Abstract
Bone marrow (BM) fibrosis is a central diagnostic and pathogenetic feature of hairy-cell leukemia (HCL). It is known that fibronectin (FN) produced and assembled by the malignant hairy cells (HCs) themselves is a major component of this fibrosis. It is also known that FN production is greatly enhanced by adhesion of HCs to hyaluronan (HA) via CD44. The aim of the present study was to establish the roles of fibrogenic autocrine cytokines (fibroblast growth factor-2 [FGF-2] and transforming growth factor β [TGFβ]) and of different isoforms of CD44 in this FN production. We show that HC adhesion to HA stimulates FGF-2, but not TGFβ, production and that HCs possess FGF-2 receptor. In a range of experiments, FN production was greatly reduced by blocking FGF-2 but not TGFβ. Moreover FN, but not FGF-2, secretion was blocked by down-regulation of the v3 isoform of CD44 and by addition of heparitinase. These results show that autocrine FGF-2 secreted by HCs is the principal cytokine responsible for FN production by these cells when cultured on HA. The central role of FGF-2 in the pathogenesis of the BM fibrosis of HCL was supported by our immunohistochemical demonstration of large amounts of this cytokine in fibrotic BM but not in HCL spleen where there is no fibrosis. As regards CD44 isoforms, the present work demonstrates that CD44v3 is essential for providing the heparan sulfate necessary for HC stimulation by FGF-2, whereas the signal for production of the cytokine was provided by HA binding to CD44H, the standard hematopoietic form of the molecule.
Introduction
Fibrosis is a feature of many tumors, including those of hemic origin. An important component of the fibrotic process is the production of extracellular matrix (ECM) by either autocrine or paracrine stimulation of tumor or tissue stromal cells by a range of growth factors.1 Thus, primary myelofibrosis (MF) is the best known example of a hemic malignancy where cytokines produced by malignant myeloid cells are thought to stimulate fibroblasts to proliferate and produce excess ECM, and this process in turn inhibits normal hematopoiesis.2-4 Fibrosis can also be a feature of lymphoid malignancies, but the role of the malignant lymphocytes in the production of this fibrosis is not clear.5-7
Among lymphoid tumors, the diffuse fine bone marrow (BM) fibrosis in hairy-cell leukemia (HCL) is one of the most distinctive appearances in hematopathology and is responsible for the typically inaspirable BM of the disease.8 Previously, we have shown that fibronectin (FN) is a major component of this fibrosis and that the protein is produced and assembled into a matrix by the infiltrating hairy cells (HCs).9 Also, during studies of the adhesive reactions of HCs, we observed that these cells readily adhere not only to FN via integrins but also to another ECM component, hyaluronan (HA), via CD44.10
The pathophysiologic importance of this CD44-HA interaction for fibrosis in HCL was strongly suggested by the demonstration that HA is abundant in the infiltrated BM but absent from splenic red pulp where there is no fibrosis despite heavy infiltration by HCs.8 That the interaction of HCs with HA via CD44 is responsible for FN production was then directly demonstrated in vitro.10 Moreover, previous studies of the pathogenesis of primary myelofibrosis had suggested that this process involves CD44-induced production of fibrogenic cytokines by MF monocytes.11
HCs spontaneously adhere to HA,10 indicating that they may express a CD44 isoform that interacts with HA without the need for cell stimulation. Alternatively, their standard hematologic isoform of CD44 (CD44H, also designated CD44s) may bind HA as a consequence of the constitutive activation that is known to be a feature of HCs.12
The above observations suggest that whatever the isoform of CD44 responsible for HA binding, this stimulates FN production either directly or indirectly through the induction of autocrine cytokines. Indeed, HCs have been shown to produce fibroblast growth factor-2 (FGF-2) (bFGF) and transforming growth factor β (TGFβ),13 2 well-known fibrogenic cytokines.2,14,15
Therefore, the aim of the present study was to examine the production of fibrogenic cytokines by HCs cultured on HA and to determine the relative contribution of these 2 cytokines, versus direct signaling via CD44, for the induction of FN production. We also examined the expression and function of the CD44v3 isoform on HCs because this isoform can bind HA constitutively16 and is also known to act as a coreceptor for FGF-2.17
Patients, materials, and methods
Patients
All patients had typical disease as determined by clinical presentation, cell morphology, tartrate-resistant acid phosphatase staining, and immunophenotype. All had typical diffuse fibrosis of the bone marrow as assessed by silver staining. All samples were studied with informed consent and with the approval of the Liverpool Research Ethics Committee.
