Abstract
Numerous reports have described the effects of interleukin-4 (IL-4) on bone biology. Previous studies, performed using complex coculture systems, demonstrated the effects of IL-4 on osteoblasts and osteoclasts. To directly test the effect of IL-4 on osteoclasts, we took advantage of a simplified system using recombinant receptor activator of nuclear factor κB ligand (RANKL) as the osteoclast differentiation factor. We analyzed the ability of IL-4 to directly regulate osteoclast differentiation and mature osteoclast function. We found that IL-4 inhibited the differentiation of osteoclasts from bone marrow precursors in an irreversible manner and also inhibited the resorptive capacity of mature osteoclasts. In the presence of IL-4, we detected the appearance of tartrate-resistant acid phosphatase (TRAP)–negative multinucleated giant (MNG) cells. Both IL-4 effects were dependent on signal transducer and activator of transcription 6 (STAT6). We found that IL-4 suppresses RANK mRNA expression in the developing precursor cells. When RANK was ectopically expressed under the cytomegalovirus (CMV) promoter in RAW264.7 macrophages, IL-4 treatment did not inhibit osteoclast development. Furthermore, when osteoclastogenesis was induced independently of RANKL by using tumor necrosis factor-α (TNF-α), IL-4 inhibited osteoclast differentiation through a STAT6-dependent mechanism. These results suggest that IL-4 regulates osteoclast development by regulating gene expression, including RANK. We propose that IL-4 irreversibly regulates the lineage commitment of precursor cells by regulating gene expression, resulting in the suppression of osteoclast development and the generation of MNG cells as an alternative pathway of differentiation.
Introduction
Cells of the monocyte-macrophage lineage derived from hematopoietic progenitors are able to differentiate into several cell types with markedly distinct morphologic and functional characteristics, depending on the extracellular environment.1-3 Some of these cell types, such as dendritic cells and mononuclear macrophages, are mononuclear whereas others are multinucleated. Among the multinucleated type there are multinucleated giant (MNG) cells and osteoclasts.3 Although they share a common origin, the signals involved in their differentiation, the spectrum of expressed genes, and their biologic functions are markedly distinct.
In several of these cell types, the immune cytokine interleukin-4 (IL-4) plays a critical role regulating differentiation and activity.2-9 This pleiotropic immunomodulatory cytokine, originally identified as a stimulator of B-cell proliferation, is produced by helper T2 (TH2) lymphocytes, mast cells, and eosinophils and can regulate proliferation, apoptosis, gene expression, and differentiation in many cell types.7,10-14 Responses to IL-4 are mediated by a cell surface IL-4 receptor complex (IL-4R). This receptor consists of at least 2 different types. The type 1 receptor contains the IL-4Rα chain and the common γ chain. The type 2 receptor includes IL-4Rα and the low-affinity receptor for IL-13 (IL-13Rα1). Both types of receptors are known to be active on monocytic cells. The binding of IL-4 to its receptor results in the activation of Janus tyrosine kinases (JAKs) and several cellular signaling molecules, including the insulin receptor substrate 1 (IRS-1) and IRS-2 and the transcription factor signal transducer and activator of transcription 6 (STAT6). The IRS pathway contributes to cellular proliferation in cell lines and in normal lymphocytes. IL-4–induced activation of STAT6 has been shown to be critical for the regulation of gene expression, the development of immunoglobulin E (IgE) producers from B cells, and the efficient development of TH2 in response to protein antigens.11
In the past 10 years, numerous reports have described an effect of IL-4 on bone biology. Initial experiments were performed in vivo or by using a complex in vitro coculture system consisting of stromal cells and bone marrow precursors.6,8,15-22 It was found that IL-4 could have potent effects on the osteoblast and the osteoclast, indicating that IL-4 could play a complex role in regulating bone homeostasis. From that first general observation of IL-4 inhibition of bone resorption6 to the establishment of the osteoclast precursor as the ultimate target of the IL-4 effect,8 the limitation of having to use coculture systems has hindered further advances in clarifying the mechanism by which IL-4 regulates osteoclast differentiation and function. The discovery of receptor activator of nuclear factor κB ligand (RANKL), a member of the tumor necrosis factor (TNF) cytokine superfamily, and its role in the bone and immune systems has further clarified the mechanism by which IL-4 regulates bone biology.23-27 Two recent reports5,7 demonstrated that IL-4 was able to directly suppress the development of osteoclasts from bone marrow precursors induced by RANKL. This IL-4–induced suppression was dependent on STAT6. These studies also provided some data suggesting that IL-4 inhibited RANKL-activated signaling, including nuclear factor κB (NF-κB) activation.5,7 One of these reports concluded that the IL-4 inhibition of osteoclastogenesis was reversible,5 whereas the other came to the opposite conclusion.7
The ability of IL-4 to directly suppress NF-κB activation per se is controversial. In many cases IL-4 enhances NF-κB activation, depending on the cell type.28 Therefore, we analyzed the ability of IL-4 to directly regulate osteoclast differentiation and mature osteoclast function in several different cell systems. In contrast to a previous report,5 we found that IL-4 inhibited the differentiation of osteoclasts from bone marrow precursors in an irreversible manner. Furthermore, we found that IL-4 was able to directly inhibit the resorptive capacity of mature osteoclasts in a stromal cell–free culture system. The effects of IL-4 on osteoclast differentiation and function of mature osteoclasts were dependent on STAT6. The IRS pathway was not activated in immature or mature osteoclasts by IL-4. We further provide direct evidence that IL-4 suppresses osteoclast differentiation in part through the regulation of RANK expression.
Materials and methods
Isolation of osteoclast precursors from different sources
Human mononuclear cells were collected from whole-blood buffy coats after informed consent from healthy donors (according to acceptable guidelines from the American Red Cross Biomedical Services Institutional Review Board), and CD14+ monocytes were immunoselected (Miltenyi Biotech, Auburn, CA). Purified CD14+ (purity was determined as 95%-98% through the use of the CellDyn 3700 Analyzer [Abbott Laboratories, Abbott Park, IL] and flow cytometry with a FACScan equipped with CellQuest software [Becton Dickinson, San Jose, CA]) were cultured at 37°C and 5% CO2 atmosphere in α-10 media (α-minimal essential medium supplemented with penicillin, streptomycin, and glutamine [all from BioWhittaker, Walkersville, MD] and 10% heat-inactivated fetal bovine serum [FBS; Invitrogen, Frederick, MD]) plus 20 ng/mL recombinant human macrophage–colony-stimulating factor (rhM-CSF) (R&D Systems, Minneapolis, MN). These cells were plated in the presence of rhM-CSF for 3 days before use in osteoclast differentiation assays.
For the murine experiments, nonadherent bone marrow mononuclear cells were isolated from femurs and tibias of 4- to 6-week-old wild-type Balb/c (Taconic Laboratories, Germantown, NY) and STAT6-/- (obtained from Dr William E. Paul, NIH) female mice. Cells were cultured overnight in α-10 media to deplete adherent stromal cells; this was followed by isolation of the mononuclear cells over Ficoll-Hypaque density gradient centrifugation, as described.29 These nonadherent bone marrow mononuclear cells were cultured for 3 days in the presence of 20 ng/mL recombinant mouse M-CSF (rmM-CSF) (R&D Systems) to generate osteoclast precursors (hereafter also called BMM).
