Abstract
Minor histocompatibility (H) antigens crucially affect the outcome of human leukocyte antigen (HLA)–identical allogeneic stem cell transplantation (SCT). To understand the basis of alloimmune responses against minor H antigens, identification of minor H peptides and their antigenicity-determining mechanisms is essential. Here we report the identification of HA-3 and its encoding gene. The HA-3 peptide, VTEPGTAQY (HA-3T), is encoded by the lymphoid blast crisis (Lbc) oncogene. We thus show for the first time that a leukemia-associated oncogene can give rise to immunogenic T-cell epitopes that may have participated in antihost and antileukemic alloimmune responses. Genotypic analysis of HA-3- individuals revealed the allelic counterpart VMEPGTAQY (HA-3M). Despite the lack of T-cell recognition of HA-3- cells, the Thr→Met substitution had only a modest effect on peptide binding to HLA-A1 and a minimal impact on recognition by T cells when added exogenously to target cells. This substitution did not influence transporter associated with antigen processing (TAP) transport, but, in contrast to the HA-3T peptide, HA-3M is destroyed by proteasome-mediated digestion. Thus, the immunogenicity of minor H antigens can result from proteasome-mediated destruction of the negative allelic peptide.
Introduction
Hematopoietic stem cell transplantation (SCT) is an important and curative therapy for a variety of malignant diseases, especially hematologic malignancies. It is the treatment of choice for patients with chronic myeloid leukemia (CML), acute myeloid leukemia (AML), and acute lymphoid leukemia (ALL) with high-risk features or relapsed disease.1,2 The therapeutic potential of human leukocyte antigen (HLA)–matched SCT relies on the graft-versus-leukemia (GVL) effect, which is mediated by allogeneic donor T cells recognizing minor histocompatibility (H) antigens and leukemia-associated antigens expressed on malignant cells.3,4 However, the GVL effect is often accompanied by graft-versus-host disease (GVHD), which is the major complication of allogeneic SCT.3,5
Minor H antigens are capable of eliciting cellular alloimmune responses in vitro and in vivo.6,7 They are peptides derived from polymorphic proteins. Their immunogenicity arises as a result of their presentation on the plasma membrane in the context of major histocompatibility complex (MHC) class I or II, where they are recognized by alloreactive MHC-restricted T cells (for review see Goulmy4,6 ). Until recently, the biochemical structures of minor H antigens in both humans and mice had remained elusive due to the technical difficulty of identifying T-cell epitopes. The application of microcapillary high-performance liquid chromatography (HPLC)–electrospray ionization tandem mass spectrometry has enabled the detection and sequencing of nonabundant peptides among a pool of HLA-bound peptides. This approach has been successfully applied for the identification of human minor H antigens encoded by autosomal genes8-10 and those encoded by the Y chromosome.11-13
Genetic polymorphisms that qualitatively or quantitatively affect the display of self-peptides at the cell surface could give rise to a minor H antigen disparity in an HLA-identical transplantation setting. These can be classified based on their impact on either T-cell recognition or peptide presentation by HLA molecules. To date, limited information has been gathered on the various mechanisms resulting in immunogenic minor H T-cell epitopes. In the case of the human minor H antigens B7-HY,11 A1-HY,13 and HB-1,14 the existence of these minor H antigens is dependent on the presence within any individual of T-cell receptors (TCRs) with an appropriate fine specificity to distinguish minor H antigen–expressing cells from their negative counterparts. Alternatively, minor H antigens might be distinguished due to polymorphisms that diminish or abolish the ability of the peptide to bind to the relevant HLA molecule. This mechanism most probably underlies the immunogenicity of HA-1.9 A third possibility is that the positive and negative peptides are antigenically similar but are handled differently by the antigen-processing machinery of the cell. This mechanism has recently been described for HA-8,10 where differential transporter associated with antigen processing (TAP) binding was observed.
Previously, HLA-A1–restricted HA-3–specific T-cells had been isolated from a patient with acute GVHD grade II after HLA-identical SCT for treatment of AML.4 GVHD was successfully treated with prednisone, whereafter durable remission was induced. HA-3 has a phenotype frequency of 88%15 and exhibits ubiquitous tissue expression.16 At present, 20 years after SCT, the patient is still disease-free and in good health. In this report, we describe the identification of the amino acid and nucleotide sequences of the immunogenic minor H antigen HA-3 and its negative allelic counterpart. The HA-3- allelic counterpart is destroyed by the presence of a proteasome cleavage site that is absent in the HA-3+ allele. Our data for the first time demonstrate that the immunogenicity of a minor H antigen can result from altered proteasomal digestion of immunologically similar peptides rather than from differences in binding affinities to MHC or the T-cell receptor, or in differential TAP translocation. Interestingly, HA-3 is encoded by the lymphoid blast crisis oncogene (Lbc). The fact that minor H antigens can be encoded by disease-related oncogenes sheds a novel light on their putative involvement in the curative effects of HLA-matched, minor H–mismatched SCT.
Patient, materials, and methods
Cell lines and clones
The HLA-A1–restricted HA-3–specific CD8+ cytotoxic T-lymphocyte (CTL) clone designated 5Ho11 was generated after SCT from the peripheral blood mononuclear cells (PBMCs) of a male acute myelogenous leukemia patient (HoRe) who had received an SCT from his HLA-identical brother (HoDo).4 The HA-3 CTLs were maintained and used in cytotoxicity and epitope reconstitution assays as described previously.17,18
The HLA-A1+ Epstein Barr virus–transformed B-lymphoblastoid cell lines (EBV-BLCL) used were HoDo (A1, A11, B8, B60, HA-3-), HoRe (A1, A11, B8, B60, HA-3+), and Rp (HLA-A1, A2, B8, B27, HA-3+). C1R-A1 is an HLA-A*0101+ transfectant of C1R. All transformed cell lines were cultured in RPMI-1640 supplemented with 4 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 5% fetal bovine serum (FBS), 0.125% SerXtend (Irvine Scientific, Santa Ana, CA), and 3 mM l-glutamine (EBV-BLCL medium). To maintain the expression of the HLA-A*0101 gene in the C1R-A1 transfectant, the medium was supplemented with 300 mg/mL G418.
Extraction and HPLC fractionation of HLA-A–associated peptides
HLA-A1 molecules were immunoaffinity purified from Rp cells and their associated peptides were extracted as previously described.13 Peptides were separated from class I heavy chains and β2-microglobulin (β2m) by elution in 10% acetic acid and passage through a 5-kDa cutoff filter. Peptides were fractionated on a narrow-bore HAISIL C18 column (2.1 × 40 mm, 5 μm particles, 30 nm [300 Å] pore size; Higgins, Winter Park, FL) on a 130A HPLC (Applied Biosystems, Foster City, CA). The elution gradient used was 0% to 10% solvent B in 10 minutes, 10% to 60% B in the next 55 minutes, and 60% to 100% B in the next 7 minutes, where solvent A was 0.1% trifluoroacetic acid (TFA; HPLC grade; Applied Biosystems) in NANOpure water (Barnstead, Dubuque, IA) and solvent B was 0.085% TFA in 60% acetonitrile (HPLC grade; Mallinckrodt, Paris, KY). Fractions were collected every 40 seconds at a flow rate of 200 μL/min. Active fractions were pooled and rechromatographed with the identical column and gradient, but using 0.1% heptafluorobutyric acid (HFBA; HPLC grade; Pierce, Rockford, IL) as the ion-pairing agent. Half of the active second-dimension material was used for a third-dimension fractionation on a microcapillary column19 (280 μm outer diameter [OD], 75 μm inner diameter [ID] packed with 25 cm of 5-μm C18 beads (YMC, Morris Plains, NJ). TFA was used as the ion-pairing agent in buffers A and B, and the column was eluted with a linear gradient of 0% to 100% B over 40 minutes at a flow rate of 300 nL/min.
