Abstract
Multiple myeloma is a disseminated neoplasm of terminally differentiated plasma cells that is incurable with currently available therapies. Although the disease is radiosensitive, external beam radiation leads to significant toxicity due to sensitive end-organ damage. Thus, genetic approaches for therapy are required. We hypothesized that the incorporation of immunoglobulin promoter and enhancer elements in a self-inactivating (SIN) lentiviral vector should lead to specific and high-level transgene expression in myeloma cells. A SIN lentivector with enhanced green fluorescent protein (EGFP) expression under the control of a minimal immunoglobulin promoter as well as the Kappa light chain intronic and 3′ enhancers transduced myeloma cell lines with high efficiency (30%-90%). EGFP was expressed at a high level in myeloma cells but silent in all nonmyeloma cell lines tested compared with the cytomegalovirus (CMV) promoter/enhancer. Transduction of myeloma cells with the targeted vector coding for the human sodiumiodide symporter (hNIS) led to hNIS expression by these cells allowing them to concentrate radioiodine up to 18-fold compared with controls. Tumor xenografts in severe combined immunodeficiency mice expressing hNIS could be imaged using iodine-123 (123I) and shown to retain iodide for up to 48 hours. These tumor xenografts were completely eradicated by a single dose of the therapeutic isotope iodine-131 (131I) without evidence of recurrence up to 5 months after therapy. We conclude that lentivectors can be transcriptionally targeted for myeloma cells and the use of hNIS as a therapeutic gene for myeloma in combination with 131I needs further exploration.
Introduction
Multiple myeloma is a systemic, clonal plasma cell disorder that is clinically characterized by a monoclonal immunoglobulin in the blood and/or urine, lytic bone lesions, anemia, renal dysfunction, and bone marrow plasmacytosis.1 Current therapies for this disease include combination chemotherapy with or without stem cell transplantation, alkylating agents, glucocorticosteroids, and thalidomide. However, none of these therapeutic modalities is curative and in most patients the disease will relapse necessitating further therapy.1,2 Therefore, new therapeutic approaches are urgently needed. Although the disease is very sensitive to radiation therapy, its disseminated nature precludes curative total body irradiation (TBI) because of unacceptable end-organ toxicity.2
In the last few years there has been considerable interest in the development of gene therapy approaches for tumor cytoreduction.3 For this approach to work in myeloma, vectors that can be delivered systemically and result in high-level and specific gene expression in myeloma cells are necessary. In most patients with myeloma, the pool of actively replicating cells is usually less than 1%.4,5 Thus, vectors than can efficiently transduce nondividing cells are required. Lentivirus-based vectors can transduce nondividing cells and the last few years have witnessed major strides in the development of multiply attenuated and safe vectors based on the human immunodeficiency virus genome.6-8 Deletion of the transcription factor binding sites in the U3 region of the lentiviral long-terminal repeat (LTR) effectively silences the viral promoter after reverse transcription leading to the term self-inactivating (SIN) vectors.6 This not only silences transcription from the viral promoter but also eliminates transcriptional interference with the internal promoter that is required to drive transcription of the therapeutic gene. Therefore it may be possible to achieve tissue-specific transgene expression with these vectors using promoter and enhancer elements with restricted expression (transcriptional targeting). Indeed, other investigators have shown that it is possible to restrict reporter gene expression in erythroid cells and cells of the monocyte/macrophage lineage.9,10
More than 90% of patients with myeloma have a detectable monoclonal immunoglobulin or light chain, which is the signature of the expanded malignant clone of plasma cells. Immunoglobulin gene expression is regulated by the presence of tissue-specific promoter and enhancer elements that allow high-level expression of these genes in plasma cells but effectively silence these genes in all other tissues.11-16 We hypothesized that immunoglobulin promoter and enhancer elements inserted in the context of a SIN lentiviral vector may result in tissue-specific and high-level transgene expression in plasma cells transfected with these vectors.
The therapeutic efficacy of any gene used for cancer cytoreduction is critically dependent on the transduction efficiency of the target tissue. In vitro, high transduction efficiency can be achieved by increasing the ratio of vector particles to target cells, (multiplicity of infection) but in vivo, this is not practical. Therefore, a therapeutic gene with a significant bystander effect is highly desirable. Such a gene will result in the destruction not only of transduced cells but also of surrounding tumor cells that escape transduction by the vector. The human sodium-iodide symporter (hNIS), the thyroidal protein responsible for concentrating iodide in the thyroid gland, can be used as a therapeutic gene in cancer.17,18 Tumors expressing hNIS can concentrate iodide isotopes such as iodine-131 (131I) that undergoes β– particle decay. The electron emitted by 131I has a path length of 0.2 to 2.4 mm (mean, 0.4 mm)19 and therefore can damage surrounding tumor cells not expressing NIS, inducing their death (bystander effect). This therapy is potentially attractive in myeloma because plasma cells are very sensitive to radiation.
Here we show that SIN lentiviral vectors with a reporter gene under the control of immunoglobulin promoter and enhancer elements led to specific and high-level transgene expression in myeloma cell lines with negligible expression in other hematopoietic and nonhematopoietic cell lines. Myeloma cells transduced with a transcriptionally targeted vector coding for hNIS concentrate iodide up to 18-fold compared with controls. Myeloma tumor xenografts expressing hNIS can be imaged with a gamma camera after a single injection of iodine-123 (123I; a gamma-emitting isotope). These tumor xenografts can be completely eradicated with a single dose of a therapeutic isotope (131I). In addition, we provide in vivo evidence of a significant bystander effect of NIS with 131I in eradicating mixed tumor populations.
Materials and methods
Cell culture
The human B-cell lines ARH-77, JJN3, MM1, and RPMI 8226 were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). ARH-77 and RPMI 8226 were purchased from the American Type Culture Collection (Manassas, VA), while JJN3 and MM1 were a generous gift from Dr R. Fonseca (Mayo Clinic, Rochester, MN). The human B-cell lymphoma lines DoHH2 and Daudi, a generous gift from Dr A.K. Fielding (Mayo Clinic), were maintained in RPMI 1640 supplemented with 10% FBS. Jurkat T cells were maintained in RPMI 1640 with 10% FBS. The human mast cell line HMC-1 was a gift from Dr Joseph H. Butterfield (Mayo Clinic) and was maintained in Iscove medium supplemented with 10% FBS and alpha-thioglycerol (1.2 mM; Sigma-Aldrich, St Louis, MO). Maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FBS were 293T embryonal kidney epithelial cells, Mel-624 (human melanoma), and HT1080 (human fibrosarcoma) as well as K562 (human erythroid leukemia) cell lines. Meg-01 cells, a human megakaryocytic cell line, were grown in RPMI 1640 with 10% FBS. All cultures were maintained at 37°C in an atmosphere with 5% CO2.