Osteoclast differentiation in vitro
Human or murine osteoclast precursor cells were plated at 1 × 106 cells/mL in α-10 in the presence of 20 ng/mL recombinant M-CSF (R&D Systems) and RANKL (150 ng/mL recombinant mouse RANKL-thioredoxin fusion protein, produced in our laboratory). With the murine macrophage cell line RAW264.7, cells were plated at 4 × 104 cells/mL in D-10 media (Dulbecco minimal essential medium, plus 10% FBS [Invitrogen], penicillin, and streptomycin [BioWhittaker]), 20 ng/mL rmM-CSF (R&D Systems), and 150 ng/mL recombinant mouse RANKL-thioredoxin fusion protein. After 6 to 7 days in culture, the cells were subjected to tartrate resistant acid phosphatase (TRAP) staining with kit 387-A (Sigma, St Louis, MO), according to the manufacturer's instructions.
Osteoclast generation with TNF-α
For these experiments, osteoclasts progenitors were treated as described elsewhere.30 Briefly, osteoclast precursors were plated in α-10 in the presence of 100 ng/mL rmM-CSF for the first 3 days. Nonadherent cells were completely removed from the culture, and the adherent cells were cultured for an additional 3 days in 100 ng/mL rmM-CSF and 20 ng/mL recombinant mouse TNF-α (rmTNF-α) (R&D Systems) or 150 ng/mL RANKL ± 10 ng/mL recombinant mouse IL-4 (rmIL-4) (R&D Systems). At the end of the 6-day culture period, the cells were subjected to TRAP staining.
Isolation of mature osteoclast from collagen films and bone resorption assays
The method for the purification of mature osteoclast from collagen films was adapted from a previous protocol.31,32 Briefly, osteoclast precursors were plated in culture plates coated with a type 1 collagen film (Vitrogen 100; Cohesion, Palo Alto, CA). Cells were fed fresh α-10, rmM-CSF (20 ng/mL) (R&D Systems), and RANKL (150 ng/mL) every other day. After 6 days in culture, the plates were washed with phosphate-buffered saline (PBS), and then the collagen film was digested with a solution of 0.2% collagenase A (Boehringer Mannheim GmbH, Mannheim, Germany). Cells were harvested by centrifugation, washed, resuspended in fresh complete α-10, and plated on hydroxyapatite-coated glass (Osteologic analysis discs; BD Biosciences, Bedford, MA) or on dentin slices. At the time of seeding on dentin or hydroxyapatite discs, rmM-CSF (20 ng/mL) (R&D Systems) and RANKL (150 ng/mL) plus 10 ng/mL rmIL-4 (R&D Systems) in PBS or control vehicle were added to the cultures. After 24 or 48 hours of incubation, the discs were washed in 5% sodium hypochlorite for 5 minutes to remove the cells. Resorbed surfaces were visualized by light microscopy and quantified by image analysis (Bioquant Image Analysis, Nashville, TN).
Signaling experiments
Cells were deprived of serum for 4 hours before the addition of 10 ng/mL rmIL-4 (R&D Systems) for 10 minutes. Subsequently, the cells were lysed in lysis buffer (50 mM Tris HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 5 mM EDTA [ethylenediaminetetraacetic acid], 10 mM Na pyrophosphate, 50 mM NaF, 0.25% Na deoxycholate, 1 mM Na orthovanadate, 1 mM phenylmethylsulfonyl fluoride [PMSF], pepstatin, leupeptin, and aprotinin) and were clarified by centrifugation. Protein concentration was determined in the samples by BCA assay (Pierce, Rockford, IL), and equal amounts of protein were incubated with the primary antibody as indicated. Complexes were collected by immunoprecipitation with protein G or A beads (Invitrogen), according to the manufacturer's instructions.
Immunoprecipitates were separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) before transfer to polyvinylidene fluoride (PVDF) membrane (Immobilon-P; Millipore, Bedford, MA). Membranes were then blocked overnight and probed with a monoclonal antiphosphotyrosine antibody (Transduction Laboratories, Lexington, KY). Membranes were then washed extensively with Tris-buffered saline plus 0.5% Tween (TBST) and were developed using Supersignal West Pico Chemiluminescent Substrate (Pierce). The antiphosphotyrosine antibody was stripped from the membranes and exposed to the corresponding primary antibody from 2 hours to overnight according to the manufacturer's instructions. Membranes were washed and exposed to a horseradish peroxidase (HRP)–conjugated secondary antibody for 1 hour at room temperature, washed again, and developed with the Supersignal West Pico Chemiluminescent Substrate (Pierce).
Antibodies
Polyclonal STAT6 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), polyclonal antibodies against IRS-2 and IRS-1 were purchased from Upstate Biotechnology (Lake Placid, NY), and monoclonal antiphosphotyrosine antibody was purchased from Transduction Laboratories. Secondary antibodies goat antimouse and goat antirabbit were purchased from Bio-Rad Laboratories (Hercules, CA). Antihuman fluorescein isothiocyanate (FITC)–CD14 monoclonal antibody was purchased from BD Biosciences PharMingen (San Diego, CA). Each antibody was used at the concentration recommended by its manufacturer.
Reverse transcription–polymerase chain reaction analysis
Total RNA was isolated from bone marrow cells using an RNeasy Mini Kit (Qiagen, Valencia, CA). Two micrograms total RNA were used to generate cDNA using Omniscript Reverse Transcriptase (Qiagen) according to the manufacturer's instructions. One microliter of this cDNA per sample was used for conventional semiquantitative polymerase chain reaction (PCR) using a Light-Cycler (Roche Molecular Biochemicals, Indianapolis, IN) and Light Cycler-Fast Start DNA Master SYBR Green I (Roche). The sequence of the primers for murine RANK have been described previously,33 and the primers for murine β-actin were: sense, 5′-CAGGGCGTGATGGTGG-3′; antisense, 5′-GGAAGGTGGACAGCGAGG-3′. Conditions for Light-Cycler reactions for both genes were denaturation at 95°C for 2 seconds, annealing at 60°C for 5 seconds, and extension at 72°C for 45 seconds for 45 cycles.
Flow cytometry
Surface expression of RANK protein on osteoclast progenitors was measured by fluorescence-activated cell sorter (FACS) analysis using FITC-RANKL, as previously described.34 Murine osteoclast precursors were cultured under various experimental conditions. Aliquots of 1 × 106 cells were suspended in PBS plus 1% FBS and stained with or without 0.5 μg FITC-RANKL for 30 minutes on ice. Cells were then washed with PBS to remove unbound FITC-RANKL, and data were acquired using a FACScan instrument and analyzed by CellQuest software, version 3.3 (Becton Dickinson Immunocytometry Systems, Bedford, MA).
Stable transfection of RAW264.7 cells with RANK
The coding sequence of mouse RANK from amino acid 31 (initiating methionine was number 1) to the stop codon was amplified by PCR and cloned into PCR-TOPO I plasmid (Invitrogen). The entire cloned cDNA was sequenced to verify that no mutations were introduced by the PCR reaction. PCR primers used were from nucleotides 115 to 1903 of the cDNA (GenBank accession no. AF019046); the sequence of the 5-prime end was 5′-gccaggcgcgccgaacaaaaactcatctcagaagaggatctgaagcttGTCACTCCTCCATGCACCCAG-3′, and that of the 3-prime end was 5′-atcgaattcgcggccgcTCATTCTGCACATTGTCCGGAC-3′ (lowercase letters represent restriction enzyme and myc-tag sequences, whereas uppercase letters represent nucleotide sequences derived from RANK cDNA). These primers introduced an AscI site followed by a myc-tag (recognition sequence for antibody 9E10) followed by a HindIII site at the 5-′ end of the cDNA and a NotI and an EcoRI site at its 3-′ end such that the entire AscI-NotI fragment could be inserted in the correct reading frame into the pSECTAG-2a mammalian expression vector (Invitrogen). With this cloning strategy, the RANK cDNA was expressed from the cytomegalovirus (CMV) promoter of pSECTAG, and the signal peptide, derived from Igκ-chain leader sequence, was also provided by the vector. The signal peptide is followed by the myc-tag, which is followed by the entire mature mouse RANK protein sequence. Therefore, the expressed RANK protein is distinguishable from the endogenous protein by the presence of the myc-tag. RAW264.7 cells were transfected with this RANK construct by Lipofectamine (Invitrogen) according to manufacturer's instructions. Subsequently, the cells were diluted in media containing 300 μg/mL hygromycin (Invitrogen) to an average density of 0.5 cells/well of 96-well microtiter plates to ensure isolation of the clonal lines. Antibiotic-resistant clones were assayed for the presence of ectopic RANK expression by immunoblots using the 9E10 anti-myc monoclonal antibody for detection. Two RANK overexpressing clonal lines, herein referred to as clones 1 and 2, were used for osteoclastogenesis assays in the presence or absence of IL-4.
