Abstract
Efficient retroviral gene transfer to pluripotential hematopoietic stem cells (PHSCs) requires ex vivo culture in multiple hematopoietic growth factors (HGFs) to promote cell division. While treatment of PHSCs with HGF can render stem cells viable targets for retroviral infection, HGFs can promote differentiation, loss of self-renewal potential, and affect the homing/engraftment capacity of PHSCs. To avoid the negative impacts observed with ex vivo transduction protocols, we developed a murine model for in vivo retroviral infection by direct intrafemoral injection (DII), thus abolishing the need for removal of cells from their native microenvironment and the signals necessary to maintain their unique physiology. Using this approach we have demonstrated in vivo retroviral gene transfer to colony-forming units–c (CFU-c), short-term reconstituting cells, and PHSCs. Moreover, direct intrafemoral injection of Jak3 knock-out mice with retroviral particles encoding the Jak3 gene resulted in reconstitution of normally deficient lymphocyte populations concomitant with improved immune function. In addition, DII can be used to target the delivery of other gene therapy vectors including adenoviral vectors to bone marrow cells in vivo. Taken together, these results demonstrate that in vivo retroviral gene transfer by direct intrafemoral injection may be a viable alternative to current ex vivo gene transfer approaches.
Introduction
The self-renewal potential of pluripotential hematopoietic stem cells (PHSCs) makes them ideal targets for retroviral gene therapy where integration of a therapeutic gene into the host cell genome could correct hematologic disease.1-3 However, retroviral integration of PHSCs requires cycling of this normally quiescent population.4-6 Ex vivo culture of PHSCs in hematopoietic growth factors (HGFs) promotes cell cycling of murine and human hematopoietic stem/progenitor cells, which results in retroviral gene transfer efficiency levels above 50%.7-10 While ex vivo retroviral gene transfer is efficient, some disadvantages have been reported, including a defect in PHSC function following culture in HGFs. Alterations in PHSC function following ex vivo gene transfer have been attributed to differentiation and loss of self-renewal capacity or defects in homing and engraftment following transplantation in vivo.11-16 With this in mind, we explored in vivo retroviral gene transfer as an approach that would not negatively impact PHSC physiology.
Recently, ex vivo retroviral gene transfer to PHSCs has been shown to be successful in ameliorating disease in model systems where a selective advantage for expansion of transduced cells exists.17-19 Among these, reconstitution of functional lymphocyte populations through restoration of the γc/Jak3 signaling axis has been demonstrated.19-22 Because signaling via this pathway is critical for proper lymphocyte development, a mutation or deletion of either the cytokine receptor common gamma chain (γc) or Jak3 results in a severe combined immunodeficient (SCID) phenotype in both humans and mice.23-27 PHSCs from γc or Jak3 knock-out mice that were transduced ex vivo with retroviral vectors that encode either murine γc or Jak3 possess a selective advantage for the expansion and long-term reconstitution of functional lymphocyte populations when transplanted in vivo.19,20,22
Because transduced PHSCs in the Jak3 and γc knock-out models show a selective repopulating advantage, we hypothesized that gene transfer to hematopoietic cells in vivo (where even fewer PHSCs would be targeted) could be accomplished. In vivo gene transfer would abolish the need to remove PHSCs from their native microenvironment and alleviate the negative side effects observed using an ex vivo approach. To test this hypothesis, we developed an approach to deliver concentrated retroviral particles directly into the bone marrow space by direct intrafemoral injection (DII). In this study, we show that DII results in retroviral gene transfer to PHSCs in vivo. Furthermore, introduction of retroviral particles encoding the human Jak3 gene by DII to Jak3 knock-out animals results in reconstitution of deficient lymphocyte populations and restores cell-mediated and humoral immunity, thereby demonstrating the usefulness of this approach in a clinically relevant disease model.
Materials and methods
Animals
Eight- to 20-week-old C57BL/6NCR and C57BL/6.Ly5.2CR mice were obtained from the animal production area at the National Cancer Institute–Frederick (NCI-Frederick). Eight- to 50-week-old homozygous Jak3-/- mice (B6.129S4, TM1Ljb) were obtained from Jackson Laboratories (Bar Harbor, ME). Animal care was provided in accordance with procedures outlined in the National Institute of Health's Guide for the Care and Use of Laboratory Animals.
Direct intrafemoral injection of PKH-26
C57BL/6 mice were anesthetized, and a small incision was made over the kneecap on each hind limb. A 22-gauge needle attached to a 1-mL syringe was lodged between the condyles at the top of the femur, and access to the intrafemoral space was gained by applying gentle twisting and pressure. Forty microliters of 1 × 10-5 M PKH-26 (Sigma, St Louis, MO) was injected. One hour following injection, bone marrow was harvested and the percentage of PKH-26 labeling was determined by fluorescence microscopy.
