Abstract
B7-1 and B7-2 are costimulatory molecules expressed on antigen-presenting cells. The CD28/B7 costimulation pathway is critical for T-cell activation, proliferation, and Th polarization. Blocking both cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) and CD28 interactions with a CTLA-4/Ig fusion protein inhibits various immune-mediated processes in vivo, such as allograft rejection and autoimmunity. However, selective blockade of CD28 may represent a better strategy for immunosuppression than B7 blockade, because CTLA-4/B7 interactions have been shown to participate in the extinction of the T-cell receptor–mediated activation signal and to be required for the induction of immunologic tolerance. In addition, selective CD28 inhibition specifically decreases the activation of alloreactive and autoreactive T cells, but not the activation of T cells stimulated by exogenous antigens presented in the context of self major histocompatibility complex (MHC) molecules. CD28 blockade cannot be obtained with anti-CD28 dimeric antibodies, which cluster their target and promote T-cell costimulation, whereas monovalent Fab fragments can block CD28 and reduce alloreactivity. In this study, we report the construction of a monovalent single-chain Fv antibody fragment from a high-affinity antihuman CD28 antibody (CD28.3) that blocked adhesion of T cells to cells expressing the CD28 receptor CD80. Genetic fusion with the long-lived serum protein α1-antitrypsin led to an extended half-life without altering its binding characteristics. The anti-CD28 fusion molecule showed biologic activity as an immuno-suppressant by inhibiting T-cell activation and proliferation in a mixed lymphocyte reaction.
Introduction
Targeting T-cell costimulation has been widely investigated to control T-cell reactivity in autoimmunity and transplantation. Inhibiting costimulation through B7 (B7-1/B7-2 or CD80/86) blockade by cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4)–Ig has now become a clinical reality.1 Blocking B7, however, might have 2 opposite effects. In addition to reducing T-cell costimulation through CD28, B7 blockade can also prevent CTLA-4 from transmitting a negative/regulatory signal. Indeed, the signal transmitted through CTLA-4 leads to the recruitment of phosphatases that dephosphorylate activated second messengers in the CD3 complex resulting in a reduction in the nuclear translocation of Rel A needed for the production of multiple cytokines produced by Th1 and Th2 cells.2,3 Moreover, CTLA-4 has been implicated in the development of regulatory T cells in several models of transplantation4 and animals lacking CTLA-4 are resistant to tolerance induction.5 Therefore, inactivation of CTLA-4 might well be detrimental to the development of transplantation tolerance and may ultimately oppose the effect of B7 blockade. This double-edged nature of B7 blockade suggests that an agent that would selectively block CD28 and not CTLA-4 may be more adapted to maneuvers aimed at inducing tolerance.
Despite the clinical promise of anti-CD28–targeted therapy, antagonists of human CD28 are currently unavailable. So far, all antibodies described as reacting against human CD28 are agonists. Indeed, CD28 is a homodimeric receptor6 whose degree of cross-linking is implicated in signal transduction through its association with phosphatidylinositol 3-kinase (PI3-kinase) via the cytoplasmic domain.7 However, monovalent fragments can inhibit CD28/B7 interactions without stimulating CD28,7 although they cannot be used therapeutically in vivo because of their rapid elimination from the body. Extension of the half-life of antibody fragments has been achieved by in vitro conjugation to one or more molecules of polyethylene glycol8,9 or by molecular fusion with serum albumin.10 Our group has recently investigated strategies aimed at developing monovalent anti-CD28 reagents by cloning antigen-combining portions and fusing them with monovalent carrier molecules. In this paper we demonstrate that an scFv recombinant fragment from a monoclonal antibody (mAb) directed against human CD28 (CD28.3 mAb11 ) fused with the monovalent and long-lived human serum protein α1-antitrypsin (HaaT), retains the avidity and the biologic properties of the original monovalent Fab antibody fragment. In addition, this genetic fusion extends the serum half-life of the recombinant protein further than that of HaaT.
Materials and methods
Cells and antibodies
Mouse antihuman CD28 hybridoma CD28.1 (IgG1), CD28.2 (IgG1), CD28.3 (IgG1), CD28.4 (IgM), CD28.5 (IgG1), and CD28.6 (IgG2a) and mouse L fibroblasts expressing human CD80 (LB7 cells) were kindly provided by D. Olive (INSERM U119).11,12 CD28.1, CD28.2, CD28.3, and CD28.5 mAbs share a common target epitope, whereas CD28.4 and CD28.6 mAbs bind to 2 other epitopes.11 Fab fragments were prepared using the Immunopure IgG1 Fab preparation kit (Pierce, Rockford, IL).
In vivo infusion and blood sampling in macacus fascicularis
This part of the study was performed by Biomatech (Lyon, France) in Cynomolgus maccaca fascicularis. Two infusions of 8 mg/kg antibodies were performed on days 0 and 2.
Amplification and assembly of VH and VL by PCR
mRNA from CD28.3 hybridoma was prepared and retrotranscribed, and cDNA for VH and VL was amplified using primers for mouse V-gene amplification of the mouse ScFv module of the recombinant phage antibody system (Pharmacia Biotech, Guyancourt, France), according to the manufacturer's instructions. The amplified V and H genes were cloned into a TA vector (Invitrogen, Cergy Pontoise, France), followed by DNA sequencing. The cloned VH gene was modified by polymerase chain reaction (PCR) amplification by introducing a 5′ BamHI site (primer sc28.3-5: 5′-ATATGGATCCGTCAAGCTGCAGCAGTCAGG-3′) and a 3′ (SGGGG)3 synthetic linker primer (primer: 5′-GACTGGGTCATCTGGATGTCCGATCCGCCACCGCCAGAGCCACCTCCGCCTGAACCGCCTCCACCTGAGGAGACGGTGACCATGG-3′). The VL gene was then assembled to the modified VH gene by PCR using a 3′ primer (primer sc28.3-3: 5′-ATATCTCGAGTTATTAGAATTCCCGTTTTATTTCCAGCTTGG-3′) introducing EcoRI and XhoI cloning sites and using the modified VH gene as the 5′ primer. Assembled VH and VL genes were then reamplified using a 3′ primer introducing a TVAAPS peptidic linker originating from the natural Fab hinge region of immunoglobulins and EcoRI and XhoI restriction sites (primer sc28.3-TV5: 5′-ATATCTCGAGTTATTAGAATTCAGATGGTGCAGCCACAGTCCGTTTTATTTCCAGCTT-3′). The construct was then sequenced to verify its identity with the original VH and VL genes. The construct was further subcloned into the BamHI/XhoI sites of the pSecTag2B eukaryotic expression plasmid (Invitrogen), 3′ to the Igκ leader and introduced into the EcoRI/EcoRV sites of the pIg6 prokaryotic expression plasmid (a gift from Dr Jean-Charles Tellier, University of Nantes, Nantes, France) 3′ to the OmpA leader.