Primary myeloma cells (CD138 enriched) were isolated from the bone marrow of a patient with advanced multiple myeloma by bone marrow aspiration under sedation. The sample was collected in anticoagulated tubes and the red blood cells were lysed. The nucleated cells were washed in RPMI for 5 minutes and after centrifugation resuspended in phosphate-buffered saline (PBS), pH 7.2, with 0.5% bovine serum albumin and 2 mM ethylene diamine tetracetic acid (EDTA). After a cell count, the nucleated cells were resuspended at a density of 5 × 106 cells in 95 μL buffer and incubated with 5 μL CD138 antibody (magnetically labeled) for 15 minutes at 4°C. The cells were then centrifuged at 400g for 5 minutes and resuspended in 4 mL PBS buffer. This was followed by separation on the AutoMACS (Miltenyi Biotec, Auburn, CA). Following purification, the cells were resuspended in RPMI with 10% FBS supplemented with 1 ng/mL interleukin-6 (R&D Systems, Minneapolis, MN) and used for lentiviral transduction at a density of 1 × 105 cells in 0.5 mL medium as described for the other cell lines. The population was 95% pure when analyzed by flow cytometry.
All cell culture media were purchased from Gibco BRL (Gaithersburg, MD) unless otherwise stated.
Production of transcriptionally targeted lentiviral vectors
The plasmid pHR′CMV-EGFP-SIN has been described previously.7 The cytomegalovirus (CMV) promoter/enhancer in this plasmid was released by digestion with PpuMI and BamHI. An immunoglobulin promoter homologous to the murine immunoglobulin heavy chain promoter (Genbank no. Z17336) was synthesized as follows. Synthesized at the Mayo Oligonucleotide Synthesis Core facility were 2 oligomers: 5′-GACCCCAATGTGACTCATGAAGCCCACCCATATGCAAATCTAGAGAAGACTTTAGAGTATAAATCTGAGGCTCACCTCACATACCAGCAAGGGAG-3′ (sense) and 5′-GATCCTCCCTTGCTGGTATGTGAGGTGAGCCTCAGATTTATACTCTAAAGTCTTCTCTAGATTTGCATATGGGTGGGCTTCATGAGTCACATTGG-3′ (antisense). Equimolar amounts were mixed together and heated to 94°C for 5 minutes after which the temperature was slowly decreased by 0.01°C per second up to 65°C using a thermocycler. The annealed product was gel purified and ligated into pHR′-CMV-EGFP-SIN digested with PpuMI and BamHI. Thus plasmid pHR′IgP-EGFP-SIN was generated. The murine Kappa light chain 3′ enhancer (referred to here as Kappa) was polymerase chain reaction (PCR) amplified from murine genomic DNA (808 bp) using primers 5′-GGGGGGACCCAGCTCAAACCAGCTTAGG-3′ (sense) and 5′-GGGGGGACCCCTAGAACGTGTCTGGGCCCCATGA-3′ (antisense) with restriction sites underlined (sequence in Meyer and Neuberger14 ). The reaction mixture was heated to 94°C for 4 minutes and followed by 25 cycles of heating to 94°C for 1 minute, annealing at 58°C and elongation for 1 minute at 72°C. A proofreading thermostable polymerase was used (Pwo; Roche Diagnostics, Mannheim, Germany). The PCR product was gel purified and digested with PpuMI. Plasmid pHR′IgP-EGFP-SIN was also digested with PpuMI and Kappa ligated in this position to obtain plasmid pHR′KIgP-EGFP-SIN. The murine kappa light chain intronic enhancer (IE, 890 bp) was PCR amplified using primers 5′-GGGACCCTCAGTTTCTGTTTTACTACCTCTGT-3′ (sense) and 5′-GGGACCCATCGATTTGAGGACGCCATTTTGTCGTTCACT-3′ (antisense) from murine genomic DNA using the same temperature conditions as above (sequence in Max et al20 ). The PCR product (IE) was gel purified and incubated with Taq polymerase (Roche Diagnostics, Mannheim, Germany) and buffer for 15 minutes at 72°C and again gel purified. The product was cloned into pCR2.1 (Invitrogen, Carlsbad, CA) to generate pCR2.1-IE. Kappa was again PCR amplified using primers with ClaI sites 5′CCATCGATAGCTCAAACCAGCTTAGGCTACACAG-3′ (sense) and 5′-CCATCGATCTAGAACGTGTCTGGGCCCCATGA-3′ (antisense) under the same reaction conditions and gel purified. The PCR product KappaCla as well as pCR2.1-IE were digested with Cla1, and Kappa was cloned downstream of IE to generate plasmid pCR2.1-IE-K. IE was released from pCR2.1-IE by digestion with PpuMI. Plasmid pHR′IgP-EGFP-SIN was also digested with PpuMI and IE cloned into it to generate pHR′-IEIgP-EGFP-SIN. The orientation of the inserted enhancer was verified by sequencing. Finally, plasmids pHR′KIEIgP-EGFP-SIN and pHR′-IEKIgP-EGFP-SIN were generated by digesting pCR2.1-IE-K with PpuMI and ligating the enhancer combination released into pHR′-IgP-EGFP-SIN at the PpuMI position. The orientation of the insert was determined by sequencing. In plasmid pHR′IEKIgP-EGFP-SIN the sequence is 5′-IE-Kappa-3′ and the reverse in pHR′KIEIgP-EGFP-SIN.