HC isolation and purification
Peripheral blood HCs were isolated by Ficoll-Hypaque density-gradient centrifugation (Lymphoprep; Nycomed, Oslo, Norway). Samples were purified either by depletion of T cells and monocytes using magnetic beads (Miltenyi Biotec, Bisley, United Kingdom) coated with antibodies against CD3 and CD14 or by positive enrichment using CD19-coated beads (Miltenyi Biotech). After purification, contaminating cells were less than 1% as measured by monoclonal antibody (mAb) staining and flow cytometry.
Antibodies
Blocking antibodies to FGF-2 (goat polyclonal) and TGFβ (mAb) (both from R & D Systems Europe, Abingdon, United Kingdom) were used in order to inhibit growth-factor function. Polyclonal antibodies (R & D Systems Europe) were used in order to stain tissue for FGF-2 (goat antibody) and TGFβ (chicken antibody). mAbs to CD44v3, CD44v6 (R & D Systems Europe), CD138 (syndecan 1; Becton Dickinson, Cowley, United Kingdom), and FGFR-1 (Abcam, Cambridge, United Kingdom), and a polyclonal antibody to CD44v10 (rabbit antibody; Calbiochem, San Diego, CA) were used to stain cells for these receptors.
Measurement of FGF-2, TGFβ, and FN production by ELISA
Commercial enzyme-linked immunosorbent assays (ELISAs; both from R & D Systems Europe) were used to detect the secretion of FGF-2 and TGFβ by HCs. Briefly, HCs 2 × 109 L (2 × 106/mL) in RPMI containing 0.1% bovine serum albumin (BSA), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Life Technologies, Paisley, United Kingdom) were cultured for 24 hours at 37°C in 5% CO2 in air, on tissue-culture plates that were either uncoated or coated with BSA (1%) or HA (100 μg/mL; ICN, Basingstoke, United Kingdom). Supernatants were harvested from triplicate cultures and assayed according to the manufacturer's protocol. For the TGFβ assay, the supernatants were tested before and after activation using acidification with HCl as specified by the suppliers (R & D Systems Europe). In order to assess the role of CD44v3 in HA-induced FGF-2 production, this receptor was down-regulated by incubating HCs in the presence of anti-CD44v3 mAbs (10 μg/mL).17 The coefficient of variation of the ELISA assays was always less than 5%.
FN production was also measured using an ELISA (Immunodiagnostics [IDS], Baldon, United Kingdom). Briefly, HCs were cultured as described above and, since the FN produced by HCs is either bound to the cells or to the culture dish, FN was harvested using 0.05% Triton X-100 and assayed according to the manufacturer's protocol. Selected cultures were also performed in the presence of anti-CD44v3 mAbs or of heparitinase (5 U/mL; Sigma, St Louis, MO).17
Detection of FGF-2 mRNA by RT-PCR
Total RNA was extracted from approximately 1 × 107 cells cultured on either plastic or on an HA surface using an RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Single-strand cDNA was synthesized from 0.5 μg total RNA using the 1st Strand cDNA Synthesis kit for reverse transcriptase–polymerase chain reaction (RT-PCR; Boehringer Mannheim, Mannheim, Germany). The synthesized cDNA was amplified by PCR using the following FGF-2 primers: 5′-GTGTGTGCTAACCGTTACCT-3′ and 5′-GCTCTTAGCAGACATTGGAAG-3′ (30 cycles, annealing at 58°C). The quality of each cDNA preparation was checked by amplification of the L27 housekeeping gene using the following primers: 5′-GACGCAAAGCTGTCATCGTG-3′ and 5′-GCAGTTTCTGGAAGAACCAC-3′ (30 cycles, annealing at 60°C). The amplified PCR products were subjected to electrophoresis and visualized by ethidium bromide staining.
Immunocyto(histo)chemical staining
For the detection of FN, HCs were cultured on coverslips coated with BSA (1%) on HA (100 μg/mL). Cells were then fixed and stained with an anti-FN mAb (3E2, Sigma) or isotype control (IgG1), followed by biotinylated goat antimouse antibody and ExtrAvidin-peroxidase staining (both from Sigma).