Genetic fusion of scFv with HaaT
The cDNA for human α1-antitrypsin (corresponding to the GenBank sequence no. X01683) was a gift from Dr D. Favre (INSERM ERM-0105, Nantes, France). Amino acids 48 to 425 were amplified by PCR with primers that add flanking EcoRI restriction sites (5′ primer: 5′-ATATGAATTCAACAAGATCACCCCCAAC-3′; 3′ primer: 5′-ATATGAATTCTTTTTGGGTGGGATTCAC-3′). The modified gene was inserted into the EcoRI site of the pSecTag2B-scFv28.3, 3′ and into the EcoRV site of the pIg6-scFv28.3 in frame with the scFv cDNA.
Expression in Escherichia coli
JM83 cells were transformed with the pIg6-scFv28.3-HaaT plasmid. Transfectants were allowed to grow in Luria-Bertani (LB) medium containing 100 μg/mL ampicillin until an OD600 of 0.5 was reached. Then, 1 mM isopropyl thiogalacto-pyranoside (IPTG) was added to the cultures and the cells were incubated at 25°C for 3 hours. The cells were then centrifuged and the periplasmic extracts were collected after hypotonic lysis and ultracentrifugation at 10 000g.
Transient transfection of Cos cells and purification of scFv28.3-HaaT
Cos cells were transfected using the Fugene lipofection kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer's instructions. Cultures were maintained for 3 days at 37°C, divided one third, and put back into culture for an additional 3 days, after which time the medium was collected. Supernatants were passed through NiNTA Sepharose columns (Amersham Pharmacia Biotech, Saclay, France) at a rate of 0.5 mL/min. The columns were rinsed with 0.02 M imidazole in phosphate-buffered saline (PBS) and proteins were eluted with 0.2 M imidazole in PBS and then dialyzed extensively against PBS at 4°C.
Enzyme-linked immunosorbent assay
Recombinant human CD28-Fc (R&D Systems, Abingdon, United Kingdom) was used at 1 μg/mL in borate buffer (pH 9.0) to coat 96-well microtiter plates (Immulon, Chantilly, VA) overnight at 4°C. Reactive sites were blocked with 5% skimmed milk in PBS for 2 hours at 37°C and supernatants or purified material were reacted for 2 hours at 37°C. Bound scFV28.3-HaaT was revealed with successive incubations (1 hour, 37°C) with rabbit antihuman α1-antitrypsin antibodies (1:500 dilution; Dako, Trappes, France) and horseradish peroxidase (HRP)–conjugated donkey antirabbit Ig antibodies (1:500 dilution; Jackson ImmunoResearch Laboratories, Bar Harbor, ME). Control scFv-M13 was revealed with an HRP-conjugated anti-M13 antibody (Amersham Pharmacia Biotech), as described.13 Bound antibody was revealed by colorimetry using the ABTS substrate (Roche, Mannheim, Germany) read at 405 nm.
EliSpot assay
CD4+ T cells (105) were mixed with irradiated allogeneic peripheral blood mononuclear cells (PBMCs; 105) in RPMI 1640 medium (Sigma, St Quentin Fallavier, France) supplemented with glutamine, nonessential amino acids, sodium pyruvate, antibiotics, and 10% heat-inactivated fetal calf serum (FCS) and plated in quadruplicate into microtiter plates coated with anti–interferon-γ (anti–IFN-γ) mAb (AID, Strassberg, Germany). After a 24-hour incubation at 37°C, wells were washed, incubated with a biotin-labeled secondary anti–IFN-γ mAb, and revealed according to the manufacturer's instructions. Spots were counted with an EliSpot Reader System (AID).
Detection of scFv28.3-HaaT by Western blotting
scFv28.3-HaaT fusion proteins were electrophoresed in 10% polyacrylamide gels and blotted onto nitrocellulose membranes. Blots were revealed with an anti–c-myc mAb (prepared in our laboratory from 9E10 hybridoma) and an HRP-conjugated donkey antimouse Ig antibody (Jackson Immuno-Research Laboratories) and developed by chemiluminescence (Amersham Pharmacia Biotech).
Adhesion assay
Adhesion assays were performed as previously described12 except that Jurkat T cells were loaded with calcein am (Molecular Probes, Eugene, OR) instead of being radiolabeled. Jurkat T cells (6 × 104) were added to a monolayer of 104 LB7 cells (expressing human CD80) and incubated at 37°C for 1 hour. Adherent cells were analyzed by fluorometry (excitation at 485 nm and emission at 430 nm).
Cytofluorometry
Jurkat T cells or U937 cells were incubated for 1 hour at 4°C with supernatants from control or transfected Cos cells, or with purified proteins. Bound scFv28.3-HaaT fusion proteins were detected with a rabbit antihuman α1-antitrypsin antibody (1:200 dilution; Dako) and a fluorescein isothiocyanate (FITC)–conjugated donkey antirabbit Ig antibody (dilution 1:200; Jackson ImmunoResearch Laboratories) for 30 minutes at 4°C. Cells were then analyzed by fluorescence-activated cell sorting (FACS). Monkey PBMCs were prepared by centrifugation of blood on Ficoll (Pharmacia Biotech) and incubated on ice in PBS/0.1% NaN3 with a mixture of anti–CD2-phycoerythrin (PE) mAb (Immunotech, Marseille, France) and FITC-labeled anti-CD28.6 mAb (prepared in our laboratory) for revelation of CD28 on T cells from monkeys treated with CD28.1, CD28.2, CD28.3, and with a mixture of anti–CD2-PE plus FITC-labeled anti-CD28.2 mAb (prepared in our laboratory), for revelation of CD28 on T cells from monkeys treated with CD28.6.
Mixed lymphocyte reactions
Human PBMCs were seeded in triplicate at a final concentration of 105 cells/well in RPMI 1640 medium (Sigma) supplemented with glutamine, nonessential amino acids, sodium pyruvate, antibiotics, and 10% heat-inactivated FCS and cultured for 5 days with 105 allogeneic PBMCs irradiated at 30 Gy. Proliferation was measured by 3H-thymidine incorporation after 16 hours of incubation with 10-6 Ci (37 KBq). Alternatively, CD4+ T cells prepared from PBMCs using the Lymph-Kwik Th system (One Lambda, Canoga Park, CA) were used as responding cells. The results were expressed as specific counts per minute per triplicate: (cpm assay — cpm stimulating cells alone — cpm responding cells alone).
Capping and immunofluorescence microscopy
Capping experiments were performed using a modified procedure of Gassmann et al.14 Jurkat T cells were incubated either at 37°C for 1 hour with mouse mAb CD28.3 (10 μg/mL), CD28.3 Fab fragments, scFv28.3-HaaT, or scFv28.3 recombinant proteins in RPMI 1640 medium (Sigma) containing 10% FCS, or at 0°C for 1 hour in the same medium containing 0.1% NaN3. After washing, cells were immediately fixed in 0.5% paraformaldeyde (wt/vol) for 30 minutes at room temperature, centrifuged onto glass slides, and allowed to dry overnight. Slides were immunolabeled with either FITC-labeled goat antimouse IgG antibodies (Jackson ImmunoResearch Laboratories), rabbit antihuman α1-antitrypsin followed by FITC-labeled donkey antirabbit IgG antibodies, or mouse anti–c-myc 9E10 mAb followed by goat antimouse IgG antibodies. After washing, the slides were mounted in Moviol and examined using the × 63 immersion lens of a Zeiss microscope equipped for epifluorescence.