For the generation of a transcriptionally targeted vector with a selectable marker, the neomycin phosphotransferase gene was cloned after an internal ribosome entry site (IRES) in pHR′KIE-EGFP-SIN by replacing enhanced green fluorescent protein (EGFP) with the IRES-NeoR sequence. IRES-NeoR (1428-bp product) was generated by PCR amplification using pIRESneo2 as template (Clontech Laboratories, Palo Alto, CA) with primers 5′-CGCGGATCCGGGCGGCCAATTCCGCCCCTC-3′ (sense) and 5′-CCGCTCGAGTCAGAAGAACTCGTCAAGAAGG-3′ (antisense) using the same temperature conditions as described. The primers hybridize at positions 1323 and 2760 of the template plasmid. The PCR product and plasmid pHR′KIE-EGFP-SIN were digested with BamHI and XhoI and ligated after purification to generate pHR′KIEIgP-IRESNeoR-SIN. Human NIS (1932 bp) was PCR amplified using primers 5′-CGCGGATCCGCCACCATGGAGGCCGTGGAGACCGGG-3′ (sense) and 5′-CGCGGATCCTCAGAGGTTTGTCTCCTG-3′ (antisense). The reaction mixture was initially heated to 94°C for 4 minutes and then annealed at 58°C for 1 minute followed by elongation at 72°C for 2 minutes. After 30 cycles of amplification, the product was gel purified and digested with BamHI. This was ligated into plasmid pHR′KIEIgP-IRESNeoR-SIN linearized with BamHI to generate pHR′KIEIgP-hNISIRESNeoR-SIN. The orientation of hNIS was determined by diagnostic digests and sequencing.
Lentivector generation
To generate the transcriptionally targeted lentiviral vectors, 293T cells were plated into T75 flasks at a density of 1.5 × 106 cells per flask and allowed to attach overnight. The next day, the cells were transfected with a 3-plasmid mixture containing the transfer plasmid that provides the genome of the vector generated (30 μg), the packaging plasmid (pCMVΔ8.91, 30 μg), and pMD.G that codes for the vesicular stomatitis G (VSV-G) membrane glycoprotein (10 μg) by calcium phosphate precipitation. The next day the cells were washed twice with warm DMEM and incubated with serum-free DMEM for another 2 days. Vector-containing supernatants were harvested after 48 hours, filtered through 45-μm pore filters, snap frozen in liquid nitrogen, and stored at –80°C. All vectors were generated simultaneously under identical conditions.
In vitro transduction of cell lines and primary myeloma cells
For target cell transfection, 1 × 105 cells were suspended in 0.5 mL growth medium with polybrene at a concentration of 8 μg/mL, and 100 μL vector supernatant was added. The cells were incubated at 37°C overnight, washed, and resuspended in fresh medium. After 72 hours at 37°C, the cells were fixed in 4% paraformaldehyde and analyzed for EGFP expression using a flow cytometer (FACScan; Becton Dickinson, San Jose, CA). Untransfected cells were used as controls to set the gates. All transfections were performed in triplicate and data were analyzed using Cell Quest software (Becton Dickinson).
Detection of integrated vectors in transduced cells
Transduced MM1 and 293T cells were expanded in culture and total genomic DNA was extracted using the DNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Using primers that hybridize to a common site upstream of the PpuMI restriction site (sense) 5′-CACCATTATCGTTTCAGACCCACC-3′ in the vectors and with the 5′ end of the EGFP coding region (antisense) 5′-CCCCGGTGAACAGCTTCCTCGCCCT-3′, PCR was used to detect the presence of vector integrants using 0.2 μg total genomic DNA. The reaction temperatures were 94°C for 4 minutes, 94°C for 45 seconds, 58°C for 45 seconds, and 72°C for 1 minute with the reaction cycle repeated 35 times. As internal controls, primers that hybridize with a segment of the dystrophin gene were included in the same reaction mixture (7632 sense primer: 5′-AATTCACAGAGCTTGCCATGCTG-3′; 6975 antisense primer: 5′-TGCCTCCCAGATCTGAGTCCTGTA-3′).21
Lentivirus-mediated hNIS expression in myeloma cells
ARH-77 cells were transduced with the vector KIEIgP-hNISIRESNeoR-SIN in the presence of polybrene. At 3 days after transduction the cells were placed in medium with G418 (Gibco BRL) at a concentration of 1 mg/mL and expanded. Human NIS expression was tested in vitro by radioiodine uptake as previously described.22 Cells (6 × 106) were washed twice in warm Hanks balanced salt solution (HBSS; Gibco BRL) supplemented with 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.3). The cells were split into 6 wells: 3 wells with 0.8 mL and 3 wells with 0.9 mL warm HBSS supplemented with cold sodium iodide (100 μM). Added to the wells with 0.8 mL medium was 100 μL potassium perchlorate (100 μM). Finally, 0.1 mL sodium iodide (125I) with an activity of 1 × 105 cpm/0.1 mL was added to each well and the cells were incubated at 37°C for 45 minutes. After the incubation, the cells were centrifuged at 250g for 5 minutes and washed twice with cold HBSS. After a final centrifugation, residual activity in the cells was measured using a gamma counter. Untransduced ARH-77 cells were used as controls.
In vivo imaging and therapy of myeloma tumor xenografts expressing hNIS
All studies involving animals were approved by the Institutional Animal Care and Use Committee (IACUC) of the Mayo Clinic.
Female CB17scid/scid mice (6-weeks old) were purchased from Harlan Sprague Dawley (Madison, WI). After a week of observation the mice were irradiated with 250 cGy, and 24 hours later, washed ARH-77 (1 × 107 per mouse, 10 mice) or ARH-NIS cells (same number of cells, 10 mice) were implanted in their right flank. The mice were fed a low iodine chow and had levo-thyroxine replacement in their water (5 mg/L) to suppress thyroidal NIS expression.17 Tumor xenograft growth was monitored 3 times a week and tumors were measured in 2 dimensions using calipers. Tumor volumes were calculated using the formula a2b/2 in which a is the shorter diameter. When the tumors reached a mean diameter of 0.6 cm, the mice were injected with sodium iodide (123I, 500 μCi [18.5 MBq]) intraperitoneally and imaged serially at 1, 2, 4, 8, 24, and 48 hours after injection using a gamma camera (Helix System; Elscint, Haifa, Israel).
