Abstract
Phosphoinositide 3-kinases (PI3Ks), a family of lipid kinases comprising 3 classes with multiple isoforms, have been shown to participate in different phases of platelet signaling. To investigate the roles that enzymes play in platelet function in vivo and determine which isoforms are important for particular signaling events, we analyzed platelet function of gene knockout mice deficient in the p85α regulatory subunit of heterodimeric class IA PI3K. The kinase activity of p85α—/— platelets was only 5% of the activity of platelets from wild-type littermates. Platelet aggregation induced by adenosine diphosphate (ADP), thrombin, U46619, phorbol 12-myristate 13-acetate (PMA), or botrocetin was not defective in p85α—/— mice, compared with wild-type animals. In contrast, aggregation induced by collagen and collagen-related peptide (CRP) was partially but readily impaired in p85α—/— mice. Both P-selectin expression and fibrinogen binding in response to CRP were also decreased to a similar extent in p85α—/— platelets. Platelets from p85α—/— mice appeared to spread poorly over a CRP-coated surface with intact filopodial protrusions. Significant attenuation of CRP-induced tyrosine phosphorylation in known PI3K effectors such as Btk, Tec, PKB/Akt, and phospholipase Cγ2 were observed with p85α—/— platelets, whereas no alteration was noted in upstream molecules of Syk, LAT, and SLP-76. Considered as a whole, these results provide the first genetic evidence that PI3K p85α plays a significant role in platelet function, almost exclusively in the glycoprotein (GP) VI/Fc receptor γ chain complex-mediated signaling pathway.
Introduction
Phosphoinositide 3-kinases (PI3Ks) constitute a family of lipid kinases that are ubiquitously expressed in many cell types. These enzymes play a key role in the regulation of a variety of cellular processes: proliferation, survival, glucose metabolism, cytoskeletal remodeling, and vesicular trafficking.1 PI3Ks phosphorylate an inositol ring of membrane-embedded inositol phospholipid at the 3′ position and generate D3 phosphoinositides (PtdIns-3-P; PtdIns-3,4-P2; PtdIns-3,5-P2; and PtdIns-3,4,5-P3), which then function as potent second messengers to relay signals by recruiting down stream molecules to the vicinity of the cellular membrane.2,3 The most favored targets of the enzyme via its phosphoinositide products are the pleckstrin homology (PH) domain-containing effector molecules. These include Tec family tyrosine kinases, serine/threonine kinases such as Akt/protein kinase B (PKB), guanosine diphosphate/guanosine triphosphate (GDP/GTP) ex change factor (GEF) families such as Vav, and phospholipase (PLC)γ subtypes.1-3 Of the 3 classes of PI3K (classes I-III), class IA and IB enzymes have been extensively studied in platelets4-6 and are positioned downstream of membrane receptor stimulation and preferentially catalyze phosphorylation of PtdIns-4,5-P2 in vivo. The class IA subclass enzyme is a heterodimer comprising p110 catalytic and regulatory subunits, and to date 3 p110 catalytic subunits (α, β, and δ) and 3 regulatory subunits (85α, 85β, and 55γ) derived from different genes have been reported.1 In addition, the 85α protein possesses 2 alternatively spliced variants, p55α and p50α.7 Regulatory subunits act as adaptor molecules to activate the p110 catalytic subunit via interactions between their Src homology 2 (SH2) domains and specific phosphorylated tyrosine residues (Y-X-X-M motif) of upstream signaling molecules. In addition, class IA enzymes are known to be activated by G proteins.8,9 A class IB subclass enzyme, PI3Kγ, is also abundant in platelets; it comprises a heterodimer complex with a p110γ catalytic subunit and a unique p101 regulatory subunit. The enzyme has been shown to be specific for G protein-coupled receptor (GPCR) activation through G protein βγ subunits.4,10 Class IA PI3K utilizes a series of nonreceptor tyrosine kinases and associated adaptor molecules when it is activated downstream of adhesion receptor engage ment to transduce signals. In fact, the collagen-induced platelet activation pathway via the glycoprotein (GP) VI/Fc receptor γ chain (FcRγ) complex evokes significant lipid kinase activity.11 Upon GP VI cross-linking by collagen, immunoreceptor tyrosine-based activation motifs (ITAMs) displayed on the FcRγ subunit are phosphorylated by the protein tyrosine kinases Lyn and Fyn, leading to binding and activation of the Syk tyrosine kinase. Following assembly with adaptor molecules such as LAT and SLP-76, PI3K is activated via its p85 adaptor subunit.12,13 With GP VI stimulation, platelet activation induced by aggregated immunoglobulin G (IgG) also involves class IA PI3K as a major signaling element associated with another ITAM-containing receptor, FcγR IIA.14,15 Class IA enzymes are also implicated in processes downstream of GP Ib/IX/V complex-mediated signaling16 and upstream of integrin αIIb/β3 (GP IIb/IIIa) complex activation (inside-out signaling),4 and in postintegrin cellular responses (outside-in signaling), including conformational changes induced by actin rearrangement.17,18 Platelet activation stimulated by GPCRs is physiologically important for platelet thrombus formation and is closely associated with PI3K activity.4 Since PI3Kγ is activated only by G protein βγ subunits, the enzyme may be responsible for causing the observed increase in PI3K lipid products in response to stimulation with thrombin, adenosine diphosphate (ADP), and the thromboxane A2 analog U46619.4,19,20 However, class IA PI3K is also activated by GPCRs, and this type of activation may involve either a nonreceptor tyrosine-phosphorylated intermediate or the direct engagement of G protein βγ subunits.9,21 Although a large body of evidence has clearly demonstrated that PI3K is intimately associated with different phases of platelet activation, the exact roles the enzyme plays in platelet functions in vivo and which isoforms are important for particular signaling events are yet to be determined. Platelets are terminally differentiated anucleate cells, and conclusions from most studies predominantly rely on indirect measurement of PI3K activity using structurally distinct inhibitors of PI3K (wortmannin and LY294002). This means that data analyses may be inherently limited in terms of specificity. To directly address these issues, we analyzed the function of platelets deficient in class IA PI3K p85α proteins in gene knockout mice.