Biosensor affinity measurement
CD28-Fc recombinant protein (R&D Systems) was immobilized onto a CM5 sensor chip. Analysis was performed with a BIAcore 2000 (Biacore, Paris, France). Antibodies or recombinant proteins were applied at concentrations ranging from 0.67 to 12.5 nM with a flow rate of 30 μL/min. An association period of 300 seconds was followed by a dissociation period of 600 seconds. Analyses and binding constants were deduced using the BIAevaluation software (Biacore).
Pharmacokinetic analysis of the conjugate
Proteins (30-60 μg) were labeled with 100 μg iodogen and 3.10-6 Ci (110 KBq)/mg 125I as described.15 Free iodine was eliminated by chromatography on Sephadex G-25M using a PD10 column (Amersham Pharmacia Biotech). Via thin layer chromatography with 10% trichloroacetic acid it was determined that 91% to 96% of the radioactivity was associated with the proteins. The specific activity of each preparation was calculated from estimates of protein concentration (measured by absorption at 280 nm) and radioactivity and was typically in the range of 0.1 to 0.3 × 10-6 Ci (4 to 10 KBq)/μg. The radiolabeled samples were used directly after labeling. Thirty or 60 μg 125I-labeled protein in PBS was injected into the tail vein of 10-week-old male Balb/c mice. Blood samples were collected periodically from the orbital sinus. Blood samples were weighed and radioactivity detected using a gamma counter (Canberra Packard Topcount, Mississauga, ON, Canada). Percent injected dose (%ID) was calculated for each individual mouse and expressed as %ID/mL total blood volume. The data were analyzed by Siphar software (Simed, Utrecht, The Netherlands) with the use of a 2-compartment model.
Results
The antihuman CD28 mAbs tested do not down-regulate their target in vivo
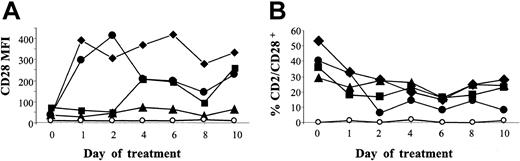
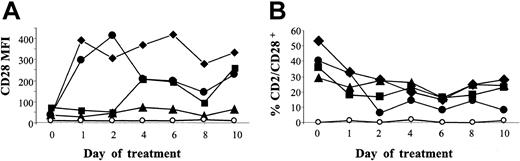
In the rat species, one mAb is known to induce CD28 capping and complete down-regulation in vivo.16 Thus, we first screened a series of antihuman CD28 mAbs for cross-reaction with macaques to investigate a possible down-regulating effect in vivo. Cross-reacting mAbs (CD28.1, CD28.2, CD28.3, and CD28.6 mAb12 ) were then injected intravenously into macacus fascicularis and CD28+CD2+ cell counts and CD28 expression intensity were monitored in the blood (Figure 1). No reduction in CD28 expression intensity was noted. Conversely, it was increased, together with an increase in the mean forward scatter (FSC) signal in flow cytometry (not shown). CD28+ cell counts were unchanged (CD28.3) or reduced by 30% for CD28.2, 50% for CD28.1, and 60% for CD28.6.
Assessment of a possible in vivo modulating activity of anti-CD28 antibodies. Anti-CD28 mAb (8 mg/kg) was infused intravenously into macacus fascicularis on days 0 and 2. Blood samples were collected before injection and every 2 days for 10 days. PBMCs were then analyzed by flow cytometry after gating of CD2+ cells. Mean fluorescence intensity (MFI) of CD28 expression (A) as well as percent of CD2+CD28+ double-positive cells within mononuclear cells (B) are represented; ⋄ indicates CD28.1; ▴, CD28.2; ▪, CD28.3; •, CD28.6; and ○, isotype control. Similar data were found in 2 monkeys per antibody, one of them being shown here.
Assessment of a possible in vivo modulating activity of anti-CD28 antibodies. Anti-CD28 mAb (8 mg/kg) was infused intravenously into macacus fascicularis on days 0 and 2. Blood samples were collected before injection and every 2 days for 10 days. PBMCs were then analyzed by flow cytometry after gating of CD2+ cells. Mean fluorescence intensity (MFI) of CD28 expression (A) as well as percent of CD2+CD28+ double-positive cells within mononuclear cells (B) are represented; ⋄ indicates CD28.1; ▴, CD28.2; ▪, CD28.3; •, CD28.6; and ○, isotype control. Similar data were found in 2 monkeys per antibody, one of them being shown here.
Selection of an anti-CD28 Fab fragment inhibiting CD28/B7 interaction
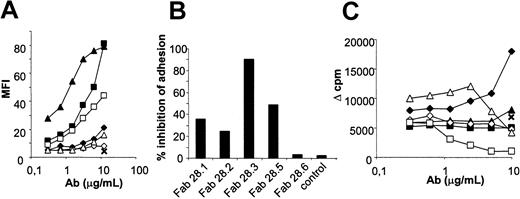
In the absence of modulating activity of the anti-CD28 antibodies tested, we screened the CD28 blocking ability of their monovalent fragments. Fab fragments were prepared and tested by flow cytometry, inhibition of adhesion of Jurkat T cells to CD80-transfected fibroblasts, and inhibition of mixed lymphocyte reactions (MLRs; Figure 2). Reactivity was compared with initial divalent IgG molecules. As determined by FACS, the CD28.1 and CD28.2 antibodies reduced their binding activity at least 100-fold after digestion into monovalent Fab fragments. In contrast, the reduction in reactivity of Fab fragments from CD28.3 was below a factor of 10 (Figure 2A). An evaluation of binding parameters performed by Biacore confirmed that Fab monovalent fragments from the CD28.3 mAb had a higher association rate (Ka, 9650/mole-second) than Fab fragments from the CD28.2 and CD28.1 mAb (Ka, 920/mole-second and below the detection limit, respectively). Fab fragments from the CD28.3 antibody fully inhibited the adhesion of CD28+ T cells to CD80-expressing cells (Figure 2B), whereas fragments from the other antibodies only partially reduced or had no effect on the adhesion of T cells. In MLRs, anti-CD28 antibodies are expected to costimulate (cross-linking of CD28) or, alternatively, to reduce T-cell proliferation (CD28/B7 interaction inhibition). In contrast, monovalent fragments are expected to inhibit T-cell proliferation. The anti-CD28 antibodies in their dimeric configuration showed no inhibition of proliferation in MLRs and some of them promoted T-cell proliferation at high doses (CD28.1, Figure 2C). Monovalent Fab fragments from the CD28.3 antibody showed a dose-response inhibition of proliferation, whereas Fab fragments from the other antibodies did not, probably as a result of their lower binding avidity. Finally, observations by immunofluorescence revealed that the divalent anti-CD28 mAb CD28.3 induced a capping of CD28 molecules on T-cell surfaces at 37°C, whereas monovalent Fab fragments did not.
