Abstract
Fibrinogen γA/γ′ results from alternative splicing of mRNA. This variant, which constitutes approximately 8% to 15% of plasma fibrinogen, contains FXIII and thrombin binding sites. Our objective was to investigate whether γA/γ′ differs in fibrin formation and structure from the more common variant γA/γA. Both variants were separated and purified by anion-exchange chromatography. Fibrin formation and clot structure of the variants and unfractionated fibrinogen were investigated by turbidity and scanning electron microscopy (SEM). Thrombin cleavage of fibrinopeptides was analyzed by high-performance liquid chromatography (HPLC). Turbidity analysis showed significantly altered polymerization rates and overall fiber thickness in γA/γ′ clots compared with γA/γA and unfractionated fibrinogen. This finding was consistent with a range of thrombin concentrations. HPLC demonstrated reduced rates of fibrinopeptide B (FpB) release from γA/γ′ fibrinogen compared with γA/γA. Delayed FpB release was associated with delayed lateral aggregation of protofibrils and significant differences were found on SEM, with γA/γ′ clots consisting of smaller diameter fibers and increased numbers of branch points compared with both γA/γA and unfractionated fibrinogen. These results demonstrate that the γA/γ′ splice variant of fibrinogen directly alters fibrin formation and structure, which may help to explain the increased thrombotic risk associated with this variant.
Introduction
Fibrinogen is a 340-kDa glycoprotein of bilateral symmetry consisting of 6 polypeptides (AαBβγ)2 held together by disulphide bonding. The molecule consists of 3 main structural regions, D-E-D, that are connected by coiled-coil regions composed of intertwining α helices of the Aα, Bβ, and γ chains.1,2 The amino termini of all 6 polypeptide chains are located in the central E region. Carboxyl termini of the Bβ and γ chains are located in the D regions, whereas those of the Aα chain extend from the D regions to form relatively flexible αC domains. Thrombin cleavage of the amino termini of the Aα and Bβ chains results in the release of fibrinopeptides A (FpA) and B, initiating fibrin formation. Release of FpA by thrombin is fast and exposes a binding sequence (Gly-Pro-Arg) in the E region for a complementary binding site of the γ chain in the D region, leading to protofibril formation of half-staggered, overlapping fibrin molecules. Cleavage of FpB by thrombin is slower than that of FpA and also exposes a binding sequence (Gly-His-Arg) in the E region that binds to a complementary site of the β chain in the D region. FpB release is associated with lateral aggregation of the protofibrils, leading to the formation of thicker fibers.3,4 Lateral aggregation involves interactions of extending αC domains between fibrin molecules in aligning protofibrils.5 The fibrin fibers thus formed branch out and build the backbone of the fibrin clot. Several factors influencing clot architecture have thus far been identified, such as altered fibrinogen plasma concentrations,6 genetic variations in fibrinogen and factor XIII,7-9 and elevated thrombin or prothrombin levels.10 There is increasing evidence that altered structure of the fibrin clot may be involved in the pathogenesis of atherosclerosis and thrombotic disease. Fatah et al11,12 found that patients with myocardial infarction formed clot structures that were denser and less permeable than those from healthy subjects. A study from our laboratory found that relatives of patients with coronary artery disease showed similar fibrin clot structures of reduced permeability independent of fibrinogen levels, suggesting that familial factors play a role in determining the structure and function of the fibrin clot.13
Approximately 8% to 15% of plasma fibrinogen contains a variant γ chain that results from alternative processing at the exon 9/exon 10 boundaries of the mRNA.14,15 The most frequent γA chain results from the splicing of intron 9, so that the polymerase translates exon 9, followed by exon 10 up to the stop codon after the only 4 residues that are coded by this last exon. The variant γ′ arises from inclusion of intron 9 in the messenger. The polymerase in this case reads from exon 9 into intron 9, where a stop codon is present after 20 residues. Therefore, a new C terminus is created in which the last 4 amino acids (AGDV) of γA are replaced by a sequence of 20 alternative residues (VRPEHPAETEYDSLYPEDDL) in γ′.14-16 This γ′-chain extension protrudes from the D region, contains 2 sulfated tyrosines17 and several Asp and Glu residues18 that provide an overall negative charge to the region. γ′ Fibrinogen contains binding sites for thrombin19 and factor XIII B subunit,20 but unlike γA it does not have a binding site for the platelet integrin αIIbβ3.21 Data collected to date suggest that the variant in vivo is found in the heterozygous form with γA(γA/γ′), with one D region containing γA and the other γ′. Although little is known about regulation of γ′ levels in vivo, there is evidence that mRNA processing resulting in γ′ is a tissue-specific and regulated event.22 Several functional studies have been performed that showed that γA/γ′ fibrin is more extensively cross-linked by activated factor XIII than γA/γA23 and has greater resistance to fibrinolysis.24 These properties, along with a recent study that demonstrated elevated levels of γ′ in patients with cardiovascular disease independent of total plasma fibrinogen,25 indicate that the proportion of γ′ in plasma may be a risk factor for disease. The direct effect of the γ′ on fibrin formation and structure has so far been unknown. The aim of this study was to compare structural properties of clots formed with purified γA/γ′ and γA/γA fibrinogen.