Direct intrafemoral injection of adenovirus
Adenovirus serotype 5 recombinant viral particles encoding the luciferase gene (rAdluc) were obtained from Robert Gerard (University of Texas Southwestern Medical Center, Dallas) and grown on 293 cells and concentrated to 1 × 1010 infectious particles per milliliter by centrifugation on 2 successive, discontinuous cesium chloride (CsCl) gradients. Purified adenovirus was eluted from PD10 columns (Amersham Pharmacia, Piscataway, NJ) in 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.3), 150 mM NaCl; and 40 μL was injected by DII. To determine luciferase activity, cells were lysed in 1% Triton X-100, 50 mM NaCl, 10 mM Tris (tris(hydroxymethyl)aminomethane) (pH 7.6), 5 mM EDTA (ethylenediaminetetraacetic acid); cell debris was removed by centrifugation and luciferase activity was determined following the manufacturer's direction (Promega, Madison, WI). The protein content of each lysate was determined by the bicinchoninic acid method (Pierce, Rockford, IL). Luciferase activity is reported as average relative light units divided by the protein content for each sample (RLU/mg protein).
Direct intrafemoral injection of retroviral vectors
Retroviral vectors encoding green fluorescent protein (GFP) or human Jak3 were packaged in the GP + E86 cell line.28 Producer cell lines were grown to 90% confluence, the supernatant was harvested, and retroviral particles were concentrated by centrifugation onto a 20% sucrose cushion at 20 000 rpm for 2 hours at 4°C. MGIN viral supernatants were concentrated to 1 × 107 particles per milliliter,29 while MND.Jak3 supernatants were 2 × 106 to 5 × 106 particles per milliliter. C57BL/6 mice were injected with 150 mg/kg 5-fluorouracil (5-FU) intraperitoneally 5 days prior to DII of retrovirus, and 4, 24, or 48 hours later bone marrow was harvested. In some experiments the entire contents of individual femurs were transplanted by tail vein injection into 8- to 12-week-old C57BL/6-Ly5.2 recipients irradiated at 10 Gy (1000 rad) from a 137Cs source. Alternatively, enhanced GFP+ (EGFP+) bone marrow cells were separated by fluorescence-activated cell sorting (FACS), and 5 × 104 EGFP+ cells were combined with 1.5 × 105 EGFP- for a total of 2 × 105 cells transplanted per animal. Peripheral blood (PB) and bone marrow were collected at various time points, and red blood cells were lysed in ACK buffer (Quality Biological, Gaithersburg, MD). Cells were analyzed by flow cytometry for EGFP expression or mature lineage markers by staining with phycoerythrin (PE)–conjugated antimouse CD45R/B220 (RA3-6B), Ly-6G (Gr-1) (BR6-8C5), or CD90.2 (Thy1.2) antibodies and PE-conjugated rat immunoglobulin G2a (IgG2a) as an isotype control (PharMingen, San Diego, CA).
Immune reconstitution of Jak3-/- mice
Beginning 2 months after DII, PB lymphocytes were monitored by flow cytometry using biotin-conjugated antimouse CD45R/B220 or CD4 (L3T4), PE-conjugated IgM, or biotin-conjugated antimouse CD8a (Ly2) (53-6.7) or CD62L (L selectin, LECAM-1, Ly-22). Isotype control antibodies included PE-conjugated antimouse IgG2a and biotin-conjugated IgG2b. Secondary staining of biotin-conjugated antibodies was carried out using cychrome-conjugated streptavidin (PharMingen). Proliferation assays were carried out as previously described.24 Briefly, mononuclear PB cells were plated in triplicate in 96-well flat bottom plates (Corning, Acton, MA) in RPMI (Life Technologies, Bethesda, MD) or RPMI plus 2 μg/mL concanavalin A (Sigma) plus 30 ng/mL interleukin-2 (IL-2). Cells were incubated for 48 hours, treated overnight with 1 μCi (0.037 MBq) per well of [3H]thymidine (Amersham, Arlington Heights, IL), and [3H]thymidine uptake was determined using a β-plate reader. To test antibody production, DII Jak3-/- animals were injected intraperitoneally with 100 μg Al(OH)3 absorbed chicken γ-globulin (Jackson ImmunoResearch Laboratories, West Grove, PA). Serum was collected 14 days later, and Nunc ImmunoMaxiSorp plates (Fisher Scientific, Pittsburgh, PA) that were coated overnight at 4°C with 20 μg/mL chicken γ-globulin were blocked and then incubated with serum dilutions ranging from 1:10 to 1:10 000. Bound chicken gamma-globulin–specific antibodies were incubated with alkaline phosphatase–conjugated antimouse IgG followed by detection with 1 mg/mL p-nitrophenyl phosphate (Sigma). Absorbance was read at 405 nm.