Materials and methods
Materials and antibodies
Adenosine 5′-triphosphate (ATP), apyrase, phorbol 12-myristate 13-acetate (PMA), Arg-Gly-Asp-Ser (RGDS) peptide, acetylsalicylic acid (ASA), prostaglandin E1 (PGE1), bovine serum albumin (BSA), and poly-l-lysine were all purchased from Sigma-Aldrich (Tokyo, Japan). Phosphatidylinositol was obtained from Funakoshi (Tokyo, Japan), U46619 and A23187 from Merck KGaA (Darmstadt, Germany), and adenosine diphosphate (ADP) from Biopool (Ventura, CA). Collagen was supplied by Nycomed (Munchen, Germany). Collagen-related peptide (CRP) was prepared as previously described.22 Botrocetin was a generous gift from Dr Yoshihiro Fujimura (Nara Medical College, Kashiwara, Japan). Human thrombin was provided by Welfide (Osaka, Japan). Alexa Fluor 488-conjugated human fibrinogen was from Molecular Probes (Eugene, OR). Fluorescein isothiocyanate (FITC)-conjugated antimouse P-selectin antibody was purchased from BD Pharmingen (San Diego, CA). Polyclonal anti-Btk antibody was kindly provided by Dr Owen Witte (University of California, Los Angeles). Anti-Syk, PLCγ2, SLP-76, p110α, p110β, p110δ and Akt/PKB polyclonal antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antiphosphotyrosine monoclonal antibody (clone 4G10) and anti-PI3K p85PAN, LAT, and Tec polyclonal antibodies were obtained from Upstate Biotechnology (Lake Placid, NY). Anti-phospho-Akt antibody was obtained from New England Biolabs (Beverly, MA). A specific antibody against p85β was prepared as described.23 Thin-layer chromatography (TLC) plates were purchased from Whatman International (Maidstone, United Kingdom).
Mice
PI3K p85α–/– mice24,25 were backcrossed to C57BL/6 mice for more than 7 generations before intercrossing heterozygous mice. The targeting strategy allowed selective disruption of p85α expression, while leaving gene products for p55α and p50α isoforms intact.25 All mice were maintained under strict pathogen-free conditions. All experiments were performed in accordance with Keio University Institutional Guidelines.
Bleeding time
At 2 to 3 months of age, mice were anesthetized using diethylether. An incision was made 1 cm from the tip of the tail, and the emerging blood was blotted onto Whatman 3M paper (Whatman International) every 15 seconds. Bleeding times were defined as the time required for all visible signs of bleeding to stop.26
Blood collection and preparation of platelets
Whole murine blood was collected in syringes containing 100 μL acid citrate dextrose (ACD; 120 mM sodium citrate, 110 mM glucose, and 80 mM citric acid) by cardiac puncture under diethylether anesthesia. Blood cell counts were determined using an automated blood cell counter. Blood from 2 to 6 mice (2 to 4 months old, both sexes) was pooled for preparing platelet samples. Platelet-rich plasma (PRP) was obtained by centrifuging whole blood at 660g for 1 minute at 22°C. The residual blood sample was diluted using 400 μL RCD solution (36 mM citric acid, 5 mM glucose, 5 mM KCl, 103 mM NaCl, and 2 μM PGE1, pH 6.5) containing 10% (volume/volume) ACD and 0.4 U/mL apyrase, then centrifuged at 660g for 1 minute to recover diluted PRP. To prepare washed platelets, PRP was combined with diluted PRP pretreated with 0.2 mM ASA at 37°C for 15 minutes and diluted using an equal volume of RCD solution containing 10% (vol/vol) ACD and 0.4 U/mL apyrase. Washed platelets were then obtained by centrifuging at 1000g for 7 minutes. Isolated platelets were resuspended at a final concentration of 5 × 105/μL in modified Tyrode-HEPES buffer (134 mM NaCl, 0.34 mM NaH2PO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES [N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid], and 5 mM glucose, pH 7.3) containing apyrase.
Aggregation studies
Platelet concentrations of PRP were adjusted to 3 × 105/μL using platelet-poor plasma (PPP). A total of 125 μL PRP was placed in siliconized glass tubes and incubated at 37°C for 10 minutes before stimulation. Aggregation was optically monitored using a platelet aggregometer (Hema Tracer TM Model 601; Niko Bioscience, Tokyo, Japan). PPP was used as a reference to indicate 100% aggregation. For studies utilizing thrombin, washed platelets without pretreatment under ASA were suspended in modified Tyrode-HEPES buffer before use.
Flow cytometry
Washed platelets suspended in modified Tyrode-HEPES buffer containing 0.4 U/mL apyrase were labeled using FITC-conjugated antimouse P-selectin antibody (with 1 mM RGDS peptide and 1 mM CaCl2) or Alexa Fluor 488-conjugated human fibrinogen (1 mM CaCl2). Following stimulation with the appropriate concentrations of CRP, labeling of murine platelets was performed for 30 minutes. Samples were then analyzed using a FACSCalibur flow cytometer (Nippon Becton Dickinson, Tokyo, Japan).