Using region-of-interest analysis, the total counts in each mouse and in the corresponding tumor were determined. The counts were corrected for gamma camera background activity and for decay of the 123I (half-life, 13.2 hours). Total counts in each mouse and in each tumor were expressed as a percentage of total body activity in the one-hour image. The cumulative tumor activity was calculated as the product of the injected dose and the area under the activity time curve.
Estimation of the radiation dose to the tumors was obtained from the product of the cumulated activity times the “S” factor for the tumor. The cumulative activity for 131I was obtained using the cumulated activity determined from 123I, but adjusted for an isotope with a physical half-life of 8 days. The S factor is the mean dose per cumulated activity. For 131I, we assumed that the target and source organ were the same (ie, all exposure to the tumor came only from the 131I trapped within the tumor) and utilized an S factor of 0.5 × 10–2 rad/μCi per hour (Gy/MBq per hour).23 Dose per gram of tumor was obtained by dividing the total dose by the weight of the tumor. For the evaluation of therapy, the mice were injected intraperitoneally with 131I (1 mCi [37 MBq]) and tumor growth was serially measured. The mice were humanely killed if the tumors reached more than 10% of their weight or if the tumors ulcerated.
In an attempt to evaluate the potential bystander effect of NIS and 131I, we implanted in irradiated severe combined immunodeficiency mice ARH-77 and ARH-NIS cells in different proportions to establish mixed tumors in the right flank. There were 5 mice each injected with 1 × 107 ARH-77 cells (negative controls), 5 mice injected with 1 × 107 ARH-NIS (positive controls), 5 mice injected with 5 × 106 ARH-NIS and 5 × 106 ARH-77 (50% of the cells expressing NIS), and another 5 mice injected with 1 × 106 ARH-NIS and 9 × 106 ARH-77 cells (10% expressing NIS). Tumor growth was monitored as described in the second paragraph of this section, and when the mean tumor diameter reached 0.6 cm, all mice were treated with a single dose of 131I (1 mCi [37 MBq], intraperitoneally). The end points for the study were as described above.
Statistical analysis
Statistical analysis was performed using StatView software (SAS Institute). For comparisons between groups, the Wilcoxon rank sum test was used with a P value less than .05 for statistical significance.
Results
Generation of transcriptionally targeted lentiviral vectors for multiple myeloma
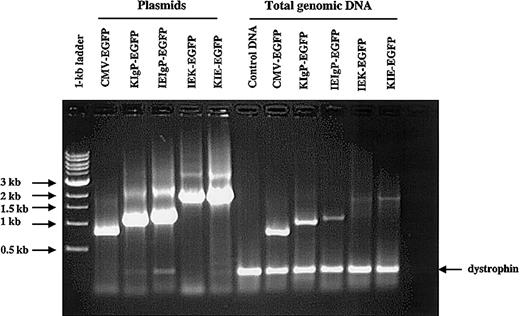
More than 90% of patients with multiple myeloma have a detectable monoclonal immunoglobulin or light chain in the blood or urine.1 We thus hypothesized that the inclusion of immunoglobulin transcriptional regulatory elements in the context of a SIN lentiviral vector might result in high-level and tissue-specific transgene expression in myeloma cells. We generated a number of lentiviral vectors (Figure 1) as described in “Materials and methods.” Our vectors were pseudotyped with the VSV-G envelope, a membrane glycoprotein with a glycolipid receptor that is widely expressed in mammalian cells. Thus, our vectors could transduce essentially all human cell lines. All vectors were generated by transient transfection in 293T cells under identical conditions. To evaluate proviral integrity, MM1 and 293T cells (1 × 105 cells) were transduced with 100 μL of each vector supernatant, expanded, and total genomic DNA extracted using the DNeasy kit. PCR primers were designed to hybridize upstream of all promoter and enhancer combinations in the vectors and within the 5′ region of the EGFP gene (Figure 1, horizontal arrows). Using these 2 primers we could detect all vectors generated using a common PCR reaction mixture. In addition, for internal load controls 2 primers that hybridize to a specific region of the human dystrophin gene were included in the reaction. The expected PCR product from the control primers was 230 bp. As can be seen from Figure 2, we could detect integration of all vectors in 293T cells as well as in MM1 cells (not shown). All proviral PCR products were of the expected length when compared with the plasmids used to generate the vectors, showing that there was no loss of any of the engineered elements by recombination. From Figure 2, it is also apparent that the titers of the vectors generated were different, with the highest titer for the control vector (CMV) and least for IEK.
Schematic representation of the genomes of the lentiviral vectors generated.The parent vector was CMV-EGFP-SIN. This vector has a 400-bp deletion in the U3 region of the LTR to inactive transcription from the lentiviral promoter. Ψrepresents the packaging signal for the vectors. The restriction enzyme sites used to generate the other vectors are marked by vertical arrows and labeled. The 2 horizontal arrows represent hybridization sites for 2 PCR primers used to detect the presence of integrands using total genomic DNA. In vector IEKIgP-EGFP-SIN, the orientation of the enhancers (IEK) is the reverse of that in KIE. R indicates repeat and P indicates immunoglobulin promoter. Gray boxes indicate EGFP.
Schematic representation of the genomes of the lentiviral vectors generated.The parent vector was CMV-EGFP-SIN. This vector has a 400-bp deletion in the U3 region of the LTR to inactive transcription from the lentiviral promoter. Ψrepresents the packaging signal for the vectors. The restriction enzyme sites used to generate the other vectors are marked by vertical arrows and labeled. The 2 horizontal arrows represent hybridization sites for 2 PCR primers used to detect the presence of integrands using total genomic DNA. In vector IEKIgP-EGFP-SIN, the orientation of the enhancers (IEK) is the reverse of that in KIE. R indicates repeat and P indicates immunoglobulin promoter. Gray boxes indicate EGFP.
PCR amplification of promoter/enhancer elements of the vectors generated. To determine that all vectors were generated, 293T and MM1 cells were transduced with these vectors, expanded, and total genomic DNA extracted. The presence of lentivector sequences was determined by PCR using primers common to all the vectors generated (Figure 1). Plasmids used to generate the vectors were used as controls. To ensure that equal amounts of genomic DNA were used, 2 PCR primers that amplify a region of the human dystrophin gene were also included in the reaction mixture. The expected product is 230 bp in length. The PCR products were run on a 1% agarose gel and stained with ethidium bromide. The data shown are for 293T cells.