Results
Direct intrafemoral injection
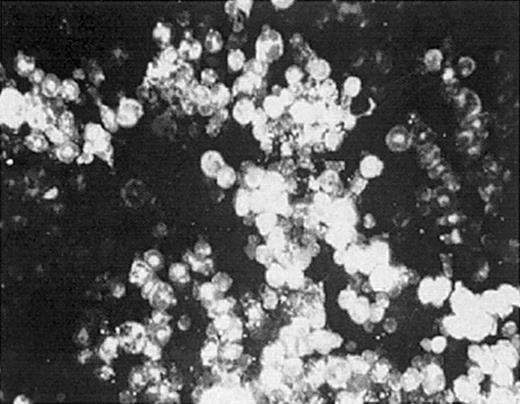
To evaluate whether we could deliver retroviral vectors to bone marrow cells (BMCs) by DII, we examined BMC labeling after injecting the fluorescent lipophilic dye PKH-26. DII was accomplished by making a small incision over the kneecap on the hind limb, followed by piercing the head of the femur with a 22-gauge needle. Upon gaining access to the intrafemoral space, 40 to 50 μL PKH-26 was slowly introduced. PKH-26–labeled BMCs were observed in more than 95% of injections 1 hour after administration (Figure 1). Furthermore, 30% to 80% of total bone marrow cells obtained from the ipsilateral femur showed PKH-26 fluorescence (Table 1), while no fluorescence was observed in control saline-injected mice. These data demonstrate that molecules introduced by DII are distributed throughout the bone marrow space, suggesting that gene therapy vectors can be delivered directly to BMCs by DII.
Direct intrafemoral injection procedure. One or both hind limbs of anesthetized mice were injected by DII with 30 μL PKH-26 dye at a concentration of 1 × 10-5 M. Bone marrow from injected femurs was harvested and analyzed for PKH-26 labeling by fluorescence microscopy (original magnification, × 400). The data shown are representative of 3 separate experiments.
Direct intrafemoral injection procedure. One or both hind limbs of anesthetized mice were injected by DII with 30 μL PKH-26 dye at a concentration of 1 × 10-5 M. Bone marrow from injected femurs was harvested and analyzed for PKH-26 labeling by fluorescence microscopy (original magnification, × 400). The data shown are representative of 3 separate experiments.
Direct intrafemoral injection of adenoviral vectors
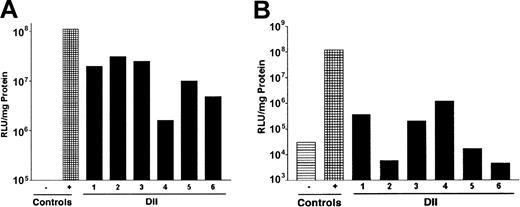
Because DII of PKH-26 was efficiently introduced to a significant fraction of the bone marrow, we evaluated whether delivery of viral particles would result in infection of BMCs. To test this, we chose to perform DII using a recombinant adenovirus encoding luciferase (rAdluc). Adenovirus was chosen for these initial studies because infection does not require target cell cycling or preconditioning of animals. We examined luciferase expression in BMCs immediately following DII of rAdluc (1) to determine how efficiently we delivered virus to intact femurs because we can visually confirm virus introduction to the bone marrow space of femurs that have been removed from the animal and (2) to look at expression in BMCs before they may have migrated from the bone marrow. We also determined luciferase expression in BMCs 24 hours after DII of rAdluc. To this end, 40 μL rAdluc (multiplicity of infection [MOI] = 20) was administered to anesthetized mice by DII. The MOI was determined by dividing the number of adenoviral particles by the femur cellularity. Immediately following injection, femurs were harvested and placed in culture for 4 to 6 hours at 37°C to allow for virus binding and entry. Afterward, BMCs were flushed from the femurs and cultured overnight in the presence of IL-3 plus IL-6 plus stem cell factor (SCF) to promote cell survival and allow time for luciferase gene expression. Six of 6 experimentally treated femurs showed increases in luciferase expression that were 2.7- to 34-fold higher than the uninfected control cells (Figure 2A). In comparison, similar levels of luciferase expression were observed when DII was performed on an intact femur that was removed from the animal prior to injection of rAdluc, which serves as the positive control because DII to the intact femur is reproducible and can be visually confirmed. Furthermore, high levels of luciferase expression were also observed when BMCs were harvested 24 hours after DII of rAdluc (Figure 2B). Taken together, DII can be used to target the delivery of gene therapy vectors to BMCs in vivo.