Results
IL-4 inhibits osteoclast differentiation from human and murine precursor cells
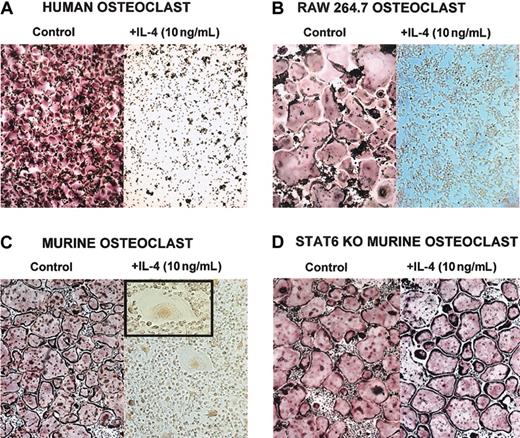
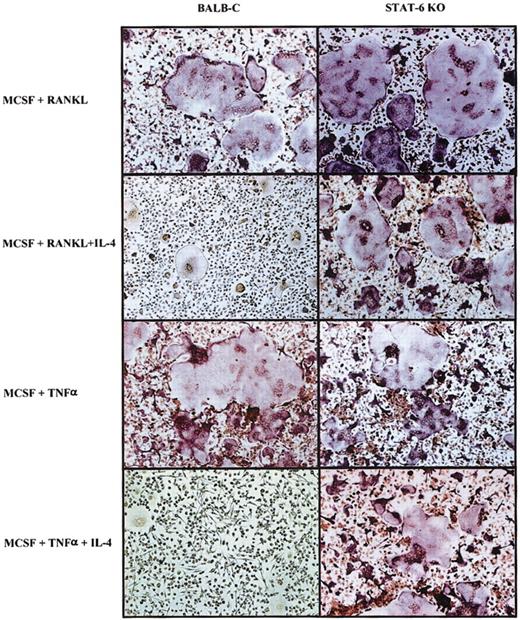
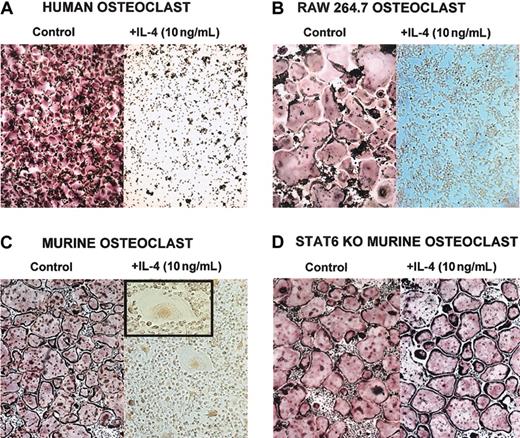
The T-cell–derived cytokine IL-4 has been reported to affect osteoblasts and osteoclasts.6,8,15-19,22,35 Recently, it has been shown that IL-4–induced suppression of osteoclast development is dependent on STAT6 expression.5,7 To fully delineate the mechanism by which IL-4 regulates osteoclast development and function, we used 3 different sources of osteoclast precursors (Figure 1)—murine BMM cells, human CD14+ monocytes, and the RAW264.7 murine macrophage cell line. Precursor cells were cultured with M-CSF (recombinant human or murine, as appropriate) and recombinant murine RANKL. This stimulation induced the differentiation of osteoclasts in the absence of osteoblasts or stromal cells. When IL-4 (recombinant human or murine, as appropriate) was added to the cultures on the same day of the seeding, it inhibited the development of mature osteoclasts characterized as TRAP-positive multinucleated cells (Figure 1). If the BMM precursors were isolated from STAT6-/- mice, IL-4 failed to inhibit osteoclast differentiation, demonstrating that STAT6 is a mediator of this process (Figure 1D).5,7 We have obtained similar results with the IL-4–related cytokine IL-13 (data not shown). These experiments demonstrate that IL-4 acts directly on the osteoclast progenitors independently of the species or the source. Interestingly, in the presence of IL-4, we detected multinucleated cells that were TRAP negative, with a morphology characteristic of MNG cells.2,36
IL-4 inhibits osteoclast differentiation by acting directly on progenitor cells. Cells were stained for TRAP, and osteoclasts were identified as multinucleated TRAP-positive (red-stained) cells. (A) CD14+ human monocytes were cultured with rhM-CSF (20 ng/mL) and RANKL (150 ng/mL) ± rhIL-4 (10 ng/mL) for 5 days. (B) RAW264.7 cells were cultured with rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) ± rmIL-4 (10 ng/mL) for 5 days. At the end of all culture periods, the plates were stained for TRAP. (C) BMMs were isolated from wild-type or (D) STAT6-/- mice and were cultured for 3 days in the presence of rmM-CSF (20 ng/mL) to obtain a homogeneous population of M-CSF–dependent adherent macrophages. They were then differentiated to osteoclasts by culturing for 7 days in the presence of rmM-CSF (20 ng/mL), RANKL (150 ng/mL), and rmIL-4 (10 ng/mL). Panel C inset shows a magnified image of an MNG (original magnification, × 20). Original magnification, × 10 for panels A-D.
IL-4 inhibits osteoclast differentiation by acting directly on progenitor cells. Cells were stained for TRAP, and osteoclasts were identified as multinucleated TRAP-positive (red-stained) cells. (A) CD14+ human monocytes were cultured with rhM-CSF (20 ng/mL) and RANKL (150 ng/mL) ± rhIL-4 (10 ng/mL) for 5 days. (B) RAW264.7 cells were cultured with rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) ± rmIL-4 (10 ng/mL) for 5 days. At the end of all culture periods, the plates were stained for TRAP. (C) BMMs were isolated from wild-type or (D) STAT6-/- mice and were cultured for 3 days in the presence of rmM-CSF (20 ng/mL) to obtain a homogeneous population of M-CSF–dependent adherent macrophages. They were then differentiated to osteoclasts by culturing for 7 days in the presence of rmM-CSF (20 ng/mL), RANKL (150 ng/mL), and rmIL-4 (10 ng/mL). Panel C inset shows a magnified image of an MNG (original magnification, × 20). Original magnification, × 10 for panels A-D.