Epitope reconstitution assays
Aliquots of each HPLC fraction were incubated with 2000 51Cr-labeled HoDo target cells and 7.5 μg/mL human β2m (Calbiochem, San Diego, CA) for 90 minutes at 26°C in 150 μl EBV-BLCL medium. HA-3 CTLs were added in 100 μL EBV-BLCL medium at an effector-target (E/T) ratio of 17:1 in a standard chromium release assay.8 Synthetic peptides were assayed using the same protocol.
Fourier transform mass spectrometry of HLA-A1–associated peptides
Mass spectrometric data were acquired on a home-built Fourier transform ion cyclotron resonance mass spectrometer (FTMS) equipped with a nanoflow-HPLC microelectrospray ionization source as described previously.20 Briefly, an aliquot of samples was loaded onto a nano-HPLC column and eluted into the FTMS using a gradient: 0% to 60% solvent B in 32 minutes and 60% to 100% solvent B in the next 3 minutes, where solvent A is 0.1 M acetic acid (Sigma Chemical, St Louis, MO) in NANOpure water (Barnstead) and solvent B is 0.1 M acetic acid in 70% acetonitrile. Full scan mass spectra were acquired at a rate of 1 scan/second. To determine the candidate masses for the antigenic peptide, active third-dimension fractions were analyzed using FTMS and nanoflow effluent splitter technology.13,21 In this analysis the column eluent was split such that one eighth went into the FTMS for mass detection and the remaining seven eighths was plated into a 96-well plate containing 25 μL NANOpure water per well for reconstitution assay and future sequencing.
Sequence analysis of candidate antigens
Collision-activated dissociation (CAD) mass spectra were recorded on selected peptide candidates using a ThermoFinnigan LCQ ion trap mass spectrometer equipped with sheathless nanoflow HPLC–electrospray ionization (ESI) (San Jose, CA).22 Targeted CAD spectra were acquired by manually switching from mass spectrometry (MS)–only mode to MS/MS mode after the chromatographic elution of a marker peptide. In MS/MS mode, the ion of interest was isolated using a 3.0 atomic mass unit (AMU) isolation window and fragmented using 35% collision energy. CAD spectra were manually sequenced and the resulting peptide sequence was searched for homology in GenBank, European Molecular Biology Laboratory (EMBL), and DNA Data Bank of Japan (DDBJ) DNA and protein databases.
Synthetic peptides
Peptides were synthesized on an AMS 1400 multiple peptide synthesizer (Gilson Medical Electronics, Middleton, WI) using solid-phase FMOC (9-fluorenylmethoxycarbonyl) chemistry and Wang resins (Calbiochem-Novabiochem, La Jolla, CA). Peptides were HPLC purified by reverse phase HPLC (RP-HPLC) to more than 90% purity (POROS R2/H, 4.6-mm × 10-cm column; Roche, Basel, Switzerland) on an Applied Biosystems (Foster City, CA) model 140 A dual-syringe pump. Peptides were eluted with a linear gradient of 0% to 80% B where solvent A was 0.1% TFA and solvent B was 0.085% TFA in acetonitrile. Effluent was monitored at 214 nm and peaks were collected manually. HPLC solvent was removed using a Savant Speed Vac (Thermo Savant, Holbrook, NY) and the crystalline peptide was weighed to make accurate solutions.
Reverse transcriptase–polymerase chain reaction (RT-PCR) amplification and sequencing of the kinase A anchoring protein 13 (AKAP13)–Lbc region encoding the minor H antigen HA-3
Poly(A)+ RNA was isolated from HA-3+ and HA-3- EBV-BLCL with the QuickPrep Micro mRNA Purification Kit (Amersham Pharmacia Biotech, Piscataway, NJ). cDNA was synthesized using a First Strand cDNA Synthesis Kit (MBI Fermentas, Hanover, MD). Amplifications were performed with 500 μM each of forward primer 5′-ACAGGAGAATACAGACCGTT-3′ and reverse primer 5′-CAGTGTCTGGGGTACTGACA-3′ (Research Genetics, Huntsville, AL) in 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates (dNTPs), and 2.5 U Taq polymerase in 1 × PCR buffer (all obtained from Life Technologies, Rockville, MD). Cycle parameters were initial denaturation at 94°C for 2 minutes, 30 cycles of denaturation at 94°C for 1 minute, annealing at 53.2°C for 1 minute, extension at 72°C for 1 minute, and final extension at 72°C for 10 minutes. PCR products were cloned using the AdvanTAge cloning kit (Clontech, Palo Alto, CA). At least 5 individual clones were sequenced and analyzed bidirectionally for each cell line examined.
RT-PCR analysis for HA-3 RNA expression
Melanocytes, fibroblasts, keratinocytes, human umbilical vein endothelial cells (HUVECs), and proximal tubular epithelial cells (PTECs) were all isolated and cultured as described elsewhere.16 PBMCs were isolated by Ficoll-Isopaque density centrifugation of whole donor blood, washed twice with phosphate-buffered saline (PBS), and used immediately. Poly(A)+ RNA was isolated as described above. For tissue-specific expression, PCR fragments corresponding to bases 4239-4536 in the Lbc sequence (GenBank accession no. AB055890) were amplified from cDNA using the forward primer 5′-TGAGCCAGCAGCAGAAATGC-3′ and the reverse primer 5′-AGAATCACTCCCAGATTCTC-3′. Primers specific for GAPDH (glyceraldehyde phosphate dehydrogenase) were used to amplify a 358–base pair (bp) positive control fragment. The conditions used were as described but with an annealing temperature of 60°C.