Direct intrafemoral injection of recombinant adenovirus. The rAdluc virus was produced, purified, and administered by DII according to the procedures described in “Materials and methods.” Following DII, BMCs were harvested from individual mice (1-6) and assayed for luciferase activity 24 hours after culture in vitro (A) or were harvested from mice 24 hours after DII in vivo (B). Saline injection was used as a negative control (-), and DII of femurs removed from host was used as a positive control (+). These results are representative of 3 separate experiments.
Direct intrafemoral injection of recombinant adenovirus. The rAdluc virus was produced, purified, and administered by DII according to the procedures described in “Materials and methods.” Following DII, BMCs were harvested from individual mice (1-6) and assayed for luciferase activity 24 hours after culture in vitro (A) or were harvested from mice 24 hours after DII in vivo (B). Saline injection was used as a negative control (-), and DII of femurs removed from host was used as a positive control (+). These results are representative of 3 separate experiments.
Direct intrafemoral injection of retroviral vectors and gene transfer to PHSCs in vivo
To determine the optimal conditions to deliver retroviral vectors by DII, we looked at the cell cycle status of BMCs in mice pretreated with a sublethal dose of 5-FU, which promotes active cycling of primitive hematopoietic cells in vivo (required for efficient retroviral infection).30,31 Over the first 4 days, we observed a decrease in the percentage of BMCs in S phase after 5-FU treatment that was followed by a rebound between day 4 and 5 after treatment as determined by propidium iodide staining and cell-cycle analysis (data not shown). Therefore, we injected concentrated MGIN retrovirus encoding both enhanced green fluorescent protein (EGFP) and neomycin resistance 5 days after 5-FU treatment by DII. Because a single femur contains roughly 5% of the total BMC mass, it was unlikely that we would obtain a sufficient level of PHSC infection to compete with the endogenous untransduced cell population after a single DII injection. Therefore, the entire content of the injected femur (roughly 2 × 106 cells) was harvested and transplanted into a lethally irradiated recipient to measure transduction of short-term reconstituting cells (STRCs), which provide short-term radioprotection and survival of animals undergoing transplantation. Two to 3 months following transplantation, EGFP+ cells were observed in 13 of 23 animals (56%) at levels ranging from 0.1% to 4% of PB mononuclear cells (Figure 3); in contrast, no EGFP expression was observed in control mice. In parallel experiments, the level of retroviral gene transfer to committed progenitors was evaluated by plating DII-injected BMCs in colony-formation assays in the presence of G418. We found that 1.3% to 14.2% of the bone marrow colony-forming unit–c (CFU-c) were G418 resistant in 4 of 4 femurs harvested 24 hours after DII. In addition, lower levels of G418-resistant colonies (2%-4%) were observed in 3 of 4 mice 48 hours after DII (Figure 3B). Thus, DII of retroviral vectors results in transduction of hematopoietic progenitor populations including STRCs and CFU-c's in vivo.
Gene transfer to bone marrow progenitors and to peripheral blood cells 2 months after transplantation. Retroviral vector was produced, purified, and injected into 5-FU–treated mice by DII according to the procedures described in “Materials and methods.” BMCs from saline (control) or MGIN virus–injected femurs were transplanted into primary irradiated recipient mice 24 hours after DII, and PB was analyzed 2 months after transplantation for EGFP expression by flow cytometry (A). In addition, BMCs were obtained from DII-treated 5-FU day-5 animals 24 and 48 hours following injection and plated at 5 × 104 cells per plate in soft agar in duplicate in the presence of IL-3, IL-6, SCF, and granulocyte colony-stimulating factor (G-CSF). Additionally, plating was carried out in the presence or absence of 600 μg/mL G418. Seven days after plating, viable colonies of 50 or more cells were scored. The percentage of G418-resistant colonies was calculated by dividing the number of colonies growing in the presence of G418 by the total number of colonies scored in the absence of G418 (B). The data presented are representative of 3 experiments.