Tissue staining for FGF-2 and TGFβ was performed on paraffin-embedded, formaldehyde-fixed material. HCL tissue was obtained with informed consent from BM trephines performed for diagnostic reasons, while therapeutic splenectomy was the source of the splenic tissue used. The “normal” BM was from diagnostic trephines that proved to be morphologically normal. The “normal” spleen was from patients with immune thrombocytopenic purpura who had undergone therapeutic splenectomy. Staining was performed as follows. After clearing and rehydration, the slides were boiled in 10 mM sodium citrate buffer (pH 6) for 10 minutes and blocked with 10 mg/mL BSA before overnight incubation with goat anti–FGF-2 antibody (0.4 μg/mL) or chicken anti-TGFβ (5 μg/mL). Sections were then incubated with biotinylated rabbit antigoat or goat antichicken antibodies (Vector Laboratories, Burlingame, CA) and then with ExtrAvidin-alkaline phosphatase before exposure to substrate (Fast Red/Naphthol AS-MX phosphate and levamisole; Sigma). The slides were counterstained with hematoxylin.
Since FGFR-1 (the main receptor for FGF-2), CD44v3, CD44v6, CD44v10, and CD138 are often expressed at low levels, a sensitive triple-layer technique was used to detect these receptors. Cells were incubated with mAbs at saturating concentrations followed by biotin-conjugated donkey antimouse or goat antirabbit immunoglobulin (Vector Laboratories) and finally by streptavidin-conjugated phycoerythrin (Becton Dickinson). Staining was analyzed by fluorescence-activated cell sorter (FACS) and compared with that obtained with isotypic control mAbs. Both the percentage of positive cells and the mean fluorescent intensity (MFI) were recorded.
Results
HCs cultured on HA secrete FGF-2
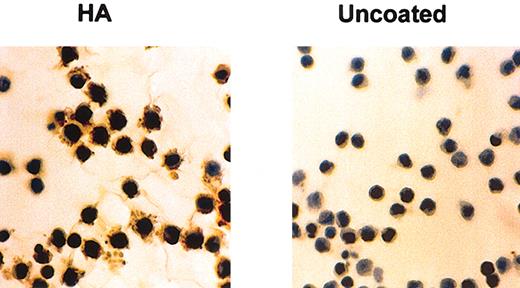
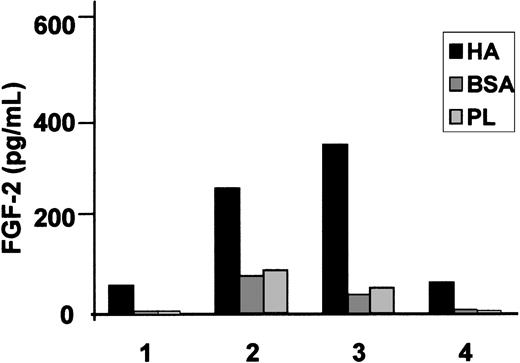
We have already shown that HCs cultured on HA produce substantial amounts of FN after 24 hours of culture (Figure 1 and Aziz et al10 ). In contrast, HCs cultured on BSA-coated or uncoated plastic produced little or no FN (Figure 1). To test whether this increased FN production is mediated by the fibrogenic cytokines implicated in the pathogenesis of HCL we measured FGF-2 and TGFβ secretion by HCs cultured for 24 hours on HA-coated plastic and compared the levels with those secreted by cells cultured on BSA-coated or uncoated plastic. On all these surfaces, the amount of secreted FGF-2 varied from case to case but on HA the production was 7.1 ± 2.6-fold and 6.8 ± 2.8-fold higher than on BSA and plastic, respectively (Figure 2). Although some basal production of latent, nonactive TGFβ was detected in the supernatants of HCs cultured on plastic or BSA (39 ± 4 pg/mL and 28 ± 6 pg/mL, respectively), interaction with HA did not increase this production (21 ± 6 pg/mL), and no activated cytokine (“Patients, materials, and methods”) was detectable in the cultures (n = 4 cases).