Allele-specific PCR for HA-3
Genomic DNA was isolated from EBV-BLCL with the High Pure Template Purification Kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's description. PCR was performed using 2 different primer sets specific for either the HA-3 peptide VTEPGTAQY (HA-3T) or the allelic counterpart VMEPGTAQY (HA-3M). HA-3T primers were 5′-CTTCAGAGAGACTTGGTCAC-3′ and 5′-GTTCATGAGCCCATGTTCCAT-3′ (129-bp fragment), and the HA-3M primers were 5′-CTTCAGAGAGACTTGGTCAT-3′ and 5′-AGACTCAGCAGGTTTGTTAC-3′ (318-bp fragment). PCR mixes contained 80 ng genomic DNA, 0.25 U Amplitaq (Perkin-Elmer, Norwalk, CT), 0.01% gelatin, 0.8 mM dNTP, 0.5 μM specific primers, 1.5 mM MgCl2; 50 mM KCl, 10 mM Tris HCl (pH 8.3), 6% sucrose, and 1 mM cresol red. The PCR program was 10 cycles of 2 minutes at 94°C, 10 seconds at 94°C, and 60 seconds at 65°C. Another 20 cycles were run using the following conditions: 10 seconds at 94°C, 50 seconds at 61°C, and 30 seconds at 72°C. Samples were analyzed on a 2% agarose gel. Internal control primers for human platelet antigen (5′-ACCTAGATAGGTGCGAGCTCACC-3′ and 5′-CAGACTGAGCTTCTCCAGCTTGG-3′; 0.125 μM each) were used for HA-3T amplifications, resulting in a product of 439 bp. For HA-3M amplifications, the human growth hormone-2 control primers 5′-CAGTGCCTTCCCAACCATTCCCTTA-3′ and 5′-ATCCACTCACGGATTTCTGTTGTGTTTC-3′ were used to amplify a product of 504 bp.
Class I MHC peptide-binding affinity assay
Streptolysin-O peptide transport assay
In vitro assays of TAP-mediated peptide transport were performed as previously described,25 with modifications. The T × B cell hybrids T1 and T2, which are positive and negative for TAP, respectively, were obtained from Dr Peter Cresswell (Yale University, New Haven, CT). T1 cells (1 × 106/sample) were permeabilized on ice for 15 minutes with streptolysin O (15 U/mL; Murex Diagnostics, Norcross, GA) and incubated for 5 minutes at 37°C with 100 ng of the iodinated reporter peptide TVNKTERAY,26 10 μL 100 mM adenosine triphosphate (ATP), and indicated dilutions of competitor peptides. The reporter peptide contains an N-linked glycosylation site (Asn-Xaa-Thr/Ser) and will become glycosylated after translocation by TAP into the endoplasmic reticulum (ER). Glycosylated reporter peptide was isolated using Con A Sepharose (Pharmacia Biotech AB, Uppsala, Sweden), eluted with 0.2 M methyl α-d-mannopyranoside (Sigma, St Louis, MO), and quantitated on a gamma counter. Reporter peptide transport in TAP-negative T2 cells was assessed as a negative control. Samples were done in duplicate; the T2 negative control and T1 cells with no inhibitor were done in triplicate.
Proteasomal digestion prediction
Proteasomal cleavage prediction of the HA-3T and HA-3M alleles was performed using the programs NetChop (http://www.cbs.dtu.dk/services/NetChop/)27 and PAProc (http://www.paproc.de/).28,29 NetChop analyses were performed with the C-term 2.0 network and a threshold of 0.5. Analyses with PAProc were performed with the human type III algorithm. For both programs input sequences were the HA-3 35-mers SLSSGDAVLQRDLVTEPGTAQYSSGGELGGISTTN (HA-3T) and SLSSGDAVLQRDLVMEPGTAQYSSGGELGGISTTN (HA-3M).
Peptide digestion assays
The 20S proteasomes from HeLa, and the EBV-BLCL JY and ROF were prepared as described.30 The purity of the proteasome preparations, checked by Coomassie-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), was more than 95%. Aliquots were frozen at -80°C until use. To determine proteasome-mediated cleavage of the HA-3 25-mers DAVLQRDLVTEPGTAQYSSGGELGG and DAVLQRDLVMEPGTAQYSSGGELGG, 15 μg of HPLC-purified polypeptide and 1 μg of purified 20S proteasomes were incubated in 100 μL assay buffer (20 mM HEPES/KOH, pH 7.8; 2 mM Mg acetate; 5 mM dithiotreitol [DTT]) at 37°C for 1, 2, 4, 8, 24, 48, and 72 hours. Samples were subsequently frozen. Electrospray ionization mass spectrometry was performed on a hybrid quadrupole time-of-flight mass spectrometer (Q-TOF; Micromass, Manchester, United Kingdom) equipped with an on-line nano-electrospray interface with an approximate flow rate of 250 nL/min. This flow rate was obtained by splitting of the 0.4 μL/minute flow of a conventional high-pressure gradient system, using an Acurate flow splitter (LC Packings, Amsterdam, The Netherlands).
Injections were done with a dedicated micro/nano HPLC autosampler (FAMOS; LC Packings). Before mass analysis, the digestions were desalted on a C18-precolumn (300 μm × 5 mm) and block-eluted into the mass spectrometer. Mass spectra were recorded from 50 to 2000 Da. The peptides were identified by their molecular masses calculated from the mass/charge (m/z) peaks of the single- or multiple-charged ions. Additionally, the sequence of some proteasome-formed fragments were confirmed by MS/MS.
Proteasome inhibition assay
EBV-BLCL HoRe were pretreated with proteasome inhibitor (PSI) N-benzyl-oxycarbonyl-Ile-Glu(O-tert-butyl)-Ala-leucinal (5 μM) for 16 hours. After labeling with 51Cr and washing, EBV-BLCL and 5Ho11 T cells were seeded in a 96-well round-bottom plate (E/T = 40:1). To control for toxic effects of PSI, HA-3T peptide (10 μg/mL) was added exogenously to PSI-pretreated cells.
Results
Mass spectrometric identification of the HA-3 epitope
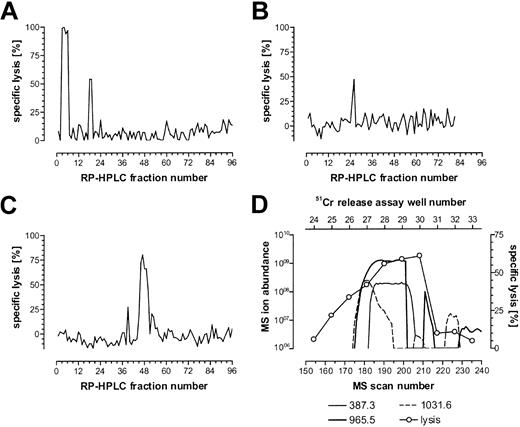
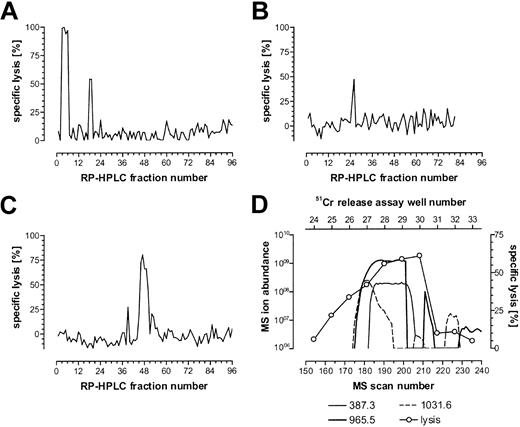
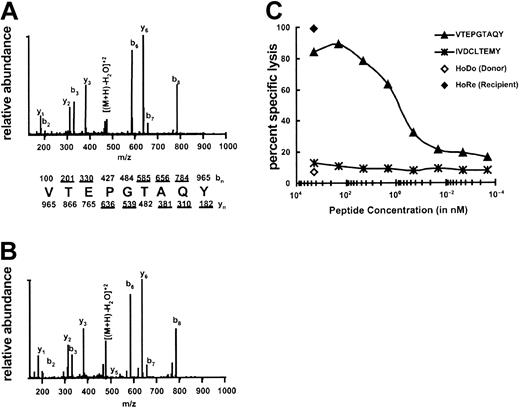
The CTL recognition of the minor H antigen HA-3 is HLA-A*0101 restricted. Therefore, HLA-A1–associated peptides were purified from the HA-3+ HLA-A1+ EBV-BLCL Rp and fractionated by RP-HPLC. Fractions were analyzed for their ability to reconstitute the HA-3 epitope using the HA-3–specific CTL clone 5Ho11 derived from HoRe as effector cells and HA-3- target cells derived from the HLA-identical sibling donor HoDo. Fractions recognized by the relevant CTLs were pooled and carried forward into another round of RP-HPLC under different conditions. Single peaks of reconstitution were observed through 3 rounds of fractionation (Figure 1A-C). Candidate masses for the minor H antigen HA-3 were identified by an online effluent splitter analysis of the third-dimension active fractions using a combination of nanoflow liquid chromatography with ESI on an FTMS.13 By comparing the abundance of peptide ions in spectra from wells that showed epitope reconstituting activity with HA-3 CTLs, 3 candidate peptides were identified (Figure 1D).