Gene transfer to bone marrow progenitors and to peripheral blood cells 2 months after transplantation. Retroviral vector was produced, purified, and injected into 5-FU–treated mice by DII according to the procedures described in “Materials and methods.” BMCs from saline (control) or MGIN virus–injected femurs were transplanted into primary irradiated recipient mice 24 hours after DII, and PB was analyzed 2 months after transplantation for EGFP expression by flow cytometry (A). In addition, BMCs were obtained from DII-treated 5-FU day-5 animals 24 and 48 hours following injection and plated at 5 × 104 cells per plate in soft agar in duplicate in the presence of IL-3, IL-6, SCF, and granulocyte colony-stimulating factor (G-CSF). Additionally, plating was carried out in the presence or absence of 600 μg/mL G418. Seven days after plating, viable colonies of 50 or more cells were scored. The percentage of G418-resistant colonies was calculated by dividing the number of colonies growing in the presence of G418 by the total number of colonies scored in the absence of G418 (B). The data presented are representative of 3 experiments.
To ascertain the potential for gene transfer to nonhematopoitic organs, brain, lung, muscle, and bone marrow tissues were analyzed 24 hours following DII treatment for the presence of EGFP gene sequences. Southern blotting of DNA obtained from the bone marrow of injected femurs showed strong hybridization to EGFP probes, while analysis of muscle tissues surrounding the femur were weakly positive. As expected, no gene transfer was observed at more distal sites including brain and lung (data not shown). Assessment of gene transfer levels was also evaluated in animals treated by DII of concentrated MGIN retrovirus 4 days following 5-FU treatment. No significant differences in gene transfer efficiency were observed. To determine whether transduced cells were lost as a result of movement out of the bone marrow, we examined retroviral gene transfer to STRCs and CFU-c's at 4 and 48 hours following DII. Again, no significant differences in gene transfer efficiency were seen compared with transplantation at 24 hours following DII (data not shown). Finally, DII of producer cell lines did not improve the efficiency of gene transfer (data not shown).
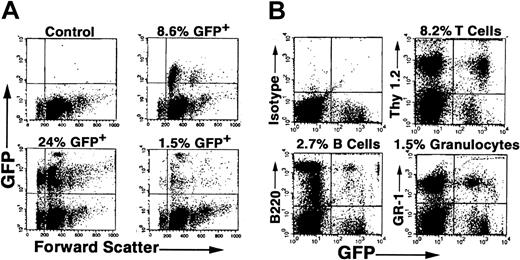
Because the percentage of GFP+ cells was low in primary recipients undergoing transplantation with BMCs from DII mice, we isolated EGFP+ BMCs from primary recipients 5 months after transplantation by FACS sorting and transplanted these cells into secondary irradiated recipient mice. We found 1.5% to 24% of PB cells expressed EGFP in 3 of 3 animals 2 months following transplantation (Figure 4A). In contrast, no EGFP expression was observed in control animals undergoing transplantation with EGFP- sorted BMCs. Furthermore, examination of PB at 4 months following secondary transplantation showed reconstitution of host animals with EGFP+ granulocytes, T lymphocytes, and B lymphocytes in all animals tested (Figure 4B). Taken together, durable long-term and trilineage reconstitution in secondary recipients demonstrates that DII results in in vivo retroviral transduction of PHSCs.
EGFP expression in hematopoietic cells in recipient mice undergoing secondary transplantation. C57BL/6 mice underwent DII of MGIN retrovirus and transplantation, and then EGFP+ BMCs were obtained from these mice after 6 months by FACS sorting and transplanted into secondary irradiated recipient mice as described in “Materials and methods.” Two to 5 months following secondary transplantation of 50 000 EGFP+ cells, PB was analyzed for the presence of donor cells in 3 separate mice (A). (B) In vivo gene transfer to PHSCs. Four months following secondary transplantation, PB was analyzed for GFP expression in T cells (Thy 1.2), B cells (B220), and granulocytes (Gr-1) from a representative animal.
EGFP expression in hematopoietic cells in recipient mice undergoing secondary transplantation. C57BL/6 mice underwent DII of MGIN retrovirus and transplantation, and then EGFP+ BMCs were obtained from these mice after 6 months by FACS sorting and transplanted into secondary irradiated recipient mice as described in “Materials and methods.” Two to 5 months following secondary transplantation of 50 000 EGFP+ cells, PB was analyzed for the presence of donor cells in 3 separate mice (A). (B) In vivo gene transfer to PHSCs. Four months following secondary transplantation, PB was analyzed for GFP expression in T cells (Thy 1.2), B cells (B220), and granulocytes (Gr-1) from a representative animal.