Morphologic analysis of adherent platelets under electron microscopy
Control discoid platelets were obtained as follows: platelets in modified Tyrode-HEPES buffer containing 2 mM MgCl2 were fixed in 1% glutaraldehyde, placed on poly-l-lysine-coated coverslips, and allowed to adhere to the surface for 60 minutes. Collagen and CRP were diluted to 10 μg/mL and 6 μg/mL, respectively, with modified Tyrode-HEPES buffer and immobilized on coverslips in culture dishes overnight at 4°C. Coverslips were washed 3 times with modified Tyrode-HEPES buffer and incubated with 2% BSA for 2 hours at room temperature for blocking. Platelets suspended in modified Tyrode-HEPES buffer containing 2 mM MgCl2 (1 × 105 platelets/μL, 100 μL) were brought into contact with collagen- or CRP-coated coverslips in culture dishes and platelets were allowed to adhere at 37°C for 10 minutes. After rinsing 3 times to remove nonadherent platelets, coverslips were further incubated at 37°C for 20, 50, and 80 minutes. Adherent platelets were fixed using 1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 20 minutes. Fixed platelets adhering to coverslips were washed 5 times in 0.1 M phosphate buffer (pH 7.4), postfixed using 1% osmium tetroxide in the same buffer for 15 minutes, dehydrated in a graded ethanol series, and then dried using a Hitachi ES-2020 freeze dryer (Hitachi, Tokyo, Japan) and t-butyl alcohol. Specimens were coated with an amorphous and continuous layer of sublimated osmium tetroxide (approximately 10 nm) using an NL-OPC80 osmium plasma coater (Nippon Laser & Electronics Lab, Nagoya, Japan). Slides were then examined under a Hitachi S-4500 field emission scanning electron microscope (Hitachi) at an accelerating voltage of 10 kV. Lengths of filopodia, defined as protrusions from the platelet body less than 130 nm in width, were measured using NIH Image software (National Institutes of Health; http://rsb.info.nih.gov/nih-image/). The area of the CRP-coated slides covered by platelets was analyzed using the same software.
Immunoprecipitation and Western blotting
Platelets in modified Tyrode-HEPES buffer containing 0.4 U/mL apyrase, 1 mM RGDS peptide, and 1 mM EGTA (ethylene glycol tetraacetic acid) were stimulated using the appropriate concentrations of collagen or CRP. Reactions were terminated with the addition of an equal volume of ice-cold lysis buffer (2% Nonidet P40 [NP-40], 20 mM Tris [tris(hydroxymethyl)aminomethane], 300 mM NaCl, 10 mM EDTA [ethylenediaminetetraacetic acid], 2 mM Na3VO4, 10 mg/mL aprotinin, 1 mg/mL pepstatin, 10 mg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride [PMSF], pH 7.3). Debris was removed by centrifugation at 20 000g for 10 minutes. Platelet lysates were incubated with antibodies for various proteins and protein A-sepharose beads (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) for 2 hours at 4°C under continuous rotation. The beads were washed extensively in 2-fold diluted lysis buffer. Precipitated proteins were extracted in Laemmli sample buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Separated proteins were transferred to Hybond-ECL membrane (Amersham Pharmacia), which was then blocked in 10% BSA in TBS-T (20 mM Tris-HCl, 137 mM NaCl, and 0.1% Tween 20) and hybridized sequentially using primary antibodies and horseradish peroxidase-conjugated secondary antibody (Amersham Pharmacia). Bound antibodies were detected using an enhanced chemiluminescence (ECL) Western blotting kit (Amersham Pharmacia).
PI3K assay
Platelets were lysed and immunoprecipitated using anti-p85PAN antibody (Upstate Biotechnology), which reacts with all 3 p85α isoforms (p85α, p55α, and p50α) in addition to p85β and p55γ subunits. Beads were washed 3 times in PI3K buffer (25 mM Tris, 0.5 mM EGTA, and 100 mM NaCl, pH 7.4) and suspended in PI3K buffer containing 200 μg/mL phosphatidylinositol. Following preincubation at 37°C for 10 minutes, the reaction was initiated with the addition of 2.5 μL start solution (200 mM MgCl2, 200 μM ATP, 3 μCi [0.111 MBq] γ-32P-ATP; Amersham Pharmacia). After incubation for 10 minutes, the reaction was terminated with the addition of 100 μL stop solution (chloroform, methanol, and 11.6 N HCl in the ratio of 50:100:1). Labeled phosphoinositides were extracted using chloroform, washed 3 times in 100 μL wash solution (mixture of 50 μL methanol and 50 μL 1 N HCl), separated by thin-layer chromatography, and analyzed using a BAS 2000 bioimaging analyzer (Fuji, Tokyo, Japan).