Reconstitution of the minor H antigen HA-3 with HPLC-fractionated peptides extracted from HLA-A*0101 molecules. HLA-A*0101–associated peptides were purified from 5 × 1010 Rp EBV-BLCLs and fractionated by RP-HPLC as described in “Patient, materials, and methods.” Aliquots of each fraction (corresponding to 4.5 × 109, 4 × 109, and 8 × 109 cell equivalents for each of the 3 respective dimensions of epitope reconstitution shown in panels A-C) were preincubated with 51Cr-labeled HoDo cells and tested for their ability to reconstitute epitope activity for the HA-3 CTL clone 5Ho11. An E/T ratio of 17:1 was used. (A) First-dimension separation of extracted peptides was achieved using TFA as the ion-pairing agent. The peak in fractions 18 and 19 is biologically active, while the peak in fractions 3 to 6 is due to the acetic acid in the peptide extract sample. (B) Fractions 18 and 19 from panel A were pooled and rechromatographed using HFBA as the ion-pairing agent. (C) Fractions 25 and 26 from panel B were pooled and rechromatographed on a microcapillary column using TFA as the ion-pairing agent. (D) Determination of candidate peptides via mass spectrometry correlated with 51Cr-release assay. Fractions 46 to 48 were pooled and chromatographed using nanoflow effluent splitter technology, and aliquots of each splitter fraction corresponding to 8 × 109 cell equivalents were incubated with 51Cr-labeled HoDo target cells and tested for their ability to reconstitute epitope activity as described in “Patient, materials, and methods.” Ion abundances of candidate masses within the MS scan window 155-215 were plotted and correlated to the percent specific 51Cr release in that same region. Background lysis of HoDo by the CTLs in the absence of any peptides was 13% in panelA, -3% in panel B, 5% in panel C, and -2% in panel D. Positive control lysis was 70% in panel A, 51% in panel B, 88% in panel C, and 60% in panel D.
Reconstitution of the minor H antigen HA-3 with HPLC-fractionated peptides extracted from HLA-A*0101 molecules. HLA-A*0101–associated peptides were purified from 5 × 1010 Rp EBV-BLCLs and fractionated by RP-HPLC as described in “Patient, materials, and methods.” Aliquots of each fraction (corresponding to 4.5 × 109, 4 × 109, and 8 × 109 cell equivalents for each of the 3 respective dimensions of epitope reconstitution shown in panels A-C) were preincubated with 51Cr-labeled HoDo cells and tested for their ability to reconstitute epitope activity for the HA-3 CTL clone 5Ho11. An E/T ratio of 17:1 was used. (A) First-dimension separation of extracted peptides was achieved using TFA as the ion-pairing agent. The peak in fractions 18 and 19 is biologically active, while the peak in fractions 3 to 6 is due to the acetic acid in the peptide extract sample. (B) Fractions 18 and 19 from panel A were pooled and rechromatographed using HFBA as the ion-pairing agent. (C) Fractions 25 and 26 from panel B were pooled and rechromatographed on a microcapillary column using TFA as the ion-pairing agent. (D) Determination of candidate peptides via mass spectrometry correlated with 51Cr-release assay. Fractions 46 to 48 were pooled and chromatographed using nanoflow effluent splitter technology, and aliquots of each splitter fraction corresponding to 8 × 109 cell equivalents were incubated with 51Cr-labeled HoDo target cells and tested for their ability to reconstitute epitope activity as described in “Patient, materials, and methods.” Ion abundances of candidate masses within the MS scan window 155-215 were plotted and correlated to the percent specific 51Cr release in that same region. Background lysis of HoDo by the CTLs in the absence of any peptides was 13% in panelA, -3% in panel B, 5% in panel C, and -2% in panel D. Positive control lysis was 70% in panel A, 51% in panel B, 88% in panel C, and 60% in panel D.
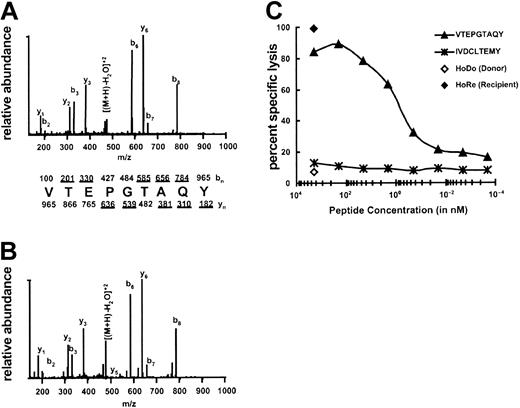
The most abundant candidate ion (m/z 965.5+1, m/z 483.5+2) was targeted for MS/MS analysis on the ion trap mass spectrometer (LCQ), and the peptide sequence was determined to be VTEPGTAQY (Figure 2A-B). The experimental MS/MS spectra were compared with the MS/MS spectra of the synthetic peptide of the same sequence to ensure that the peptides matched. The synthetic peptide was also coeluted with the sample to further ensure that the peptides were identical (data not shown). To determine whether VTEPGTAQY represented HA-3, the peptide was tested for its ability to sensitize HoDo target cells lysis by HA-3 CTLs. Target cells pulsed with VTEPGTAQY were lysed by HA-3 CTLs, with half-maximal activity seen at a peptide concentration of 1 nM (Figure 2C). Thus, the peptide VTEPGTAQY represents the HLA-A1–restricted HA-3 epitope.
