Abstract
The platelet receptor for the von Willebrand factor (VWF) glycoprotein Ib-IX-V (GPIb-IX-V) complex mediates platelet adhesion at sites of vascular injury. The cytoplasmic tail of the GPIbα subunit interacts with the actin-binding protein, filamin, anchoring the receptor in the cytoskeleton. In motile cells, the second messenger phosphatidylinositol 3,4,5 trisphosphate (PtdIns(3,4,5)P3) induces submembraneous actin remodeling. The inositol polyphosphate 5-phosphatase, Src homology 2 domain-containing inositol polyphosphate 5-phosphatase-2 (SHIP-2), hydrolyzes PtdIns(3,4,5)P3 forming phosphatidylinositol 3,4 bisphosphate (PtdIns(3,4)P2) and regulates membrane ruffling via complex formation with filamin. In this study we investigate the intracellular location and association of SHIP-2 with filamin, actin, and the GPIb-IX-V complex in platelets. Immunoprecipitation of SHIP-2 from the Triton-soluble fraction of unstimulated platelets demonstrated association between SHIP-2, filamin, actin, and GPIb-IX-V. SHIP-2 associated with filamin or GPIb-IX-V was active and demonstrated PtdIns(3,4,5)P3 5-phosphatase activity. Following thrombin or VWF-induced platelet activation, detection of the SHIP-2, filamin, and receptor complex decreased in the Triton-soluble fraction, although in control studies the level of SHIP-2, filamin, or GPIb-IX-V immunoprecipitated by their respective antibodies did not change following platelet activation. In activated platelets spreading on a VWF matrix, SHIP-2 localized intensely with actin at the central actin ring and colocalized with actin and filamin at filopodia and lamellipodia. In spread platelets, GPIb-IX-V localized to the center of the platelet and showed little colocalization with filamin at the plasma membrane. These studies demonstrate a functionally active complex between SHIP-2, filamin, actin, and GPIb-IX-V that may orchestrate the localized hydrolysis of PtdIns(3,4,5)P3 and thereby regulate cortical and submembraneous actin.
Introduction
Following vascular injury, platelet adhesion and aggregation mediate the immediate arrest of bleeding; however, in pathologic states this response may contribute to arterial blockage leading to cerebrovascular and coronary arterial occlusion. The interaction of the platelet membrane glycoprotein Ib-IX-V (GPIb-IX-V) complex with its adhesive ligand, von Willebrand factor (VWF), in the subendothelial matrix initiates platelet adhesion under conditions of rapid blood flow in the arterial circulation. The GPIb-IX-V complex can also bind plasma VWF under conditions of pathologic shear stress, leading to platelet aggregation and thrombosis.1-3 Following binding of VWF, the GPIb-IX-V complex initiates intracellular signals inducing platelet activation, spreading, and activation of the integrin αIIbβ3 receptor from a low- to high-affinity state (inside-out signaling). The molecular mechanisms regulating VWF–GPIb-IX-V–induced signaling, however, remain to be fully delineated.4
The GPIb-IX-V complex is composed of 4 subunits: GPIbα disulphide linked to GPIbβ, GPV, and GPIX. The extracellular N-terminal globular domain of GPIbα contains the binding site for VWF. The 96–amino acid cytoplasmic tail of GPIbα contains no overt signaling domains but directly binds the actin-binding protein, filamin 1a, and the signaling adapter protein, 14-3-3ζ.5,6 Filamins are large actin-binding proteins that stabilize submembraneous actin webs and link them to cellular membranes. Filamins promote high-angle actin-filament branching and thereby actin gelation, although the molecular mechanisms mediating this effect are incompletely understood. Three filamin isoforms, A, B, and C, have been identified, with A and B expressed in platelets.7 The interaction of filamin with GPIb-IX-V is essential for regulating the shape of resting platelets, the spreading of activated platelets, and the anchoring of the receptor complex to the membrane skeleton.5,8-10 The stabilization of the platelet membrane resulting from the interaction of filamin with GPIb-IX-V may not only prevent platelet membrane deformation under conditions of high fluid shear stress but also regulates the adhesive function of the receptor complex itself and the ability of the receptor complex to maintain adhesion to VWF under conditions of high shear stress.10,11 Following platelet adhesion, VWF binding to GPIb-IX-V induces the cytoskeletal translocation and activation of the p85/p110 form of phosphoinositide 3-kinase (PI 3-kinase).12 Under shear conditions, PI 3-kinase is essential for VWF-induced calcium release.13 A potential mechanism for activation of PI 3-kinase via GPIb-IX-V has been demonstrated in studies from our laboratory, which have shown that the p85 subunit of PI 3-kinase forms a complex with both 14-3-3ζ and the receptor complex.6
