Abstract
Eotaxin-3 (CCL26) belongs to the group of CC chemokines that attract eosinophils, basophils, and Th2 lymphocytes. Like eotaxin (CCL11) and eotaxin-2 (CCL24), eotaxin-3 mediates its activity through CCR3. Here we show that eotaxin-3 also binds to CCR2 on monocytes and CCR2-transfected cells. In contrast to monocyte chemotactic protein 1 (MCP-1; CCL2), eotaxin-3 does not trigger intracellular calcium mobilization, enzyme release, or phosphorylation of the mitogen-activated protein (MAP) kinase ERK and induces a weak chemotaxis in monocytes. Instead, eotaxin-3 inhibits MCP-1–mediated responses, thus acting as a natural antagonist for CCR2. This study also demonstrates that eotaxin-3 promotes active movement of monocytes away from a gradient of eotaxin-3 in vitro. This repellent effect is amplified when an additional gradient of MCP-1 is applied, demonstrating that the 2 mechanisms are synergistic. Eotaxin-3 effects on monocytes are largely abolished when cells are pretreated with MCP-1 or CCR2 antagonists. Like MCP-1–mediated migration, repulsion is sensitive to Bordetella pertussis toxin, indicating the involvement of Gi protein–coupled receptors. However, using transfected cells expressing CCR2 we could not detect F-actin formation or an active movement away induced by eotaxin-3, suggesting that either expression of a single receptor type is not sufficient to mediate cell repulsion or that the used transfected cell lines lack additional interaction molecules that are required for reverse migration. Eotaxin-3 was expressed by vascular endothelial cells and was essential for endothelial transmigration of eosinophils. Our data provide a mechanism by which 2 chemokine gradients that are oriented in opposite directions could cooperate in efficiently driving out monocytes from blood vessels into tissue.
Introduction
Like eotaxin (CCL11) and eotaxin-2 (CCL24), eotaxin-3 (CCL26) has been reported as a selective stimulus for eosinophilic granulocytes by interacting with the chemokine receptor CCR3.1-4 This receptor is highly expressed on eosinophils, basophils, and Th2 lymphocytes,5-9 and is therefore involved mainly in allergic inflammation.10,11 CCR3-recruiting chemokines and monocyte chemotactic protein 1 (MCP-1) were found to be expressed in several tissues, especially in pathologic reactions, in which Th2-type cytokines are produced.12-16 In addition to eosinophils, basophils, and Th2 lymphocytes, which are typically found in allergic inflammations and express CCR3, these infiltrates also contain monocytes that lack CCR3.17
Eotaxin-3 is expressed in vascular endothelial cells and dermal fibroblasts after interleukin 4 (IL-4) and IL-13 stimulation,18,19 and in bronchial tissue on allergen challenge.20,21 In a current report, its relevance for transendothelial migration of eosinophils has been elucidated,22 whereas the significance of the constitutive expression in the reproductive system and the heart2 remains to be clarified. Eotaxin-3 is as efficient as eotaxin in attracting eosinophils and inducing shape changes in basophils,23 indicating that it is an important effector chemokine in allergic conditions. The present study reveals that eotaxin-3 acts also as an antagonist on CCR2, a chemokine receptor that is widely expressed on leukocytes and is essential for inflammatory reactions.24-26
We reported recently that eotaxin is also a CCR5 agonist and a CCR2 antagonist and affects the responses of monocytes, which express both receptors.27 Similar to the antagonistic activity of eotaxin on CCR2, eotaxin-3 inhibits MCP-1–mediated responses on monocytes and CCR2-transfected cells. Natural chemokine antagonists could represent a new mechanism orchestrating the fine-tuning of cellular responses at sites of inflammation, in which more than one chemokine is produced.
Furthermore, we show here that eotaxin-3 has the exceptional property to actively repulse monocytes. Exposure of monocytes to a combination of a gradient of the agonist MCP-1 and an oppositely oriented eotaxin-3 gradient results in significantly higher migration efficacy. This indicates that both stimuli synergize. A previous report on T lymphocytes shows that the active movement away from a gradient of stromal-derived factor 1 (SDF-1) is CXCR4 dependent, as forward migration.28 We show that monocyte repulsion by eotaxin-3 is inhibited by MCP-1 and CCR2 antagonists, indicating that CCR2 availability is necessary for this effect. Nevertheless, the effect was to the same extent sensitive to Bordetella pertussis toxin as chemotaxis in response to agonists, indicating that both events involve Gi protein–coupled receptors. Our data suggest that eotaxin-3 plays a role in the subtle regulation of monocyte responses in inflammation and might contribute to the precise positioning of monocytes in vivo by providing a repulsive guidance cue.