Characterization of the Fab fragments of anti-CD28 antibodies. (A) CD28+ Jurkat T cells were incubated with the indicated amounts of anti-CD28 mAb or their Fab fragments and stained with antimouse-FITC antibody. MFI was determined by flow cytometry. (B) Inhibition of CD28/CD80 interactions was assessed by incubating calcein-labeled CD28+ Jurkat T cells on monolayers of mouse fibroblasts expressing human CD80 in the presence of saturating doses of anti-CD28 Fab fragments or control Fab fragments. After washing, adherent cells were collected and associated fluorescence was measured by fluorometry. (C) PBMCs (105) were mixed with 105 allogeneic irradiated PBMCs in the presence of the indicated amount of anti-CD28 mAb and Fab fragments or with 10 μg/mL control antibody. Proliferation on day 5 was measured by addition of 10-6 Ci (37 KBq) 3H-thymidine and measurement of incorporation after 16 hours; ⋄ indicates IgG CD28.1; ▴, IgG CD28.2; ▪, IgG CD28.3; ⋄, Fab CD28.1; ▵, Fab CD28.2; □, Fab CD28.3; and ×, isotype control. The results shown are representative of more than 3 independent experiments.
Characterization of the Fab fragments of anti-CD28 antibodies. (A) CD28+ Jurkat T cells were incubated with the indicated amounts of anti-CD28 mAb or their Fab fragments and stained with antimouse-FITC antibody. MFI was determined by flow cytometry. (B) Inhibition of CD28/CD80 interactions was assessed by incubating calcein-labeled CD28+ Jurkat T cells on monolayers of mouse fibroblasts expressing human CD80 in the presence of saturating doses of anti-CD28 Fab fragments or control Fab fragments. After washing, adherent cells were collected and associated fluorescence was measured by fluorometry. (C) PBMCs (105) were mixed with 105 allogeneic irradiated PBMCs in the presence of the indicated amount of anti-CD28 mAb and Fab fragments or with 10 μg/mL control antibody. Proliferation on day 5 was measured by addition of 10-6 Ci (37 KBq) 3H-thymidine and measurement of incorporation after 16 hours; ⋄ indicates IgG CD28.1; ▴, IgG CD28.2; ▪, IgG CD28.3; ⋄, Fab CD28.1; ▵, Fab CD28.2; □, Fab CD28.3; and ×, isotype control. The results shown are representative of more than 3 independent experiments.
Construction and expression of sc28.3HaaT from the CD28.3 hybridoma
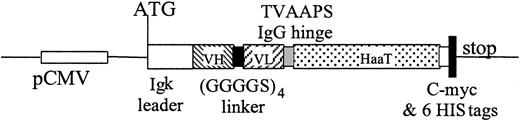
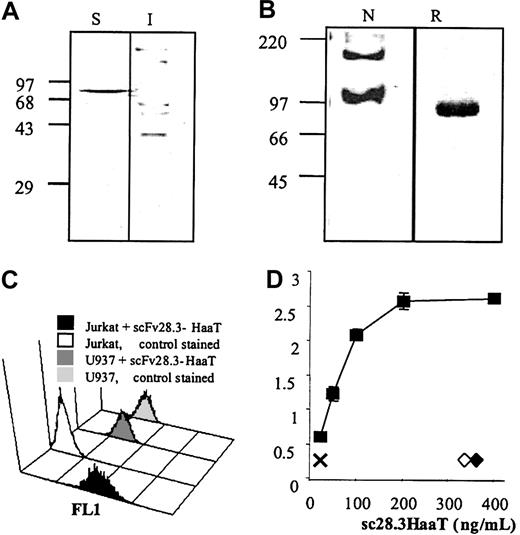
VH and VL chain fragments were amplified from CD28.3 hybridoma cDNA and assembled using a (GGGGS)4 linker. The nucleotide sequence of this scFv was registered in GenBank under the accession no. AF451974. To load the scFv with a carrier molecule that would increase the molecular weight of the complex and give an extended bioavailability in vivo, we selected a series of monovalent macromolecules that are abundant in plasma. We first fused the coding sequence of serum albumin to the C-terminus of the scFv because this had already been used successfully in a rabbit system.17,18 In the meantime, feasibility was reported with human albumin.10 In our hands, the genetic fusion of scFv 28.3 with serum albumin resulted in the production of a misfolded, insoluble complex without antibody activity in prokaryotic and eukaryotic expression systems (not shown). In contrast, the genetic fusion of scFv28.3 with a TVAAPS Fab hinge sequence and with amino acids 53 to 425 (referring to GenBank accession no. K01396) of the human α1-antitrypsin resulted in the production of a soluble and active molecule, as illustrated in Figure 3. The scFv28.3-HaaT cDNA was first subcloned into the EcoRV/XhoI sites of the pIg6 prokaryotic expression plasmid (a generous gift from Dr Jean-Charles Tellier, University of Nantes, Nantes, France) 3′ to an ompA signal sequence, enabling accumulation of recombinant proteins in the periplasm of JM83 E coli cells, under the control of the lac promoter (Figure 4A). However, periplasmic extracts contained only low levels of soluble protein. The scFv28.3-HaaT cDNA was subsequently subcloned into the BamHI/EcoRI sites of the pSecTag2B pCMV-based eukaryotic expression plasmid, enabling a fusion at the C-terminus with the Igκ leader sequence and at the N-terminus with a 6-HIS tag. After transfection in Cos cells, anti-CD28 activity was found in the supernatant and the recombinant protein was purified on NiNTA affinity columns with a yield of 1.5 mg/L supernatant (Figure 4B-D).
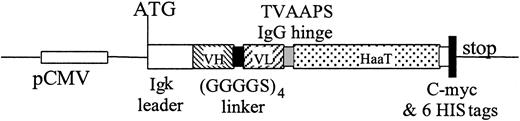
Construction for expression of scFv28.3-HaaT in eukaryotic cells. cDNA fragments coding for variable regions of VH and VL chains of the CD28.3 antibody were assembled with a (GGGGS)4 linker. A cDNA coding for the TVAAPS hinge peptide from the IgG heavy chain was added 3′ and fused in frame with a cDNA coding for amino acids 53 to 425 of HaaT cDNA. The complex was then introduced into the pSecTag2B expression plasmid by adding an Igκ leader to the N-terminus and a stretch sequence containing c-myc and 6HIS flag plus a stop codon to the C-terminus.
Construction for expression of scFv28.3-HaaT in eukaryotic cells. cDNA fragments coding for variable regions of VH and VL chains of the CD28.3 antibody were assembled with a (GGGGS)4 linker. A cDNA coding for the TVAAPS hinge peptide from the IgG heavy chain was added 3′ and fused in frame with a cDNA coding for amino acids 53 to 425 of HaaT cDNA. The complex was then introduced into the pSecTag2B expression plasmid by adding an Igκ leader to the N-terminus and a stretch sequence containing c-myc and 6HIS flag plus a stop codon to the C-terminus.