HA induces FN production by HCs. HCs were cultured for 24 hours at 37°C on glass coverslips that were either untreated or coated with HA. FN was stained by an indirect immunocytochemical method involving anti-FN mAbs and peroxidase-labeled goat antimouse antibody as first and second layers, respectively. This is a representative example of 5 experiments involving 3 different cases of HCL. Original magnification, × 40.
HA induces FN production by HCs. HCs were cultured for 24 hours at 37°C on glass coverslips that were either untreated or coated with HA. FN was stained by an indirect immunocytochemical method involving anti-FN mAbs and peroxidase-labeled goat antimouse antibody as first and second layers, respectively. This is a representative example of 5 experiments involving 3 different cases of HCL. Original magnification, × 40.
HA induces FGF-2 secretion by HCs. HCs were cultured for 24 hours at 37°C in 24-well plates coated with HA; control substrata were BSA-coated or uncoated plastic (PL). FGF-2 was measured by a commercial ELISA. A culture time of 24 hours was chosen because we have previously shown that, during this period of culture, HA induces substantial FN production by HCs. The results represent the means of triplicate measurements involving the cells of 4 different HCL patients.
HA induces FGF-2 secretion by HCs. HCs were cultured for 24 hours at 37°C in 24-well plates coated with HA; control substrata were BSA-coated or uncoated plastic (PL). FGF-2 was measured by a commercial ELISA. A culture time of 24 hours was chosen because we have previously shown that, during this period of culture, HA induces substantial FN production by HCs. The results represent the means of triplicate measurements involving the cells of 4 different HCL patients.
We also examined the effect of HA on FGF-2 mRNA expression. Preliminary time-course experiments (data not shown) showed peak induction of FGF-2 message at between 1 and 3 hours of culture. Figure 3 shows that this HA-induced increase of FGF-2 mRNA measured at 3 hours was highly significant (n = 5 separate cases; P = .0005).
HA induces FGF-2 mRNA expression by HCs. Panel A shows RT-PCR analysis of FGF-2 and L27 (a housekeeping gene) mRNA from the malignant cells of 5 patients with HCL. The cells were cultured for 3 hours on HA-coated (HA) or uncoated plastic (PL). Panel B is a graphic representation of the data shown in panel A, where the intensities of the FGF-2 bands (mean ± 1 SD) were measured by densitometry and normalized against the loading control (L27).
HA induces FGF-2 mRNA expression by HCs. Panel A shows RT-PCR analysis of FGF-2 and L27 (a housekeeping gene) mRNA from the malignant cells of 5 patients with HCL. The cells were cultured for 3 hours on HA-coated (HA) or uncoated plastic (PL). Panel B is a graphic representation of the data shown in panel A, where the intensities of the FGF-2 bands (mean ± 1 SD) were measured by densitometry and normalized against the loading control (L27).
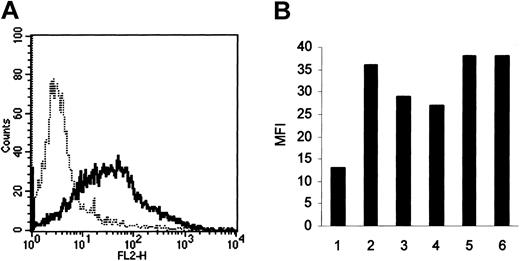
Because these results suggest that HA-induced FN production by HCs may be the result of the action of autocrine FGF-2, we next used FACS analysis to determine whether these cells express the principal FGF-2 receptor, FGFR-1. In all 6 cases examined, the HCs possessed this receptor (Figure 4).
FGFR-1 expression by HCs. Receptor levels were measured by FACS using a triple-layer technique involving a mAb against FGFR-1, biotinylated antimouse antibody, and streptavidin-phycoerythrin (—). In all 6 cases, HCs were positively stained compared with the class-specific (IgG1) control (- - - -; representative result shown in panel A). Panel B shows the extent of positivity expressed as the mean fluorescence intensity (MFI) of the reactive cells.
FGFR-1 expression by HCs. Receptor levels were measured by FACS using a triple-layer technique involving a mAb against FGFR-1, biotinylated antimouse antibody, and streptavidin-phycoerythrin (—). In all 6 cases, HCs were positively stained compared with the class-specific (IgG1) control (- - - -; representative result shown in panel A). Panel B shows the extent of positivity expressed as the mean fluorescence intensity (MFI) of the reactive cells.