Materials and methods
Cells
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of donor blood (Central Laboratory of the Swiss Red Cross, Basel, Switzerland) by Fycoll-Paque density centrifugation. Monocytes (95% purity with 5% contaminating lymphocytes), CD14+, were isolated by a positive immunoselection procedure (CD14 MicroBeads, Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions or by Percoll gradient.29 Stable transfection of human CCR1, CCR2, CCR5, CCR8, and CXCR4 in murine pre-B 300.19 cells was performed as described before.27
Chemokines
All chemokines and their analogs were chemically synthesized as previously described.30
Receptor binding
125I-MCP-1 was purchased from Amersham Pharmacia Biotech (Uppsala, Sweden). For competition binding studies, 4 × 106 freshly isolated monocytes or CCR2-transfected 300.19 cells were incubated for 90 minutes on ice with 0.8 nM 125I-MCP-1 in the presence of increasing concentrations of unlabeled MCP-1 or eotaxin-3 as described in detail previously.27 Unbound radioactivity was removed by centrifugation through 6% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and cell-bound radioactivity was determined by γ counting. The data were analyzed by PRISM software (GraphPad Software, San Diego, CA), using nonlinear regression in a one binding-site model.
Chemokine receptor expression and internalization
CCR2 expression in freshly isolated monocytes was analyzed by flow cytometry (FACScan, Becton Dickinson, San Jose, CA). The cells were incubated for 30 minutes in fluorescence-activated cell sorting (FACS) buffer (2% fetal calf serum [FCS], 0.1% sodium azide in PBS) with phycoerythrin (PE)–conjugated anti-CCR2b (FAB151P; R&D Systems, Oxon, United Kingdom). Isotype-matched immunoglobulins were used as controls. For determination of chemokine-induced receptor internalization, the cells were incubated at 37°C with MCP-1 (10 nM) or eotaxin-3 (1 μM) in FACS buffer without sodium azide before staining. Controls were performed under the same conditions at 4°C. To remove receptor-bound eotaxin-3 that interfered with anti-CCR2 antibody recognition, cells were washed in ice-cold acidic glycine buffer (50 mM glycine in 100 mM NaCl, pH 3.0) for 30 seconds before staining. To assess the inhibition of MCP-1–induced internalization by eotaxin-3 on monocytes, eotaxin-3 (for 20 minutes) and MCP-1 (10 nM for 10 minutes) were sequentially applied before receptor expression evaluation.
Calcium transients
[Ca++]i changes were measured in fura-2–loaded cells as previously described.31 Cells were sequentially stimulated with eotaxin-3 and MCP-1 at 90-second intervals. The rate of the [Ca2+]i rise (percentage of fura-2 saturation per second) induced by 10 nM MCP-1 was set to 100% and the percentage rate obtained after pretreatment with eotaxin-3 was calculated.
Actin polymerization
F-actin formation was determined with fluorescein isothiocyanate (FITC)–coupled phalloidin (Sigma, Munich, Germany) in chemokine-stimulated CCR1-, CCR2-, CCR5-, CCR8-, and CXCR4-transfected 300.19 murine pre-B cells and in freshly isolated human monocytes. The reaction was stopped after 10 seconds and cells fixed with cold paraformaldehyde (4%) in PBS. For inhibition studies cells were pretreated with eotaxin-3 for 3 minutes and then stimulated with MCP-1. After paraformaldehyde fixation, cells were permeabilized with 0.1% Triton X-100, stained with FITC-conjugated phalloidin (6 μg/mL), and analyzed by FACS. All stimulations were performed in duplicate. Relative F-actin formation was calculated as the ratio of the mean fluorescence intensity between stimulus-induced and basal phalloidin loading.
N-acetyl-β-d-glucosaminidase release
Chemokine-induced release of N-acetyl-β-D-glucosaminidase in cytochalasin-B–treated monocytes was detected as described before.32
Migration
Quantitative transmigration assays were performed using a 24-transwell system (Corning Costar, Corning, NY; 6.5-mm diameter, 5-μm pore size, polycarbonate membrane). Then 400 × 103 cells were added to the upper well. In some cases, as indicated in the figures, the cells were preincubated for 10 minutes with 1 μM eotaxin-3 and then applied in the upper wells without removing the chemokine from the medium. Various concentrations of the different chemokines were added to the bottom chamber of the transwell. Transwells were incubated for 1 hour at 37°C in 5% CO2, and migrated cells collected with cold PBS. The cell count was performed for 120 seconds using a FACScan. Results are shown as chemotaxis index, which represents the ratio between cells migrated in the presence of a chemokine and cells migrated in response to medium alone.
To perform a standard “checkerboard” analysis of positive, negative, and absent gradients, different concentrations of eotaxin-3 were placed in the lower well of a 24-transwell system. Monocytes were applied in the upper well with different eotaxin-3 concentrations (10 nM, 100 nM, or 1 μM). Transwells were incubated for 1 hour at 37°C in 5% CO2, and migrated cells evaluated as described.