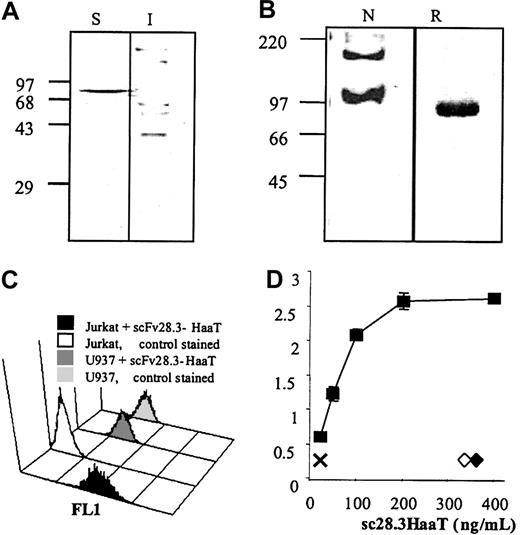
Expression and activity of scFv28.3-HaaT. (A) Prokaryotic expression: 10 μg protein extract from the periplasmic, soluble (S), and insoluble (I) fractions of E coli JM83 expressing scFv28.3-HaaT were resolved under reducing conditions on 10% polyacrylamide electrophoresis gels and blotted onto nylon membranes. Recombinant proteins were revealed by incubation of the membranes with HRP-conjugated anti–c-myc antibody and by enhanced chemiluminescence (ECL). (B) Fifty nanograms scFv28.3-HaaT produced in Cos cells, under native (N) or reducing (R) conditions, was analyzed by Western blotting as described in panel A. Molecular size markers (kDa) are shown on the left. (C) CD28+ Jurkat T cells were incubated with supernatant from transfected Cos cells containing 2 μg/mL scFv28.3-HaaT (black histogram, first row) or with supernatant from control Cos cells (white histogram, second row), revealed with anti-HaaT antibody plus FITC-conjugated donkey antirabbit Ig antibody (DAR) and analyzed by cytofluorometry. CD28- U937 cells were analyzed in parallel with supernatant containing scFv28.3-HaaT (dark gray histogram, third row) or with control supernatant (light gray histogram, last row). (D) The reactivity of Cos-produced scFv28.3-HaaT was examined by ELISA. CD28-Fc molecules were coated onto microtiter plates. Purified scFv28.3-HaaT at different dilutions in PBS-Tween was added and incubated for 1 hour at 37°C. After washing, bound scFv28.3-HaaT molecules were revealed with a rabbit anti-HaaT antibody followed by a peroxidase-conjugated DAR as a third-step reagent. Colorimetric analysis was performed after reaction of ABTS with peroxidase. X indicates signal without sc28.3-HaaT; ♦ and ⋄, signals obtained with incubations of HaaT alone and with an irrelevant scFv-M13 fusion antibody, revealed with a peroxidase-labeled anti-M13 antibody. Data are presented as means of triplicates.
Expression and activity of scFv28.3-HaaT. (A) Prokaryotic expression: 10 μg protein extract from the periplasmic, soluble (S), and insoluble (I) fractions of E coli JM83 expressing scFv28.3-HaaT were resolved under reducing conditions on 10% polyacrylamide electrophoresis gels and blotted onto nylon membranes. Recombinant proteins were revealed by incubation of the membranes with HRP-conjugated anti–c-myc antibody and by enhanced chemiluminescence (ECL). (B) Fifty nanograms scFv28.3-HaaT produced in Cos cells, under native (N) or reducing (R) conditions, was analyzed by Western blotting as described in panel A. Molecular size markers (kDa) are shown on the left. (C) CD28+ Jurkat T cells were incubated with supernatant from transfected Cos cells containing 2 μg/mL scFv28.3-HaaT (black histogram, first row) or with supernatant from control Cos cells (white histogram, second row), revealed with anti-HaaT antibody plus FITC-conjugated donkey antirabbit Ig antibody (DAR) and analyzed by cytofluorometry. CD28- U937 cells were analyzed in parallel with supernatant containing scFv28.3-HaaT (dark gray histogram, third row) or with control supernatant (light gray histogram, last row). (D) The reactivity of Cos-produced scFv28.3-HaaT was examined by ELISA. CD28-Fc molecules were coated onto microtiter plates. Purified scFv28.3-HaaT at different dilutions in PBS-Tween was added and incubated for 1 hour at 37°C. After washing, bound scFv28.3-HaaT molecules were revealed with a rabbit anti-HaaT antibody followed by a peroxidase-conjugated DAR as a third-step reagent. Colorimetric analysis was performed after reaction of ABTS with peroxidase. X indicates signal without sc28.3-HaaT; ♦ and ⋄, signals obtained with incubations of HaaT alone and with an irrelevant scFv-M13 fusion antibody, revealed with a peroxidase-labeled anti-M13 antibody. Data are presented as means of triplicates.
Detection of sc28.3HaaT binding activity by flow cytometry, ELISA, and biosensor
The binding of the scFv28.3-HaaT construct was then analyzed by flow cytometry using CD28+ Jurkat T cells and control U937 cells. The binding activity was tested by adding the scFv28.3-HaaT construct to Jurkat cells followed by staining with a rabbit anti–α1-antitrypsin antibody and an FITC-conjugated donkey antirabbit Ig antibody as a third-step reagent and analysis by flow cytometry. The results indicated that scFv28.3-HaaT bound specifically to CD28 expressed on Jurkat cells with no binding to U937 cells, which do not express CD28 (Figure 4). Binding was also confirmed in an enzyme-linked immunosorbent assay (ELISA), performed by adding various concentrations (25-400 ng/mL) of scFv28.3-HaaT to plates coated with recombinant CD28-Fc molecules (Figure 4C). Negative controls where plates were coated with irrelevant molecules (gelatin, bovine serum albumin, ovalbumin) gave no signal (not shown). In addition, an unrelated scFv (fused with the G3p protein from M13 bacteriophage) and α1-antitrypsin alone did not bind to recombinant CD28-Fc. From this analysis, the inhibitory concentration of 50% (IC50) was estimated as being 0.4 nM. The avidity of the scFv28.3-HaaT was evaluated via a biosensor analysis. A detector chip was coated with CD28-Fc at high and low densities. Recombinant proteins were applied at concentrations ranging from 0.67 to 12.5 nM with a flow rate of 30 μL/min, an association period of 300 seconds followed by a dissociation period of 600 seconds. The analysis of the avidity, deduced from the BIA evaluation software from 6 determinations at different doses, revealed a Kd (M) of 7.1 ± 1.8 10-9. Similar values were obtained in 3 independent analyses using either Cos cell supernatants or purified material. A similar analysis of the initial CD28.3 Fab fragments revealed avidity in the same order of magnitude (Kd of 8.5 × 10-9).