Materials and methods
Purification of γA/γA and γA/γ′ fibrinogen
The γA/γAand γA/γ′ fibrinogen variants were purified from human plasminogen-free fibrinogen (Calbiochem, San Diego, CA) by anion-exchange chromatography on a DE52 column (30 × 0.9 cm) using a BioCad Sprint chromatography system (Perseptive Biosystems, Framingham, MA), based on a method previously described by Siebenlist et al.20 Before chromatography, lyophilized fibrinogen was dissolved in and dialyzed with 3 changes at 4°C against buffer A (0.039 M Tris [tris(hydroxymethyl)aminomethane], 0.005 M H3PO4, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM benzamidine, and 5 mM ϵACA [aminocaproic acid], pH 8.6). After 1 column volume (CV) equilibration with buffer A, the fibrinogen sample was injected, and the DE52 column was washed with buffer A for 5 CVs. Flow rate was 1.3 mL/min throughout the chromatography protocol. A 13-segment concave gradient from buffer A to buffer B (0.5 M Tris, 0.5 M H3PO4, 0.5 mM PMSF, 1 mM benzamidine, and 5 mM ϵACA, pH 4.2) was established over 13.4 CVs and 10 mL fractions were collected. The column was washed with 5 CVs of buffer B and then equilibrated with buffer A for 5 CVs. Absorbency was read at 280 nm and protein peaks were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The γA fibrinogen eluted around 10% to 15% and γ′ around 15% to 20% buffer B. Fractions containing γA and γ′ fibrinogen were concentrated using Amicon centriplus YM-100 centrifugal devices (Millipore, Bedford, MA). Fibrinogen γ′ was also purified from 30 mL of plasma obtained from one healthy volunteer. Informed consent was obtained according to the Declaration of Helsinki, and the research protocol was approved by the Leeds Teaching Hospitals Trust Research Ethics Committee. Blood was withdrawn from the anticubital vein with minimal stasis into a siliconized tube with 15 IU/mL lithium heparin as anticoagulant. The sample was processed by centrifugation at 2540g for 20 minutes at room temperature. Platelet-poor plasma was separated and immediately used for purification. Fibrinogen was purified to homogeneity by affinity chromatography with a calcium-dependent monoclonal antibody (IF-1) coupled to Sepharose 4B as described by Takebe et al.26 In brief, plasma was filtered through 0.2 μm and 2 mL was applied to the IF-1 column (8 mL column volume) equilibrated with 0.02 M Tris-Cl, 0.3 M NaCl, 1 mM CaCl2, and 0.2% NaN3. The column was washed with 6 CVs 0.02 M Tris-Cl, 1 M NaCl, 1 mM CaCl2, pH 7.4 and 6 CVs 0.05 M sodium acetate, 0.3 M NaCl, 1 mM CaCl2, pH 6.0. Fibrinogen was eluted with 3 CVs 0.2 M Tris-Cl, 0.3 M NaCl, 5 mM EDTA (ethylenediaminetetraacetic acid), pH 7.4. The purified fibrinogen was concentrated, dialyzed against buffer A and γ-chain variants were separated by DE52 chromatography as described in the first part of this paragraph. Purity of all preparations was examined by SDS-PAGE, and concentration was determined with absorbency at 280 nm using a SP-75 spectrophotometer (Sanyo Gallenkamp, Loughborough, United Kingdom) and an extinction coefficient of
SDS-PAGE
Gel electrophoresis was performed using a Miniprotean 3 (Biorad, Hercules, CA) electrophoresis unit. Gels were cast at a polyacrylamide concentration of 8% (bis-acrylamide ratio of 1:37.5) in 1.5 M Tris-Cl, pH 8.8, and run at 150 V for 80 minutes. Gels were stained with Gelcode blue protein stain (Pierce, Rockford, IL) and photographed digitally using an Alpha Innotech (San Leandro, CA) gel-documentation system.