Additional transmigration assays were performed using 48-well Boyden chambers (Neuro Probe, Cabin John, MD) with 5-μm pore polycarbonate membranes.32 In some cases, as indicated in the figures, the cells were preincubated for 10 minutes with 1 μM eotaxin-3 and then applied in the upper wells without removing the chemokine from the medium. Migration was allowed to proceed for 60 minutes at 37°C in 5% CO2. The membrane was then removed, washed on the upper side with PBS, fixed, and stained. All assays were done in triplicate, and the migrated cells were counted in 5 randomly selected fields at 1000-fold magnification. Spontaneous migration was determined in the absence of chemoattractant.
For inhibition studies, cells were pretreated with 1 μg/mL B pertussis toxin (Calbiochem, Schwalbach, Germany) at 37°C for 30 minutes or with 1 μg/mL genistein (Alexis, Lausen, Switzerland) at 37°C for 20 minutes.
To abolish CCR2-mediated responses on monocytes, cells were preincubated with 1 μM MCP-1, MCP-1 (9-76), or RANTES (9-68) and then applied in the upper wells without removing the chemokine from the medium.33
Western blot analysis
Freshly isolated monocytes (106 cells/time point) were incubated in RPMI/HEPES (N-2-hydroxyethylpiperazine-N′-ethanesulfonic acid) for 10 minutes at 37°C, stimulated with MCP-1 (100 nM or 10 nM), macrophage inflammatory protein 1β (MIP-1β; 100 nM), or eotaxin-3 (300 nM). The reaction was stopped by addition of 10% tricloricacetic acid (TCA). For desensitization studies, monocytes were treated with MCP-1 (10 nM) or eotaxin-3 (300 nM) for 3 minutes and then stimulated with MCP-1 (10 nM) for 1 minute. Whole cell lysates were separated on 11% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. Activated ERK was detected with an antidiphospho-ERK antibody (1:10 000-M8159, Sigma). Enhanced chemiluminescence was used for detection of horseradish peroxidase–conjugated secondary antibody (Bio-Rad Laboratories, Hercules, CA). Equal loading was confirmed by reprobing with an antibody against total ERK-2 (C-14; Santa Cruz Biotechnology, Santa Cruz, CA) as described previously.34
Statistical analysis
Statistical analysis of the results obtained was performed using the ANOVA test to compare differences in cell migration.
Results
Eotaxin-3 binds to CCR2 and acts as a CCR2 antagonist
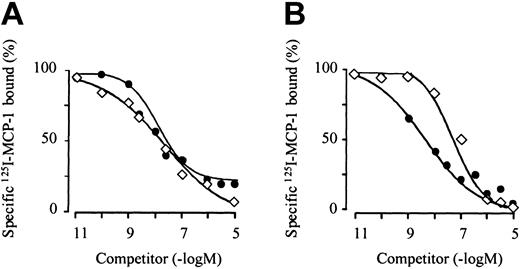
As shown in Figure 1, eotaxin-3 competes for the binding of 125I-MCP-1 on monocytes and CCR2-transfected cells. The receptor affinity of eotaxin-3 on monocytes (Figure 1A) is comparable to that of the selective agonist MCP-1, whereas on CCR2-transfected cells (Figure 1B) it is about one order of magnitude lower. Median effective concentration (EC50) values for MCP-1 and eotaxin-3 on monocytes were 8.3 nM and 17.9 nM, respectively. EC50 values for MCP-1 and eotaxin-3 on CCR2-transfected cells were 3.5 nM and 97.6 nM, respectively.
Eotaxin-3–binding competition with 125I-MCP-1 on monocytes and CCR2-transfected cells. Freshly isolated monocytes (A) or CCR2-transfected cells (B) were incubated with 0.8 nM 125I-MCP-1 in the presence of increasing concentrations of unlabeled MCP-1 (•) or eotaxin-3 (⋄) and cell-bound radioactivity was counted. The curves (means of duplicate determinations) are representative for 1 of 3 experiments with monocytes from different donors and 1 of 3 experiments performed with CCR2-transfected cells.
Eotaxin-3–binding competition with 125I-MCP-1 on monocytes and CCR2-transfected cells. Freshly isolated monocytes (A) or CCR2-transfected cells (B) were incubated with 0.8 nM 125I-MCP-1 in the presence of increasing concentrations of unlabeled MCP-1 (•) or eotaxin-3 (⋄) and cell-bound radioactivity was counted. The curves (means of duplicate determinations) are representative for 1 of 3 experiments with monocytes from different donors and 1 of 3 experiments performed with CCR2-transfected cells.