HA-induced FN production by HCs is mediated by FGF-2
Having demonstrated that HA stimulates FGF-2 production by HCs and that the cells possess receptors for this growth factor, we next examined whether the supernatants of HCs cultured on HA were able to stimulate FN production by the cells cultured on BSA.
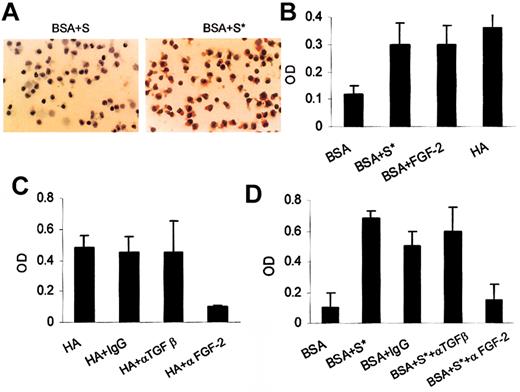
Figure 5 shows that when 24-hour supernatants of cells cultured on HA were added to fresh cultures of HCs on BSA, these supernatants stimulated far greater FN production than did the control 24-hour supernatants of cells cultured on BSA. This increase in FN production on BSA induced by the supernatant from HCs cultured on HA was then quantitated by ELISA. These results were compared with the production of FN by HCs on HA and with the production of FN induced by addition of recombinant FGF-2 to cells cultured on BSA, using the cytokine at a concentration (200 pg/mL) comparable to that found in the supernatant of cells cultured on HA (Figure 2). Under all 3 sets of culture conditions, HCs produced similarly increased amounts of FN compared with cells on BSA (Figure 5B). Moreover, the stimulation of FN production by HA (Figure 5C) or by the supernatants of HCs on HA (Figure 5D) was almost completely abolished by a blocking anti–FGF-2 antibody, whereas blocking of TGFβ had no effect (Figure 5C-D). Also, the addition of active TGFβ at a concentration comparable to that of inactive cytokine found in HC culture supernatants (25 pg/mL) did not increase FN production. A 10-fold higher concentration of active TGFβ also had no effect on FN production by HCs in 2 of 3 cases studied but caused an increase (∼3 fold) in FN production in the third case (data not shown). These results indicate that TGFβ is unlikely to be involved in the stimulation of FN production by HCs cultured on HA.
The role of FGF-2 in HA-dependent FN production by HCs. Panel A shows the FN production by HCs after culture for 24 hours on BSA in the presence of supernatants obtained from either an equivalent culture (S) or from HCs cultured for 24 hours on HA (S*). This is a representative of 3 experiments involving cells from 3 different patients. Original magnification, × 40. Panel B shows the relative amounts of FN produced by HCs cultured on BSA or HA or on BSA in the presence of recombinant FGF-2 (200 pg/mL) or of supernatants (S*) from cells cultured on HA. Panel C shows the blocking of FN production by anti–FGF-2 antibody, but not by anti-TGFβ or IgG control antibodies, in cultures of HCs on HA. Panel D shows the effect of anti–FGF-2 or anti-TGFβ antibodies on FN production induced by supernatants of HCs cultured on HA (S*). The amounts of FN produced in the presence of BSA alone or in the presence of supernatant (S*) and IgG control antibody are shown for comparison. In panels B-D, the results represent the means (± SEM) of 3 experiments involving 3 different patients.
The role of FGF-2 in HA-dependent FN production by HCs. Panel A shows the FN production by HCs after culture for 24 hours on BSA in the presence of supernatants obtained from either an equivalent culture (S) or from HCs cultured for 24 hours on HA (S*). This is a representative of 3 experiments involving cells from 3 different patients. Original magnification, × 40. Panel B shows the relative amounts of FN produced by HCs cultured on BSA or HA or on BSA in the presence of recombinant FGF-2 (200 pg/mL) or of supernatants (S*) from cells cultured on HA. Panel C shows the blocking of FN production by anti–FGF-2 antibody, but not by anti-TGFβ or IgG control antibodies, in cultures of HCs on HA. Panel D shows the effect of anti–FGF-2 or anti-TGFβ antibodies on FN production induced by supernatants of HCs cultured on HA (S*). The amounts of FN produced in the presence of BSA alone or in the presence of supernatant (S*) and IgG control antibody are shown for comparison. In panels B-D, the results represent the means (± SEM) of 3 experiments involving 3 different patients.