Results
The p85α protein is the major class IA PI3K isoform expressed in platelets
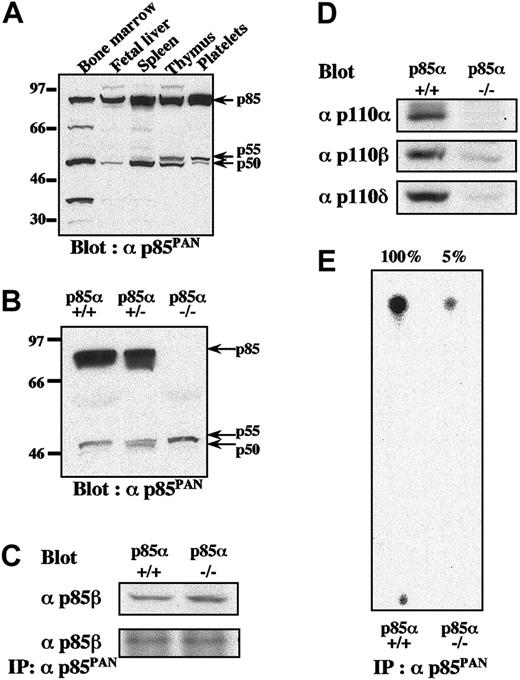
To explore the relative contributions of the various isoforms of class IA PI3K, we first examined the expression of various isoforms in murine platelets by Western blotting using anti-p85PAN antiserum, which has been shown to recognize all variants of p85α (p85α, p55α, and p50α), p85β, and p55γ.23-25 Among the variety of hematopoietic tissues examined, expression levels of p85α isoform far exceeded those of p55α and p50α in platelets, spleen, thymus, and fetal liver, whereas bone marrow expressed similar amounts of all 3 isoforms (Figure 1A). In platelets from p85α–/– mice, selective disruption of the p85α isoform did not result in significant up-regulation of p55α, p50α, and p85β proteins to compensate for the lack of p85α protein (Figure 1B).24,25 In addition, expression patterns of p85α, p55α, and p50α isoforms in p85α+/– platelets did not significantly differ from those of p85α+/+ platelets (Figure 1B). The p85β protein was detected in p85α–/– platelets only when the film was overexposed (data not shown). When expression of the p85β protein in platelets was examined using a specific antibody, the levels of p85β in p85α–/– and p85β+/+ platelets were found to be not significantly different (Figure 1C). It was also found that the p85PAN antiserum equally and efficiently captured p85β proteins in platelet lysates from either p85α+/+ or p85α–/– mice (Figure 1C). In addition, the expression of catalytic subunit p110α in p85α–/– compared with p85α+/+ platelets was almost undetectable, while expression of p110β and p110δ subunits were greatly reduced in amount, consistent with the instability of the p110 proteins in the absence of sufficient adaptor subunit concentrations (Figure 1D).27 Consistent with these observations, remaining class IA PI3K activity in p85α–/– platelets was only 5% of the activity in wild-type littermates (Figure 1E).
Differential expression patterns of regulatory and catalytic subunits of PI3K class IA and their kinase activities in platelets from p85α—/— and wild-type mice. (A-B) Expression patterns of regulatory subunits of PI3K class IA in wild-type hematopoietic organs (A) and in platelets from wild-type p85α+/+, p85α+/–, and p85α–/– mice (B), shown by Western blot with αp85PAN. (C-D) The amount of p85β protein in platelet lysate and its immunoprecipitate with anti-p85PAN antiserum (C) and the amount of p110 catalytic subunits (p110α,p110β, and p110δ) in platelet lysate from p85α+/+ and p85α–/– mice (D), shown by Western blot with p85β-specific antiserum and anti-p110 polyclonal antibodies, respectively. Whole-cell lysate (50 μg per lane) or its immunoprecipitate was resolved using SDS-PAGE. (E) PI3K activity of wild-type and p85α–/– platelets measured using anti-p85PAN antiserum and resolved by thin-layer chromatography. The wild-type response was set to 100% and the activity of p85α–/– platelets was estimated to be 5% of the wild-type response.
Differential expression patterns of regulatory and catalytic subunits of PI3K class IA and their kinase activities in platelets from p85α—/— and wild-type mice. (A-B) Expression patterns of regulatory subunits of PI3K class IA in wild-type hematopoietic organs (A) and in platelets from wild-type p85α+/+, p85α+/–, and p85α–/– mice (B), shown by Western blot with αp85PAN. (C-D) The amount of p85β protein in platelet lysate and its immunoprecipitate with anti-p85PAN antiserum (C) and the amount of p110 catalytic subunits (p110α,p110β, and p110δ) in platelet lysate from p85α+/+ and p85α–/– mice (D), shown by Western blot with p85β-specific antiserum and anti-p110 polyclonal antibodies, respectively. Whole-cell lysate (50 μg per lane) or its immunoprecipitate was resolved using SDS-PAGE. (E) PI3K activity of wild-type and p85α–/– platelets measured using anti-p85PAN antiserum and resolved by thin-layer chromatography. The wild-type response was set to 100% and the activity of p85α–/– platelets was estimated to be 5% of the wild-type response.
PI3K p85α-deficient mice displayed perturbation of platelet aggregation response to collagen and CRP, but no bleeding disorders
In contrast to Syk-, SLP-76-, or PLCγ2-deficient mice, PI3K p85α–/– mice were born intact, with no bleeding disorders.28-30 Bleeding times for PI3K p85α–/– mice (178 ± 89 seconds, n = 5) were not significantly prolonged compared with littermate controls (216 ± 97 seconds, n = 5), and peripheral blood cell counts were indistinguishable among wild-type, p85α+/–, and p85α–/– mice (data not shown). These results suggest that p85α deficiency does not cause profound defects in platelet production or function in vivo. These observations prompted us to examine the effect of PI3K p85α deficiency on platelet function in vitro.
Since many studies have suggested that the involvement of PI3K in signaling cascades in platelets is stimulated by various types of agonists, we investigated the platelet aggregation response in knockout mice. As shown in Figure 2, p85α deficiency led to an approximate 40% to 60% reduction of platelet aggregation in response to suboptimal or optimal concentrations of collagen (10 and 20 μg/mL, respectively) or the GP VI-specific agonist CRP (2.5 and 5 μg/mL). In particular, responses to lower dose stimuli, such as 5 μg/mL collagen or 1 μg/mL CRP, which induced 60% to 80% aggregation in wild-type platelets, were completely abrogated in p85α–/– mice. In contrast, responses to ADP, the thromboxane A2 analog U46619, thrombin, PMA, A23187, or botrocetin were all intact in p85α–/– mice compared with littermate controls, even at subthreshold concentrations. The same responses to collagen and CRP were observed in both p85α+/– mice and wild-type littermates (data not shown). These results are consistent with previous in vitro observations in humans that class IA PI3K plays an important role in collagen-induced platelet signaling through GP VI engagement.12,13
Platelet aggregation responses to various kinds of agonists. PRPs (3 × 105/μL) were incubated at 37°C for 10 minutes prior to stimulation. Changes to morphology and aggregation of platelets from wild-type (WT, left column) and p85α–/– (right column) were measured using an aggregometer after stimulation with collagen, CRP, ADP, U46619, thrombin, PMA, A23187, and botrocetin at the indicated concentrations. Bars indicate 1 minute. Results are from 1 experiment but are representative of at least 4 separate experiments.