To further assess the effects of eotaxin-3 on monocytes, we performed several functional assays. In contrast to the CCR2 agonist MCP-1, eotaxin-3 does not induce a rapid increase of intracellular free calcium ([Ca++]i; Figure 2A), release of N-acetyl-β-D-glucosaminidase (Figure 2C), or CCR2 internalization (data not shown). When eotaxin-3 is applied prior to stimulation of monocytes with MCP-1, [Ca++]i increase (Figure 2B), enzyme release (Figure 2D), receptor internalization (Figure 2F), and actin polymerization (data not shown) are inhibited. Different doses of eotaxin-3 are necessary for inhibiting MCP-1–induced monocyte functions; whereas [Ca++]i increase and enzyme release are already affected by 100 nM, 1 μM eotaxin-3 is required to inhibit receptor internalization (Figure 2F) and actin polymerization. Eotaxin-3 inhibits monocyte migration to MCP-1 when applied to the lower well of the chamber (Figure 2E). The inhibition of MCP-1–induced migration by eotaxin-3 is statistically significant (P < .05).
Eotaxin-3 is a CCR2 antagonist on monocytes. (A) Changes of [Ca++]i in fura-2–loaded cells sequentially stimulated with eotaxin-3 and MCP-1 at 90-second intervals were monitored. (B) The rate of [Ca++]i rise (% fura saturation/second) induced by MCP-1 was set to 100% and the rate after eotaxin-3 prestimulation was calculated. The results of 1 of 3 independent experiments are shown. (C) MCP-1 (•) or eotaxin-3 (▴)–induced N-acetyl-β-d-glucosaminidase release was assessed in cells treated with cytochalasin-B. Mean values (± SD) of 3 experiments performed with monocytes from different donors are shown. (D) Inhibition of MCP-1–induced N-acetyl-β-d-glucosaminidase release by eotaxin-3 was detected in monocytes sequentially stimulated with increasing concentrations of eotaxin-3 and 10 nM MCP-1. Mean values (± SD) of 3 experiments performed with monocytes from different donors are shown. (E) Monocyte chemotaxis induced by MCP-1 (10 nM) or MCP-1 (10 nM) plus eotaxin-3 (1 μM) was evaluated in Boyden microchambers. Chemotaxis index (mean ± SD) of 3 experiments performed with monocytes from different donors is shown. (F) Eotaxin-3 inhibits MCP-1–induced CCR2 internalization. The histograms represent CCR2 expression on unstimulated cells (dark-filled), cells stimulated with MCP-1 (10 nM; thin line), and cells sequentially stimulated with eotaxin-3 (1 μM) and MCP-1 (10 nM). One representative of 3 experiments performed with monocytes from different donors is shown.
Eotaxin-3 is a CCR2 antagonist on monocytes. (A) Changes of [Ca++]i in fura-2–loaded cells sequentially stimulated with eotaxin-3 and MCP-1 at 90-second intervals were monitored. (B) The rate of [Ca++]i rise (% fura saturation/second) induced by MCP-1 was set to 100% and the rate after eotaxin-3 prestimulation was calculated. The results of 1 of 3 independent experiments are shown. (C) MCP-1 (•) or eotaxin-3 (▴)–induced N-acetyl-β-d-glucosaminidase release was assessed in cells treated with cytochalasin-B. Mean values (± SD) of 3 experiments performed with monocytes from different donors are shown. (D) Inhibition of MCP-1–induced N-acetyl-β-d-glucosaminidase release by eotaxin-3 was detected in monocytes sequentially stimulated with increasing concentrations of eotaxin-3 and 10 nM MCP-1. Mean values (± SD) of 3 experiments performed with monocytes from different donors are shown. (E) Monocyte chemotaxis induced by MCP-1 (10 nM) or MCP-1 (10 nM) plus eotaxin-3 (1 μM) was evaluated in Boyden microchambers. Chemotaxis index (mean ± SD) of 3 experiments performed with monocytes from different donors is shown. (F) Eotaxin-3 inhibits MCP-1–induced CCR2 internalization. The histograms represent CCR2 expression on unstimulated cells (dark-filled), cells stimulated with MCP-1 (10 nM; thin line), and cells sequentially stimulated with eotaxin-3 (1 μM) and MCP-1 (10 nM). One representative of 3 experiments performed with monocytes from different donors is shown.
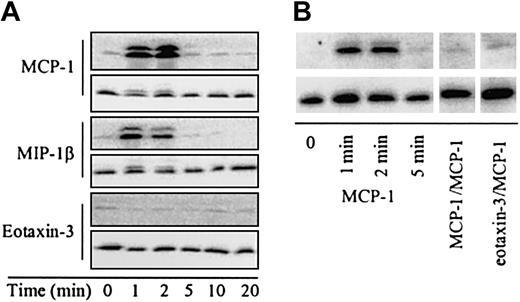
In contrast to MCP-1 and MIP-1β, which induce ERK phosphorylation by the triggering of CCR2 or CCR5, respectively, on monocytes, eotaxin-3 does not lead to ERK activation (Figure 3A).