Identification of the minor H peptide HA-3. (A) CAD mass spectrum of candidate peptide (M+2H)2+ ion with monoisotopic m/z of 965.5 as eluted from Rp EBV-BLCL. (B) CAD mass spectrum of synthetic peptide VTEPGTAQY. Mass spectra were recorded on a Finnigan LCQ ion trap MS operating with a 3.0 atomic mass unit isolation window and 35% collision energy. The b and y ions are labeled above and below the amino acid sequence, respectively. Ions observed in the spectrum are underlined. (C) Minor H antigen HA-3 epitope reconstitution with synthetic peptides. A standard 51Cr-release assay was performed by incubating the indicated quantities of synthetic peptides with 51Cr-labeled HoDo target cells and then adding HA-3–specific CTLs. An E/T ratio of 17:1 was used. IVDCLTEMY corresponds to the minor H antigen A1-HY13 , and serves as a negative control. Background lysis of HoDo by the CTLs in the absence of any peptides was 7%; positive control lysis was 99%.
Identification of the minor H peptide HA-3. (A) CAD mass spectrum of candidate peptide (M+2H)2+ ion with monoisotopic m/z of 965.5 as eluted from Rp EBV-BLCL. (B) CAD mass spectrum of synthetic peptide VTEPGTAQY. Mass spectra were recorded on a Finnigan LCQ ion trap MS operating with a 3.0 atomic mass unit isolation window and 35% collision energy. The b and y ions are labeled above and below the amino acid sequence, respectively. Ions observed in the spectrum are underlined. (C) Minor H antigen HA-3 epitope reconstitution with synthetic peptides. A standard 51Cr-release assay was performed by incubating the indicated quantities of synthetic peptides with 51Cr-labeled HoDo target cells and then adding HA-3–specific CTLs. An E/T ratio of 17:1 was used. IVDCLTEMY corresponds to the minor H antigen A1-HY13 , and serves as a negative control. Background lysis of HoDo by the CTLs in the absence of any peptides was 7%; positive control lysis was 99%.
The HA-3 epitope is encoded by the polymorphic Lbc oncogene
A search of known protein and DNA sequence databases with the sequence VTEPGTAQY identified a single precise match with residues 451-459 of the predicted protein sequence of the guanine nucleotide exchange factor Lbc (GenBank accession no. AB055890), which maps to chromosome 15 region q24-q25.31,32
To elucidate the basis for the differential phenotypic expression of HA-3 in the population, we next searched the GenBank DNA and protein databases with the entire Lbc sequence and identified a homolog, isoform 2 of AKAP13 (GenBank accession no. NM_007200). AKAP13 isoform 2 exhibits 99.6% identity to Lbc over 2813 amino acid residues, but it is predicted to encode VMEPGTAQY instead of VTEPGTAQY. These results were consistent with the hypothesis that Lbc and AKAP13 isoform 2 are alleles of the same genetic locus and that these alleles encode the HA-3 epitope and its counterpart in HA-3- cells, respectively.
To provide additional support for the hypothesis that Lbc and AKAP13 isoform 2 are alleles of a genetic locus encoding HA-3, RT-PCR primers that amplified a 341-bp cDNA product surrounding the epitope were generated from sequences that were identical between Lbc and AKAP13 isoform 2. Sequences identical to this segment of the Lbc gene were amplified from all 4 EBV-BLCLs that were recognized by HA-3 CTLs (Table 1). Of 5 RT-PCR products amplified from the 5 EBV-BLCLs that were not recognized by HA-3 CTLs, all were identical to AKAP13 isoform 2 sequence.
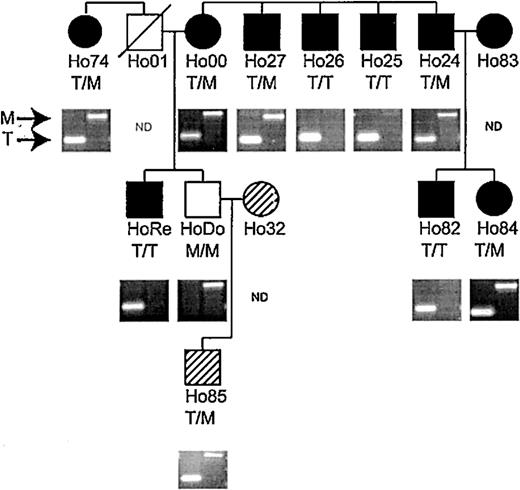
In addition, we used an allele-specific PCR assay that distinguishes between the nucleotide difference at position 1568 in the Lbc and AKAP-13 cDNA sequences to genotype all individuals in 3 consecutive generations of a family with 11 HLA-A1+ members that had been previously phenotyped for HA-3 using the HA-3 CTL clone 5Ho11. EBV-BLCLs derived from each individual in the Ho family were tested for HA-3 expression with HA-3 CTLs (Figure 3). Only the M-encoding gene sequence (identical to AKAP-13 isoform 2) was amplified from the single HA-3- member of this family, while HA-3+ family members were typed as either homozygous for the T-encoding sequence (identical to Lbc) or heterozygous for both. The exact correlation between RT-PCR and HA-3 CTL typing supports the hypothesis that the Lbc gene encodes HA-3, while the pattern of inheritance supports the hypothesis that AKAP13 isoform 2 is an allelic homolog. The HA-3 peptide and its allelic counterpart encoded by Lbc and AKAP13 isoform 2 were designated HA-3T and HA-3M, respectively.
HA-3 phenotyping and genotyping of family Ho. All members except Ho32 and Ho85 (hatched symbols) are HLA-A1 positive. • (females) or ▪ (males) indicate strong lysis by HA-3–specific CTLs (phenotype positive); open symbols indicate no lysis (phenotype negative). Hatched symbols indicate HLA-A*0101- individuals, which were not typed phenotypically. Material from Ho01 was not available. Genotyping was determined by PCR on genomic DNA as described in “Patient, materials, and methods.” A band in the left lane indicates the presence of the HA-3T allele, while the HA-3M allele is represented by a band in the right lane. The results of the genotyping are show as T/T (HA-3T homozygous), T/M (HA-3T/M heterozygous), or M/M (HA-3M homozygous).
HA-3 phenotyping and genotyping of family Ho. All members except Ho32 and Ho85 (hatched symbols) are HLA-A1 positive. • (females) or ▪ (males) indicate strong lysis by HA-3–specific CTLs (phenotype positive); open symbols indicate no lysis (phenotype negative). Hatched symbols indicate HLA-A*0101- individuals, which were not typed phenotypically. Material from Ho01 was not available. Genotyping was determined by PCR on genomic DNA as described in “Patient, materials, and methods.” A band in the left lane indicates the presence of the HA-3T allele, while the HA-3M allele is represented by a band in the right lane. The results of the genotyping are show as T/T (HA-3T homozygous), T/M (HA-3T/M heterozygous), or M/M (HA-3M homozygous).