Platelet aggregation responses to various kinds of agonists. PRPs (3 × 105/μL) were incubated at 37°C for 10 minutes prior to stimulation. Changes to morphology and aggregation of platelets from wild-type (WT, left column) and p85α–/– (right column) were measured using an aggregometer after stimulation with collagen, CRP, ADP, U46619, thrombin, PMA, A23187, and botrocetin at the indicated concentrations. Bars indicate 1 minute. Results are from 1 experiment but are representative of at least 4 separate experiments.
PI3K p85α deficiency led to impaired P-selectin expression or fibrinogen binding in response to CRP
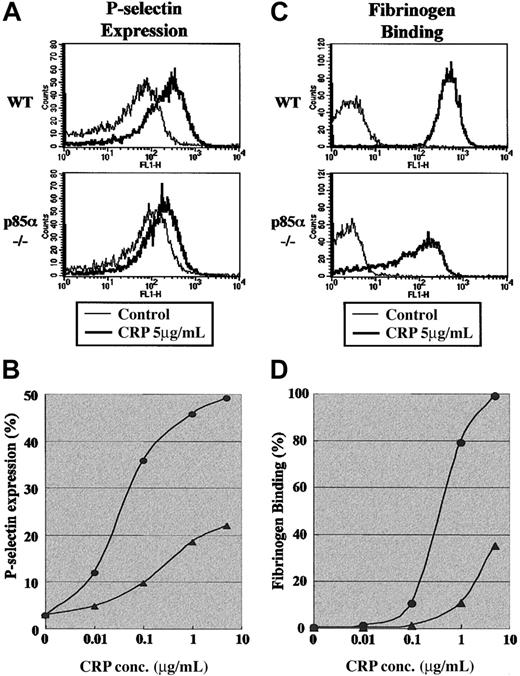
Next we analyzed the impact of p85α deficiency on platelet signaling events induced by GP VI activation. We first investigated whether p85α deficiency affects P-selectin expression or fibrinogen binding in response to CRP stimulation. As shown in Figure 3, panels A and B, 5 μg/mL CRP induced an approximately 10-fold increase in P-selectin expression in wild-type platelets, while expression in p85α–/– platelets was approximately 50% of the wild-type level. The impaired P-selectin expression in p85α–/– platelets was observed at CRP concentrations ranging from 0.01 to 5 μg/mL. Similarly, CRP-induced fibrinogen binding to p85α–/– platelets was significantly impaired (Figure 3C-D).
Surface expression of P-selectin on platelets and fibrinogen binding to platelets induced by GP VI stimulation. (A-B) P-selectin expression was detected using FITC-conjugated antimouse P-selectin antibody (A) and analyzed by flow cytometry (B). (C-D) Fibrinogen binding was detected using activated integrin αIIbβ3 and binding of Alexa Fluor 488-conjugated human fibrinogen (C) and analyzed by flow cytometry (D). Washed wild-type (WT) and p85α–/– platelets suspended in modified Tyrode-HEPES buffer containing apyrase and RGDS peptide with 1 mM CaCl2 (A-B) or modified Tyrode-HEPES buffer containing apyrase with 1 mM CaCl2 (C-D) were stimulated by CRP. Data are from 1 experiment but are representative of 3 independent experiments. In panels B and D, • indicates WT platelets and ▴ indicates p85α–/– platelets.
Surface expression of P-selectin on platelets and fibrinogen binding to platelets induced by GP VI stimulation. (A-B) P-selectin expression was detected using FITC-conjugated antimouse P-selectin antibody (A) and analyzed by flow cytometry (B). (C-D) Fibrinogen binding was detected using activated integrin αIIbβ3 and binding of Alexa Fluor 488-conjugated human fibrinogen (C) and analyzed by flow cytometry (D). Washed wild-type (WT) and p85α–/– platelets suspended in modified Tyrode-HEPES buffer containing apyrase and RGDS peptide with 1 mM CaCl2 (A-B) or modified Tyrode-HEPES buffer containing apyrase with 1 mM CaCl2 (C-D) were stimulated by CRP. Data are from 1 experiment but are representative of 3 independent experiments. In panels B and D, • indicates WT platelets and ▴ indicates p85α–/– platelets.
PI3K p85α-deficient platelets displayed impaired spreading over collagen- or CRP-coated surfaces
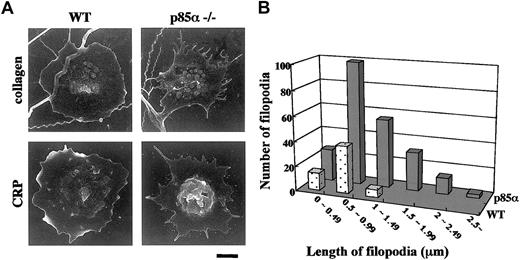
In order to further elucidate the role of p85α in collagen-induced platelet signaling, the adhesive response of platelets from knockout mice was investigated. Platelets were placed on collagen- or CRP-coated plates and allowed to adhere to the surface; then morphology was analyzed under scanning electron microscopy. As shown in Figure 4A, wild-type platelets adhered and spread over collagen- or CRP-coated surfaces after 90 minutes of incubation. In contrast, platelets from p85α–/– mice demonstrated reduced spreading, although filopodial protrusions were relatively intact (Figure 4A). Compared with the collagen-coated plates, poor lamellae formation in p85α–/– platelets was pronounced on the CRP-coated plates, consistent with the relative specificity of this isozyme to GP VI pathways among the multiple signaling cascades stimulated by collagen. Indeed, compared with wild-type platelets, filopodia on adhered platelets from p85α–/– mice had increased numbers and length (Figure 4B). These data suggest that p85α plays an essential role in platelet lamellipodia formation during adhesive responses triggered by GP VI engagement.