Detection of sc28.3HaaT aggregation by Western blotting and absence of capping induction
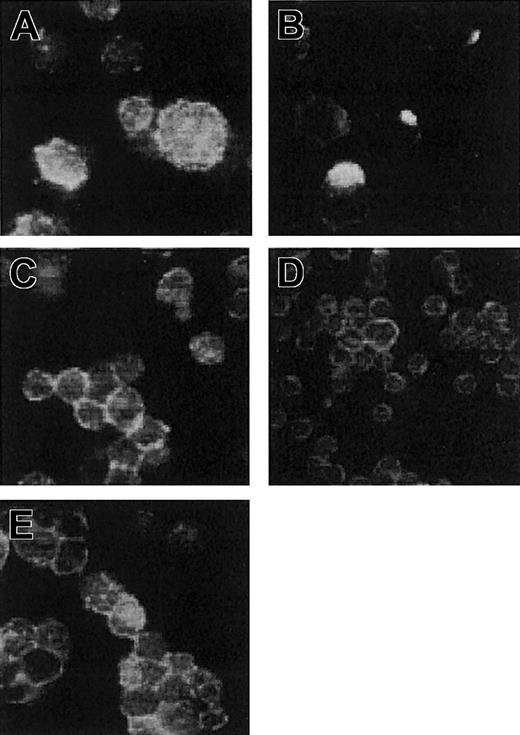
Recombinant scFv antibodies often tend to aggregate or form dimers in solution19 ; thus, even after the fusion with a monovalent carrier such as HaaT, our scFv28.3 may dimerize and induce clustering of CD28 molecules on T-cell membranes. To characterize the nature of the recombinant protein produced in Cos cells, supernatants were analyzed by electrophoresis under native and denaturant conditions. After reduction and denaturation in sodium dodecyl sulfate (SDS), the protein had an apparent molecular mass of 90 kDa (Figure 4B). The fact that the same protein produced in E coli had an apparent molecular mass of 78 kDa (Figure 4A) indicated that approximately 12 kDa could be attributed to glycosylation after production in eukaryotic cells. Under native conditions, without SDS, the protein was mostly monomeric with an apparent molecular weight similar to that of the denatured protein. Material with a molecular weight of approximately 180 kDa was also detected, indicating the presence in the solution of dimeric proteins (Figure 4). The capping assay, however, revealed that the scFv28.3-HaaT produced in Cos cells, unlike divalent IgG molecules, did not induce formation of CD28 clusters on T-cell membranes, and neither did an scFv28.3 nonfused to the HaaT (Figure 5). This suggests that the dimeric proteins contained in the preparation could not stimulate CD28 either due to their association being too weak or due to competition for binding with quantitatively dominant monovalent fragments.
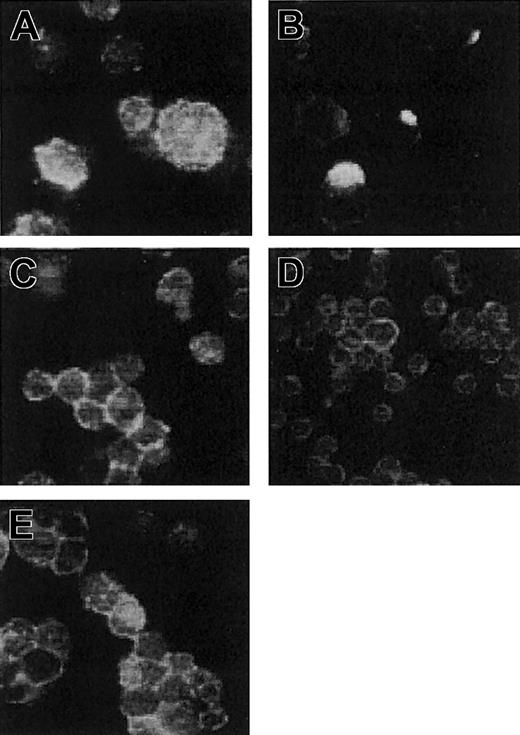
Immunofluorescent analysis of antibody-induced capping of CD28. Jurkat T cells expressing CD28 were incubated in culture medium with 2 μg/mL divalent CD28.3 IgG antibody (A-B) or 2 μg/mL CD28.3 Fab fragments, with 2 μg/mL scFv28.3-HaaT (E) or with 50% Cos cell supernatant containing approximately 4 μg/mL scFv28.3 (D). Cells were incubated for 1 hour at 0°C (A) or at 37°C (B-E), washed in ice-cooled PBS containing NaN3, and centrifuged onto microscope slides. After fixation with 0.5% paraformaldehyde (PFA), CD28 molecules were revealed using FITC-labeled CD28.6, a mAb reacting against an epitope on CD28 other than CD28.3.11 Slides were observed by fluorescence microscopy, and magnification is × 63 for all panels.
Immunofluorescent analysis of antibody-induced capping of CD28. Jurkat T cells expressing CD28 were incubated in culture medium with 2 μg/mL divalent CD28.3 IgG antibody (A-B) or 2 μg/mL CD28.3 Fab fragments, with 2 μg/mL scFv28.3-HaaT (E) or with 50% Cos cell supernatant containing approximately 4 μg/mL scFv28.3 (D). Cells were incubated for 1 hour at 0°C (A) or at 37°C (B-E), washed in ice-cooled PBS containing NaN3, and centrifuged onto microscope slides. After fixation with 0.5% paraformaldehyde (PFA), CD28 molecules were revealed using FITC-labeled CD28.6, a mAb reacting against an epitope on CD28 other than CD28.3.11 Slides were observed by fluorescence microscopy, and magnification is × 63 for all panels.
scFv28.3-HaaT modulates T-cell allogeneic responses
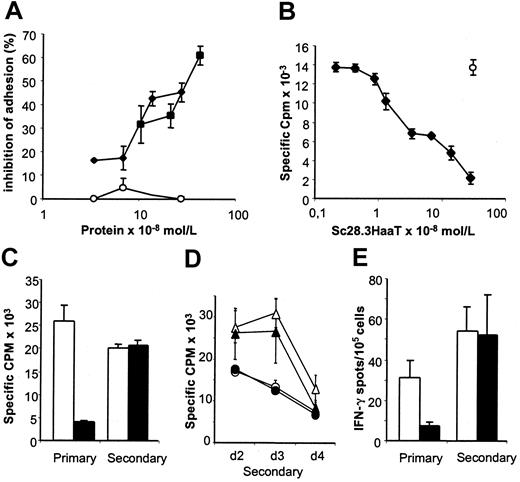
Our group20 has previously demonstrated that monovalent Fab fragments from CD28 antibodies reduce proliferation of alloreactive T cells but are ineffective in modulating proliferation of T cells stimulated by heterologous antigens presented in the context of self major histocompatibility complex (MHC) molecules. To investigate whether scFv28.3-HaaT could similarly reduce T-cell alloresponses, we first checked whether this construct inhibited CD28/B7 interactions and inhibited an MLR. As shown in Figure 6, scFv28.3-HaaT dose dependently inhibited adhesion of Jurkat T cells to CD80-expressing fibroblasts (Figure 6A) and reduced proliferation of allogeneic T cells in vitro (Figure 6B). CD28 blockade also reduced the frequency of IFN-γ–producing cells in MLRs 24 hours after stimulation (Figure 6E).