In vivo reconstitution of Jak3 function in Jak3-/- SCID mice
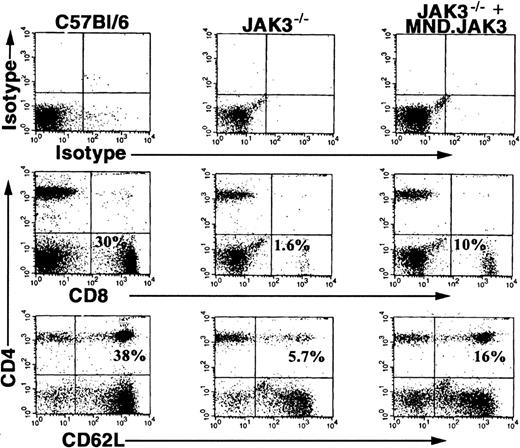
Having demonstrated in vivo PHSC transduction, we wanted to examine the usefulness of DII in a clinically relevant disease model. Specifically, we determined if deficits in lymphocyte populations found in the Jak3-/- mouse model (CD8+, CD4+/CD62L+ T cells, and B cells) could be rescued by in vivo transduction using retroviral vectors encoding Jak3.20,23,24 The retroviral vector encoding the human Jak3 gene (MND.Jak3) driven by a modified myeloproliferative sarcoma virus promoter was concentrated and administered to Jak3-/- mice by DII.32 To demonstrate increases in specific lymphocyte populations, we examined PB at 8 weeks following DII for the presence of CD8+ and CD4+/CD62L+ T cells (Figure 5, C57BL/6 mice, left column; JAK3-/- mice, middle column; JAK3-/--injected mice, right column). Five of 12 treated animals showed increases in specific lymphocyte populations compared with control animals. The percentage of CD8+ lymphocytes was significantly increased in treated mice to 4% ± 1.25% versus 1.8% ± 1.1% in control animals (P = .0149 by Student t test). A similar increase in CD4+/CD62L+ T cells was observed in treated mice up to 6% ± 2.1% compared with 3.6% ± 2.3% in controls (P = .0144 by Student t test). The increases in CD8+ and CD4+/CD62L+ T lymphocytes were 40% to 50% of the levels seen in wild-type (WT), age-matched control animals (23.2% ± 4.3% CD8+ and 33.2% ± 3.0% CD4+/CD62L+) demonstrating partial reconstitution of T-cell populations in Jak3-/- mice. An increase in B220+ but not B220+/IgM+ B cells was observed in one animal. However, reconstitution of the B-cell compartment is not expected because these animals are maintained in a pathogen-free environment on antibiotics (low or no antigen challenge).
Direct intrafemoral injection of MND.Jak3 virus into Jak3-/- mice. MND.Jak3 retrovirus (JAK3 RV) was administered to Jak3-/- and control mice by DII according to the procedures described in “Materials and methods.” Two months following treatment, PB cells from control C57BL/6 mice (column 1), mock-injected Jak3-/- mice (column 2), and MND.Jak3-injected mice (Jak3-/- + JAK3 RV) (column 3) were analyzed for the presence of CD8+ and CD4+/62L+ T cells (% of total population indicated in quadrant).
Direct intrafemoral injection of MND.Jak3 virus into Jak3-/- mice. MND.Jak3 retrovirus (JAK3 RV) was administered to Jak3-/- and control mice by DII according to the procedures described in “Materials and methods.” Two months following treatment, PB cells from control C57BL/6 mice (column 1), mock-injected Jak3-/- mice (column 2), and MND.Jak3-injected mice (Jak3-/- + JAK3 RV) (column 3) were analyzed for the presence of CD8+ and CD4+/62L+ T cells (% of total population indicated in quadrant).
Functional restoration of cell-mediated and humoral immunity
In vitro T-cell proliferation assays were performed to determine if lymphocytes in the MND.Jak3-transduced Jak3-/- mice were functional. Mononuclear PB cells were stimulated with concanavalin A and IL-2 24 weeks following DII. Two of 8 injected animals showed a proliferative response similar to WT controls (P < .001 by Student t test). In contrast, no proliferation was observed in mock-infected control animals (Figure 6A). Proliferation in response to mitogenic stimulation demonstrates reestablishment of T-lymphocyte development and function through restoration of the Jak3 signaling pathway.
Functional analysis of lymphoid cell populations following DII of Jak3-/-. For T-cell proliferation, PB cells from control C57/BL6 mice (WT), Jak3-/- mice (KO), DII-injected Jak3-/- mice (KO + DII), or Jak3-/- mice undergoing transplantation with ex vivo–infected BMCs (Ex Vivo) were plated in the presence of either media alone or with ConA plus IL-2, cultured for a total of 48 hours in vitro, pulsed with [3H]thymidine, and then harvested according to the procedures described in “Materials and methods.” Data are plotted as the means ± SDs counts per minute (cpm) of triplicate wells (A). Error bars indicate standard deviations. (B) For B-cell function, mice were challenged with chicken IgG, and 14 days after inoculation serum plasma was collected to measure for the presence of chicken IgG–specific antibodies (absorbance at 405 nm in ELISA assay according to the procedures described in “Materials and methods”).