IL-4 inhibits the bone resorption activity of mature osteoclasts
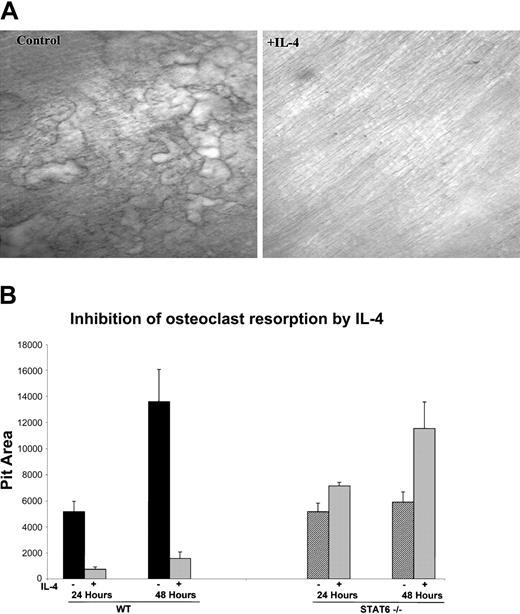
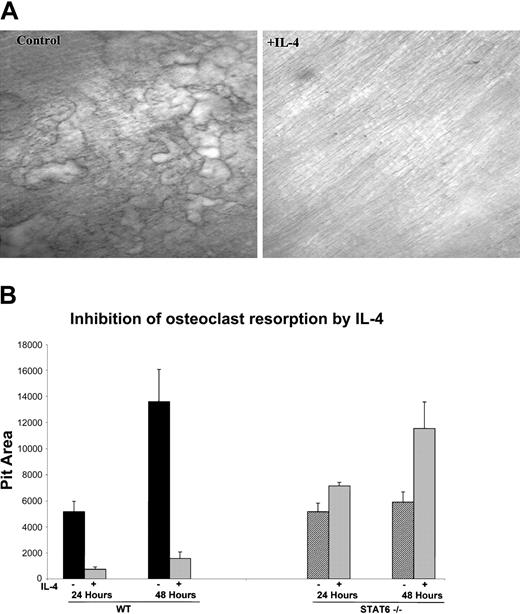
In addition to the ability of IL-4 to inhibit in vitro osteoclast formation from human and murine progenitors, we also investigated whether IL-4 was able to regulate the resorbing activity of the mature osteoclast. We focused on the murine system because of easy access to the cells and to reduce heterogeneity in age and variability between individual donors. Bone marrow cells from wild-type mice were plated on collagen films in the presence of M-CSF and RANKL for 6 days, at which time they became mature and developed the ability to resorb bone. The cells were lifted from the plate with collagenase and were then cultured with M-CSF and RANKL on top of dentin slices or on hydroxyapatite-coated discs in the presence or absence of IL-4. The addition of IL-4 at the time of plating on bone substrate strikingly inhibited the bone resorptive capacity of primary murine-derived mature osteoclasts (Figure 2A). These results indicate that IL-4 inhibits bone resorption not only by inhibiting the differentiation of the progenitors but also by inhibiting the function of the fully differentiated osteoclasts. To determine whether STAT6 is also critical for this IL-4 effect, we performed the resorption assays with mature osteoclasts derived from STAT6-/- mice. Osteoclasts were cultured on collagen films and then lifted and plated on hydroxyapatite-coated discs with or without IL-4 to quantify the IL-4 effect on the resorptive activity. IL-4 inhibited the resorptive activity of mature osteoclasts derived from wild-type mice by 86% after 24 hours and by 88% after 48 hours, reflecting a strong and constant inhibition of osteoclast resorptive activity by this cytokine. Mature osteoclasts derived from STAT6-/- mice showed an increase in the resorptive activity over control of 138% and 196% at 24 and 48 hours, respectively. The increased activity of STAT6-deficient osteoclasts may be a reflection of slightly more robust osteoclastogenesis from the mutant mice. These results demonstrate that IL-4 inhibits the activity of mature osteoclasts by a STAT6-dependent mechanism.
IL-4 inhibits the bone resorption activity of mature osteoclasts. (A) BMMs were isolated from wild-type mice and differentiated to osteoclasts by culturing with rmM-CSF (20 ng/mL) and RANKL (150 ng/mL), as in the previous experiments. Cells were plated on a layer of type 1 collagen. Six days later, when mature multinucleated osteoclasts were abundant, the plates were treated with collagenase to release the osteoclasts. These osteoclasts were then cultured on dentin slices with or without 10 ng/mL rmIL-4 for 24 hours. Resorption pits were visualized by staining with toluidine blue; original magnification, × 10 for both panels. (B) Cells from wild-type and STAT6-/- mice were isolated and cultured as described in panel A and were plated on hydroxyapatite-coated discs with or without 10 ng/mL rmIL-4. Areas of resorbed substrate were quantified by image analysis. Data represent the mean ± SD of 3 independent experiments.
IL-4 inhibits the bone resorption activity of mature osteoclasts. (A) BMMs were isolated from wild-type mice and differentiated to osteoclasts by culturing with rmM-CSF (20 ng/mL) and RANKL (150 ng/mL), as in the previous experiments. Cells were plated on a layer of type 1 collagen. Six days later, when mature multinucleated osteoclasts were abundant, the plates were treated with collagenase to release the osteoclasts. These osteoclasts were then cultured on dentin slices with or without 10 ng/mL rmIL-4 for 24 hours. Resorption pits were visualized by staining with toluidine blue; original magnification, × 10 for both panels. (B) Cells from wild-type and STAT6-/- mice were isolated and cultured as described in panel A and were plated on hydroxyapatite-coated discs with or without 10 ng/mL rmIL-4. Areas of resorbed substrate were quantified by image analysis. Data represent the mean ± SD of 3 independent experiments.
Mechanism of IL-4 action
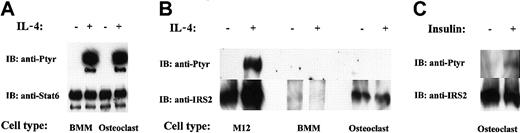
Because IL-4 inhibited the differentiation of osteoclast progenitors and the activity of mature osteoclasts, we analyzed IL-4 signaling in BMM precursors and mature cells. It is well known in other systems that IL-4 activates 2 major signaling pathways, namely STAT6 and IRS.11 We determined the activation status of these signal transducers in response to IL-4 in osteoclast progenitors and mature osteoclasts (Figure 3). As shown in Figure 3A, IL-4 stimulation of osteoclast progenitors and mature osteoclasts resulted in tyrosine phosphorylation of STAT6. The relative amounts of STAT6 protein in the progenitors and in the mature cells were similar. Next, we investigated whether IRS also becomes activated on IL-4 stimulation. We focused our analysis on IRS-2 because we detected IRS-2, but not IRS-1, protein in osteoclasts and their precursors. In addition, RT-PCR experiments by Ogata et al37 confirm that only IRS-2 is expressed in osteoclasts. We applied the same methodology for IRS-2 as was used in the previous experiments with STAT6. Despite numerous attempts, we did not detect IRS-2 phosphorylation after IL-4 stimulation of mature osteoclasts or their precursors (Figure 3B). Surprisingly, analysis of the protein blots showed that osteoclast precursors express very low levels of IRS-2 protein, though its expression is readily detectable in mature osteoclasts (Figure 3B). Insulin and insulinlike growth factor-1 (IGF-1) are known to signal by activating IRS proteins. As a positive control, we stimulated mature osteoclasts with insulin, followed by IRS-2 immunoprecipitation and phosphotyrosine immunoblot. As expected, IRS-2 became phosphorylated, indicating that there is no inherent anomaly in the cells to signal through the IRS pathway (Figure 3C). We also routinely tested the activity of our IL-4 (and antibodies) in parallel experiments by stimulating M12 cells (a murine B-cell lymphoma line), which are known to activate IRS-2 in response to the cytokine (Figure 3B). These results demonstrated that IL-4 signals through the phosphorylation of STAT6, but not IRS-2, in progenitors and mature osteoclasts. Consistent with our results, Akune et al38 have reported that the deletion of IRS-2 in mice does not affect osteoclast differentiation or function.
IL-4–induced signaling in osteoclasts. The indicated cells were treated with rmIL-4 (10 ng/mL) or insulin, as indicated for 10 minutes. Lysates were immunoprecipitated with anti-STAT6 or anti–IRS-2 antibody, run on a 4% polyacrylamide gel and probed first with antiphosphotyrosine antibody. Membranes were stripped and reprobed with anti-STAT6 or anti–IRS-2 antibody. (A) Analysis of STAT6 tyrosine phosphorylation and protein level. (B) IRS-2 tyrosine phosphorylation and protein level. (C) IRS-2 phosphorylation on mature osteoclasts in response to insulin.