The gene encoding HA-3 contains multiple amino acid polymorphisms
To determine the degree of amino acid polymorphism between AKAP13 isoform 2 and Lbc, the full GenBank-derived sequences were aligned (Figure 4). In this way, 11 nonsynonymous mutations leading to amino acid polymorphisms besides the HA-3 epitope-encoding sequence were identified. Seven of these polymorphisms were confirmed in a sequence analysis of a 3347-bp fragment of the gene encoding HA-3 from cDNA of HA-3+ and HA-3- individuals (data not shown). Four other amino acid differences between the Lbc and AKAP13 reported in GenBank could not be confirmed by the present sequence analysis.
Amino acid sequence alignment of the predicted amino acid sequence of Lbc and AKAP13 isoform 2. Polymorphisms confirmed by analysis of PCR products from typed individuals in the current study are indicated by bold underlined characters. The HA-3 CTL epitope is marked by a box. Bold amino acids that are not underlined represent polymorphisms reported in databases but not confirmed by the current analysis.
Amino acid sequence alignment of the predicted amino acid sequence of Lbc and AKAP13 isoform 2. Polymorphisms confirmed by analysis of PCR products from typed individuals in the current study are indicated by bold underlined characters. The HA-3 CTL epitope is marked by a box. Bold amino acids that are not underlined represent polymorphisms reported in databases but not confirmed by the current analysis.
Tissue-specific expression of HA-3
Previous analyses showed recognition of cells of hematopoietic origin, fibroblasts, keratinocytes, melanocytes, PTECs, and HUVECs by HA-3–specific CTLs.16 To confirm these data at the mRNA level, an epitope-spanning (set 1) and an intron-spanning (set 2) RT-PCR was developed. As shown in Figure 5, all cell lines tested express Lbc/AKAP-13 mRNA. These results confirm the CTL recognition analyses and are in concordance with the previous finding that Lbc and AKAP13 exhibit a wide tissue distribution.33,34
Tissue distribution of Lbc Expression of Lbc/AKAP13 was analyzed in various cell types using epitope spanning primers (HA-3, set 1) and intron spanning primers (HA-3, set 2) as described in “Patient, materials, and methods.” GAPDH-specific primers were used as positive control. A sample without DNA (–) functioned as negative control.
Tissue distribution of Lbc Expression of Lbc/AKAP13 was analyzed in various cell types using epitope spanning primers (HA-3, set 1) and intron spanning primers (HA-3, set 2) as described in “Patient, materials, and methods.” GAPDH-specific primers were used as positive control. A sample without DNA (–) functioned as negative control.
Effect of HA-3 polymorphism on HLA-A*0101 binding and CTL recognition
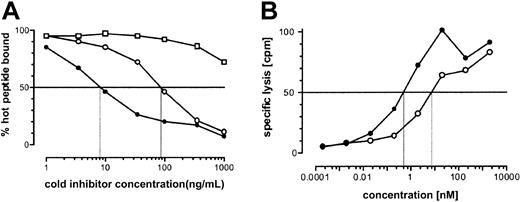
To gain insight into the mechanisms governing the lack of CTL recognition of the cells of HA-3- individuals, we tested the hypothesis that the substitution of Met for Thr at P2 in VTEPGTAQY had a detrimental effect on binding to HLA-A*0101. Using a quantitative, cell-free peptide binding assay, we determined that VTEPGTAQY half-maximally inhibited the binding of an iodinated indicator peptide to HLA-A*0101 at a concentration of 10 nM, while comparable inhibition by VMEPGTAQY required 10-fold more peptide (Figure 6A). Thus, substitution of Met for Thr at P2 of HA-3 reduces peptide binding, but this modest effect appeared unlikely to account for the complete lack of recognition of HA-3- cells. We next compared the ability of the 2 polymorphic peptides to reconstitute the epitope for the HA-3 CTL clone when pulsed exogenously onto HoDo target cells (Figure 6B). HA-3 CTL recognition of VMEPGTAQY required only 7-fold more peptide (half-maximal lysis of 5 nM) than that of VTEPGTAQY (half-maximal lysis of 0.7 nM). Taking into account the 10-fold difference in binding affinity, this suggests that HA-3 CTLs recognize VMEPGTAQY and VTEPGTAQY comparably when both are presented at the cell surface. These results suggested that differences in MHC binding and CTL recognition do not account for the failure of HA-3 CTLs to recognize cells that are homozygous for HA-3M, and that differences in antigen processing might instead be responsible.
Binding of HA-3T and HA-3M peptides to HLA-A*0101 and recognition by HA-3–specific T cells. (A) Binding of VTEPGTAQY (•) and VMEPGTAQY (○)to HLA-A*0101. HPLC-purified synthetic peptides were assayed for their ability to inhibit the binding of the iodinated peptide YTAVVPLVY to affinity-purified HLA-A*0101 molecules in a cell-free peptide binding assay (see “Patient, materials, and methods”). The HLA-A*0201–binding peptide IP30 (□) was used as negative control. (B) VTEPGTAQY (•) and VMEPGTAQY (○) were tested for their ability to reconstitute the epitope for the HA-3 CTL clone. Epitope reconstitution assay conditions are described in “Patient, materials, and methods.” An E/T ratio of 17:1 was used. Background CTL lysis in the absence of any peptide was 17%. Lysis of HoRe EBV-BLCL was 95%.
Binding of HA-3T and HA-3M peptides to HLA-A*0101 and recognition by HA-3–specific T cells. (A) Binding of VTEPGTAQY (•) and VMEPGTAQY (○)to HLA-A*0101. HPLC-purified synthetic peptides were assayed for their ability to inhibit the binding of the iodinated peptide YTAVVPLVY to affinity-purified HLA-A*0101 molecules in a cell-free peptide binding assay (see “Patient, materials, and methods”). The HLA-A*0201–binding peptide IP30 (□) was used as negative control. (B) VTEPGTAQY (•) and VMEPGTAQY (○) were tested for their ability to reconstitute the epitope for the HA-3 CTL clone. Epitope reconstitution assay conditions are described in “Patient, materials, and methods.” An E/T ratio of 17:1 was used. Background CTL lysis in the absence of any peptide was 17%. Lysis of HoRe EBV-BLCL was 95%.
Effect of the HA-3 polymorphism on cell surface presentation
To directly assess whether the HA-3T and HA-3M peptides are differentially presented at the surface of HA-3+ and HA-3- cells, respectively, HLA-A1–associated peptides were extracted from either Rp (HA-3T/T homozygous) or HoDo (HA-3M/M homozygous) cells and separated by HPLC. Fractions that could have contained either HA-3T or HA-3M peptides were identified based on the elution position of synthetic peptides in parallel HPLC runs, and these fractions were analyzed by mass spectrometry to identify peptide ions m/z of 965.5+1 and 995.5+1, corresponding to the +1 ions of HA-3T and HA-3M, respectively. The m/z 965.5+1 ion corresponding to naturally processed HA-3T was identified and targeted for MS/MS analysis. By comparing the magnitudes of the ion current of several fragment ions from naturally processed HA-3T with those of a known quantity of synthetic material, we calculated that HA-3T was present in the Rp peptide sample in an amount corresponding to approximately 640 copies per cell (data not shown). However, no naturally processed HA-3M, corresponding to the m/z 995.5+1 ion, could be detected in the extracts from HA-3M homozygous HoDo EBV-BLCL above a detection limit of 5 copies/cell (data not shown). We also found no evidence of masses corresponding to HA-3M with an oxidized Met residue. We conclude that HA-3M peptide was present at fewer than 5 copies per cell on the surface of HoDo EBV-BLCL, despite the expression of mRNA encoding HA-3M in this cell.