Effect of eotaxin-3, MCP-1, and MIP-1β on ERK activation. Activated ERK was determined by Western blot analysis. Freshly isolated monocytes were stimulated with chemokines for the indicated times and phosphorylated ERK was detected with an antibody against diphospho-ERK. (A) Eotaxin-3 does not induce ERK phosphorylation. Cells were stimulated with MCP-1 (100 nM), MIP-1β (100 nM), or eotaxin-3 (300 nM) for the indicated times (upper blots). (B) Eotaxin-3 inhibits MCP-1–induced ERK phosphorylation. ERK phosphorylation in monocytes on MCP-1 (10 nM) stimulation for the indicated times is shown in the left panel. In the central and right panels the cells were treated with MCP-1 (10 nM) or eotaxin-3 (300 nM) for 3 minutes, and then stimulated with MCP-1 (10 nM) for 1 minute. To confirm equal loading, the blots were subsequently stripped and reprobed with an antibody directed against total ERK (lower blots).
Effect of eotaxin-3, MCP-1, and MIP-1β on ERK activation. Activated ERK was determined by Western blot analysis. Freshly isolated monocytes were stimulated with chemokines for the indicated times and phosphorylated ERK was detected with an antibody against diphospho-ERK. (A) Eotaxin-3 does not induce ERK phosphorylation. Cells were stimulated with MCP-1 (100 nM), MIP-1β (100 nM), or eotaxin-3 (300 nM) for the indicated times (upper blots). (B) Eotaxin-3 inhibits MCP-1–induced ERK phosphorylation. ERK phosphorylation in monocytes on MCP-1 (10 nM) stimulation for the indicated times is shown in the left panel. In the central and right panels the cells were treated with MCP-1 (10 nM) or eotaxin-3 (300 nM) for 3 minutes, and then stimulated with MCP-1 (10 nM) for 1 minute. To confirm equal loading, the blots were subsequently stripped and reprobed with an antibody directed against total ERK (lower blots).
Eotaxin-3 pretreatment of monocytes, as well as MCP-1/MCP-1 homologous desensitization, was able to almost completely abolish MCP-1–induced ERK phosphorylation, as shown in Figure 3B.
In addition, we performed migration analysis of CCR2-transfected cells. Eotaxin-3 is able to inhibit MCP-1–induced migration if applied to the lower well with MCP-1 (Figure 4A) or to the upper well together with the cells (Figure 4B). Together with the competition for MCP-1 binding, these data indicate that eotaxin-3 acts as a natural antagonist on CCR2.
Eotaxin-3 acts as an antagonist on CCR2-transfected cells. (A) MCP-1 induced chemotaxis of CCR2-transfected cells in the presence (▴) or absence (•) of eotaxin-3 at 1 μM. Cell migration was evaluated in Boyden microchambers. All determinations were performed in triplicate. The control indicates the number of cells migrating in medium alone. (B) Chemotaxis of CCR2-transfected cells was evaluated in 24-transwell chambers. Where indicated, eotaxin-3 (1 μM) was applied to the cells in the upper well, and 10 nM MCP-1–induced migration was assessed. Data are expressed as chemotaxis index.
Eotaxin-3 acts as an antagonist on CCR2-transfected cells. (A) MCP-1 induced chemotaxis of CCR2-transfected cells in the presence (▴) or absence (•) of eotaxin-3 at 1 μM. Cell migration was evaluated in Boyden microchambers. All determinations were performed in triplicate. The control indicates the number of cells migrating in medium alone. (B) Chemotaxis of CCR2-transfected cells was evaluated in 24-transwell chambers. Where indicated, eotaxin-3 (1 μM) was applied to the cells in the upper well, and 10 nM MCP-1–induced migration was assessed. Data are expressed as chemotaxis index.
We described previously that eotaxin acts as a natural antagonist for CCR2 and as an agonist for CCR5. Because CCR5 is expressed on monocytes, we analyzed the ability of eotaxin-3 to induce chemotaxis or intracellular calcium rise in CCR5-transfected cells. Eotaxin-3, in contrast to eotaxin, does not induce any response (data not shown).
Eotaxin-3 is repulsive for human monocytes
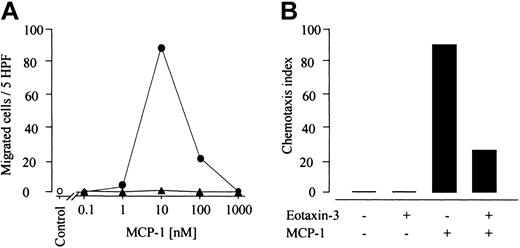
Unexpectedly, we observed that high concentrations of eotaxin-3 in the upper well of the chemotaxis chamber, providing a gradient that is oriented oppositely to the classical chemotaxis assay, induces monocyte migration in a statistically significant manner (Figure 5A). It is of note that the effect is specific for eotaxin-3. Neither eotaxin, which was previously characterized as a CCR2 antagonist on monocytes,27 nor the agonist MCP-1 (not shown) exhibit this repellent property.
