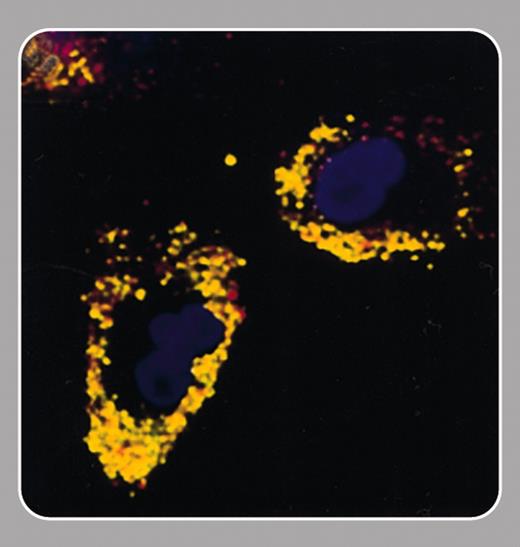
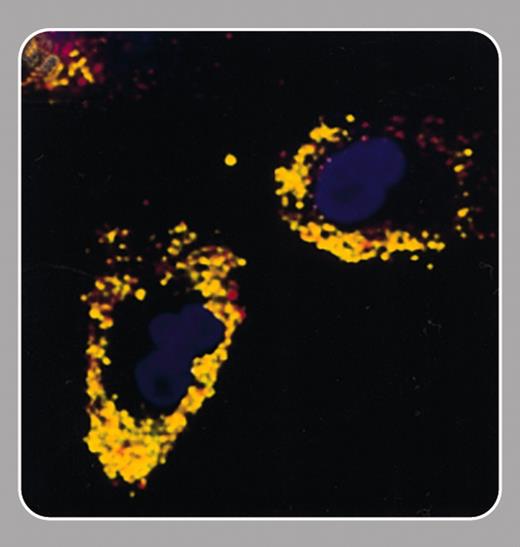
Recent progress in stem cell biology has made cell-based therapies for the treatment of tissue damage a realistic prospect. The ability to track noninvasively the migration of these cells after implantation could aid the development of this therapeutic approach. In this issue Hinds and colleagues (page 867) describe a cell-labeling method that will allow cell tracking using an imaging modality, magnetic resonance imaging (MRI), that is widely used in the clinic.
The idea of labeling cells with MRI-detectable paramagnetic complexes is not new (Hawrylak et al, Exp Neurol. 1993;121: 181-192). Studies over the last 10 years have defined the requirements of such labels. They should be very sensitive to MRI detection, cell uptake should be rapid and uniform, there should be even distribution to daughter cells, and they should have no effect on cell viability, proliferation, or function. The agent described by Hinds et al fits the bill on all counts. An important attribute, which is shared by some other particles of this type, is that it permits detection of single cells (Dodd et al, Biophys J. 1999; 76:103-109). This is possible since, although MR image resolutions are rarely comparable with cell dimensions, the particles distort the magnetic field far beyond the cell. Thus water molecules moving within the vicinity of a cell are affected, and the effect of the label is amplified enormously. A distinguishing feature of the agent described by Hinds et al is that it is relatively large, and this should allow detection of single cells at the lower image resolutions that can be achieved in vivo.
MRI detection of labeled cells is one of a growing number of noninvasive methods for investigating biologic processes in vivo (Weissleder and Mahmood, Radiology. 2001;219:316-333). These techniques are a valuable tool in the laboratory, but perhaps more importantly, they can also be used to help translate our understanding of these processes into new treatments in the clinic.