Morphologic examination of platelets adhering to collagen- or CRP-coated surfaces. (A) Washed platelets suspended in modified Tyrode-HEPES buffer containing apyrase and 2 mM MgCl2 were exposed to surfaces coated with collagen (top row) or CRP (bottom row). Scanning electron images show wild-type (WT; left panels) and 85α–/– (right panels) platelets after 90 minutes of incubation. Scale bar indicates 1 μm. (B) Frequency analysis of the length of remnant filopodia from wild-type (dotted columns) and p85α–/– (solid columns) platelets (n = 50) adhering to CRP-coated surface after 60 minutes. Analysis was performed using NIH Image software.
Morphologic examination of platelets adhering to collagen- or CRP-coated surfaces. (A) Washed platelets suspended in modified Tyrode-HEPES buffer containing apyrase and 2 mM MgCl2 were exposed to surfaces coated with collagen (top row) or CRP (bottom row). Scanning electron images show wild-type (WT; left panels) and 85α–/– (right panels) platelets after 90 minutes of incubation. Scale bar indicates 1 μm. (B) Frequency analysis of the length of remnant filopodia from wild-type (dotted columns) and p85α–/– (solid columns) platelets (n = 50) adhering to CRP-coated surface after 60 minutes. Analysis was performed using NIH Image software.
Collagen and CRP induce a reduction in tyrosine phosphorylation of PLCγ2 and several other downstream molecules in PI3K p85α-deficient platelets
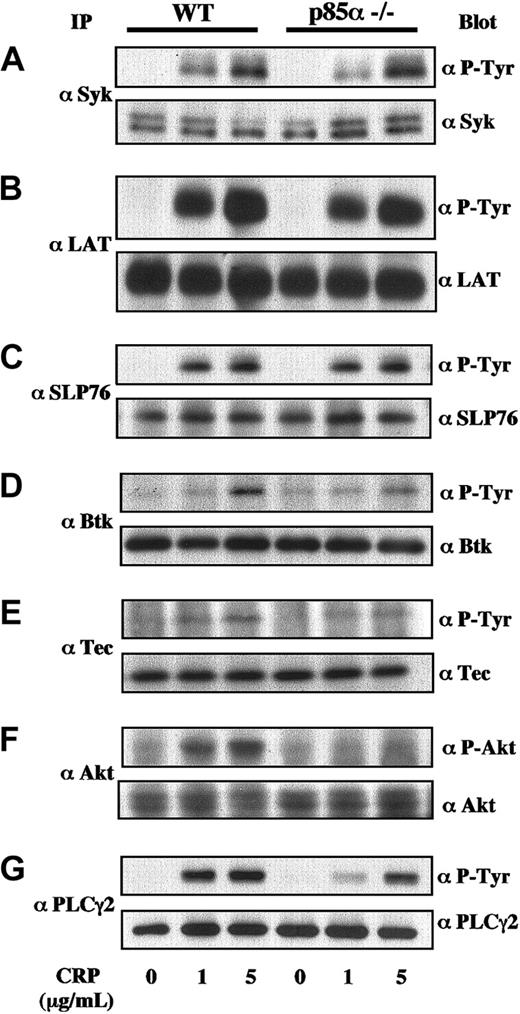
Collagen and CRP induced tyrosine phosphorylation of various signaling molecules, including Syk, LAT, SLP-76, Btk, Tec, Akt/protein kinase B (PKB), and PLCγ2 in platelets.11,13,28-34 To investigate the underlying molecular mechanisms of impaired platelet function mediated by the GP VI/FcRγ complex in PI3K p85α-deficient mice, we analyzed tyrosine phosphorylation of these signaling molecules in response to collagen and CRP. Key molecules, Syk, LAT, and SLP-76, initially recruited and activated in the vicinity of the GP VI/FcRγ complex, were equally phosphorylated by CRP in both wild-type and p85α–/– platelets (Figure 5A-C). In contrast, phosphorylation of Btk, Tec, or Akt in downstream PH domain-containing effectors for PI3K in response to CRP was partially abrogated in p85α–/– mice (Figure 5D-F). Similar results were also obtained using collagen as the GP VI stimulator (data not shown). It is well known that PLCγ2 is one of the critical targets of collagen- and CRP-induced signaling in platelets. As shown in Figure 5G, phosphorylation of PLCγ2 was also clearly decreased in p85α–/– platelets.
Protein phosphorylation in GP VI-stimulated wild-type and PI3K p85α—/— platelets. Murine platelets were treated using 0.2 mM acetylsalicylic acid and suspended in modified Tyrode-HEPES buffer containing 0.4 U/mL apyrase, 1 mM RGDS peptide, and 1 mM EGTA. The platelets were stimulated with CRP at 0, 1, and 5 μg/mL on an aggregometer with constant stirring and were lysed 90 seconds after stimulation. Then they were subjected to immunoprecipitation using anti-Syk (A), anti-LAT (B), anti-SLP-76 (C), anti-Btk (D), anti-Tec (E), anti-Akt (F), and anti-PLCγ2 (G) antibodies. Proteins were resolved using SDS-PAGE, transferred to nitrocellulose membrane, immunoblotted with antiphosphotyrosine (P-Tyr) antibody 4G10 or anti-phospho-Akt-specific antibody, and reprobed with the antibodies used for immunoprecipitation to demonstrate equal amounts of immunoprecipitated proteins in each lane. Identical results were obtained in at least 4 separate experiments.