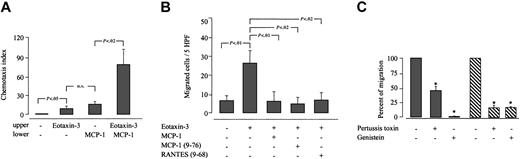
Eotaxin-3 is a repulsive factor for human monocytes. (A) Monocyte chemotaxis was evaluated in 24-transwell chambers. Cells were preincubated with or without 1 μM eotaxin-3, and migration induced by MCP-1 (10 nM) was assessed. Data are expressed as chemotaxis index (mean ± SD) of 8 experiments performed with monocytes from different donors. (B) Inhibition of the repulsive effect of eotaxin-3 by MCP-1 and truncated analogs of MCP-1 and RANTES was assessed in Boyden microchambers. Cells were preincubated with 1 μM eotaxin-3 and 1 μM of the following chemokines: MCP-1, MCP-1 (9-76) and RANTES (9-68) as indicated. Data are expressed as chemotaxis index (mean ± SD) of 3 experiments performed with monocytes from different donors. Differences that are statistically significance are indicated. (C) Inhibition of the repulsive effect of eotaxin-3 (filled bars) and of the synergistic effect of opposing gradients of MCP-1 and eotaxin-3 (hatched bars), by the Gαi inhibitor B pertussis toxin, and the tyrosine kinase inhibitor genistein. Migration of untreated cells = 100%. Percent of migration ± SD of 5 experiments performed with monocytes from different donors. Statistically significant differences (P < .01) between untreated and monocytes treated with B pertussis toxin or genistein are indicated with an asterisk.
Eotaxin-3 is a repulsive factor for human monocytes. (A) Monocyte chemotaxis was evaluated in 24-transwell chambers. Cells were preincubated with or without 1 μM eotaxin-3, and migration induced by MCP-1 (10 nM) was assessed. Data are expressed as chemotaxis index (mean ± SD) of 8 experiments performed with monocytes from different donors. (B) Inhibition of the repulsive effect of eotaxin-3 by MCP-1 and truncated analogs of MCP-1 and RANTES was assessed in Boyden microchambers. Cells were preincubated with 1 μM eotaxin-3 and 1 μM of the following chemokines: MCP-1, MCP-1 (9-76) and RANTES (9-68) as indicated. Data are expressed as chemotaxis index (mean ± SD) of 3 experiments performed with monocytes from different donors. Differences that are statistically significance are indicated. (C) Inhibition of the repulsive effect of eotaxin-3 (filled bars) and of the synergistic effect of opposing gradients of MCP-1 and eotaxin-3 (hatched bars), by the Gαi inhibitor B pertussis toxin, and the tyrosine kinase inhibitor genistein. Migration of untreated cells = 100%. Percent of migration ± SD of 5 experiments performed with monocytes from different donors. Statistically significant differences (P < .01) between untreated and monocytes treated with B pertussis toxin or genistein are indicated with an asterisk.
Moreover, when applying a reverse gradient of eotaxin-3 together with an optimal concentration of MCP-1 in the lower well of the chamber, we detected a significant increase of migration efficacy (Figure 5A). This indicates that the synergistic effect of opposing gradients of MCP-1 and eotaxin-3 is stronger than the inhibitory potential of eotaxin-3 on MCP-1–mediated migration.
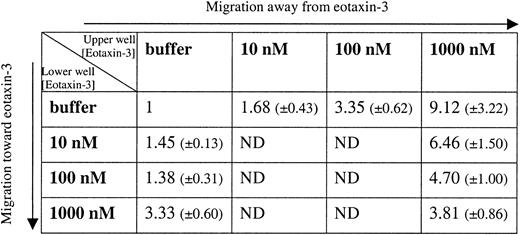
To further investigate the repulsive effect of eotaxin-3 on human monocytes, we performed a checkerboard study, analyzing monocyte migration to a positive, negative, or absent eotaxin-3 gradient. As shown in Figure 6, monocytes migrate away from eotaxin-3 in a dose-dependent fashion. The repulsive capability is decreased in the absence of a gradient (1 μM in the lower well and in the upper well). Eotaxin-3 reveals a weak chemotactic activity only at 1 μM (3.33 chemotaxis index). It is of note, that under the same conditions, 10 nM MCP-1 induces a much higher monocyte migration (26.33 chemotaxis index). Eotaxin-3 repulsive effect gives an index of 9.12 (Figure 6).
Transmigration analysis of chemotaxis for human monocytes in response to eotaxin-3. Migration in response to eotaxin-3 was measured in human monocytes using a 24-transwell chamber system. Chemotaxis index, mean ± SEM, of 8 experiments performed with cells from different donors is shown. MCP-1 at 10 nM was used as positive control, giving a chemotaxis index of 26.33 (± 9.79). ND indicates not determined.
Transmigration analysis of chemotaxis for human monocytes in response to eotaxin-3. Migration in response to eotaxin-3 was measured in human monocytes using a 24-transwell chamber system. Chemotaxis index, mean ± SEM, of 8 experiments performed with cells from different donors is shown. MCP-1 at 10 nM was used as positive control, giving a chemotaxis index of 26.33 (± 9.79). ND indicates not determined.