TAP translocation of the HA-3 locus peptides
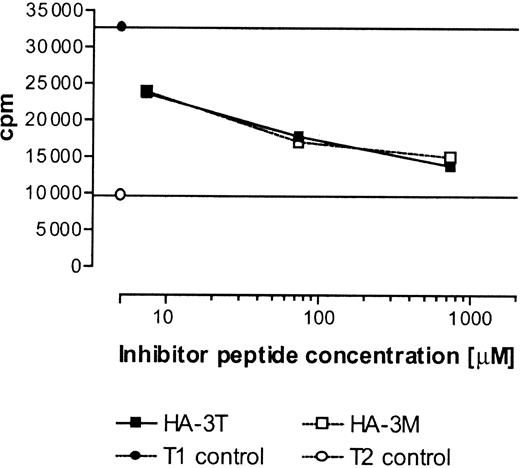
To gain additional insight into the failure of HA-3M to be presented at the cell surface, we compared the ability of HA-3T and HA-3M to inhibit TAP-dependent transport of the radiolabeled reporter peptide TVNKTERAY in streptolysin O–permeabilized T1 cells. Both HA-3 peptides were transported equally well (Figure 7). While it is possible that the actual substrates for TAP transport may correspond to precursor peptides rather than the mature 9-mer epitope, these data suggest that factors other than TAP transport determine the differential expression of the HA-3T and HA-3M peptides in association with HLA-A1.
In vitro binding of HA-3T and HA-3M peptides to TAP. T1 cells were permeabilized with streptolysin O (15 U/mL) and incubated with radioiodinated reporter peptide TVNKTERAY plus the indicated concentration of test peptides. Reporter peptide binding in TAP-negative T2 cells was assessed as a negative control. Samples were done in duplicate except for T2 negative control and T1 cells with no inhibitor, which were done in triplicate.
In vitro binding of HA-3T and HA-3M peptides to TAP. T1 cells were permeabilized with streptolysin O (15 U/mL) and incubated with radioiodinated reporter peptide TVNKTERAY plus the indicated concentration of test peptides. Reporter peptide binding in TAP-negative T2 cells was assessed as a negative control. Samples were done in duplicate except for T2 negative control and T1 cells with no inhibitor, which were done in triplicate.
Proteasome-mediated digestion of the HA-3 locus products
One possible explanation for the differential cell-surface expression of HA-3T and HA-3M is that the HA-3M peptide is not appropriately generated by the proteasome. When 35-mer peptides centered on the HA-3T and HA-3M sequences were analyzed with the NetChop27 and PAProc29 proteasomal cleavage prediction algorithms, only a few cleavage sites were predicted within the 9-mer epitope (Table 2). Correct C-terminal cleavage after the Tyr at P9 in the epitope, a prerequisite for proper peptide generation, is highly predicted by both programs. However, both programs predicted a strong cleavage preference after the Met at position P2 in the HA-3M sequence (P = .56 in NetChop, +++ in PAProc), while no cleavage is predicted after the Thr at the same position in HA-3T (P = .008 in NetChop, negative in PAProc).
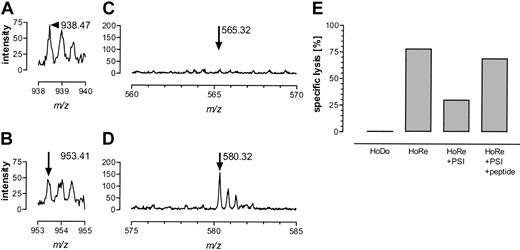
To test this cleavage prediction analysis directly, HPLC-purified 25-mers were digested with proteasomes purified from EBV-BLCL JY and ROF, representing mainly immunoproteasomes, and with constitutive proteasomes derived from HeLa cells. The resulting cleavage products were analyzed by mass spectrometry. Although cleavage of both 25-mers was reproducibly low, both constitutive proteasomes and immunoproteasomes generated correct C-termini to create HA-3T and HA-3M, yielding peaks at m/z 938.46+2 for DAVLQRDLVTEPGTAQY and m/z 953.41+2 for DAVLQRDLVMEPGTAQY, as shown for the J-Y–derived proteasomes in Figure 8A-B. A peptide ion with m/z = 580.32+2 was detected in all HA-3M peptide digestions after one hour (Figure 8D). MS/MS analysis confirmed its sequence as DAVLQRDLVM (data not shown). This indicates that proteasomes are able to cleave and destroy a potential HA-3M peptide. No peptide was observed that matched the analogous HA-3T–derived peptide DAVLQRDLVT (Figure 8C, m/z = 565.32+2), demonstrating the inability of proteasomes to cleave and destroy the HA-3T peptide at this position. Results for HeLa-derived constitutive proteasomes were identical to those obtained with JY immunoproteasomes and prolonged incubation up to 72 hours yielded similar results for all proteasomal preparations. PSI pretreatment induced a significant inhibitory effect on antigen presentation of HA-3 by HoRe EBV-BLCL (Figure 8E). Target lysis could completely be restored by addition of exogenous HA-3T peptide, showing that PSI had no toxic effect on the EBV-BLCL target cells. Collectively, these results indicate that the failure of HA-3M to be expressed at the cell surface in association with HLA-A1 is due to its destruction by proteasomes.
Proteasome-mediated digestion of HA-3T and HA-3M. The 25-mer HA-3 peptides were cleaved with J-Y–derived immunoproteasomes as described in “Patient, materials, and methods.” Correct C-terminal cleavage was observed for both HA-3T (panel A, m/z 938.46+2) and HA-3M (panel B, m/z 953.41+2). Arrows indicate the correct cleavage product. Destruction of the HA-3T peptide could not be observed, as no peptide ion with m/z 565.32+2 (DAVLQRDLVT) was detectable (C). The HA-3M peptide was cleaved behind P2 of the HA-3 9-mer as indicated by the presence of DAVLQRDLVM (panel D, m/z 580.322+). (E) Proteasome inhibitor PSI was able to inhibit antigen-specific lysis of HA-3+ EBV-BLCL HoRe significantly. Target cell lysis was restored after adding exogenous HA-3T peptide.
Proteasome-mediated digestion of HA-3T and HA-3M. The 25-mer HA-3 peptides were cleaved with J-Y–derived immunoproteasomes as described in “Patient, materials, and methods.” Correct C-terminal cleavage was observed for both HA-3T (panel A, m/z 938.46+2) and HA-3M (panel B, m/z 953.41+2). Arrows indicate the correct cleavage product. Destruction of the HA-3T peptide could not be observed, as no peptide ion with m/z 565.32+2 (DAVLQRDLVT) was detectable (C). The HA-3M peptide was cleaved behind P2 of the HA-3 9-mer as indicated by the presence of DAVLQRDLVM (panel D, m/z 580.322+). (E) Proteasome inhibitor PSI was able to inhibit antigen-specific lysis of HA-3+ EBV-BLCL HoRe significantly. Target cell lysis was restored after adding exogenous HA-3T peptide.