Protein phosphorylation in GP VI-stimulated wild-type and PI3K p85α—/— platelets. Murine platelets were treated using 0.2 mM acetylsalicylic acid and suspended in modified Tyrode-HEPES buffer containing 0.4 U/mL apyrase, 1 mM RGDS peptide, and 1 mM EGTA. The platelets were stimulated with CRP at 0, 1, and 5 μg/mL on an aggregometer with constant stirring and were lysed 90 seconds after stimulation. Then they were subjected to immunoprecipitation using anti-Syk (A), anti-LAT (B), anti-SLP-76 (C), anti-Btk (D), anti-Tec (E), anti-Akt (F), and anti-PLCγ2 (G) antibodies. Proteins were resolved using SDS-PAGE, transferred to nitrocellulose membrane, immunoblotted with antiphosphotyrosine (P-Tyr) antibody 4G10 or anti-phospho-Akt-specific antibody, and reprobed with the antibodies used for immunoprecipitation to demonstrate equal amounts of immunoprecipitated proteins in each lane. Identical results were obtained in at least 4 separate experiments.
These results suggest that the impaired response to collagen and CRP seen in p85α-deficient platelets is, at least partially, based on reduced phosphorylation and/or activation of PLCγ2.
Discussion
This report provides the first direct evidence of the effect of PI3K p85α deficiency on platelet function in vivo, demonstrating that class IA PI3K functions exclusively as a major element in the GP VI/FcRγ complex-mediated signaling cascade in mice. In contrast, the fact that this enzyme subclass did not exert any significant effect on other relevant platelet signaling pathways, such as those triggered by thrombin, ADP, U46619, and botrocetin, was rather unexpected.
GP VI ligation by collagen or CRP initiates intracellular signals through phosphorylated ITAMs on the FcRγ chain, leading to activation of serially connected downstream molecules in a phosphotyrosine-dependent manner.13 At the end of this signaling cascade, the fully activated PLCγ2 is assembled at or in the vicinity of membrane rafts with signaling complexes such as tyrosine kinases Lyn/Fyn, Syk, Btk/Tec, and adaptors of LAT and SLP-76.35-37 Evidence of the participation of class IA PI3K in this particular signaling cascade is that in the absence of PI3K p85α, platelets become defective in the following GP VI-induced signaling events: platelet aggregation, degranulation of α granules, integrin activation, lamellipodia formation, and tyrosine phosphorylation of putative effector molecules. Consistent with this conclusion, Btk mutations in humans32,33 and Syk, LAT, SLP-76, and PLCγ2 mutations in mice28-31 all produce platelet phenotypes similar to those seen in p85α-deficient mice.
The finding that GP VI-induced tyrosine phosphorylation of Syk, LAT, and SLP-76 is not defective in p85α-deficient platelets might indicate that these molecules are active upstream or are independent of the PI3K-pathway. In fact, the p85 subunit of class IA PI3K has been shown to associate with tyrosine-phosphorylated LAT and tyrosine-phosphorylated ITAM of the FcRγ chain through each of the tandem SH2 domains following GP VI stimulation.12 This association then triggers PI3K-pathway activation, resulting in the liberation of D3 phosphoinositides. LAT is tyrosine-phosphorylated by Syk following binding of the kinase to the phosphorylated ITAM of the FcRγ chain. LAT then forms a complex with SLP-76 via the adaptor protein Gads, becoming a scaffold for PLCγ2 recruitment.13,31 The PI3K lipid products target the Btk PH domain and the PLCγ2 SH2 or PH domains to induce subcellular localization.13,38 Therefore, 2 independent pathways exist—one mediated by membrane protein LAT and the other by PI3K membrane lipid products—for the full activation of PLCγ2at specific membrane microdomains. Keeping this model in mind, the finding that the loss of the p85α protein does not exert a profound effect on platelet function is not particularly surprising. Although PLCγ2 activity in p85α-deficient platelets was not measured in this study, we identified partial but substantial reduction in GP VI-induced tyrosine phosphorylation of PLCγ2 in p85α-deficient platelets, which may be due to the incomplete defect in platelet cellular responses following GP VI stimulation. Other groups have reported that treatment with pharmacologic PI3K inhibitors strongly suppresses GP VI-induced PLCγ2 activation but has a minimal effect on tyrosine phosphorylation in human platelets.11,17 This discrepancy might be attributed to species differences or the methods of inducing PI3K disruption. The lipid kinase activity of class IA PI3K is almost completely deleted in p85α-deficient platelets. Only trace amounts of activity may be attributed to the very low levels of the other kinase isoforms associated with the p85α splice variants p55 and p50, p85β, or p55γ. Nevertheless, complete loss of p85α activity does not result in the severe platelet phenotype seen in response to GP VI stimulation, supporting the hypothesis that full activation of PLCγ2 may involve a PI3K-independent pathway, possibly via the scaffold complex formed by LAT, Gads, and SLP-76.13,39 However, this concept is challenged by the finding that the extent and spectrum of immune deficiency resulting from the loss of p85α in mice B lymphocytes closely approximates those seen in PLCγ2-deficient mice.25,30,40 In addition, mice with the Btk deficiency Xid (a naturally occurring point mutation in the PH domain of the kinase) are unable to bind PI3K products PtdIns-3,4,5-P3 or SLP-65/BLNK (an adaptor connecting Btk and PLCγ2 and thought to be a counterpart of the LAT/SLP-76 adaptor complex in T lymphocytes) and also display remarkably similar immune phenotypes. This indicates that p85α and PLCγ2 are serially connected through Btk and SLP-65/BLNK.41-43 Although this discrepancy may simply be due to differences in cell type, careful comparisons of the platelet phenotypes of p85α-deficient mice and PLCγ2-deficient mice of identical genetic backgrounds are required to address the issue.