Turbidity measurements at 350 nm
Initial rates of fibrin polymerization of each γ-chain isoform as well as that of the unfractionated starting material (Calbiochem plasminogen-free human fibrinogen) were studied by turbidity analysis.27 Samples were diluted to a final concentration of 1 mg/mL in 50 mM Tris, 100 mM NaCl, pH 7.5. Immediately upon addition of human α thrombin (American Diagnostica, Greenwich, CT) and calcium (final concentrations 0.5 IU/mL and 10 mM, respectively), absorbency was read every 10 seconds at 350 nm for 4 minutes on a MRX Microtiterplate Reader (Dynex Technologies, Ashford, United Kingdom). Twenty-eight replicate measurements were performed per sample. To investigate the possibility of a rate-limiting effect of the thrombin concentration, further experiments were carried out with higher thrombin concentrations of 1 and 2 IU/L.
Analysis of fibrinopeptide release by HPLC
Fibrinopeptide release was measured by reverse-phase high-performance liquid chromatography (HPLC) as previously described.9 Clotting of 0.77 mg/mL γA/γA, γA/γ′, or unfractionated fibrinogen was initiated by the addition of 1 IU/mL human α thrombin (American Diagostica). Reaction mixtures were incubated at 37°C until polymerization was stopped at 0.5, 2, 5, and 10 minutes with 1:10 (vol/vol) of 3 M HClO4. Samples were spun for 15 minutes in a microcentrifuge, and the supernatant was applied to a 0.46 × 25 cm silica C18 (bead size, 5 μm; pore size, 30 nm [300 Å]) column (Pepmap C18) on a BioCad Sprint chromatography system (both from Perseptive Biosystems). Peptides were eluted with a linear gradient from 100% buffer C (10% acetonitrile and 90% 0.083 M NaH2PO4, pH 3.1) to 100% buffer D (40% acetonitrile and 60% 0.083 M NaH2PO4, pH 3.1.), and UV absorbency was measured at 205 nm. All reagents were HPLC grade and solutions were filtered through 0.22 μm to eliminate particulates. For each sample, the area under the curve of the peak on the chromatogram was integrated and converted to molar concentration by comparison to the amount of fibrinopeptides obtained at maximum release (45 minutes). A minimum of 4 replicates were performed for each time point and data were expressed as mean and SD of the ratio of fibrinopeptide concentration at a given time point over that at maximum release ([Fp]/[Fp]max).
Scanning electron microscopy of fibrin clots
Scanning electron microscopy was used to further investigate the structure of clots formed from γA/γA and γA/γ′ fibrinogen as well as the unfractionated starting material. Human α thrombin (American Diagnostica, final concentration 1 IU/mL) and calcium chloride (final concentration 10 mM) in 50 mM Tris-Cl, 100 mM NaCl, pH 7.5, were added to purified γA/γA and γA/γ′ fibrinogen (final concentration 0.7 mg/mL). Samples were extensively washed with cacodylate buffer and prepared for microscopy by fixation with 2% glutaraldehyde solution, serial stepwise acetone dehydration, critical point drying, and sputter coating with gold palladium, as described previously.9 Clots were observed and photographed digitally in at least 5 different areas per clot, using a Camscan series IV scanning electron microscope (Obducat Camscan, Waterbeach, United Kingdom). Average fiber diameters were measured from 50 random fibers of each sample using ImageJ 1.29x software (National Institutes of Health, Bethesda, MD). The number of branch points was determined in 5 areas of 4 μm2 per micrograph.