The inositol lipids phosphatidylinositol 4,5 bisphosphate (PtdIns(4,5)P2) and phosphatidylinositol 3,4,5 trisphosphate (PtdIns(3,4,5)P3) play a central role in the regulation of actin polymerization and thereby cell motility.14,15 Many recent studies have demonstrated in motile cells that PtdIns(3,4,5)P3 spatially localizes to the leading edge of the cell at membrane ruffles (lamellipodia). Production of this lipid determines where and when submembraneous actin polymerization takes place. PtdIns(3,4,5)P3 localizes many signaling and actin-regulatory proteins to the inner wall of the plasma membrane and allosterically regulates their function (reviewed by Vanhaesebroeck et al16 ). In this manner, PtdIns(3,4,5)P3 mediates agonist-induced actin-dependent extension of lamellipodia and cell migration. The agonist-induced transient accumulation of PtdIns(3,4,5)P3 at the leading edge of motile cells results from a balance between its synthesis by the PI 3-kinase and hydrolysis by specific lipid phosphatases.17 Recent studies have demonstrated that the SH2-inositol polyphosphate 5-phosphatase, SHIP-2 (Src homology 2 domain-containing inositol 5-phosphatase), hydrolyzes the 5-position phosphate from PtdIns(3,4,5)P3 forming PtdIns(3,4)P2 and is localized to the leading edge of the cell, whereby the 5-phosphatase regulates submembraneous actin and membrane ruffling.18 This 5-phosphatase is a widely expressed enzyme that contains an amino-terminal SH2 domain, a central 5-phosphatase domain, and a carboxyl-terminal proline-rich domain.19 It bears significant sequence identity with the hematopoietic-specific 5-phosphatase, SHIP-1, except in the proline-rich domain where the 2 5-phosphatases demonstrate significant sequence diversity.20 SHIP-2 localization to membrane ruffles is mediated via complex formation through its C-terminal proline-rich domain with the actin-binding protein, filamin.18 Further evidence for the role SHIP-2 plays in regulating submembraneous actin has been demonstrated by studies that have shown SHIP-2 forms a complex with p130Cas at focal adhesions and regulates cell adhesion.21 Collectively, these studies suggest that SHIP-2 regulates the localized changes in PtdIns(3,4,5)P3 that instruct submembraneous actin remodeling, instruct membrane ruffling, and promote cell adhesion.
As SHIP-2 appears to significantly influence cytoskeletal remodeling, we investigated the localization and expression of SHIP-2 in platelets. Platelets are highly motile cells with a complex cytoskeleton. At early time points in platelets spreading on a VWF matrix, SHIP-2 localized initially to filopodia and the central actin ring, and with filamin and submembraneous actin at lamellipodia. Actin, GPIb-IX-V, SHIP-2, and filamin were shown in complex in the Triton-soluble fraction of platelets; however, following platelet activation the level of this complex decreased significantly. No complex was detected in the detergent-extracted actin cytoskeleton of resting or activated platelets; however, immunofluorescence colocalization studies demonstrate SHIP-2 and filamin colocalized at the submembraneous actin cytoskeleton of activated platelets spreading on a VWF matrix, suggesting the SHIP-2 filamin complex may relocate to the submembraneous actin cytoskeleton upon platelet activation. Collectively, these studies provide evidence that SHIP-2 localizes in spreading platelets to sites of active actin remodeling and thereby may regulate localized concentrations of PtdIns(3,4,5)P3.
Materials and methods
Reagents
Monoclonal antibodies raised to human platelet filamin, SHIP-1, cortactin, and hemagglutinin (HA) were obtained from Chemicon (Temecula, CA), Santa Cruz Biotechnology (Santa Cruz, CA), Auspep (Parkville, Australia), Molecular Probes (Eugene, OR), and Silenus (Melbourne, Australia). Rabbit polyclonal antibodies to glycocalicin (a soluble fragment of GPIbα) and SHIP-2 were raised as previously described.6,18 von Willebrand Factor (VWF) and botrocetin were purified as previously described.9 α-32P]dCTP (deoxycytidine triphosphate) and γ-32P]ATP (adenosine triphosphate) were from NEN Life Science Products (Boston, MA). HA–SHIP-1 cDNA was a kind gift from Dr Gerry Krystal (British Columbia Cancer Agency, BC, Canada). All other reagents were purchased from Sigma (St Louis, MO) unless otherwise stated.