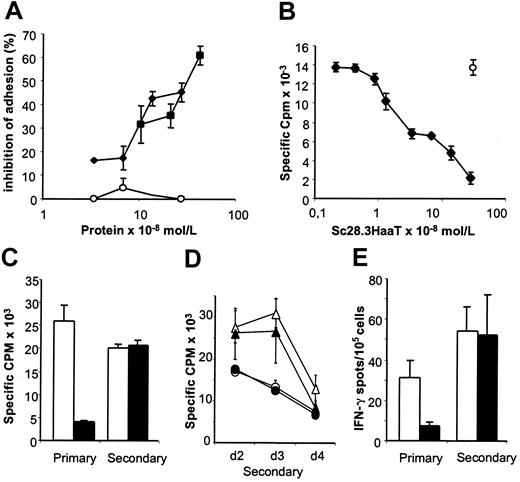
Biologic activity of scFv28.3-HaaT in a binding assay and MLRs. (A) CD28+ Jurkat T cells stained with calcein am dye were incubated on a monolayer of CD80-expressing fibroblasts, as in Figure 2B, in the presence of dilutions of supernatant from Cos cells transfected with pSecTag2B-scFv28.3-HaaT (▪) or pSecTag2B-scFv28.3 (⋄), in which recombinant protein was quantified by ELISA. Control-transfected supernatant (○) was used at similar dilutions. The percentage of inhibition of adhesion after washing was evaluated by luminescence. (B) CD4+ T cells from healthy volunteers were incubated in microtiter plates with irradiated allogeneic PBMCs for 5 days in the presence of dilutions of purified sc28.3-HaaT. Proliferation was measured by incorporation of 3H-thymidine for the last 16 hours of culture; m indicates proliferation obtained after addition of a control mAb (mouse IgG1) to the cultures. Data shown here are means ± SDs of triplicates and are representative of 5 experiments. (C-E) MLRs similar to panel B were performed with (filled bars and symbols) or without (empty bars and symbols) saturating amounts of sc28.3-HaaT. After 5 days an aliquot of cells was assessed for proliferation (“primary” in panel C) and for frequency of IFN-γ secretion (“primary” in panel E). The remaining cells were washed and cultured for a resting period of 7 days, after which time cells were collected and restimulated (“secondary”) with the same (C, circles in panels D and E) or third-party (triangles in panel D) stimulatory cells. Proliferation was measured 2 (C-D), 3, and 4 (D) days later. The frequency of IFN-γ–secreting cells during secondary stimulation was measured 24 hours after restimulation (“secondary” in panel E).
Biologic activity of scFv28.3-HaaT in a binding assay and MLRs. (A) CD28+ Jurkat T cells stained with calcein am dye were incubated on a monolayer of CD80-expressing fibroblasts, as in Figure 2B, in the presence of dilutions of supernatant from Cos cells transfected with pSecTag2B-scFv28.3-HaaT (▪) or pSecTag2B-scFv28.3 (⋄), in which recombinant protein was quantified by ELISA. Control-transfected supernatant (○) was used at similar dilutions. The percentage of inhibition of adhesion after washing was evaluated by luminescence. (B) CD4+ T cells from healthy volunteers were incubated in microtiter plates with irradiated allogeneic PBMCs for 5 days in the presence of dilutions of purified sc28.3-HaaT. Proliferation was measured by incorporation of 3H-thymidine for the last 16 hours of culture; m indicates proliferation obtained after addition of a control mAb (mouse IgG1) to the cultures. Data shown here are means ± SDs of triplicates and are representative of 5 experiments. (C-E) MLRs similar to panel B were performed with (filled bars and symbols) or without (empty bars and symbols) saturating amounts of sc28.3-HaaT. After 5 days an aliquot of cells was assessed for proliferation (“primary” in panel C) and for frequency of IFN-γ secretion (“primary” in panel E). The remaining cells were washed and cultured for a resting period of 7 days, after which time cells were collected and restimulated (“secondary”) with the same (C, circles in panels D and E) or third-party (triangles in panel D) stimulatory cells. Proliferation was measured 2 (C-D), 3, and 4 (D) days later. The frequency of IFN-γ–secreting cells during secondary stimulation was measured 24 hours after restimulation (“secondary” in panel E).
To determine whether the reduced alloreactivity in MLRs with CD28 blockade was due to immune ignorance, anergy, or deletion of alloreactive T cells, we measured secondary responses of cells initially primed in the presence of sc28.3-HaaT. No modifications in the frequency of IFN-γ–secreting cells (Figure 6E) or in proliferation (Figure 6C) were noted after secondary stimulation, indicating that alloreactive cells had not been deleted in the primary stimulation. Inhibiting CD28 in primary MLRs with Fab fragments from the CD28.3 antibody instead of sc28.3HaaT led to similar conclusions (data not shown). However, proliferation in recall stimulation was maximal on day 2, whereas it peaked on day 3 when third-party stimulating PBMCs were used (Figure 6D), indicating that donor-specific proliferating cells had been primed in the primary reaction despite CD28 blockade.
Pharmacokinetics in murine plasma
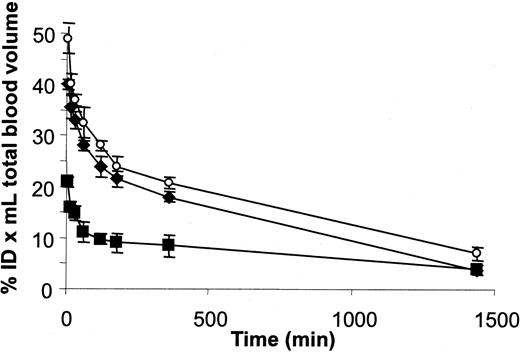
To address the question of whether the genetic fusion of the scFv fragment with HaaT actually resulted in an extended half-life in vivo, we followed the distribution in mice of iodinated scFv28.3-HaaT, Fab fragments from the CD28.3 antibody, and HaaT. Figure 7 shows that the distribution and elimination half-lives of the Fab fragments were rapid, as expected for a 50-kDa protein (14.5 ± 3.3 minutes and 7.4 ± 1.7 hours, respectively). HaaT distribution was similar (17.4 ± 10.8 minutes) but it was eliminated much more slowly (10.8 ± 0.6 hours) despite having a slightly lower molecular weight (46.6 kDa). The distribution and the elimination half-lives were significantly extended for the scFv28.3-HaaT recombinant molecule (26.6 ± 2.3 minutes and 11.8 ± 0.5 hours, respectively). Finally, an analysis of the biodistribution of the labeled molecules 48 hours after injection confirmed the accumulation of scFv28.3-HaaT in the reticuloendothelial compartment (liver, kidneys, intestine, spleen; not shown), whereas Fab fragments were lost, probably through renal elimination. Furthermore, the distribution volume increased from 3 mL for Fab and HaaT to 8 mL for scFv28.3-HaaT, indicating that the conjugate left the blood to penetrate organs and tissues more rapidly.