IL-4–induced signaling in osteoclasts. The indicated cells were treated with rmIL-4 (10 ng/mL) or insulin, as indicated for 10 minutes. Lysates were immunoprecipitated with anti-STAT6 or anti–IRS-2 antibody, run on a 4% polyacrylamide gel and probed first with antiphosphotyrosine antibody. Membranes were stripped and reprobed with anti-STAT6 or anti–IRS-2 antibody. (A) Analysis of STAT6 tyrosine phosphorylation and protein level. (B) IRS-2 tyrosine phosphorylation and protein level. (C) IRS-2 phosphorylation on mature osteoclasts in response to insulin.
Dose response to IL-4
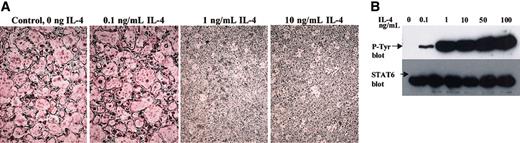
We tested the sensitivity of the precursor cells by adding increasing amounts of rmIL-4 to the cultures, keeping the concentrations of M-CSF and RANKL at optimal levels. As shown in Figure 4A, as little as 1 ng/mL IL-4 completely blocked osteoclastogenesis, whereas 0.1 ng/mL cytokine did not block differentiation. Interestingly, the level of STAT6 phosphorylation (Figure 4B) correlated with the inhibitory activity of IL-4 on osteoclastogenesis such that submaximal phosphorylation of STAT6 was attained at 0.1 ng/mL, whereas IL-4 at concentrations of 1 ng/mL or higher induced maximal STAT6 phosphorylation. These results are consistent with the notion that STAT6 activation by tyrosine phosphorylation is necessary and sufficient for the IL-4–mediated inhibition of osteoclastogenesis.
Dose response of osteoclast precursors to IL-4. (A) BMMs from wild-type mice were differentiated to osteoclasts in the presence rmM-CSF (20 ng/mL), RANKL (150 ng/mL), and the indicated concentrations of IL-4 for 7 days and were stained for TRAP expression. TRAP stain; original magnification, × 10. (B) BMMs were stimulated with the indicated concentrations of IL-4 for 10 minutes and lysed. STAT6 was immunoprecipitated from each culture, blotted, and probed with an antiphosphotyrosine antibody to determine its activation state. Blots were subsequently probed with anti-STAT6 to ensure equal loading.
Dose response of osteoclast precursors to IL-4. (A) BMMs from wild-type mice were differentiated to osteoclasts in the presence rmM-CSF (20 ng/mL), RANKL (150 ng/mL), and the indicated concentrations of IL-4 for 7 days and were stained for TRAP expression. TRAP stain; original magnification, × 10. (B) BMMs were stimulated with the indicated concentrations of IL-4 for 10 minutes and lysed. STAT6 was immunoprecipitated from each culture, blotted, and probed with an antiphosphotyrosine antibody to determine its activation state. Blots were subsequently probed with anti-STAT6 to ensure equal loading.
Mechanism of IL-4–mediated inhibition of osteoclast differentiation
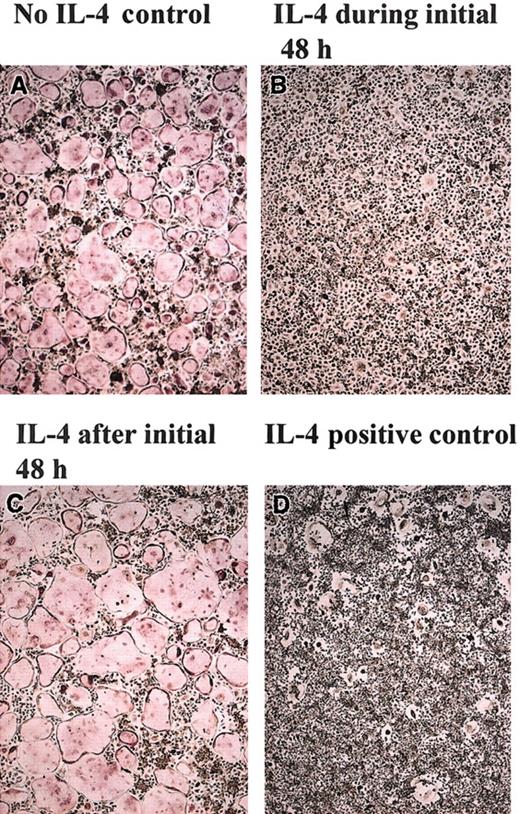
To understand the mechanism by which IL-4 targets the osteoclast precursor during the differentiation process and to determine whether the actions of IL-4 occur before or after the commitment of the precursor cell to the osteoclast lineage, we cultured primary BMM precursors with M-CSF and RANKL for varying amounts of time before the addition of IL-4 (Figure 5). We found that osteoclast precursors are responsive to IL-4 only during the first 48 hours of culture. After this “sensitive” period, the cells are no longer responsive to IL-4 and continue along the osteoclastogenic pathway, as evidenced by the presence of TRAP-positive multinucleated, mature osteoclasts. In the converse experiment, osteoclast precursors were cultured with M-CSF, RANKL, and IL-4 for an initial period, after which IL-4 was removed and the cells were further cultured with M-CSF and RANKL (Figure 5). Subsequent culture of the cells in the absence of IL-4 (but the continued presence of M-CSF and RANKL) failed to generate osteoclasts if IL-4 was present throughout the first 48 hours. However, we detected the presence of TRAP-negative MNG cells, similar to our findings when IL-4 was present throughout the entire culture period (Figure 1C). These data are consistent with the notion that during this critical 2-day period, each cytokine (RANKL or IL-4) induced the expression of groups of genes that forced differentiation toward (RANKL) or away from (IL-4) osteoclasts.
Irreversible commitment of BMMs to osteoclasts during the first 48 hours. BMMs from wild-type mice were cultured in the presence of rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) in the presence or absence of rmIL-4 (10 ng/mL) for various time periods, as indicated. (A) BMMs without rmIL-4. (B) rmIL-4 was present only during the first 48 hours. Then rmIL-4 was removed by washing the wells 3 times with α-10 media, and the cultures continued in this media and were complemented with rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) for 5 days. (C) After 2 days of culture in the presence of RANKL and rmM-CSF, rmIL-4 (10 ng/mL) was added to the cultures for 5 days. (D) rmIL-4 was present throughout the culture period. At the end of the culture period, cells were fixed and stained for TRAP. TRAP stain; original magnification, × 10 for all panels.
Irreversible commitment of BMMs to osteoclasts during the first 48 hours. BMMs from wild-type mice were cultured in the presence of rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) in the presence or absence of rmIL-4 (10 ng/mL) for various time periods, as indicated. (A) BMMs without rmIL-4. (B) rmIL-4 was present only during the first 48 hours. Then rmIL-4 was removed by washing the wells 3 times with α-10 media, and the cultures continued in this media and were complemented with rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) for 5 days. (C) After 2 days of culture in the presence of RANKL and rmM-CSF, rmIL-4 (10 ng/mL) was added to the cultures for 5 days. (D) rmIL-4 was present throughout the culture period. At the end of the culture period, cells were fixed and stained for TRAP. TRAP stain; original magnification, × 10 for all panels.