Partial reduction of GP VI-induced tyrosine phosphorylation of Btk and Tec in p85α-deficient platelets supports the previously proposed hypothesis that 2 PH domains containing tyrosine kinases are located proximally to class IA PI3K and function as redundant PI3K effectors in human platelets.33 B lymphocytes from Tecdeficient mice exhibit no apparent immune phenotype, but the double deficiency of Btk and Tec results in a more severe disorder than the Btk deficiency alone.44 Whether this is also true for murine platelets is yet to be determined. Since the immune phenotype of Xid mice (with normal levels of protein expression) is comparable to that of Btk-deficient animals, Btk activation should be virtually PI3K-dependent.45 Despite nearly total loss of PI3K activity and expression in p85α-deficient platelets, minimal tyrosine phosphorylation of both kinases remains inducible by GP VI stimulation. This implies the existence of an alternate pathway for Btk/Tec activation that is independent of PI3K.46,47
The defective spreading of p85α-deficient platelets over collagen- or CRP-coated surfaces is most likely caused by the loss of PI3K products. Lamellipodia formation in adherent cells is mediated by Rac-1, a member of the Rho family of small guanosine triphosphatases (GTPases). This protein is regulated by a PH domain-containing GEF Vav family.48,49 In fact, Vav-1, a member of this family, is present in high quantities in platelets.48 In contrast, filopodial protrusion is mediated through the RhoA small GTPase with the help of cdc42 GEF, which is regulated in a PI3K-independent manner.49 The observation that p85α-deficient platelets show defective lamellipodia formation while retaining intact filopodial protrusions is therefore unsurprising. In agreement with these observations, treatment of human platelets with wortmannin and LY294002 followed by contact with a collagen- or CRP-coated surface reportedly results in similar phenotypic changes to platelet morphology.17
In p85α-deficient mice, the apparent lack of alteration in platelet aggregation response to other important platelet stimulators (thrombin, ADP, U46619, PMA, A23187, and botrocetin) was unexpected, since class IA PI3K involvement has been proposed to activate αIIbβ3 integrin (inside-out signaling) or assist in the postaggregation response through integrin engagement (outside-in signaling)4,50 in the final common pathways. For example, thrombin- or PMA-induced accumulation of PtdIns-3,4-P2 or PtdIns-3,4,5-P3 and the concomitant up-regulation of αIIbβ3 integrin receptor function in human platelets are effectively inhibited by low nanomolar ranges of wortmannin, indicating that class IA PI3K is downstream of protein kinase C in the induction of integrin activation.4,51,52 Following thrombin stimulation, the p85α subunit is reportedly translocated into the focal contact area of human platelets by association with the SH3 domain and the proline-rich region of the focal adhesion kinase p125FAK.18 However, these observations disagree with our finding that p85α-deficient platelets display intact aggregation responses to thrombin and PMA, even at suboptimal concentrations. One possible explanation for the discrepancy may be the existence of a class II PI3K, which is also sensitive to wortmannin, downstream of αIIbβ3 integrin.53 Activation of GPCRs triggered by weak agonists such as ADP and U46619 readily induces PI3K activity in platelets.4,19,20 G protein βγ-specific PI3Kγ is the most plausible candidate for this particular pathway, and class IA PI3K is also postulated to be activated directly by G protein βγ subunits or tyrosine-phosphorylated intermediates following GPCR engagement.9,21 Indeed, a recent study has shown that PI3K 110γ-deficient platelets exhibit impaired response to ADP, and the defect is limited to the Gi-coupled ADP receptor (P2Y12).54 This finding, together with the observations of p85α-deficient platelets described herein, suggests that other PI3K species with involvement in the signaling pathway elicited by thrombin and U46619 or with redundant functions may exist.
The snake venom-derived botrocetin induces binding of von Willebrand factor via the receptor GP Ib/IX/V complex and mediates platelet-platelet interactions that may be accompanied by activation of class IA PI3K through either the cytoplasmic tail of the complex or ITAM-containing FcR (FcRγ in humans and mice and FcγRIIA in humans) colocalized with the complex.16,55,56 However, we were unable to observe any perturbed aggregatory response to botrocetin, even at suboptimal concentrations. The role of p85α in the GP Ib/IX/V pathway thus requires further study.
In conclusion, absence of PI3K p85α in mice leads to compromised platelet responses to GP VI stimulation in vitro, but no significant bleeding disorder. Although class IA PI3K is reportedly involved in multiple signaling pathways or different stages of cellular process in platelets, the p85α isoform functions exclusively as a major component of the ITAM-mediated signaling pathway. Platelets lack a nucleus but retain a similar set of intracellular machinery for immune receptor signaling. The cells therefore represent appropriate targets for research to elucidate the mechanisms of immediate immune responses.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-11-3327.
Supported in part by grants from Keio Gijuku Academic Department Funds and Keio University Grant-in-Aid for Encouragement of Young Medical Scientists (N.W.) and by Health Science Research Grants for Pharmaceutical and Medical Safety (Y.I. and M.H.) from the Ministry of Health, Labor and Welfare, Tokyo, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Dr Owen Witte and Dr Yoshihiro Fujimura for providing anti-Btk antibody and botrocetin, respectively. We would also like to thank Mari Fujiwara for technical assistance in the PI3K assay.