Statistical analysis
Data of repeated experiments were described with mean and standard deviation. Differences in polymerization lag phase and maximum absorbency were analyzed by unpaired t test. Fiber diameters were tested by analysis of variance (ANOVA) with post hoc Bonferroni analysis. All analyses were performed using SPSS for Windows software version 9.0 (SPSS, Chicago, IL). P values lower than .05 were considered to indicate statistical significance.
Results
Purification of γ-chain splice variants
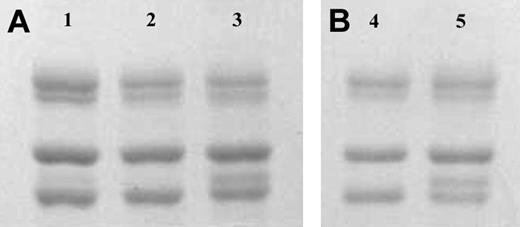
Fibrinogen γA and γ′ were separated on anion-exchange chromatography from human fibrinogen purified from a mixed plasma pool (Calbiochem; Figure 1A) and also from fibrinogen purified from the plasma from one individual (Figure 1B). The γ′ preparations showed a doublet for the γ chain of approximately equal intensity (Figure 1, lanes 3 and 5), in agreement with the concept that most of γ′ occurs in heterozygous form with γA(γA/γ′), with one of the γ-chain splice variants on each of the 2 D regions in the fibrinogen molecule. A faint band for γ′ chains could also be observed in the unfractionated fibrinogen before its application to anion exchange chromatography (Figure 1, lane 1).
SDS-PAGE of fibrinogen γ-chain variants. Panel A shows unfractionated (Calbiochem; lane 1), γA/γA (lane 2), and γA/γ′ (lane 3) fibrinogen preparations purified by DE52 anion-exchange chromatography. From top to bottom, bands for theAα, Bβ, and γA chains can be observed. An additional band for the heavier γ′ chain migrates between the γA and Bβ chain in the γA/γ′ preparation as can be observed in lane 3 and 5, and more faintly in lane 1. Panel B shows γA/γA (lane 4) and γA/γ′ (lane 5) fibrinogen purified from one individual.
SDS-PAGE of fibrinogen γ-chain variants. Panel A shows unfractionated (Calbiochem; lane 1), γA/γA (lane 2), and γA/γ′ (lane 3) fibrinogen preparations purified by DE52 anion-exchange chromatography. From top to bottom, bands for theAα, Bβ, and γA chains can be observed. An additional band for the heavier γ′ chain migrates between the γA and Bβ chain in the γA/γ′ preparation as can be observed in lane 3 and 5, and more faintly in lane 1. Panel B shows γA/γA (lane 4) and γA/γ′ (lane 5) fibrinogen purified from one individual.
Fibrin polymerization
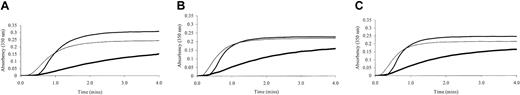
Fibrin polymerization by thrombin was investigated using turbidity at 350 nm. Polymerization of the γ-chain fibrin splice variants occurred at markedly different rates (Figure 2). The γA/γ′ fibrin displayed much-reduced polymerization rates compared with γA/γ. This effect was regardless of the thrombin concentration. As thrombin concentration was increased from 0.5 to 1 and 2 IU/mL, polymerization rate of γA/γ′ fibrin remained slower than that of γA/γA (Figure 2). The maximum absorbency, which at equal fibrinogen concentration is directly related to fiber diameter, was significantly lower for γA/γ′ fibrin compared with γA/γA (Table 1). At higher thrombin concentrations, γA/γ′ fibrin fibers remained thinner than those of γA/γA fibrin (Figure 2), although overall fiber diameters of the fibrin preparations decreased with increasing thrombin, consistent with previous findings on the effect of (pro)thrombin on fibrin structure.10 Fibrin made from unfractionated fibrinogen demonstrated intermediate fiber diameters (maximum absorbency) and shorter lag phase (Figure 2). There was no significant difference between the lag phase of polymerizing γA/γ′ and γA/γA fibrin (Table 1). To reduce possible confounding effects of other fibrinogen heterogeneities, fibrin polymerization by turbidity was analyzed using γA/γ′ and γA/γA fibrinogen purified from fibrinogen from one individual only. Similar effects of this γA/γ′ preparation on polymerization rate and maximum absorbency were observed (data not shown).