IL-4 regulates the expression of RANK mRNA
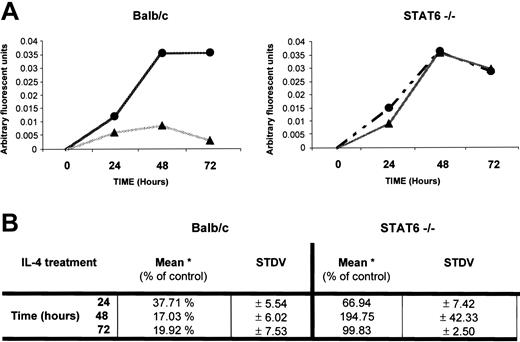
To better understand how IL-4 inhibits osteoclast differentiation, we focused on the first 48 hours, when lineage commitment appeared to be taking place. It is well established that the expression of RANK and signaling through this receptor is critical for osteoclast differentiation.39-41 Therefore, we analyzed whether IL-4 regulates RANK expression. We limited our analysis of RANK expression to the first 72 hours of culture because our results demonstrated that the first 48 hours are critical to osteoclast determination. Osteoclast progenitors from wild-type and STAT6-/- mice were analyzed for RANK expression using quantitative RT-PCR (Figure 6). The levels of RANK mRNA increased rapidly in precursor cells derived from bone marrow of wild-type or STAT6-/- mice between 0 and 48 hours after exposure to M-CSF and RANKL. In contrast, the addition of IL-4 to wild-type osteoclast precursors resulted in the inhibition of RANK mRNA expression. Although there was some elevation of RANK mRNA levels at early time points, levels in the wild-type cells exposed to IL-4 were significantly reduced after 48 hours (Figure 6A-B). However, in STAT6-/- precursors exposed to IL-4, RANK mRNA levels increased in parallel to the samples cultured in the absence of IL-4 (Figure 6A-B). These results suggest that IL-4 suppressed osteoclast development at least in part by inhibiting the induction of RANK mRNA by M-CSF and RANKL in a STAT6-dependent manner.
IL-4 regulation of RANK mRNA expression. BMMs were isolated from wild-type and STAT6-/- mice. These cells were cultured with rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) in the presence (▴) or absence (•) of rmIL-4 (10 ng/mL) for up to 72 hours, as indicated, and cDNA was generated from total RNA. Using Light-cycler PCR, we measured the expression of RANK and β-actin in the samples and calculated the ratio RANK versus actin values in arbitrary fluorescent units for each sample. (A) Expression of RANK in 1 representative experiment. (B) Levels of RANK normalized to that of β-actin (*) mRNA were expressed as a percentage of the control (no IL-4). Average value from 3 independent experiments is shown.
IL-4 regulation of RANK mRNA expression. BMMs were isolated from wild-type and STAT6-/- mice. These cells were cultured with rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) in the presence (▴) or absence (•) of rmIL-4 (10 ng/mL) for up to 72 hours, as indicated, and cDNA was generated from total RNA. Using Light-cycler PCR, we measured the expression of RANK and β-actin in the samples and calculated the ratio RANK versus actin values in arbitrary fluorescent units for each sample. (A) Expression of RANK in 1 representative experiment. (B) Levels of RANK normalized to that of β-actin (*) mRNA were expressed as a percentage of the control (no IL-4). Average value from 3 independent experiments is shown.
IL-4 inhibits surface expression of RANK receptor
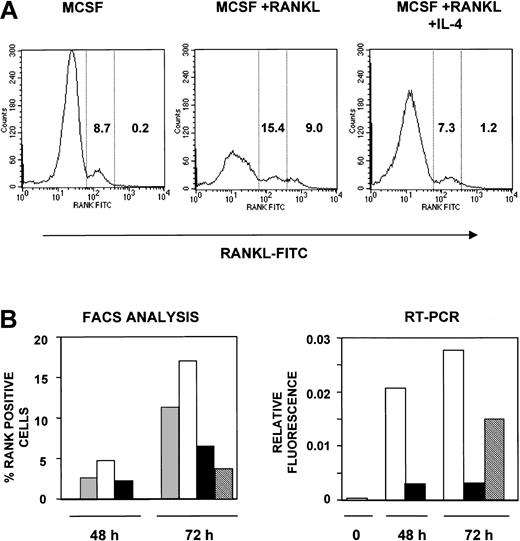
To analyze the cell surface expression of RANK and to enumerate the RANK-positive (RANK+) osteoclast precursors under various conditions, we used FITC-RANKL as previously described34 to detect RANK (Figure 7). Binding of this reagent to osteoclast precursor cells is dependent on RANK expression and is inhibited by OPG34 (and data not shown). Osteoclast precursors from wild-type animals cultured for 72 hours in the presence of M-CSF alone showed 8.7% RANK+ cells. The addition of RANKL to the culture resulted in an increase in the percentage of RANK+ cells to 24.4%, with 9% of these staining with greater intensity (greater than 500 mean fluorescence intensity [MFI]). However, culture in the presence of IL-4 suppressed the increase in RANK+ cells to levels seen in M-CSF alone (8.5% total, 1.2% greater than 500 MFI). These results suggest that IL-4 suppressed the increase in the percentage of RANK+ cells but that it does not eliminate all RANK+ precursor cells. Similar results were obtained after a 48-hour culture period, though the percentage of RANK+ cells observed at this time was lower (Figure 7B, left panel).
IL-4 inhibits the surface expression of RANK in osteoclast precursors. (A) Osteoclast precursors from wild-type mice were cultured for 72 hours in the presence of rmM-CSF (20 ng/mL), rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) or rmM-CSF (20 ng/mL), RANKL (150 ng/mL), and 10 ng/mL rmIL-4, respectively. Cells were stained using FITC-RANKL and were analyzed by flow cytometry for RANK expression. These data reflect RANK expression on the cell surface in one representative experiment. RANK-positive cells were divided into low- and high-expressing cells based on fluorescence intensity; the numbers in each column indicate the percentages of cells expressing low and high levels of RANK. (B) Osteoclast precursors from wild-type mice were cultured in the presence or absence of rmM-CSF (20 ng/mL), RANKL (150 ng/mL), and rmIL-4 (10 ng/mL) for 48 hours. For one group, media were removed. After several washes with complete media, new media were added to the cells containing rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) for another 24 hours, as indicated. After that, cells were harvested and analyzed. The left graph represents the percentage of RANK-expressing cells analyzed by flow cytometry using FITC-RANKL at 48 and 72 hours. Mean percentages from 2 independent experiments are shown. The right graph represents the ratio values in arbitrary fluorescent units for RANK versus actin mRNA using Light-cycler PCR, with the same methodology as used in Figure 6. ▦ indicates rmM-CSF; □, rmM-CSF + RANKL; ▪, rmM-CSF + RANKL + IL-4; and ▧, IL-4 washout at 48 hours.
IL-4 inhibits the surface expression of RANK in osteoclast precursors. (A) Osteoclast precursors from wild-type mice were cultured for 72 hours in the presence of rmM-CSF (20 ng/mL), rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) or rmM-CSF (20 ng/mL), RANKL (150 ng/mL), and 10 ng/mL rmIL-4, respectively. Cells were stained using FITC-RANKL and were analyzed by flow cytometry for RANK expression. These data reflect RANK expression on the cell surface in one representative experiment. RANK-positive cells were divided into low- and high-expressing cells based on fluorescence intensity; the numbers in each column indicate the percentages of cells expressing low and high levels of RANK. (B) Osteoclast precursors from wild-type mice were cultured in the presence or absence of rmM-CSF (20 ng/mL), RANKL (150 ng/mL), and rmIL-4 (10 ng/mL) for 48 hours. For one group, media were removed. After several washes with complete media, new media were added to the cells containing rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) for another 24 hours, as indicated. After that, cells were harvested and analyzed. The left graph represents the percentage of RANK-expressing cells analyzed by flow cytometry using FITC-RANKL at 48 and 72 hours. Mean percentages from 2 independent experiments are shown. The right graph represents the ratio values in arbitrary fluorescent units for RANK versus actin mRNA using Light-cycler PCR, with the same methodology as used in Figure 6. ▦ indicates rmM-CSF; □, rmM-CSF + RANKL; ▪, rmM-CSF + RANKL + IL-4; and ▧, IL-4 washout at 48 hours.