PCR amplification of promoter/enhancer elements of the vectors generated. To determine that all vectors were generated, 293T and MM1 cells were transduced with these vectors, expanded, and total genomic DNA extracted. The presence of lentivector sequences was determined by PCR using primers common to all the vectors generated (Figure 1). Plasmids used to generate the vectors were used as controls. To ensure that equal amounts of genomic DNA were used, 2 PCR primers that amplify a region of the human dystrophin gene were also included in the reaction mixture. The expected product is 230 bp in length. The PCR products were run on a 1% agarose gel and stained with ethidium bromide. The data shown are for 293T cells.
Lentivectors with targeted expression in myeloma cells
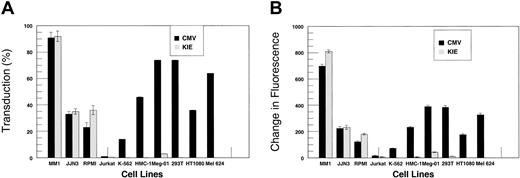
To test specificity of expression from our targeted vectors, we transduced a number of myeloma and nonmyeloma cell lines. Cells (1 × 105) were suspended in 0.5 mL medium in 24-well plates. Vector-containing supernatant (100 μL) was added to the cells with polybrene and incubated at 37°C overnight. The next day, the cells were resuspended in fresh medium and incubated for another 48 hours, after which they were fixed and analyzed for EGFP expression using flow cytometry (Figure 3). Since different cell lines have variable intrinsic fluorescence, we gated each cell line with untransduced cells from the same line (Figure 3A-C). We analyzed the cells for transduction efficiency (%) as well as change in mean fluorescence to evaluate the relative strength of the promoter/enhancer combinations; stronger promoter/enhancer combinations should lead to larger shifts in mean fluorescence. The flow cytometry data are summarized in Figure 4A-B. The untargeted vector (CMV) led to high-level EGFP expression in all cell lines tested (Figure 3D-F) except Jurkat T cells, although there was considerable variability in reporter gene expression between the cell lines. In contrast, EGFP expression from KIEIgPEGFPSIN was restricted to myeloma cell lines, where it was expressed at high levels. There was no expression in nonmyeloma cell lines (Figure 3G-I). There were no statistically significant differences in percent transduction and change in mean fluorescence between the CMV promoter and KIEIgP promoter/enhancer combination (P = .1797, Wilcoxon rank sum test). Although EGFP expression from vectors containing a single immunoglobulin (Ig) enhancer (KIgPEGFPSIN and IEIgPEGFPSIN) was restricted to myeloma cells, gene expression was not very high (data not shown). This is compatible with previous reports indicating that while immunoglobulin promoters with one enhancer element allow tissue-specific expression, the addition of a second enhancer element increases reporter gene expression several fold.14,16 Therefore, we conclude that the combination of an immunoglobulin promoter with the murine Kappa light chain intronic and 3′ enhancers in the context of a SIN lentivector results in high-level and specific transgene expression in B-cell lines with the highest expression in myeloma cell lines.
Flow cytometry analysis for EGFP expression in transduced cell lines. With the vectors generated, 10 different cell lines were transduced. EGFP expression was determined by flow cytometry. Gating was set using untransduced control cells (A-C). The CMV promoter led to high-level expression in all cell lines tested (D-F), but expression from the targeted vector was restricted to myeloma cells (G versus H and I).
Flow cytometry analysis for EGFP expression in transduced cell lines. With the vectors generated, 10 different cell lines were transduced. EGFP expression was determined by flow cytometry. Gating was set using untransduced control cells (A-C). The CMV promoter led to high-level expression in all cell lines tested (D-F), but expression from the targeted vector was restricted to myeloma cells (G versus H and I).
EGFP expression in myeloma and nonmyeloma cell lines. The cell lines tested were transduced and expressed EGFP to different extents. (A) The percentage of cells expressing EGFP after transduction with the CMV (▪) and KIE (▦) vectors (mean of 3 experiments ± 1 SD). (B) The change in mean fluorescence of cells transduced with the 2 vectors compared with controls. While the CMV promoter/enhancer led to EGFP expression in virtually all cell lines tested, EGFP expression from the KIE vector was restricted to the myeloma cell lines. The 2 vectors were statistically equivalent in myeloma cells (error bars indicate SD;P = .1797).
EGFP expression in myeloma and nonmyeloma cell lines. The cell lines tested were transduced and expressed EGFP to different extents. (A) The percentage of cells expressing EGFP after transduction with the CMV (▪) and KIE (▦) vectors (mean of 3 experiments ± 1 SD). (B) The change in mean fluorescence of cells transduced with the 2 vectors compared with controls. While the CMV promoter/enhancer led to EGFP expression in virtually all cell lines tested, EGFP expression from the KIE vector was restricted to the myeloma cell lines. The 2 vectors were statistically equivalent in myeloma cells (error bars indicate SD;P = .1797).
However, B cells also express low levels of antibodies on their surface. Thus, we tested the targeted vectors for expression in 2 B-cell lines (DoHH2 and Daudi) as well as primary myeloma cells (CD138 enriched). The targeted vector (KIEIgPEGFPSIN) was expressed in the B-cell lines as well as primary myeloma cells, but GFP expression was weaker than in myeloma cell lines (data not shown).
Targeted lentiviral vector with hNIS and in vitro iodide uptake in myeloma cells
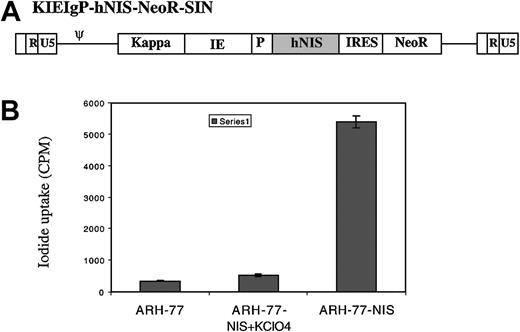
Myeloma cells are very radiosensitive; thus, we wanted to evaluate the potential use of the hNIS as a therapeutic gene for this disease. Since we were interested in proof-of-principle studies, we elected to generate a targeted vector coding for hNIS as well as a selectable marker as shown in Figure 5A. The vector was generated as described and vector-containing supernatant used to transduce ARH-77 cells in the presence of polybrene. After 3 days in culture, the cells were switched to a selection medium containing G418 (1 mg/mL) and the cells expanded.