Accordingly, we could detect eotaxin-3–induced actin polymerization in monocytes. As summarized in Table 1, treatment of monocytes with the chemotactic stimulus MCP-1 as well as with the repellent stimulus eotaxin-3 results in a rapid conversion of globular into filamentous actin (F-actin), a prerequisite of site-directed migration. This indicates that the observed reverse migration of monocytes is an active migration that requires similar cytoskeletal remodeling processes as chemotaxis.
To further characterize the distinctive phenomenon of monocyte repulsion by eotaxin-3, we assessed reverse-gradient migration of monocytes pretreated with B pertussis toxin, which prevents coupling of Gi proteins to G protein–coupled receptors, or with genistein, a tyrosine kinase inhibitor. It was shown previously that genistein inhibits migration toward SDF-1 and MCP-1, but not SDF-1–induced repulsive effect on T lymophocytes.28 Like MCP-1–induced chemotaxis, monocyte migration away from an eotaxin-3 gradient and the synergistic effect of opposing gradients of MCP-1 and eotaxin-3 are significantly decreased in the presence of B pertussis toxin and genistein (Figure 5C). These data suggest that, like chemotaxis, the reverse migration of monocytes depends on functional Gi protein–coupled receptors and on tyrosine kinase activity.
To determine whether the observed effect of eotaxin-3 is the result of triggering a single chemokine receptor on monocytes, we tested cells transfected with a single type of chemokine receptor. Human monocytes express CCR1, CCR2, CCR5, CCR8, and CXCR4. Measuring F-actin formation in cells transfected with a single type of receptor, we could show that all corresponding agonists (MIP-1α, MCP-1, MIP-1β, I-309, and SDF-1α) induced actin polymerization, whereas eotaxin-3 failed to mediate any actin polymerization even at high concentrations (Table 1).
To assess the involvement of CCR2 in the reverse migration induced by eotaxin-3 of monocytes, cells were preincubated with MCP-1 or with the CCR2 antagonists MCP-1 (9-76) and RANTES (9-68).33 When monocytes were preincubated with CCR2 ligands, the eotaxin-3–induced reverse migration is diminished. The ANOVA analysis of the data reveals that this reduction is of statistical significance (Figure 5B).
Together, these findings indicate that the observed effect of eotaxin-3 on monocytes is mediated by a Gi protein–coupled receptor, requires tyrosine kinase activity, and depends on CCR2 availability. The underlying reorganization of the cytoskeleton seems to be similar to that of classical chemotaxis. However, unlike monocyte chemotaxis in response to agonists such as MCP-1 and MIP-1β, eotaxin-3–induced reverse migration is not accompanied by a rapid [Ca++]i increase and phosphorylation of ERK.
Discussion
Chemokine receptor antagonism by naturally occurring ligands was discovered only recently.27,35-38 It constitutes a novel and potentially important regulatory principle of chemokine-driven reactions. In this study we demonstrate that eotaxin-3 acts as a natural antagonist for CCR2. It competes with MCP-1 for CCR2 binding on monocytes at equimolar concentrations and, thus, displaces MCP-1 more potently than the previously described CCR2 antagonist eotaxin.27 CCR2 is expressed on monocytes, macrophages, IL-2–stimulated T lymphocytes, natural killer (NK) cells, and basophils. It has been characterized as a major receptor for the release of inflammatory enzymes by monocytes32 and NK cells,39 as well as for histamine and leukotriene secretion by basophils.7 By virtue of its antagonistic property, eotaxin-3 efficiently inhibits CCR2-mediated inflammatory responses of monocytes, such as enzyme release.
Unexpectedly, we found that monocytes are actively repulsed by eotaxin-3. Moreover, we show that the 2 migration stimuli synergize; monocyte migration along the gradient of MCP-1 is significantly increased by the simultaneous application of a reverse gradient of eotaxin-3.
Prior to migration, leukocytes undergo shape changes forming a leading and a rear edge.40 Acquisition and maintenance of this spatial and functional asymmetry is a prerequisite of site-directed migration. It has been demonstrated previously that leukocytes can integrate coincident chemoattractant signals.41 Competing stimuli lead to an orientation of the cell that reflects the vector sum of the integrated signals. In our experimental setting, monocytes encounter an MCP-1 gradient and an oppositely oriented eotaxin-3 gradient. This renders polarized monocytes migrating more efficiently to these combined stimuli than to one of the applied gradients alone. In vivo this might reflect a situation in which eotaxin-3 production in vascular endothelium and MCP-1 expression at the site of inflammation synergistically direct monocytes from the vessel into the tissue.