Because the ability of IL-4 to suppress osteoclastogenesis was irreversible, we tested whether the suppression of RANK expression or mRNA induction is also irreversible (Figure 7B). Osteoclast precursors were cultured in the presence of M-CSF with or without RANKL or IL-4 for 48 or 72 hours. After 48 hours, cells were washed to remove IL-4 and were cultured in the presence of M-CSF and RANKL for an additional 24 hours (total time, 72 hours). By cell surface staining (Figure 7B, left panel), we found that the removal of IL-4 after 48 hours did not restore the enhanced levels of RANK expression seen in M-CSF+RANKL–stimulated cells. Using highly sensitive RT-PCR analysis (Figure 7B, right panel), we found that the removal of IL-4 resulted in the detection of RANK mRNA to levels half those seen in M-CSF+RANKL–stimulated cells. RANK mRNA levels remained the same even after further incubation (more than 96 hours) in the absence of IL-4. These results suggest that the inhibition of RANK expression on the surface of cells is irreversible but that the suppression of mRNA expression may be partially reversible. However, the significance of this mRNA expression is unclear because the cells in the population are still unable to develop into osteoclasts in the presence of M-CSF+RANKL.
IL-4 fails to inhibit osteoclastogenesis in RANK-overexpressing cells
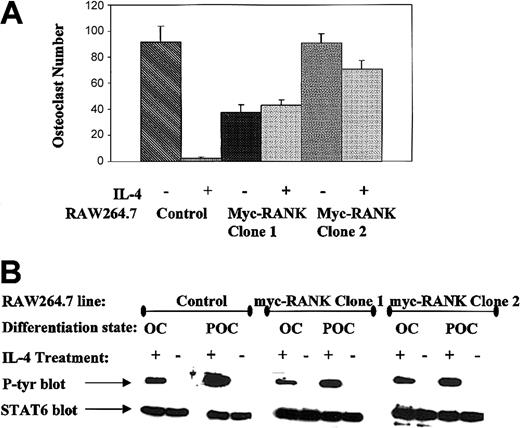
To directly test whether IL-4 suppresses osteoclast development by inhibiting the induction of RANK mRNA by M-CSF and RANKL, we asked whether IL-4 could suppress osteoclast development in the presence of ectopically driven RANK. We stably transfected murine RANK under the CMV promoter in the macrophage cell line RAW264.7. Two RANK-transfected cell lines, along with controls, were cultured under osteoclastogenic conditions in the presence or absence of murine IL-4 (Figure 8A). In the RAW264.7 cells expressing RANK as a result of transfection, IL-4 treatment did not inhibit osteoclast differentiation, even though it could clearly still signal the tyrosine phosphorylation of STAT6 (Figure 8B). These results indicate that IL-4 does not inhibit osteoclast development by simply blocking RANK signal transduction, as has been previously suggested.5,7
IL-4 fails to inhibit osteoclastogenesis in RANK-overexpressing cells. (A) Control RAW264.7 cells or RANK-transfected clones 1 and 2 were differentiated to osteoclasts in the presence of rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) ± 10 ng/mL rmIL-4. TRAP-positive multinucleated (more than 3 nuclei) cells were counted. The average number of osteoclasts generated from 3 independent experiments is presented with their respective standard deviation. (B) To ensure that transfected clones were not impaired in IL-4 signaling, growing RAW264.7 (POC) or mature osteoclasts (OC) derived from control, RANK-transfected clone 1 or 2 were stimulated with 0 or 10 ng/mL IL-4 as indicated for 10 minutes, lysed, STAT6 immunoprecipitated, blotted, and probed with an antiphosphotyrosine antibody. To ensure equal protein loading, the blots were stripped and reprobed with anti-STAT6 antibody.
IL-4 fails to inhibit osteoclastogenesis in RANK-overexpressing cells. (A) Control RAW264.7 cells or RANK-transfected clones 1 and 2 were differentiated to osteoclasts in the presence of rmM-CSF (20 ng/mL) and RANKL (150 ng/mL) ± 10 ng/mL rmIL-4. TRAP-positive multinucleated (more than 3 nuclei) cells were counted. The average number of osteoclasts generated from 3 independent experiments is presented with their respective standard deviation. (B) To ensure that transfected clones were not impaired in IL-4 signaling, growing RAW264.7 (POC) or mature osteoclasts (OC) derived from control, RANK-transfected clone 1 or 2 were stimulated with 0 or 10 ng/mL IL-4 as indicated for 10 minutes, lysed, STAT6 immunoprecipitated, blotted, and probed with an antiphosphotyrosine antibody. To ensure equal protein loading, the blots were stripped and reprobed with anti-STAT6 antibody.
TNF osteoclastogenesis
Recently it has been shown that osteoclastogenesis promoted by TNF-α is independent of RANKL-induced signaling.30 We cultured osteoclast progenitors from wild-type and STAT6-/- animals with 100 ng/mL M-CSF for 3 days, after which we added either TNF-α (20 ng/mL) or TNF-α and IL-4 (10 ng/mL) to the cultures for the next 3 days, keeping the concentration of M-CSF constant. Subsequently, the plates were fixed and stained for TRAP expression to identify osteoclast. As shown in Figure 9, IL-4 inhibited osteoclast differentiation induced by TNF-α in wild-type precursors but not in STAT6-/- precursors. Interestingly, we observed large numbers of TRAP-negative MNG cells in the wild-type cultures treated with TNF-α and IL-4, strongly supporting a role for IL-4 in promoting an alternative differentiation pathway. The inhibition of osteoclastogenesis by IL-4 in a RANKL-independent context further argued against the notion that IL-4 blocks osteoclast differentiation by simply blocking RANK signaling.
IL-4 inhibition of TNF-α–induced osteoclastogenesis. Bone marrow cells from wild-type and STAT6-/- animals were cultured with rmM-CSF (100 ng/mL) for 3 days. Subsequently, RANKL (150 ng/mL) ± rmIL-4 (10 ng/mL) or rmTNF (20 ng/mL) ± rmIL-4 (10 ng/mL) was added to the BMM, keeping the concentration of M-CSF (100 ng/mL) constant until the end of the experiment 3 days later. Cells were fixed and stained for TRAP activity. TRAP stain; original magnification, × 10.
IL-4 inhibition of TNF-α–induced osteoclastogenesis. Bone marrow cells from wild-type and STAT6-/- animals were cultured with rmM-CSF (100 ng/mL) for 3 days. Subsequently, RANKL (150 ng/mL) ± rmIL-4 (10 ng/mL) or rmTNF (20 ng/mL) ± rmIL-4 (10 ng/mL) was added to the BMM, keeping the concentration of M-CSF (100 ng/mL) constant until the end of the experiment 3 days later. Cells were fixed and stained for TRAP activity. TRAP stain; original magnification, × 10.