Targeted hNIS expression in myeloma cells with radioiodide uptake. A transcriptionally targeted lentivector coding for hNIS was generated as described in “Materials and methods.” (A) A single mRNA transcript under the control of the targeted promoter and enhancers is generated, coding for hNIS as well as neomycin phosphotransferase to allow selection of transduced cells. ARH-77 cells were transduced with the vector and selected in G418. (B) In vitro 125I uptake studies were performed on untransduced as well as transduced cells in the presence and absence of potassium perchlorate. The cells transduced with the vector concentrate radioiodine 18-fold compared with controls. Error bars indicate SD.
Targeted hNIS expression in myeloma cells with radioiodide uptake. A transcriptionally targeted lentivector coding for hNIS was generated as described in “Materials and methods.” (A) A single mRNA transcript under the control of the targeted promoter and enhancers is generated, coding for hNIS as well as neomycin phosphotransferase to allow selection of transduced cells. ARH-77 cells were transduced with the vector and selected in G418. (B) In vitro 125I uptake studies were performed on untransduced as well as transduced cells in the presence and absence of potassium perchlorate. The cells transduced with the vector concentrate radioiodine 18-fold compared with controls. Error bars indicate SD.
Expression of hNIS in transduced cells was evaluated using an in vitro iodide uptake assay.22 Potassium perchlorate, a specific inhibitor of hNIS, as well as untransduced ARH-77 cells were used as controls. As can be seen from Figure 5B, cells transduced with this vector and selected in G418 express hNIS and concentrate radioiodine compared with untransduced controls (300 cpm vs 5400 cpm). Transduced cells concentrated radioiodine 100-fold relative to the extracellular medium. This compares favorably to iodide uptake in normal thyroid cells. Iodide uptake is inhibited by perchlorate proving that uptake is due to hNIS expression.
In vivo uptake and imaging of myeloma tumor xenografts expressing hNIS
Radiation induces damage to cells by energy deposition that leads to damage to DNA (mainly) and proteins. Thus, we wanted to determine in vivo the amount of isotope that is taken up by myeloma tumor xenografts expressing NIS and for how long the isotope is retained. This allows a calculation of energy absorbed by the tumor (dosimetry). Figure 6 shows scintigraphic images of CB17/scid mice that were injected with either ARH-77 or ARH-NIS cells. The myeloma tumor xenografts could be clearly seen because of selective iodide uptake, whereas control tumors were not visualized. As can be seen from Figure 7, there is rapid iodide uptake by the tumors expressing NIS (20%). While most of the iodide leaks out within the first 4 hours, a residual activity of 4% to 5% can be clearly detected up to 48 hours after injection of the isotope. From the area under the activity time curve (Figure 7), cumulative activity in the tumor was calculated as 8670 μCi (320.79 MBq) per hour. Thus, we conclude that myeloma tumor xenografts concentrate and retain radioiodine in vivo.
In vivo uptake and retention of radioiodide in myeloma tumor xenografts. ARH-77 and ARH-77-NIS cells were implanted into SCID mice. When the tumors reached a mean diameter of 0.6 cm, the mice were injected with 123I intraperitoneally (500 μCi [18.5 MBq]) and imaged using a gamma camera. The image is taken 4 hours after isotope injection. The mouse on the left (A) has a tumor not expressing hNIS, while the 2 mice on the right (B-C) have tumors expressing hNIS. Iodide uptake in the tumors can be clearly seen in their left flank. Normal iodide uptake in the thyroid, stomach, and in the bladder due to renal excretion is seen in all.
In vivo uptake and retention of radioiodide in myeloma tumor xenografts. ARH-77 and ARH-77-NIS cells were implanted into SCID mice. When the tumors reached a mean diameter of 0.6 cm, the mice were injected with 123I intraperitoneally (500 μCi [18.5 MBq]) and imaged using a gamma camera. The image is taken 4 hours after isotope injection. The mouse on the left (A) has a tumor not expressing hNIS, while the 2 mice on the right (B-C) have tumors expressing hNIS. Iodide uptake in the tumors can be clearly seen in their left flank. Normal iodide uptake in the thyroid, stomach, and in the bladder due to renal excretion is seen in all.
In vivo retention of iodide by myeloma tumor xenografts expressing hNIS. Mice bearing myeloma tumor xenografts expressing hNIS were imaged serially starting one hour after injection of the isotope. Initial image acquisition was 3 minutes, but this increased to 5 minutes as the isotope decayed. After correction for the physical half-life of the isotope, acquisition time, and background activity, percentage iodide uptake in the tumors was calculated. There is rapid and high-level uptake of iodide by the tumors. Although most of it leaks out within the first few hours, low-level activity (4%-5%) remains up to 48 hours after injection.
In vivo retention of iodide by myeloma tumor xenografts expressing hNIS. Mice bearing myeloma tumor xenografts expressing hNIS were imaged serially starting one hour after injection of the isotope. Initial image acquisition was 3 minutes, but this increased to 5 minutes as the isotope decayed. After correction for the physical half-life of the isotope, acquisition time, and background activity, percentage iodide uptake in the tumors was calculated. There is rapid and high-level uptake of iodide by the tumors. Although most of it leaks out within the first few hours, low-level activity (4%-5%) remains up to 48 hours after injection.
Therapy of myeloma tumor xenografts expressing NIS with 131I
Once we had established that tumors retain iodide in vivo, we wanted to evaluate the use of 131I, a β–-emitting isotope for therapy of myeloma tumor xenografts. In the CB17/scid mice who were implanted with ARH-77 or ARH-77-NIS cells, we found that tumor xenografts that expressed NIS were completely destroyed in fewer than 2 weeks by a single dose of 131I (1 mCi [37 MBq]). All other tumors continued to grow and the mice had to be humanely killed because the tumors ulcerated (Figure 8A). Therapy with the isotope was well tolerated. The mice with complete tumor regression were observed for up to 5 months after therapy; the tumors never recurred and the mice appeared healthy. From the dosimetry calculations outlined in the previous paragraph, we estimated that the tumors absorbed 400 rad/g (400 cGy), although this is a lower estimate because of the half-life of 123I used for dosimetry.