It is therefore concluded that the HA-induced FN production by HCs is a secondary effect of FGF-2 secretion induced by stimulation of the HA receptor CD44, rather than by direct signaling through this receptor.
HCs express CD44v3 and this receptor is involved in FN induction by autocrine FGF-2
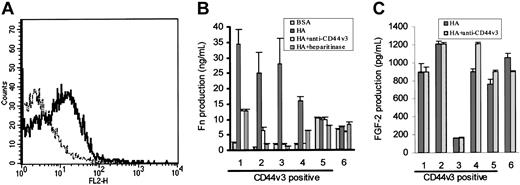
Because CD44v3,18 and CD138 (syndecan-1)19 have been shown to be coreceptors for FGF-2, we next looked for these structures on HCs using FACS analysis. This showed CD44v3 positivity on the HCs of 5 of 6 patients studied (representative example shown in Figure 6A); the expression was variable and low. In all 6 cases, the HCs were uniformly negative for CD138 (not shown).
Role of CD44v3 in HA-induced FN production by HCs. Panel A shows a representative FACS profile of HCs stained for CD44v3 (—) using a triple-layer technique identical to that used for FGFR-1 detection (- - - -; IgG2b class-specific control). In panel B, HCs were cultured on BSA-coated or HA-coated plastic and FN production at 24 hours was measured by an ELISA. In each case, the effect on FN production of coincubation with either anti-CD44v3 mAbs (or with IgG2b class-specific control, which had no effect; not shown) or with heparitinase was tested. In panel C, the effect of the anti-CD44v3 mAbs on HA-induced FGF-2 production (as estimated by ELISA) was measured.
Role of CD44v3 in HA-induced FN production by HCs. Panel A shows a representative FACS profile of HCs stained for CD44v3 (—) using a triple-layer technique identical to that used for FGFR-1 detection (- - - -; IgG2b class-specific control). In panel B, HCs were cultured on BSA-coated or HA-coated plastic and FN production at 24 hours was measured by an ELISA. In each case, the effect on FN production of coincubation with either anti-CD44v3 mAbs (or with IgG2b class-specific control, which had no effect; not shown) or with heparitinase was tested. In panel C, the effect of the anti-CD44v3 mAbs on HA-induced FGF-2 production (as estimated by ELISA) was measured.
We next examined the role of CD44v3 in FGF-2–mediated FN production by HCs. To do this, HCs from the same 6 cases were cultured for 24 hours on HA in the presence or absence of the anti-CD44v3 mAb, which is known to down-regulate this receptor.17 We also used heparitinase, the enzyme that hydrolyzes the heparan sulfate necessary for FGF-2 binding to proteoglycans.
In 4 of 6 cases studied, FN production was, as expected, increased upon cell culture on HA (Figure 6B). The cells in these 4 cases expressed the CD44v3 isoform and the increase in FN production on HA was markedly diminished (40%-97% reduction) by treatment of cells with anti-CD44v3 mAbs or with heparitinase (Figure 6B, cases 1-4). The absence of complete inhibition of FN production was to be expected since it has been reported that these treatments do not completely abolish FGF-2 binding to other cell types in which the heparan sulfate required for FGF-2 binding is supplied by CD44v3.17
The v3 isoform of CD44, in addition to acting as a coreceptor for FGF-2, can bind HA independently of cell activation.16 We therefore explored whether this isoform on HCs plays a key role in the stimulation by HA of FGF-2 secretion, as well as serving as coreceptor for the cytokine. However, the CD44v3 mAb had no effect on HA-induced FGF-2 production (Figure 6C). We therefore conclude that this isoform of CD44 is not responsible for the generation of the HA-induced signal for FGF-2 production but, rather, it acts as a coreceptor for FGF-2 and consequently is involved at the stage of induction of FN by this autocrine cytokine.