Turbidity generation from thrombin-treated γA/γA, γA/γ′ and unfractionated fibrinogen. Unfractionated (thin line), γA/γA (normal weight line) and γA/γ′ (thick line) fibrinogen was incubated at 1 mg/mL with 0.5 (A), 1 (B), and 2 (C) IU/mL human α thrombin and 10 mM calcium. Generation of turbidity at 350 nm was measured every 10 seconds for 4 minutes. Fibrinogen γA/γ′ demonstrated altered polymerization rates.
Turbidity generation from thrombin-treated γA/γA, γA/γ′ and unfractionated fibrinogen. Unfractionated (thin line), γA/γA (normal weight line) and γA/γ′ (thick line) fibrinogen was incubated at 1 mg/mL with 0.5 (A), 1 (B), and 2 (C) IU/mL human α thrombin and 10 mM calcium. Generation of turbidity at 350 nm was measured every 10 seconds for 4 minutes. Fibrinogen γA/γ′ demonstrated altered polymerization rates.
Fibrinopeptide release by thrombin
Since turbidity analysis demonstrated marked differences in fibrin polymerization rates for γA/γ′ fibrin, we further investigated the variations in the initial stages of fibrin formation by analyzing the kinetics of fibrinopeptide cleavage by thrombin using HPLC. The rate of FpB cleavage from γA/γ′ fibrinogen by thrombin was reduced compared with that from γA/γA fibrinogen (Figure 3). There was no difference in rate of FpA release from γA/γ′ and γA/γA fibrinogen. Rate of FpA release from unfractionated fibrinogen was similar to that of γA/γ′ and γA/γA, whereas FpB release rate from unfractionated fibrinogen showed intermediate behavior, more closely related to that of γA/γA (data not shown).
Fibrinopeptide release from thrombin-treated γA/γA and γA/γ′ fibrinogen. Purified fibrinogen γA/γA and γA/γ′ (0.77 mg/mL each) were incubated with 1 IU/mL thrombin and release of FpA and FpB was analyzed by reverse-phase HPLC using a silica C18 column. The fraction of the concentration of the fibrinopeptides at a given time point divided by that at maximum release ([Fp]/[Fp]max, so that full release equals 1) is plotted against time. FpA released from γA/γA fibrinogen is represented by •, FpB from γA/γA by ▵, FpA from γA/γ′ by ⋄, and FpB from γA/γ′ by ▪. Release of FpB was slower in γA/γ′ compared with γA/γA fibrinogen. Inset in the figure shows a typical chromatogram from HPLC, with the injection peak followed by 2 major peaks representing FpA and then FpB.
Fibrinopeptide release from thrombin-treated γA/γA and γA/γ′ fibrinogen. Purified fibrinogen γA/γA and γA/γ′ (0.77 mg/mL each) were incubated with 1 IU/mL thrombin and release of FpA and FpB was analyzed by reverse-phase HPLC using a silica C18 column. The fraction of the concentration of the fibrinopeptides at a given time point divided by that at maximum release ([Fp]/[Fp]max, so that full release equals 1) is plotted against time. FpA released from γA/γA fibrinogen is represented by •, FpB from γA/γA by ▵, FpA from γA/γ′ by ⋄, and FpB from γA/γ′ by ▪. Release of FpB was slower in γA/γ′ compared with γA/γA fibrinogen. Inset in the figure shows a typical chromatogram from HPLC, with the injection peak followed by 2 major peaks representing FpA and then FpB.