Therapy of myeloma tumor xenografts with radioiodine. (A) Myeloma tumor xenografts expressing hNIS and treated with a single dose of 131I (1 mCi [37 MBq] intraperitoneally) regressed completely within 2 weeks of therapy. Tumor xenografts not expressing hNIS continued to grow, as did untreated control tumors. (B) NIS with 131I has a significant bystander effect. Mixed tumors were established in SCID mice and all were treated with a single dose of 131I (1 mCi, [37 MBq] intraperitoneally). All tumors in which 50% to 100% of the cells expressed NIS regressed completely, while tumors with only 10% of the cells expressing NIS were slowed transiently. Vertical arrows indicate time of therapy with radioiodine.
Therapy of myeloma tumor xenografts with radioiodine. (A) Myeloma tumor xenografts expressing hNIS and treated with a single dose of 131I (1 mCi [37 MBq] intraperitoneally) regressed completely within 2 weeks of therapy. Tumor xenografts not expressing hNIS continued to grow, as did untreated control tumors. (B) NIS with 131I has a significant bystander effect. Mixed tumors were established in SCID mice and all were treated with a single dose of 131I (1 mCi, [37 MBq] intraperitoneally). All tumors in which 50% to 100% of the cells expressed NIS regressed completely, while tumors with only 10% of the cells expressing NIS were slowed transiently. Vertical arrows indicate time of therapy with radioiodine.
Finally, we wanted to evaluate the potential bystander effect of NIS in combination with 131I. Mixed tumor xenografts were established in the right flank of SCID mice as described in “Materials and methods.” It was assumed that as the tumors develop, the proportion of cells expressing NIS will remain as that of the injected population. The mixed tumors grew at very similar rates (Figure 8B), and all the mice were treated with a single dose of 131I (1 mCi [37 MBq]). As expected, ARH-77 tumors did not respond, while the tumors with 50% or 100% of the cells expressing NIS resolved completely within 2 weeks. The tumors in which only 10% of the cells expressed NIS slowed down after therapy but then continued to grow. We believe that a higher dose of 131I in these mice could lead to complete tumor eradication by depositing more energy in the tumor. Thus, NIS and 131I have a significant bystander effect that can be exploited to treat radiosensitive tumors. We conclude that the combination of hNIS and 131I is an attractive strategy for therapy of multiple myeloma.
Discussion
Multiple myeloma is at present an incurable disorder that leads to the death of up to 14 000 patients per year in the United States.24 The disease is very radiosensitive and in the past investigators have devised various approaches to irradiate the bone marrow in attempts to control the disease.25 In the last few years many patients have been offered high-dose chemotherapy with stem cell support. The most popular conditioning regimens have been either lower dose melphalan (140 mg/m2) combined with 8 Gy of TBI or melphalan alone (200 mg/m2). There is no survival difference between these 2 regimens and TBI is associated with higher toxicity.26 Since the pool of replicating cells in myeloma is small, we opted to work with lentiviral-based vectors for efficient transduction of the target cells. Our hypothesis was that expression of hNIS by myeloma cells could result in significant iodide uptake and retention by tumors, leading to their eradication.
The ideal vector for gene therapy applications should combine targeted entry into the tissue of interest as well as specific and high-level expression. The membrane glycoproteins of retro and lentiviruses have a dual function: binding to the cognate receptor followed by triggering of membrane fusion. Engineering these envelope glycoproteins to target entry into specific cells is mechanistically difficult since the natural tropism of the envelope must be eliminated while the protein must still be able to undergo the conformational changes necessary for the fusion event that allows entry.27 While there have been major conceptual advances in the area of targeted entry, such vectors remain to be developed. Therefore, we have developed a transcriptionally targeted lentiviral vector in which the reporter or therapeutic gene is under the control of immunoglobulin promoter and enhancer elements.
Immunoglobulin gene expression is limited to cells of the B-cell lineage because of the presence of specific cis-acting elements within promoter and enhancer elements that restrict gene transcription to these cells. The immunoglobulin promoter contains an octameric sequence (ATGCAAAT) that has been shown to be sufficient for lymphoid-specific expression.12 This octameric sequence binds to Oct-1 and Oct-2, proteins belonging to the POU family of DNA-binding proteins.28,29 While Oct-1 is ubiquitously expressed, Oct-2 has a more limited expression and is found in lymphoid cells, the nervous system, kidneys, and testis.30 A highly B lineage–specific coactivator, Oct binding factor 1 (OBF-1, also known as OCA-B or Bob-1) interacts with Oct-1 and Oct-2 in B-lineage cells and is recruited to specific octamer sites where it coactivates specific gene transcription.31 Transcription from immunoglobulin promoters is greatly enhanced by the presence of 2 enhancer elements within the Kappa light chain locus. One enhancer is located within the J-C intron and the other is 3′ to the constant region gene.14,32 The presence of the enhancer elements either singly or in combination increases the efficiency of transcription from immunoglobulin promoters.14,16 When present together, the 2 enhancers act synergistically.16 The exact mechanisms of how these enhancers work are unclear, but they work independent of their orientation and distance relative to the promoter.33 A number of transcription factors bind to these elements, including a nuclear factor (NF)–AT–related protein, NF-κB, NF-E1 (YY-1), and NF-κE2.34-37
We have used the known tissue specificity of these immunoglobulin transcriptional regulatory elements to generate a lentivector that exhibits high-level and specific transgene expression in myeloma cells. The lentiviral promoter sequences are in the U3 region of the LTR. In our vector, 400 bp of the U3 region has been eliminated to prevent transcription from the 5′ LTR.7 Therefore, the generation of reporter gene transcripts depends on an internal promoter, in our case the immunoglobulin kappa light chain enhancer elements and a minimal immunoglobulin promoter. Despite transduction of many nonmyeloma cell lines, there was no EGFP expression in these cells. Cell lines transduced with these vectors were maintained in culture for 6 months without any change in reporter gene expression. This proves not only stable integration of our vectors in the transduced cells but also the long-term specificity of gene expression achieved (data not shown). Our work provides further evidence for the feasibility of transcriptionally targeting lentivectors as gene delivery vehicles.9,10
The disease burden in multiple myeloma is in the range of 1012 cells.38 It is therefore very difficult to transduce every tumor cell in the body. Thus, for a maximal cytoreductive effect, a therapeutic gene with a significant bystander effect would be ideal. Since myeloma is a radiosensitive tumor, we studied the potential use of hNIS as a therapeutic gene for the disease. Myeloma cells expressing hNIS concentrate radioiodide in vivo and retain the isotope for at least 48 hours, leading to significant energy absorption by the tumor. 131I decays by emitting a β– particle (electron) as well as a high-energy gamma photon. The electron has a path length in tissue of 0.2 to 2.4 mm (mean, 0.4 mm).19 Assuming a mean cell diameter of 10 μm, the emitted electrons travel several cell diameters away and deposit energy in surrounding tissues rather than the cell expressing hNIS. Since myeloma cells in the bone marrow tend to form plasmacytomas, it is possible that transduction of a few cells with this targeted vector could lead to significant destruction of surrounding (untransduced) myeloma cells (bystander effect). Indeed, our mixed tumor studies suggest that such a bystander effect exists and can be exploited to treat myeloma. We have provided proof of principle that myeloma cells expressing hNIS concentrate radioiodine and retain it long enough for a therapeutic effect. Various investigators have evaluated the use of NIS as a therapeutic gene for a variety of tumors, including prostate and gliomas, and it may also be of use in some breast tumors that express NIS.17,18,39 Our work further expands on the potential use of hNIS as a therapeutic gene for cancer.