Recently it was shown that T lymphocytes also migrate away from the source of a chemotactic stimulus.28,42 Different from our observation in monocytes, which almost exclusively exhibit reverse migration in response to eotaxin-3, T lymphocytes respond in a biphasic mode to the CXCR4 ligand SDF-1; although attracted by low concentrations of SDF-1, the cells get repulsed at high SDF-1 concentrations. In this system, both effects are attributed to the chemokine receptor CXCR4 and evidence for different underlying signal transduction mechanisms at low and high stimulus concentrations has been provided. Our data show that monocyte migration away from the eotaxin-3 gradient, and the synergistic effect of opposing gradients of MCP-1 and eotaxin-3, are both sensitive to B pertussis toxin and genistein. We tested all chemokine receptors for which expression on monocytes was identified so far.43-47 We did not detect actin polymerization in response to eotaxin-3 analyzing cells transfected with a single type of receptor. In contrast to that, monocytes convert globular to filamentous actin on eotaxin-3 stimulation, indicating that repulsion requires the same cytoskeletal reshaping processes as chemotaxis. The desensitization or blockade of CCR2 on monocytes diminishes this eotaxin-3 effect. Together with the data obtained on chemokine receptor–transfected cells, we conclude that either expression of a single receptor type is not sufficient to mediate cell repulsion or that the used transfected cell lines lack additional interaction molecules that are required for reverse migration. Indirect evidence for a distinct signaling mechanism is provided by the fact that reverse migration of monocytes is not accompanied by an increase of intracellular free calcium or activation of the MAP kinase ERK. These signaling events usually coincide with chemotaxis although they are functionally not required for migration.48
Our data on eotaxin-3 further reveal the complexity of the chemokine system. While promoting allergic reactions by recruitment and activation of eosinophils and basophils, eotaxin-3 at the same time regulates CCR2-mediated responses of monocytes and provides a repulsive stimulus. Because of these various abilities, eotaxin-3 might play a versatile role in coordinating inflammatory processes in vivo.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2002-09-2773.
Ian Clark-Lewis died on December 30, 2002.
Supported by the Helmut Horten Foundation and by grant OG8/1-1 from the Deutsche Forschungsgemeinschaft (P.O.).
P.O. and S.P. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We dedicate this work to Prof Ian Clark-Lewis, who passed away December 30, 2002. We thank Prof Marco Baggiolini, Dr Federica Sallusto, and Dr Basil Gerber for instructive discussion; Dr Bernhard Moser and Dr Pius Loetscher for providing chemokine receptor-transfected cells; Andrea Blaser and Gabriela Danelon-Sargenti for expert technical assistance.

![Figure 2. Eotaxin-3 is a CCR2 antagonist on monocytes. (A) Changes of [Ca++]i in fura-2–loaded cells sequentially stimulated with eotaxin-3 and MCP-1 at 90-second intervals were monitored. (B) The rate of [Ca++]i rise (% fura saturation/second) induced by MCP-1 was set to 100% and the rate after eotaxin-3 prestimulation was calculated. The results of 1 of 3 independent experiments are shown. (C) MCP-1 (•) or eotaxin-3 (▴)–induced N-acetyl-β-d-glucosaminidase release was assessed in cells treated with cytochalasin-B. Mean values (± SD) of 3 experiments performed with monocytes from different donors are shown. (D) Inhibition of MCP-1–induced N-acetyl-β-d-glucosaminidase release by eotaxin-3 was detected in monocytes sequentially stimulated with increasing concentrations of eotaxin-3 and 10 nM MCP-1. Mean values (± SD) of 3 experiments performed with monocytes from different donors are shown. (E) Monocyte chemotaxis induced by MCP-1 (10 nM) or MCP-1 (10 nM) plus eotaxin-3 (1 μM) was evaluated in Boyden microchambers. Chemotaxis index (mean ± SD) of 3 experiments performed with monocytes from different donors is shown. (F) Eotaxin-3 inhibits MCP-1–induced CCR2 internalization. The histograms represent CCR2 expression on unstimulated cells (dark-filled), cells stimulated with MCP-1 (10 nM; thin line), and cells sequentially stimulated with eotaxin-3 (1 μM) and MCP-1 (10 nM). One representative of 3 experiments performed with monocytes from different donors is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/3/10.1182_blood-2002-09-2773/6/m_h81534731002.jpeg?Expires=1769320568&Signature=wSKqjH5RXouBHzY6uO-gu2mwob6FjsI30au5TKpckLTUm6BlbGqRxBiZ3OxwWplLdqr5uujfxFcl80Xauaadkxakwial8UWsPg3G5wtD8OnuSifigaRRMSj0xrde8XCX28pa-2--sevmvRmFs6EMY1pvJJJcTFmBWXLiClPSirL4DvrjMFsOsRhpHo0s4J7sIPPpDIGPr6ZnxufrhC6m7Ke2A61VzOulWt4RnM0zpwudg058jzOUxq1cUOmQR8ZfgJgFQAMgAOrzaDWwqHTd7oE3Pjhb~RG-rTjm4ydky4es3i1GouZdVdZJVj13gfN3c0Rh9GW55NXPkHQwYoVinw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)