Pharmacokinetics of 125I-labeled HaaT, CD28.3 Ab Fab fragments, and scFv28.3-HaaT in mice. Purified sc28.3-HaaT, HaaT, and CD28.3 Fab fragments were labeled with 125I and injected intravenously into mice. Blood samples were collected periodically and radioactivity was measured with a gamma counter. %ID indicates percent injected dose; ▪, scFv28.3-HaaT; ○, HaaT; and ⋄, Fab. For each, n = 3. Data are presented as means ± SDs.
Pharmacokinetics of 125I-labeled HaaT, CD28.3 Ab Fab fragments, and scFv28.3-HaaT in mice. Purified sc28.3-HaaT, HaaT, and CD28.3 Fab fragments were labeled with 125I and injected intravenously into mice. Blood samples were collected periodically and radioactivity was measured with a gamma counter. %ID indicates percent injected dose; ▪, scFv28.3-HaaT; ○, HaaT; and ⋄, Fab. For each, n = 3. Data are presented as means ± SDs.
Discussion
Antibody-induced modulation of CD28 prolongs allograft survival in rats16 and administration of anti-CD28 Fab fragments reverses the induction of experimental autoimmune encephalomyelitis21 and uveoretinitis22 in mice. In a murine model of graft-versus-host disease (GVHD), the selective blockade of CD28 was reported as being more immunosuppressive than blocking B7 with CTLA-4/Ig.23 CD28 blockade also synergizes with cyclosporine in the rat resulting in immune tolerance in transplantation.24 In CD28-/- mice, immune responses to viral antigens or autoantigens are impaired,25,26 whereas responses to exogenous antigens remain normal.27 In rats treated with the modulating anti-CD28 antibody JJ319, allogeneic T-cell responses are blunted but responses to exogenous antigens are unaffected.20 Collectively, these data suggest that T-cell responses to autoantigens or alloantigens are more dependent on costimulation through CD28 than T-cell responses to exogenous antigens. Thus, the blockade of CD28 may represent an immunosuppression for the selective inhibition of pathologic T cells in autoimmunity and in transplantation without inhibition of other, protective, T-cell responses.
Costimulation through CD28 in conjunction with a triggering of the T-cell receptor (TCR) leads to high-level production of interleukin 2 (IL-2) and provides an essential survival signal for T cells. Signals through CD28 also regulate T-cell cycle entry and progression through the G1 phase in an IL-2–independent manner,28 resulting in the activation of cyclines.29 At the molecular level, a selective blockade of CD28 with an unmodified CTLA-4/B7 interaction would theoretically allow signals through the TCR and CTLA-4 to be transmitted to antigen-challenged T cells. Resting T cells express a relatively low level of CTLA-4; however, when engaged on resting T cells, CTLA-4 transmits a signal that blocks transition from G0 to G1.30 Once activated, T cells further increase their membrane expression of CTLA-4, which transmits a signal leading to a Fas-independent cell death,31 contributing to the down-regulation of the immune response. Therefore, allowing CTLA-4/B7 interactions to proceed in the absence of CD28 costimulation will combine the effect of the lack of costimulation with a cell growth arrest, or a clonal deletion, depending on whether blockade is initiated before or after the initiation of T-cell activation. In addition, as agonistic anti-CD28 antibodies promote Th1 differentiation in vitro,32 inhibition of CD28 is likely to modify the Th1/Th2 balance.
This study was undertaken to obtain an anti-CD28 antagonist that would allow an in vivo investigation into whether recognition of alloantigens or autoantigens by CD4+ T cells in the absence of CD28 costimulation, but with an unmodified CTLA-4/B7 interaction, induces an immune regulation. The available anti-CD28 antibodies appear not to be antagonistic in vivo in monkeys, as shown by the absence of CD28 down-regulation. On the contrary, 3 of the 4 antibodies we tested induced a strong up-regulation of CD28 expression on CD2+ cells (Figure 1), a phenomenon that has previously been associated with T-cell activation.33
In the absence of identified modulating anti-CD28 antibodies, we constructed a recombinant monovalent antibody from the hybridoma of CD28.3. This clone was chosen because (1) it retains its high affinity even after digestion into monovalent Fab fragments, (2) it prevents CD28 from binding CD80, (3) its Fab fragments dose dependently reduce T-cell proliferation in MLRs, and (4) it retains its binding properties after recombinant expression in scFv format. The extension of its molecular weight through a genetic fusion with α1-antitrypsin resulted in a soluble macromolecule with an extended bioavailability and a preserved activity. α1-Antitrypsin is an abundant (3 g/L) monovalent plasma protein that has already been used for genetic fusion.34 In humans it has a half-life of about 8 days,35 although its half-life is much shorter in rodents.36 To demonstrate the immunosuppressive potential of scFv28.3-HaaT, we reproduced the biologic activities of the CD28.3 antibody Fab fragments in vitro. This included a preserved avidity for CD28, the absence of induction of CD28 capping on target cells at 37°C, a competition with CD80 for binding to CD28, and a dose-dependent inhibition in MLRs. In addition, sc28.3HaaT reduced the frequency of allogeneic cells producing IFN-γ 24 hours after the initiation of an MLR.
Restimulation of T cells primed in the presence of the CD28 inhibitor resulted in intact secondary responses, suggesting that one mechanism of action of sc28.3-HaaT in MLRs that results in an inhibition of T-cell proliferation and activation is related to immune ignorance rather than anergy or alloreactive cell deletion. However, the earlier kinetics of T-cell proliferation following stimulation by isogeneic antigen-presenting cells (APCs) as compared with third-party APCs in secondary responses (proliferation peak on day 2 instead of day 3) suggests that T cells were primed in the primary reaction whether or not CD28 had been blocked. Additional mechanisms to ignorance might, however, play a role in vivo because a short-course administration of Fab fragments or of a modulating anti-CD28 mAb in rodents increased apoptosis of alloreactive cells20 and, in the long term, improved disorders related to alloreactivity23 and autoimmunity.21,22 Thus, deletion of alloreactive cells or the development of regulatory mechanisms might also result from selective CD28 blockade. The possibility that a selective blockade of CD28 in humans could improve disorders such as GVHD or allograft survival, however, remains to be demonstrated.
In summary, we have produced a reagent that, for the first time, can specifically inhibit CD28/B7 interactions without interfering with those of CTLA-4/B7. This agent is efficient at inhibiting several CD28-mediated processes in vitro. We believe that this new strategy may have clinical applications in humans.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-08-2480.
Supported in part by the “Fondation Progreffe,” by the Post-Genome Program, grant 109 from the French government (Ministère de l'Education Nationale de la Recherche et de la Technologie [MENRT]), and by the association “Vaincre La Mucoviscidose.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank J. Naulet and J. Lafond for assistance in protein production and purification, S. Iyer for the preparation of Fab fragments, and Alain Faivre-Chauvet for assistance in protein iodination.