In summary, we describe the development of a transcriptionally targeted lentivector that results in high-level and specific expression of a transgene in myeloma cells. Transduction of myeloma cells with this targeted vector coding for hNIS leads to expression of the sodium-iodide symporter and iodide concentration in these cells. Myeloma tumor xenografts expressing hNIS can be imaged in vivo using 123I, and a single dose of a therapeutic isotope (131I) leads to complete and long-lasting remission of these tumor xenografts in SCID mice. NIS with 131I has a significant bystander effect and to our knowledge, this is the first time this observation has been documented in an in vivo system. The possible use of hNIS in combination with 131I as a therapy for multiple myeloma requires further study.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-11-3390.
Supported by grant CA 83181 (S.J.R.), a fellowship award from the Multiple Myeloma Research Foundation (D.D.), and the Harold W. Siebens Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ``advertisement'' in accordance with 18 U.S.C. section 1734.
The authors express their thanks to Drs Rafael Fonseca, Adele K. Fielding, and Joseph H. Butterfield (Mayo Clinic, Rochester, MN) for various cell lines; Dr Didier Trono (University of Geneva, Switzerland) for the lentivector generation plasmids; Dr Christine Spitzweg for many helpful suggestions; Christy Finke for primary myeloma cell isolation; and Mary Harvey for assistance with the animal experiments.



![Figure 6. In vivo uptake and retention of radioiodide in myeloma tumor xenografts. ARH-77 and ARH-77-NIS cells were implanted into SCID mice. When the tumors reached a mean diameter of 0.6 cm, the mice were injected with 123I intraperitoneally (500 μCi [18.5 MBq]) and imaged using a gamma camera. The image is taken 4 hours after isotope injection. The mouse on the left (A) has a tumor not expressing hNIS, while the 2 mice on the right (B-C) have tumors expressing hNIS. Iodide uptake in the tumors can be clearly seen in their left flank. Normal iodide uptake in the thyroid, stomach, and in the bladder due to renal excretion is seen in all.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/2/10.1182_blood-2002-11-3390/6/m_h81434650006.jpeg?Expires=1769530520&Signature=TtAyw3u-BDyrAHwZJRLUtxIi8xsRhT3WcgdfL6fEpqsXgY1HjnByFr69ruj1NeQhsaXCJJUL7RLZLPVvi1xBSnOxr72LiRHXOAsKOCnTRI6KQ~bOZ0HhalCAyBk1xtpKjxiGwWiaLLonAEJVhA-4Rmta-8Z5187bt~rUf-4pklSeiEdVQih3xftXvHB1r4Nur~OBGqYeJ6MN6v~bHWD8o8GJb83pnVEbIeuFC8rZPA97wEmQX88d3v6KXOYwqTO8Ve0GV6Z0gS7qQbQrjNS~Aqhno532uMEbgEY63fSTnI4um~CO5-1XGb0wl6NEd4pXATmngn~51mfjnsEbwQqfKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 8. Therapy of myeloma tumor xenografts with radioiodine. (A) Myeloma tumor xenografts expressing hNIS and treated with a single dose of 131I (1 mCi [37 MBq] intraperitoneally) regressed completely within 2 weeks of therapy. Tumor xenografts not expressing hNIS continued to grow, as did untreated control tumors. (B) NIS with 131I has a significant bystander effect. Mixed tumors were established in SCID mice and all were treated with a single dose of 131I (1 mCi, [37 MBq] intraperitoneally). All tumors in which 50% to 100% of the cells expressed NIS regressed completely, while tumors with only 10% of the cells expressing NIS were slowed transiently. Vertical arrows indicate time of therapy with radioiodine.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/2/10.1182_blood-2002-11-3390/6/m_h81434650008.jpeg?Expires=1769530520&Signature=BNqCcJHAJZw~QSCtOk4Lub3AxJ0GtbDwWjr4sm-UK7ejWIzBbhGBolTn4~lzh9AVexWVev3LTvI51I2YykLiO6iYB~MonGSiIYJQ5okMOVZXm6Dt90qYnaYf79W2kHaAfWU5tJorV2sGZgW0BXw-0YiajiAKWgv~QVXicZFQU~BoYes6uarVgeFc87AkdF0uhn41fHEieIcixj1TxPbcFxexKZStBgP6QinAhAfwR-X2spqeMJKFCXgciBnkj519v6vL1t5XvwsKEDr~hsLurcxOVyrONSfA-yow3QmhqyKNFAKueaQDPK88QOYXeGxoOq~E5ocXt8F2gCepKcxuLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