Fibrin clot ultrastructure
Maximum absorbency in the turbidity experiments indicated the presence of thinner fibers in clots formed with γA/γ′ fibrinogen. We therefore investigated structural differences between clots formed from γA/γ′, γA/γA, and unfractionated fibrinogen using scanning electron microscopy. Clots made from γA/γ′ fibrinogen demonstrated a very different structure compared with clots made from γA/γA and unfractionated fibrinogen (Figure 4). The γA/γ′ fibrin clots had thinner fibers and smaller pores, consistent with the findings in turbidity analysis. Structure of fibrin made from unfractionated fibrinogen was more similar to that of clots made from γA/γA fibrinogen. Average fiber diameter was significantly smaller for γA/γ′ fibrin compared with γA/γA or unfractionated fibrin clots (Table 2). Fiber diameter of unfractionated fibrin was smaller than γA/γA fibrin but larger than that of γA/γ′ fibrin (Table 2). There was a higher proportion of branch points per area of the clot in γA/γ′ compared with γA/γA or unfractionated fibrin (Table 2).
Scanning electron micrographs of clots made from fibrinogen γ-chain splice variants. Clots were made from purified fibrinogen γ-chain splice variants and observed by scanning electron microscopy. Scale bars indicate 2 μm. There is a marked difference in fiber size and density of clot, with thinner fibers and smaller pores for clots made from γA/γ′ fibrinogen (A) compared with the more frequent variant γA/γA (B). Unfractionated fibrinogen (C) produces clots with an appearance that is more similar to that of γA/γA.
Scanning electron micrographs of clots made from fibrinogen γ-chain splice variants. Clots were made from purified fibrinogen γ-chain splice variants and observed by scanning electron microscopy. Scale bars indicate 2 μm. There is a marked difference in fiber size and density of clot, with thinner fibers and smaller pores for clots made from γA/γ′ fibrinogen (A) compared with the more frequent variant γA/γA (B). Unfractionated fibrinogen (C) produces clots with an appearance that is more similar to that of γA/γA.
Discussion
Functional studies of the fibrinogen γ′ splice variant have been conducted in the past, such as FXIII-induced cross-linking,23 thrombin19 and platelet21 binding, and clot lysis experiments,24 but this is the first report on the direct effect of the γA/γ′ variant on the formation and structure of fibrin clots. Both turbidity and fibrinopeptide release experiments showed that heterodimers consisting of γ′ and γA chains differed strongly during the early events of polymerization from fibrinogen homodimers containing γA chains only, ultimately leading to altered fibrin structure. Fibrin produced from purified γA/γ′ fibrinogen showed a structure with reduced average fiber diameter, increased branching, and reduced pore size. Similar structures have been related in the past to an increased risk for thrombotic disease.8,11,12 Our findings could therefore represent a possible mechanism by which γA/γ′ fibrinogen predisposes toward an increased risk for thrombosis.25
Practically all of the γ′ splice variant in vivo occurs as a heterodimer with the γA variant whereby one D region contains a γ′ carboxyl terminus and the other γA. This is confirmed by the finding that γ′ fibrinogen purified from human plasma shows an approximately equal amount of γA and γ′ chains,18 as also observed in the present study. As far as known to date, heterozygous γA/γ′ fibrinogen is normally present in all individuals but at different levels between 8% and 15%. It is important to note that it is the level of γA/γ′ in circulation rather than its presence that determines risk for thrombosis.25 In the current study we have investigated the effects of purified γA/γ′ on fibrin formation and structure, in comparison with purified γA/γA fibrinogen and unfractionated fibrinogen. Fibrinogen γA/γ′ produced a clot with a distinctly more thrombotic phenotype of thinner fibers and smaller pores. We hypothesize that this effect in vivo is less pronounced, as in the circulation γA/γ′ exists in a mixture with γA/γA but will be dependent on the concentration of γA/γ′ fibrinogen, in line with the associated risk for thrombosis. This hypothesis is supported by our findings that unfractionated fibrinogen showed fiber diameters that were larger than in γA/γ′ and smaller than in γA/γA fibrinogen, with an average that was more similar to γA/γA, of which there is expected to be around 85% to 92% present in unfractionated fibrinogen. It will be interesting to investigate the structural properties of clots made from homozygous γ′/γ′ fibrinogen, although the physiologic relevance of this may not be entirely clear, as, based on a plasma concentration of 8% to 15% for γA/γ′, the level of homozygous γ′/γ′ fibrinogen could be expected to be lower than 0.5%.