Regarding the 2 cases of HCL in which HA did not induce FN production (Figure 6B, cases 5-6), the HCs constitutively produced up to 10 times more FN on BSA than did the cells from the other cases, and neither anti-CD44v3 mAbs nor heparitinase had an effect on this production (Figure 6B, cases 5-6). These observations suggest that in these 2 cases the HCs had already received a stimulus via FGF-2 for FN production (perhaps through in vivo exposure to HA) and become refractory to further stimulation, explaining why blocking CD44v3 did not have an effect. Moreover, in case 6 where CD44v3 was not detected on the malignant cells it is likely that the receptor was lost as a consequence of shedding upon in vivo cell stimulation. This absence of CD44v3 would in itself explain why in this case anti-CD44v3 antibody and heparitinase had no effect on the FGF-2–induced FN production.
As regards other CD44 isoforms, HCs also expressed CD44v6 at levels similar to those of CD44v3 (data not shown), while CD44v10 was barely detectable (MFI for v10 = 6.3 ± 0.7; control = 4.0 ± 0.5; n = 3 cases). Moreover, when HCs were cultured on surfaces coated with different antibodies to induce CD44 signaling, only the antibody against CD44H caused clear stimulation of FN production. We therefore conclude that the signal for FGF-2 production by HCs is mainly, if not exclusively, provided by stimulation of CD44H.
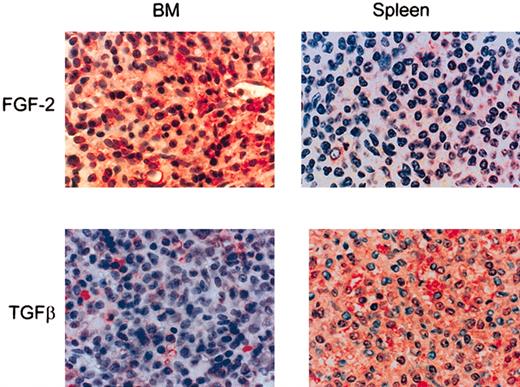
HCs in HA-rich BM, but not those in spleen, produce FGF-2
In order to examine the possible in vivo relevance of our observations, we next examined the production of FGF-2 in normal and HCL-infiltrated BM and spleen. When HCL bone marrow was stained immunohistochemically for FGF-2, the HCs were strongly reactive for the cytokine and some reactivity was also observed in the ECM (Figure 7). In contrast, in normal BM and in noninvolved areas of HCL BM (not shown) staining was largely confined to stromal cells. In spleen, where HCs are found in the red pulp, they either lacked FGF-2 staining or were only weakly reactive (Figure 7).
FGF-2 and the active from of TGFβ in HCL BM and spleen. Paraffin-embedded sections of HCL BM (n = 5) and spleen (n = 3) were stained immunohistochemically for FGF-2 or TGFβ by a triple-layer technique using ExtrAvidin-alkaline phosphatase as the final layer. Original magnification, × 40.
FGF-2 and the active from of TGFβ in HCL BM and spleen. Paraffin-embedded sections of HCL BM (n = 5) and spleen (n = 3) were stained immunohistochemically for FGF-2 or TGFβ by a triple-layer technique using ExtrAvidin-alkaline phosphatase as the final layer. Original magnification, × 40.
To confirm our hypothesis that TGFβ is not involved in the BM fibrosis of HCL, we also examined BM and spleen for the presence of the active form of this cytokine. In contrast to FGF-2, active TGFβ was distinctly more abundant in HCL spleen than in involved BM (Figure 7) (ie, its tissue distribution was the reciprocal to those of FGF-2 and tissue fibrosis).
We therefore conclude that, as in vitro, HA in vivo stimulates HCs to produce FGF-2, which then induces the malignant lymphocytes to produce the FN responsible for the reticulin fibrosis of HCL BM. TGFβ is unlikely to be involved in this process because in the splenic red pulp, where HA is absent and TGFβ is plentiful, there is no fibrosis and the HCs produce little or no FGF-2.
Discussion
BM is a major site of malignant-cell invasion in HCL and this BM infiltration is invariably accompanied by distinctive fibrosis. Previously, we have shown that FN is a major component of this fibrosis and is produced and assembled by the HCs themselves.9 Subsequently, we have demonstrated that the production of FN by HCs is likely to be a consequence of malignant-cell interaction (via CD44) with HA present in the ECM of the BM.10 The aim of the present study was to elucidate the mechanism of the HA-induced FN production by HCs. In particular, we have considered the role of FGF-2 and TGFβ, 2 fibrogenic cytokines known to be produced by HCs.13 We also examined the role of CD44 isoforms in both induction and action of these cytokines.