Northern blot analysis
A membrane containing approximately 20 μg poly(A) RNA isolated from various cell lines was probed with the C-terminal proline-rich domain of human SHIP-2 cDNA (encompassing nucleotides 3017-3989) labeled with [α-32P]dCTP by random priming (Prime-a-gene; Promega, Madison, WI) and hybridized to the membrane overnight at 42°C. Blots were washed using standard procedures.22 The membranes were allowed to decay and subsequently hybridized to an actin probe.
Platelet immunoprecipitations
Platelets obtained from healthy volunteers who were medication free for at least 10 days were washed using a previously described method.23 Washed platelets were either not stimulated or stimulated with 1 U/mL thrombin or VWF (10 μg/mL) and botrocetin (3 μg/mL) for 5 minutes. Following activation with thrombin or VWF, the platelets were lysed with 1 volume of Triton X-100 lysis buffer (200 mM Tris-HCl [tris(hydroxymethyl)aminomethane-HCl], pH 7.4; 10% Triton X-100; 50 mM EGTA [ethylene glycol tetraacetic acid]; 4 mM leupeptin; 4 mM aprotinin; 2.5 mg/mL PMSF [phenylmethylsulfonyl fluoride]) to 9 volumes of platelets and gently agitated at 4°C for 1 hour. Lysates were centrifuged at 15 400g for 10 minutes separating the Triton X-100–soluble and –insoluble (actin cytoskeletal) extracts. The supernatant represents the Triton-soluble lysate. The cytoskeletal pellet was washed twice with 20 mM Tris, pH 7.4; 150 mM NaCl; 400 μM leupeptin; 400 μM aprotinin; and 250 μg/mL PMSF, solubilized by incubation with RIPA buffer (10 mM NaH2PO4, pH 7.0; 150 mM NaCl; 2 mM EDTA; 250 μg/mL PMSF; 400 μM leupeptin; 400 μM aprotinin; 1% Nonidet P-40; 1% sodium deoxycholate; 0.1% sodium dodecyl sulfate [SDS]) for 1 hour at 4°C and centrifuged at 15 400g for 10 minutes. The supernatant represents the RIPA-extracted actin cytoskeleton. Immunoprecipitation of GPIb-IX, filamin, or SHIP-2 was performed as follows: 5 μg of polyclonal antisera directed against GPIb (antiglycocalicin antibody), 5 μg of monoclonal filamin antibody, 5 μg of polyclonal antisera against SHIP-2, or 5 μL of preimmune or nonimmune sera were incubated overnight at 4°C with 600 μL of Triton-soluble lysate and 60 μL of a 50% slurry of protein-A–Sepharose that was pre-equilibrated with 20 mM Tris, pH 7.4; 150 mM NaCl. The protein-A–Sepharose pellets were washed extensively with 20 mM Tris, pH 7.4; 150 mM NaCl; and either analyzed by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting or used for 5-phosphatase assays as described in “PtdIns(3,4,5)P3 5-phosphatase assays.” For DNase I treatment experiments, platelets were lysed with 1 volume of Triton X-100 lysis buffer (without EGTA) and fractionated in the presence of DNase I (final concentration of 2 mg/mL). The Triton X-100–soluble fraction was isolated and immunoprecipitated as described earlier in this paragraph with either nonimmune sera or affinity-purified SHIP-2 antibodies.
Intracellular localization in platelets
Coverslips were coated with VWF (10 μg/mL) blocked as previously described.24 Platelets obtained from healthy volunteers as previously described23 were applied to VWF-coated coverslips and incubated at 37°C. Following this, coverslips were washed with Tyrodes buffer (12 mM NaHCO3, 10 mM Hepes [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 137 mM NaCl, 2.7 mM KCl, 5.5 mM glucose), fixed with 4% formaldehyde, and permeabilized with 0.5% Triton X-100. Polymerized actin was detected using phalloidin–Texas Red conjugate. Proteins were detected using polyclonal antibodies raised to GPIbα diluted 1:300 (final concentration of 0.76 μg/mL), GPIbβp diluted 1:1000 (final concentration of 0.40 μg/mL), and affinity-purified SHIP-2 used neat, detected with fluorescein isothiocyanate (FITC)–conjugated goat antirabbit IgG. Filamin, cortactin, and vinculin were localized using monoclonal antibodies diluted 1:1000, 1:100, and 1:800, respectively, and detected with FITC/TRITC (FITC/tetramethylrhodamine-5 (and 6)-isothiocyanate)–conjugated goat antimouse IgG.
