially produced. Compared with the time course of erythropoiesis in their report, the 2 megakaryopoieses observed in our study appeared rather slowly, maybe indicating that megakaryocyte maturation needed more time until platelet production. In our preliminary study, reverse transcription PCR (RT-PCR) analysis of hemoglobin types in erythrocytes obtained from ES-derived mixed colonies containing the megakaryocytes showed that embryonic hemoglobin was detected in the first wave and that adult type was detected in the second wave (not shown). However, molecular markers that define primitive and definitive megakaryocytes need to be identified.
Xu et al35 reported the presence of murine primitive megakaryopoiesis in the early yolk sac. When hematopoietic cells from the yolk sac were cultured, megakaryocyte colonies were observed that corresponded with the first-wave megakaryocytes in our study, because megakaryocytes from yolk sacs rapidly produced proplatelets as early as day 3 of culture, much earlier than those from adult bone marrow, and their ploidy class was lower than that of bone marrow megakaryocytes. Furthermore, these megakaryocytes disappeared by 13.5 days postcoitum. The similarities between megakaryocytes from yolk sacs and early megakaryocytes from ES cells further suggest the presence of ES-derived primitive megakaryopoiesis in vitro. We observed that early megakaryocytes disappeared by day 12 and that large megakaryocytes developed in the other colonies thereafter. Therefore, it is also suggested that the 2 types of megakaryocytes developed from different precursors by distinct differentiation pathways.
We could obtain as many as 108 platelets in the culture supernatant from 104 ES cells. The number was below the theoretical range proposed as the number of platelets released from mature megakaryocytes,36 but was possibly an underestimate of the capacity of ES-derived megakaryocytes since not a few platelets were observed on or under the stromal layers in the case of GFP+ platelets.
A report has shown that the thrombin/antithrombin III (AT III) complex can stimulate proplatelet formation of megakaryocytes.37 However, addition of AT III in the culture medium in the late phase of differentiation did not dramatically increase the number of platelets produced (not shown). The discovery of factors that stimulate proplatelet formation and platelet release from megakaryocytes would thus improve the efficiency of platelet production in this system.
Another approach might be the recovery of the unactivated platelets in the medium of this culture system. Some platelets were still observed on the stromal layers even after the addition of PGE1 to the culture medium to suppress platelet activation. The precise mechanism by which bone marrow megakaryocytes shed platelets in physiologic conditions is not fully understood. It was postulated that the circulatory shear force within the marrow supports the fragmentation of proplatelets into platelets.38 Some reports show that proplatelets projected through the endothelial cell layer of marrow venous sinusoids and into circulation.38,39 The bone marrow environment is composed of a complex adherent cell population, which regulates platelet release and might help to keep platelets unactivated just after release from proplatelets. We also tested coculture with endothelial cell lines instead of OP9 at a late phase of differentiation, but did not obtain a higher number of platelets than with OP9 cells. The combination of our culture system with an artificial capillary culture system with rheostatic shear forces or a 3-dimensional culture system with other types of cell lines might be useful.
The hematopoietic differentiation of ES cells has important therapeutic implications, including the derivation of platelets for transfusion. For clinical purposes, the utility of human ES cells has advantages. Kaufman et al12 demonstrated that the coculture of human ES cells with murine stromal cell lines led to differentiation into hematopoietic cells. Human platelets will possibly be generated from human ES cells in vitro by a similar approach. If in the future cloned ES cells were available from patients' somatic cells, platelets from such ES cells could be applied to autologous platelet transfusion. Further, combined with the methods to generate gene-transferred platelets described here, platelets of self-origin expressing a necessary gene would be an ideal substitutive therapy for platelet disorders.
Further, the forced expression of the genes of interest in functional platelets could be a useful method for basic research into platelet biology. So far, such gene-transferred functional platelets have been obtained only from gene-modified animals such as transgenic or targeted mice,40 a process that needs complicated techniques and timing. The method we describe here could make it possible to generate gene-transferred platelets in easier ways and shorter times.
In conclusion, the method established here to produce functional platelets from ES cells in vitro will facilitate functional studies using gene-transferred platelets and might provide a future approach for clinical treatments of thrombocytopenia and platelet dysfunctions.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-06-1773.
Partially supported by the Ministry of Health, Labour and Welfare, and the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the Research Center for Molecular Medicine, Faculty of Medicine, Hiroshima University, for the use of its facilities. The authors also would like to thank Dr T. Nakano (Osaka University) for providing OP 9 cells.