Cell culture confirmed that under standard in vitro conditions HCs are indeed capable of producing both TGFβ and FGF-2. However, when the cells were cultured on HA, FGF-2 secretion was increased by several-fold, while that of TGFβ was unaffected. To determine whether either or both of these cytokines are responsible for the increased FN production by HCs on HA, we used specific blocking antibodies. These blocking studies demonstrated that FGF-2, rather than TGFβ or direct signaling via CD44, is responsible for stimulation of FN production. This was confirmed by showing that the supernatants of cells cultured on HA induce an increase in FN production by cells cultured on plastic or BSA and that this effect is blocked by anti–FGF-2 antibody. In all these experiments, anti-TGFβ antibody had no effect, a finding in accord with our observation that the TGFβ produced in the HC cultures was largely in its nonactivated form.
These in vitro experiments suggest that the principal cytokine responsible for FN deposition in HCL BM is likely to be FGF-2 and this was further supported by our immunohistochemical studies of FGF-2 and TGFβ in affected tissues. Thus, large amounts of FGF-2 were found in HC-infiltrated fibrotic bone marrow compared with only faint or absent staining of HC-infiltrated spleen, in which FN deposition does not take place. In contrast, the activated form of TGFβ showed a reciprocal distribution, being more abundant in spleen than in bone marrow. This leads us to conclude that FGF-2, rather than TGFβ, is responsible for the BM fibrosis of HCL. Our results explain the different immunohistologic appearances of BM matrix proteins in MF versus HCL. In MF, where TGFβ is thought to play a central role,3 FN is much less conspicuous and patchy,2 while collagen is far more prominent than in HCL.9
In our previous studies of HCs cultured on HA, we have observed that the malignant cells are spontaneously motile on this substrate. With regard to other cell types, this effect has been ascribed to the expression of the v3 isoform of CD44.20 Moreover, in addition to its role in cell motility, this heparan sulfate–containing isoform is also known to be an important coreceptor for cell stimulation by FGF-2.18 We therefore next examined the expression of CD44v3 by HCs and the potential roles of this protein in HA-induced, FGF-2–dependent, FN production. We showed that CD44v3 was uniformly present on HCs from the majority of our patients. Moreover, down-regulation of the receptor or removal of heparan sulfate by heparitinase markedly inhibited HA-induced FN production by HCs in 4 of 6 cases studied. In the 2 cases in which this treatment was ineffective, the HCs constitutively produced relatively large amounts of FN, and production was not increased by culture on HA. We propose that this constitutive production of FN is the result of in vivo stimulation that may have desensitized the cells to further stimulation by the FGF-2 produced by HC adhesion to HA in vitro. In this context, we have recently shown that a number of effects of in vivo stimulation of adhesion and cytokine receptors can persist in HCs for up to 72 hours under conditions of complete in vitro deprivation of such stimuli.21
The inhibition of FN production by antibody-induced shedding of CD44v3 and by heparitinase in the 4 cases in which these treatments were effective was not the result of an effect on FGF-2 production but rather of the inhibition of cell stimulation by FGF-2. We therefore conclude that the signal for FGF-2 production is provided by other isoforms of CD44, most likely the standard hematopoietic form known to be strongly expressed by HCs.10 This proposition was confirmed by our demonstration that antibody cross-linking of CD44H, but not other isoforms (CD44v6 and v10), induced FN production by HCs. Therefore, in the stimulation of FN synthesis, CD44H provides the signal for FGF-2 production, whereas CD44v3 is required as a coreceptor for FGF-2–induced stimulation of FN production.
In conclusion, by defining the role of CD44H, CD44v3, and FGF-2 in HA-induced FN synthesis by HCs, the present study establishes the likely mechanism of BM fibrosis in HCL. In particular, our present study highlights the importance of autocrine FGF-2 in this process. It has been previously suggested that the increased FGF-2 levels observed in HCL play a role in the disease by autocrine promotion of malignant-cell survival.13 By demonstrating the involvement of autocrine FGF-2 in the BM fibrosis of the disease, the present study suggests another role for this cytokine central to the pathogenesis of HCL.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2002-12-3737.
Supported by the Leukaemia Research Fund, United Kingdom.
K.A.A. and K.J.T. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.