PtdIns(3,4,5)P3 5-phosphatase assays
The preparation of32P-labeled PtdIns(3,4,5)P3 substrate was undertaken as follows: 25 μg phosphatidylserine and 65 μg PtdIns(4,5)P2 were mixed, dried under nitrogen, then resuspended in 400 μL lipid resuspension buffer (20 mM Hepes, pH 7.5; 1 mM MgCl2; 1 mM EGTA) and sonicated for 5 minutes. Fifty microliters of this suspension was added to 5 nmol of unlabeled ATP and 20 μL of [32P]ATP (2 mCi [7.4 × 107 Bq], 3000 mCi/mmol [1.11 × 1011 Bq/mmol]), 5 μL of 20 × kinase buffer (400 mM Hepes, pH 7.5; 100 mM MgCl2; 20 mM EGTA), and 1 μg affinity-purified recombinant PI 3-kinase, in a final reaction volume of 100 μL.25 Following incubation overnight at room temperature, the reaction was terminated by the addition of 1 M HCl, and following the addition of 5 μg phosphatidylserine, the lipid was extracted with chloroform/methanol (1:1) and 2MKCl saturated with chloroform and dried. PtdIns(3,4,5)P3 5-phosphatase assays were performed on immunoprecipitates in a buffer containing 50 mM Tris, pH 7.2; and 5 mM MgCl2 as described previously.26 Following the reaction, lipids were extracted and analyzed by thin-layer chromatography (TLC). The phosphoinositide standards used for the migration of PtdIns(3,4,5)P3 and PtdIns(3,4)P2 on TLC were verified by deacylation and high-performance liquid chromatography (HPLC) analysis.27
Immunoblotting HA-tagged SHIP-2 and SHIP-1 recombinant proteins in COS-1 cells
COS-1 cells were transiently transfected with either HA empty vector, HA–SHIP-2, or HA–SHIP-1 as previously described18 and lysates immunoblotted with either affinity-purified polyclonal SHIP-2 antibodies or monoclonal SHIP-1 antibodies.
Results
SHIP-2 is expressed in platelets
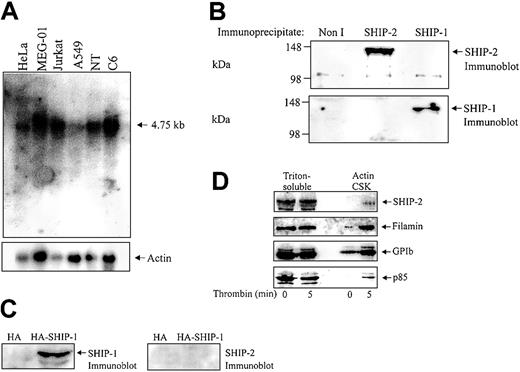
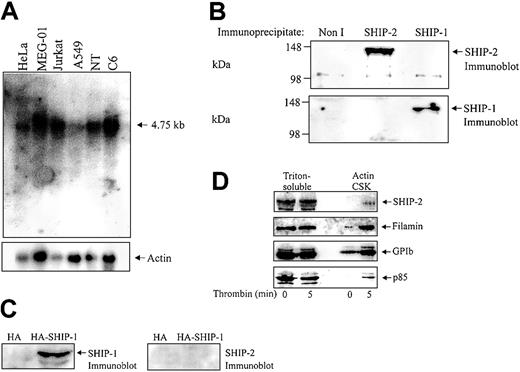
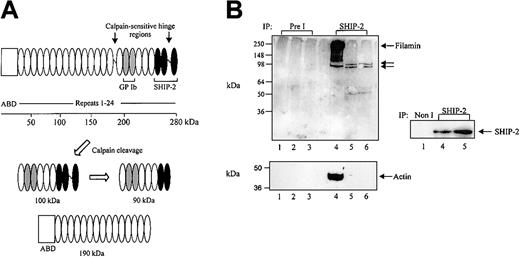
Although SHIP-2 has been strongly implicated in regulating actin dynamics at the leading edge of the cell, this signal-terminating enzyme has not been characterized in highly motile cells, such as platelets. Analysis of the expression profile of SHIP-2 has revealed widespread expression in skeletal muscle, heart, brain, lung, and some hematopoietic cells including T and B cells.20,28 However, expression of SHIP-2 in platelets has yet to be fully characterized. Northern blot analysis was therefore performed using a probe encoding sequences unique to SHIP-2 (nucleotides 3017 to 3989) that have no homology to SHIP-1. Analysis of various cancer cell lines including adenocarcinoma (Hela), a human megakaryocytic cell line (MEG-01), T-cell leukemia (Jurkat), lung cancer (A549), neuroteratocarcinoma (NT), and glioma (C6) demonstrated expression of a 4.75-kb message, consistent with the predicted size of the SHIP-2 transcript (Figure 1A). The expression level of SHIP-2 in the megakaryocyte cell line, MEG-01, when standardized by reprobing the Northern blot with an actin probe, was comparable to that observed in the other cell lines.