Discussion
In this study we have identified the peptide sequence of the minor H antigen HA-3 as VTEPGTAQY. This sequence is encoded by the Lbc gene located on chromosome 15q24-q25. The HA-3- allelic counterpart VMEPGTAQY differs from the HA-3 antigen by a single amino acid Met at P2 and is encoded by the gene identified as AKAP13 isoform 2. We here demonstrate that Lbc and AKAP13 are 2 alleles of the same gene. At least 3 variant transcripts are derived from Lbc, including the lymphoid blast crisis oncogene onco-Lbc35,36 and the protooncogene proto-Lbc,31 the splice variant Brx, which is expressed in testis and estrogen-sensitive tissues,37 and the largest splice variant AKAP-Lbc,34 also designated Ht31,32 which functions as a protein kinase A anchoring protein. The AKAP-Lbc transcript is the only transcript of Lbc that encodes the HA-3 sequence.
It is of particular interest that the minor H antigen HA-3 is encoded by the lymphoblast crisis oncogene Lbc, a gene with transforming activity that was identified using CML cells.31,35 HA-3 is, to our knowledge, the first identified oncogene-encoded T-cell epitope potentially involved in GVH alloresponses after HLA-identical SCT. The HA-3–specific T cells were isolated after HLA-identical SCT from an AML patient.38 The patient suffered from GVHD grade II, which was treated with prednisone. At present, 20 years after SCT, the patient is in good clinical condition. As yet, a causal relationship between these clinical results and the isolation of CTLs specific for the Lbc-encoded minor H antigen HA-3 cannot be drawn. The role of this oncogene-encoded minor H antigen in GVHD and GVL is currently under investigation.
The genes that encode the autosomal human minor H antigens identified to date, display only 1 or 2 nonsynonymous single nucleotide polymorphism (SNP) per gene (http://www.ncbi.nlm.nih.gov/SNP/). Interestingly, comparison of the sequences of AKAP13 isoform 2 with Lbc and the Lbc/AKAP13-related PCR products demonstrated the existence of at least 6 other nonsynonymous SNPs in addition to the one giving rise to HA-3. These additional polymorphisms may well encode for immunogenic peptides that bind to other HLA class I alleles and thus increase the importance of HA-3 disparity with regard to GVH activities. Moreover, HA-3 may contain polymorphic HLA class II–binding peptides. Indeed, in human SCT, the presence of CD4+ T cells has been demonstrated to correlate with GVHD.39,40 Therefore, the HA-3–encoding Lbc gene is an interesting target to study the molecular basis of the role of CD4+ T cells in GVHD and GVL.
Previous work has established that allelic amino acid sequence differences between minor H peptides and their homologs can lead to a failure in T-cell recognition for several reasons, including failure to be recognized by the T-cell receptor,11,13,14 failure to form stable HLA-peptide complexes, and failure to bind to TAP.10 In the present work, we demonstrate that the HA-3–specific CTL clone recognizes HA-3M very well when this peptide is added exogenously. In addition, although its binding to HLA-A*0101 is only 10-fold lower than that of HA-3T, it is not detected on the cell surface. The HA-3M peptide is therefore not an endogenously produced self-peptide that can be recognized by autologous T cells in the context of HLA-A1. On the basis of the proteasomal digestion results and the elution/mass spectrometric detection experiments of peptides from HA-3- individuals, we conclude that HA-3- individuals do not present any HA-3M epitope at all. The failure to present the negative allelic counterpart of a minor H peptide, despite its ability to bind to relevant MHC molecules and be recognized by T cells in vitro, has been observed in 2 other minor H systems: HA-2 and HA-8.10,41 Thus, these minor H antigen disparities likely exist because of differential antigen processing of immunologically similar peptides. In the case of the HLA-A2–restricted minor H antigen HA-8, it was shown that minigene products encoding either the antigen or its allelic counterpart were recognized similarly if they were expressed directly in the ER, but not if they were expressed in the cytosol.10 In vitro experiments demonstrated that peptides containing the HA-8R sequence bound to TAP more efficiently than those containing the negative counterpart. In contrast, our results show no measurable difference between HA-3T and HA-3M regarding TAP binding, ruling out differential TAP translocation as the factor responsible for the immunogenicity of HA-3. Instead, our data suggest that the differential display of HA-3T and HA-3M by HLA-A1 arises from differential cleavage by the proteasome.
Differential proteasome–mediated cleavage was not only predicted by 2 different proteasomal digestion algorithms, but was confirmed by in vitro digestion. Analysis of the relevant HA-3 25-mers predicted that substitution in HA-3 of Thr→Met would facilitate cleavage after the Met, resulting in epitope destruction, which was confirmed by the in vitro digest. Differential cleavage was observed both with immunoproteasomes and constitutive proteasomes. As a consequence, the HA-3 epitope VTEPGTAQY remains intact and can be presented at the cell surface. The negative counterpart VMEPGTAQY, however, is destroyed by proteasome-mediated cleavage and therefore cannot be expressed in the context of HLA-A1 on the cell surface, thus prohibiting cellular recognition. It is particularly interesting that we were unable to identify the fully processed VTEPGTAQY peptide after in vitro proteasome-mediated digestion, either by mass spectrometry or cellular assays. It has been well established in other systems (reviewed in Rock and Goldberg42 and Shastri et al43 ) that N-terminal trimming of precursors, either in the cytosol or in the ER, may be required to complete processing of 9-mer peptide epitopes. This mechanism does not take place in our in vitro assays but presumably does in vivo. Similar differential proteasome–mediated digestion has been reported in different murine systems.44-46 The present study reports the first demonstration of this mechanism for the generation of minor H antigens.
In conclusion, the biochemical identification of HA-3 led to a full analysis of processes that are potentially involved in causing antigenic disparity. Our data demonstrate that the immunogenicity of human minor H antigens can be the result of differential proteasome–mediated digestion. HA-3T represents the first minor H antigen that has been shown to escape from proteasome-mediated cleavage due to a single amino acid difference from its allelic counterpart. Thus, this study demonstrated that differential proteasome–mediated digestion represents an important mechanism in the generation of minor H antigens into immunogenic peptides.
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2003-01-0260.
Supported by National Institutes of Health (NIH) grants AI44134 and AI20963 (V.H.E.), AI 33993 (D.F.H.), by a grant from the J. A. Cohen Institute for Radiopathology and Radiation Protection (E.G.), and by a grant from the Leiden University Medical Center (E.G.). A.G.B. was a Kirby Foundation Postdoctoral Fellow of the American Cancer Society.
E.S., A.G.B., and J.A.C. made equal contributions to this work and the order of their listing should be considered arbitrary.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Drs T. Mutis and C. J. M. Melief for critically reading the manuscript and C. Vermeulen and J. Kessler for technical support with the proteasome inhibition assay.