Discussion
Numerous reports have indicated that immune cytokines can influence bone biology. In particular, IL-4 has been shown to have effects on osteoblasts and osteoclasts. Transgenic mice expressing IL-4 under the control of the lck proximal promoter demonstrated profound osteoporosis likely caused by the suppression of osteoblast function.35 Because IL-4 had such diverse effects on monocytic precursors and osteoblasts, the precise mechanism for the IL-4 effects in vivo and in complex in vitro coculture systems was unclear. With the discovery of RANKL as an obligate factor to regulate osteoclast development from monocytic precursors in the absence of stromal cells, it has become possible to define the molecular target for IL-4 effects on osteoclastogenesis and function. Previous reports have demonstrated that IL-4 prevents the generation of osteoclasts induced by M-CSF and RANKL.5,7 Herein we demonstrate that IL-4, in addition to inhibiting the differentiation of precursors to mature osteoclasts, directly inhibits the bone resorption activity of fully differentiated, mature osteoclasts. We found that the IL-4–induced inhibition of osteoclast differentiation and functional activity is STAT6 dependent. The minimal concentration of IL-4 required to prevent osteoclast differentiation (1 ng/mL in our experiments) correlated with maximal tyrosine phosphorylation of STAT6, whereas lower concentrations led to suboptimal levels of STAT6 phosphorylation and to only partial inhibition of osteoclastogenesis. Similar observations were made using precursor cells of different origins, including human CD14+ monocytes, murine bone marrow precursors, and the macrophage cell line RAW264.7.
In addition to STAT6, activation of the IL-4 receptor in most cell types analyzed leads to activation of the IRS pathway. Our analysis of IL-4 signaling in osteoclasts and their precursors demonstrates that though the cells express IRS-2, the addition of IL-4 does not activate this signal transduction pathway. These results suggest that the IRS-2 signaling pathway is not critical to the IL-4 effect on precursors or mature cells. Consistent with our results, IRS-2 knockout mice do not have any osteoclast defects.38
Using a stromal-cell–free culture system, we have shown that the IL-4 effect on osteoclast differentiation is irreversible. The ability of IL-4 to suppress osteoclastogenesis was dependent on the timing of the addition of IL-4. Adding IL-4 during the first 48 hours of culture with M-CSF and RANKL was necessary and sufficient to inhibit osteoclast development. In contrast to other reports, we found that removing IL-4 after 48 hours did not reverse the inhibition. Furthermore, the addition of IL-4 after 48 hours of culture in the presence of M-CSF and RANKL did not suppress osteoclast development. Our results are consistent with older studies showing that the treatment of M-CSF–dependent macrophages with increasing concentrations of IL-4 for 48 hours before the introduction of stromal cells using 1,25-(OH)2D3 and dexamethasone was sufficient to impact irreversibly the osteoclastic cell-forming potential of these cells in a concentration-dependent manner.8 In addition, it has been observed that brief (30-minute) pre-exposure to IL-4 or simultaneous exposure of cells to IL-4 and RANKL was sufficient to completely block osteoclastogenesis and that an initial, single exposure of osteoclast progenitor cells to IL-4 attenuated osteoclastogenesis despite subsequent multiple additions of RANKL.7 Interestingly, though the generation of TRAP-positive multinucleated cells was suppressed by IL-4 if added during the first 48 hours of culture, we detected the appearance of TRAP-negative MNG cells. Consistent with our findings, Lewis et al35 observed TRAP-negative multinucleated cells in bone sections derived from transgenic animals expressing IL-4 under the lck promoter. It was shown many years ago that IL-4 promotes the formation of MNG cells from monocytic precursors and that this promotion became irreversible after a 48-hour exposure to IL-4.36 These results suggest that IL-4 alters the developmental program of the monocytic precursor cells away from osteoclasts and promotes the developmental program of MNG cells. Furthermore, the similarities of the IL-4 effects on osteoclastogenesis induced by a RANKL-independent process, namely TNF-α, blocking and inducing the formation of MNG cells in those cultures, points toward a role for IL-4 in the establishment of an alternative differentiation pathway for cells of the monocyte-macrophage lineage.
Our results demonstrating that the inhibition of osteoclastogenesis by IL-4 is mediated by STAT6 are consistent with those of 2 other reports.5,7 However, we provide further evidence that the STAT6-dependent regulation of RANK mRNA and protein expression may be a dominant mechanism of action. This is distinct from the conclusions presented in the previous reports. Wei et al5 report that when osteoclast precursors were treated for 3 days with IL-4, they failed to respond to RANKL as measured by RANK-mediated activation of NF-κB and mitogen-activated protein (MAP) kinases p38, JNK, and ERK. Our results provide a rationale for the absence of RANK-generated signals under these culture conditions. Specifically, IL-4 profoundly prevents the expression of RANK on differentiating osteoclast precursors in a STAT6-dependent manner. Given that after 3 days in cytokine culture the cells would not express sufficient RANK, treatment with RANKL would not generate the activation of NF-κB or MAP kinase. Our observation that IL-4 fails to inhibit the differentiation of RAW264.7 cells overexpressing RANK is strong evidence that IL-4 and STAT6 do not block RANK signal transduction. In this regard, we did not detect any differences in TRAF6 levels or in NF-κB activation in cells incubated in the presence or absence of IL-4 (data not shown), consistent with the observations reported by Wei et al5 but in contrast to those reported by Abu-Amer.7 Abu-Amer7 reported that a short (10-minute) treatment of precursor cells with IL-4 could suppress the RANKL-induced activation of NF-κB and subsequent osteoclastogenesis in a STAT6-dependent manner. Based on the known biochemistry of STAT6 activation and the relatively long period of time needed to regulate gene expression by STAT6, it is unclear how such brief IL-4 treatment could have a profound effect on cellular differentiation.
Our results suggest that IL-4 suppresses osteoclastogenesis by inhibiting the expression of RANK mRNA in developing precursor cells induced in the presence of M-CSF and RANKL. This suppression of RANK mRNA is mediated by STAT6. On the basis of our observations, we propose that IL-4 inhibits osteoclast differentiation by regulating gene expression through the transcription factor STAT6. One such target gene is RANK itself. STAT6 is known not only to increase gene expression but to suppress gene expression in response to IL-4. Its action is mediated by its ability to bind DNA sequences in promoters of genes. In some cases STAT6 promotes gene transcription, such as CD23,11 and in other cases it inhibits gene transcription by blocking the binding of other critical transcription factors to the appropriate promoter region, such as E-selectin.42 It is interesting to speculate that STAT6 blocks transcription of the RANK promoter induced by M-CSF and RANKL in precursor cells. If this is indeed the case, it might suggest that the IL-4–induced suppression of RANK mRNA is reversible but that the sum effect of IL-4 on the developmental status of the precursor represents an irreversible cell-fate choice.
In addition to RANK, it is likely IL-4 also regulates the expression of a number of other genes in the monocytic precursor cells. Interestingly, a number of different cell types can be derived from the same precursor population. McInnes and Rennick36 described the formation of MNG cells from bone-marrow–derived precursors cultured in the presence of M-CSF and IL-4. They proposed that IL-4 promoted the irreversible development of these cells. Addition of an anti–IL-4 antibody after 2 days of culture did not inhibit the differentiation of the MNG cells already committed. In addition, it has been shown that dendritic cells and osteoclasts share a common origin from the same precursor cell (c-Kit+ c-fms+ RANK- cell) by a lineage bifurcation process, in which the regulation of c-fos by M-CSF and GM-CSF is able to direct the precursor cell toward 2 different cell types with different functions.1 The inhibitory effect of GM-CSF on osteoclastogenesis was observed only when GM-CSF was added on day 0, and the inhibition was decreased as the addition of GM-CSF was delayed. Thus, after the cells were exposed initially to the signals of M-CSF and RANKL, they became committed to the osteoclast pathway and were no longer competent to respond to GM-CSF. We propose that, in a similar fashion, IL-4 regulates the lineage commitment of precursor cells by regulating gene expression, resulting in the generation of cell types morphologically and functionally different— that is, osteoclasts versus MNG cells.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2002-11-3437.
Supported by National Institutes of Health (NIH) grants AI38985 and R21CA84636 and by the American Red Cross.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Christina Celluzzi for supplying us with the human cells and Dr Robert Hawley for critical reading of this manuscript and for support during the course of this work.