SHIP-2 is expressed in a megakaryocyte cell line and in human platelets. (A) Membranes containing approximately 20 μg mRNA from the indicated cell lines, adenocarcinoma of the cervix (HeLa), megakaryoblast (MEG-01), T-cell leukemia (Jurkat), lung carcinoma (A549), neuroteratocarcinoma (NT,) and glioma (C6), were hybridized with SHIP-2 cDNA (nucleotides 3017-3989) and washed as described in “Materials and methods.” After exposure, the membrane was allowed to decay and was subsequently hybridized to an actin probe. (B) The Triton X-100– soluble fraction of platelets was isolated and immunoprecipitated with either nonimmune (Non I) sera, affinity-purified SHIP-2 antipeptide, or SHIP-1 antibodies and immunoblotted with antibodies to SHIP-2 (top), or SHIP-1 (bottom). (C) COS-1 cells transiently transfected with HA empty vector, or HA–SHIP-1 were harvested and the lysates immunoblotted with either SHIP-1 (left), or SHIP-2 (right) antibodies. (D) Platelets were either left untreated or treated with thrombin (1 U/mL, final concentration) for 5 minutes at room temperature and then lysed. The Triton-soluble and actin cytoskeletal (Actin CSK) fractions were isolated and immunoblotted with antibodies to SHIP-2, filamin, glycocalicin (GPIb), or p85 as indicated. Results shown are typical of 3 separate experiments.
SHIP-2 is expressed in a megakaryocyte cell line and in human platelets. (A) Membranes containing approximately 20 μg mRNA from the indicated cell lines, adenocarcinoma of the cervix (HeLa), megakaryoblast (MEG-01), T-cell leukemia (Jurkat), lung carcinoma (A549), neuroteratocarcinoma (NT,) and glioma (C6), were hybridized with SHIP-2 cDNA (nucleotides 3017-3989) and washed as described in “Materials and methods.” After exposure, the membrane was allowed to decay and was subsequently hybridized to an actin probe. (B) The Triton X-100– soluble fraction of platelets was isolated and immunoprecipitated with either nonimmune (Non I) sera, affinity-purified SHIP-2 antipeptide, or SHIP-1 antibodies and immunoblotted with antibodies to SHIP-2 (top), or SHIP-1 (bottom). (C) COS-1 cells transiently transfected with HA empty vector, or HA–SHIP-1 were harvested and the lysates immunoblotted with either SHIP-1 (left), or SHIP-2 (right) antibodies. (D) Platelets were either left untreated or treated with thrombin (1 U/mL, final concentration) for 5 minutes at room temperature and then lysed. The Triton-soluble and actin cytoskeletal (Actin CSK) fractions were isolated and immunoblotted with antibodies to SHIP-2, filamin, glycocalicin (GPIb), or p85 as indicated. Results shown are typical of 3 separate experiments.