Functional analysis of lymphoid cell populations following DII of Jak3-/-. For T-cell proliferation, PB cells from control C57/BL6 mice (WT), Jak3-/- mice (KO), DII-injected Jak3-/- mice (KO + DII), or Jak3-/- mice undergoing transplantation with ex vivo–infected BMCs (Ex Vivo) were plated in the presence of either media alone or with ConA plus IL-2, cultured for a total of 48 hours in vitro, pulsed with [3H]thymidine, and then harvested according to the procedures described in “Materials and methods.” Data are plotted as the means ± SDs counts per minute (cpm) of triplicate wells (A). Error bars indicate standard deviations. (B) For B-cell function, mice were challenged with chicken IgG, and 14 days after inoculation serum plasma was collected to measure for the presence of chicken IgG–specific antibodies (absorbance at 405 nm in ELISA assay according to the procedures described in “Materials and methods”).
To confirm restoration of humoral immunity, functional analysis of emergent B-cell populations was carried out by specific antigen challenge. Jak3-reconstituted animals were immunized with chicken γ-globulin; 14 days following injection, circulating antichicken IgG levels in the serum were detected by enzyme linked immunosorbent assay (ELISA) (Figure 6B). Two of 5 mice analyzed mounted an immune response following specific challenge comparable to that observed in WT control animals. As expected, Jak3-/- control mice were unable to generate an antibody response. The ability to mount an antibody response to specific antigens requires functional interaction of both B and T cells. Thus, our results indicate that in vivo retroviral gene transfer of the Jak3 gene to Jak3-/- mice is sufficient to restore B cell–mediated immunity.
Discussion
Several disadvantages have been associated with the use of ex vivo approaches for retroviral gene delivery to PHSCs. For example, hematopoietic cells removed from their native microenvironment are cut off from the endogenous signals necessary to maintain their unique physiology. In addition, following removal from the microenvironment, BMCs undergo enforced cycling in the presence of multiple cytokines that, while rendering them viable targets for retroviral infection, also promote differentiation and loss of self-renewal capacity. Finally, removal from the bone marrow in combination with enforced cycling is known to have negative effects on the homing and engraftment capacity of transduced cells following transplantation.11-16 In an effort to develop an alternative approach for retroviral gene transfer to PHSCs, we investigated whether introduction of concentrated retroviral particles to the bone marrow space by DII would result in in vivo transduction of PHSCs and other hematopoietic progenitors. We have shown in vivo retroviral transduction of up to 4% of hematopoietic progenitor cells in the PB 2 to 5 months following DII in more than 50% of animals treated. Furthermore, transplantation of 50 000 EGFP+ cells isolated from primary recipients resulted in long-term hematopoietic reconstitution of 100% of secondary recipients with EGFP expression observed in T cells, B cells, and granulocytes 4 months following transplantation. Sustained expression of the transferred gene in both primary and secondary recipients concomitant with the presence of multiple mature hematopoietic lineages derived from transduced cells demonstrates that DII results in in vivo retroviral transduction of PHSCs and suggests that this approach may be a viable alternative to current ex vivo approaches.
Applying this methodology to a relevant human disease model, we investigated DII for treatment of the SCID phenotype observed in Jak3 knock-out mice. Extensive work has shown that correction of the SCID disease is readily accomplished through reconstitution of the Jak3 signaling pathway by ex vivo retroviral gene transfer of the murine Jak3 gene. In addition, this work has also supported an apparent in vivo selective advantage for corrected cells.19-21 DII of concentrated MND.Jak3 retroviral particles resulted in an increase in total lymphocyte counts 8 to 20 weeks following treatment. Flow cytometric analysis of cell-surface phenotype revealed the increased lymphocyte numbers were a direct result of increases of up to 30% of that seen in WT animals in the CD8+, CD4+/CD62L+, and B220+/IgM+ populations. Functionality of the reconstituted T-cell populations was demonstrated by their proliferative response to mitogenic stimuli. B-cell function was confirmed through specific antigen challenge with chicken γ-globulin with levels of antichicken IgG antibodies comparable to that observed in WT controls in 2 of 5 treated animals. Taken together, these data demonstrate that treatment of animals by DII was sufficient to partially restore both cell-mediated and humoral immunity and ameliorate disease symptoms.