The role of thrombin binding sites in γA/γ′ fibrinogen is complex. Earlier studies on γA/γ′ fibrinogen suggested that the thrombin binding site on the γ′ carboxyl terminus functions as an inhibitor of thrombin, and it has been suggested that γ′ is responsible for antithrombin I activity in plasma.28,29 As thrombin concentrations are known to directly influence fibrin structure and polymerization events, with increasing concentration of thrombin leading to a reduction in fiber diameter,10 we performed turbidity analysis of γA/γ′ fibrinogen using thrombin at various concentrations. While the overall polymerization pattern changed as expected, with lower maximum absorbency related to thinner fibers at higher thrombin concentration, the difference in fiber size between γA/γ′ heterodimers and γA/γA homodimers remained, with reduced fiber diameter for γA/γ′ fibrinogen within the range of thrombin concentrations tested. However, the reduction in fiber thickness associated with γA/γ′ fibrin is not what would be expected as a result of γA/γ′ functioning as a thrombin inhibitor. Inhibition of thrombin would be comparable to lowering thrombin concentrations and would hence be expected to cause an increase in fiber diameter. Since the effect of γA/γ′ fibrinogen on fiber diameter was found to be the opposite, we can conclude that changes in fibrin structure induced by γA/γ′ fibrinogen are not caused by an inhibitory effect of γA/γ′ fibrinogen on thrombin. A recent study has demonstrated that γ′ binds to exosite II of thrombin via a high-affinity binding site without interfering with thrombin's proteolytic activity.30 Consequently, clot-bound thrombin remains active until cleavage of the γ′ chain by plasmin. Thrombin also binds to the N-termini of the fibrinogen α and β chain via a low-affinity binding site, which facilitates cleavage of the fibrinopeptides.30
It is interesting to speculate on a mechanism to explain the observed effects of γA/γ′ fibrinogen on fibrin structure. Thrombin cleavage of γA/γ′ fibrinogen showed a decreased rate of FpB cleavage compared with γA/γA fibrinogen, and in association with this we found a marked effect on fiber diameter. The diameter of the fibrin fiber is strongly determined by the degree of lateral aggregation of (proto)fibrils to produce thicker fibrin bundles. Associations between FpB cleavage rates and lateral aggregation rates have been described before,3,4 but the causal relationships between these 2 events in fibrin formation are not entirely understood. Using an ingenious computer model of fibrin polymerization, Weisel and Nagaswami27 reported that several parameters may affect the thickness of a fibrin fiber. The model in this study that fits our findings with γA/γ′ fibrinogen best is one whereby fiber growth is inhibited, which leads to decreased fiber size, decreased maximum rate of fiber assembly, increased lag phase, increased number of fibers, and increased fiber length. Unfortunately, changes in rates of FpB release were not modeled in the study. We can hypothesize 2 possible models that could form a possible explanation for the observed changes in fibrin structure. In a first model, binding of thrombin to the γ′ carboxyl terminus could orient the active site of thrombin in a manner that reduces the affinity of the enzyme for the FpB, but not FpA, cleavage site, diminishing the relative rate of FpB release and consequently leading to less lateral aggregation and thinner fibrin fibers. In a second model, the negatively charged 20-residue carboxyl extension of the γ′ chain directly interferes with the D-E or D-D interactions involved in fibrin polymerization leading to reduced fiber growth and thinner fibers. Reduced fibrin polymerization would then cause reduction in the rate of FpB release.