Our findings and those of others support the potential for DII as a potential clinically relevant approach to transfer genes to PHSCs in vivo.33,34 Nonetheless, our findings are comparable to the marking levels observed in ex vivo protocols in both large animal and human studies.9,10,35,36 Importantly, our approach entails only a single injection to each femur of 40 to 50 μL of concentrated retroviral particles. This is in sharp contrast to ex vivo retroviral gene transduction in which maximally stimulated cells undergo multiple rounds of exposure to retrovirus containing supernatants to improve infection efficiency. By incorporating a selective advantage for transduced cells via the Jak3 knock-out model, we were able to partially overcome the low efficiency of transduction observed using the MGIN retrovirus. While functional lymphocyte reconstitution lagged using DII compared with ex vivo studies, this obstacle may be overcome by increasing the concentration of retroviral particles in combination with multiple injections. In this regard, we feel that multiple in vivo injections in a large animal model might result in improved gene transfer. Initially, we chose intrafemoral injection to minimize the possibility of immune rejection and maximize viral transduction to BMCs. In addition, we would propose to compare intrafemoral with intra-arterial injection in larger animal models. Thus, we can envision a number of improvements to this approach. First, to make this methodology feasible for treatment of diseases where a natural selective advantage is absent (eg, hemophilia, thalassemia), we would propose the use of bicistronic vectors that incorporate a drug selection gene into the therapeutic expression cassette, thereby providing a mechanism for selection and expansion of transduced cells. Secondly, lentiviral vectors may represent an improvement over MSCV vectors in that they do not require cell cycling to achieve viral DNA integration. Additionally, psuedotyping with the vesicular stomatitis virus envelope would provide extra stability such that retroviral particles may be concentrated more efficiently, thus increasing the multiplicity of infection. Also, in large animal models and human applications, multiple injections could increase the virus-to-cell ratio and presumably improve infection efficiency. Finally, improvements with regard to the safety of retroviral vectors are always a consideration. In this regard, it has recently been reported that 1 patient of 10 that received retrovirally transduced BMCs in a clinical trial to correct their SCID disorder developed a leukemia. Studies are underway to determine whether this patient's leukemia is the result of a retroviral insertional mutagenesis event.37
In conclusion, our data demonstrate that in vivo retroviral gene transfer is not only possible but that we can achieve infection efficiencies comparable to those observed in large animal and human models. While many obstacles have yet to be overcome for effective gene transfer by DII to become a reality in humans, this approach represents an interesting alternative to current ex vivo transduction protocols. Studies are in progress to investigate the use of bicistronic vectors for selection of transduced cells. Furthermore, our current studies also address preservation of stem cell function following DII.
Prepublished online as Blood First Edition Paper, April 10, 2003; DOI 10.1182/blood-2002-12-3859.
Supported by the National Cancer Institute, National Institutes of Health, under contract no. NO1-CO-12400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial, products, or organizations imply endorsement by the US government.


![Figure 6. Functional analysis of lymphoid cell populations following DII of Jak3-/-. For T-cell proliferation, PB cells from control C57/BL6 mice (WT), Jak3-/- mice (KO), DII-injected Jak3-/- mice (KO + DII), or Jak3-/- mice undergoing transplantation with ex vivo–infected BMCs (Ex Vivo) were plated in the presence of either media alone or with ConA plus IL-2, cultured for a total of 48 hours in vitro, pulsed with [3H]thymidine, and then harvested according to the procedures described in “Materials and methods.” Data are plotted as the means ± SDs counts per minute (cpm) of triplicate wells (A). Error bars indicate standard deviations. (B) For B-cell function, mice were challenged with chicken IgG, and 14 days after inoculation serum plasma was collected to measure for the presence of chicken IgG–specific antibodies (absorbance at 405 nm in ELISA assay according to the procedures described in “Materials and methods”).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/3/10.1182_blood-2002-12-3859/6/m_h81534693006.jpeg?Expires=1769132515&Signature=jHJDYIlUa0al3KH6KPOlVQ9UxNjUlJJvLjeekVv3JvqI3zh3Mrn6o5PVDCZW~kUsbFSXY-3fTlGQ3v7YWnwQwTe6KztBKTp-TyGq0v0WLkGpYYCEZuklzIc6keZ-B6vCT9OLCkQXJklbgA6~UvloNCxGWAr-U2g69qC4~gfy8l78ndvS53~kak8khu1xvIoKX2GXP1aDTZ2gs3LIgvs4lATjXwpWiKb6pkJl2OnWHbR4oEapjzh7XgjgozbCDSOhgmxa3mSFvnU3nE2M7BP-aLxBCynEg-6-asZWLHhX2KTBnRLVd7TzTNH6wE8PxER1Zofjj7B~xRel0ZpThcFcBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