A possible limitation of our study is that we have used fibrinogen purified from pooled plasma as starting material from which to isolate the γ′ and γA splice variants. The fibrinogen in this starting material could be heterogeneous in nature, possibly including genetic polymorphisms in the coding region, differences in glyc(osyl)ation, phosphorylation, tyrosine sulfation, or oxidation,31 which could have had an influence on the outcome of fibrin structure experiments. However, although not impossible, it seems unlikely that other heterogeneities would separate preferentially with either the γA/γA or γA/γ′ splice variants on the anion-exchange chromatography to cause major differences between the 2 preparations. Our conclusions are based on the assumption that the separated γA/γ′ and γA/γA fractions would not be different in consistency other than that caused by the γ′ splice variation. In support of this, we have purified γA/γ′ and γA/γA fibrinogen from one individual to reduce presence of other heterogeneities and confirmed our findings obtained with fibrinogen variants purified from a more heterogeneous starting material. Future studies using recombinant γA/γ′ fibrinogen would be advantageous to confirm the findings presented here with γA/γ′ fibrinogen purified from human plasma. Another potential limitation of our study is that fibrinogen purified from plasma may be subject to carboxyl-terminal degradation of the Aα chain by proteases. It is interesting to note in this regard that the lag phase of fibrin polymerization was shorter for unfractionated fibrinogen compared with γA/γ′ or γA/γA fibrinogen. It is possible that this effect could have been caused in part by degradation, either proteolytically, chemically or physically, of the fibrinogen preparations during anion-exchange chromatography. However, protease inhibitors were added to the purification buffers to reduce proteolysis to the minimum possible, and there is no evidence to suggest that, if it is present in the preparations, carboxyl-terminal degradation would have occurred to a different degree in the γA/γ′ and γA/γA fractions.
Alterations of fibrin structure have been demonstrated to play a role thrombotic disease. Tightly packed clots with thin fibers have been shown to have an increased resistance to fibrinolysis,32 a property that has previously been attributed to γ′ fibrin clots.24 Lovely et al25 recently described an increase of γA/γ′ fibrinogen in subjects with cardiovascular disease, but relatively little was known to date whether increased γA/γ′ levels are cause or marker of disease. Our study has shown that γA/γ′ fibrinogen directly alters fibrin structure/function and as such provides a possible mechanism by which γA/γ′ fibrinogen may contribute to vascular risk.
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2002-10-3150.
Supported in part by the British Heart Foundation and the Medical Research Council.
A.V.C. and K.F.S. contributed equally.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ``advertisement'' in accordance with 18 U.S.C. section 1734.

![Figure 3. Fibrinopeptide release from thrombin-treated γA/γA and γA/γ′ fibrinogen. Purified fibrinogen γA/γA and γA/γ′ (0.77 mg/mL each) were incubated with 1 IU/mL thrombin and release of FpA and FpB was analyzed by reverse-phase HPLC using a silica C18 column. The fraction of the concentration of the fibrinopeptides at a given time point divided by that at maximum release ([Fp]/[Fp]max, so that full release equals 1) is plotted against time. FpA released from γA/γA fibrinogen is represented by •, FpB from γA/γA by ▵, FpA from γA/γ′ by ⋄, and FpB from γA/γ′ by ▪. Release of FpB was slower in γA/γ′ compared with γA/γA fibrinogen. Inset in the figure shows a typical chromatogram from HPLC, with the injection peak followed by 2 major peaks representing FpA and then FpB.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/2/10.1182_blood-2002-10-3150/6/m_h81434607003.jpeg?Expires=1765930722&Signature=Gr37fE4gunJiYATgSJBsDKD2Yb5AZuQAEZO8zJq461q3mspdsVabBbSERx8CJYUux6a-4ptLmBH6SpEJsiqQE6-nEhWzeRrIAFwPHhC3LYNJ0ysbP9e8yKCOS1iXiKM2DEhbncpZWnqBMH8HDopcItLCYTOK99pbAN0K60fEMdu2PzdKUKE72AZw08nNdnxAOjBwALNq7EX1DsUooLdMHqIBHsd8NXi~xyfQ0BqYCYALqlevkhMcoh1boPalcQ6HNG8XL~UKKv~isEifdIlQcggjsmS4OGlR2kxvqnlHNWrhadHjisbemj5O4fdwfvvtgTWuMvRkwi7kanXVb9lJ8Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