To further characterize SHIP-2 in platelets, we immunoblotted and immunoprecipitated SHIP-2 from Triton-soluble platelet lysates using a previously characterized affinity-purified SHIP-2–specific antipeptide antibody.18 This SHIP-2 antibody is raised to peptide sequences, unique to SHIP-2, not found in the highly related 5-phosphatase SHIP-1 or any other 5-phosphatase. To confirm specificity of the SHIP-2 antibody, SHIP-1 or SHIP-2 immunoprecipitates from Triton-soluble platelet lysates were immunoblotted with specific antibodies to each 5-phosphatase. SHIP-2 antipeptide antibodies detected a 148-kDa polypeptide in SHIP-2, but not SHIP-1, immunoprecipitates consistent with SHIP-2 expression in platelets (Figure 1B). Furthermore, SHIP-1 antibodies were not immunoreactive with SHIP-2 immunoprecipitates. SHIP-2 antibodies also did not detect SHIP-1 when hemagglutinin (HA)–tagged SHIP-1 was expressed at high levels in COS-7 cells (Figure 1C).
We also investigated if SHIP-2 translocated to the actin cytoskeleton in thrombin-stimulated platelets, as has been shown for SHIP-1. The subcellular compartmentalization of SHIP-2, filamin, and GPIb in resting versus thrombin-stimulated platelets was compared with that of the p85 subunit of PI 3-kinase. In unstimulated platelets, SHIP-2 was detected in the Triton-soluble fraction but not in the actin cytoskeleton (Figure 1D). Minor proteolysis of SHIP-2 was demonstrated by the presence of a slightly faster migrating polypeptide, which we and others have previously detected in various cell lysates.29 In resting platelets, both filamin and GPIb were present in the unstimulated platelet actin cytoskeleton. Following thrombin activation, the level of SHIP-2 expression in the Triton-soluble fraction remained relatively unchanged and SHIP-2 was detected only very faintly in the actin cytoskeleton (Figure 1D). However, we noted increased amounts of GPIb and filamin in the actin cytoskeleton following 5 minutes thrombin stimulation.
Intracellular localization of SHIP-2 in VWF-stimulated platelets
Many motile cells respond to agonists and chemokines by localized actin remodeling or membrane ruffling (lamellipodia) in the direction of the stimulus.30 Platelets are terminally differentiated anucleate cells with a highly specialized cytoskeleton, and the formation of filopodia and lamellipodia in response to platelet activation is well described. We investigated the intracellular localization of SHIP-2 in platelets spreading on a VWF matrix using confocal microscopy. In addition, in order to improve the size of the platelet images and the resolution, high-powered confocal microscopy was performed using an original magnification of × 400.
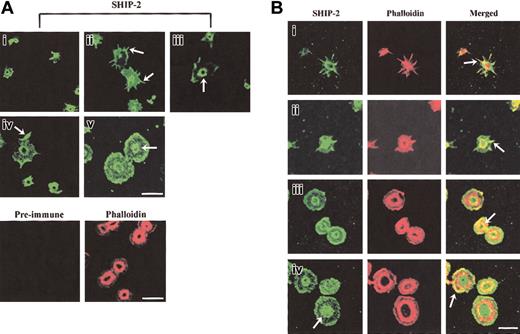
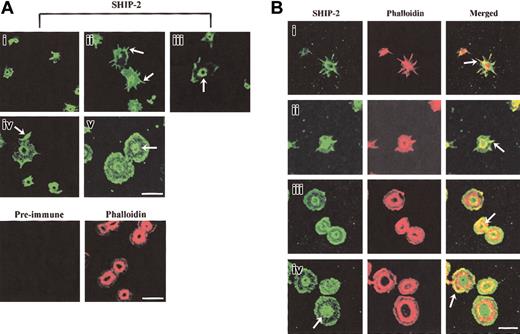
The intracellular localization of SHIP-2 in platelets spreading on a VWF matrix was dynamic with respect to specific phases of platelet activation and was different from the localization that we previously reported in resting and epidermal growth factor (EGF)–stimulated COS-7 cells.18 At early phases of platelet adhesion, SHIP-2 staining was intense in small unspread platelets and did not specifically localize to any discernable structure; however, staining was absent from the center of the platelet (Figure 2Ai). As platelets spread, SHIP-2 localized to filopodia (Figure 2Aii arrow) and SHIP-2 antibodies also stained a central ring structure, with no staining within the ring (Figure 2Biii arrow). SHIP-2 also localized to lamellipodia (Figure 2Aiv arrow). In spread platelets, SHIP-2 staining was diffuse at the plasma membrane and staining at the central ring was maintained but was less clearly demarcated (Figure 2Av). Preimmune staining was nonreactive. The outline of the platelets used for preimmune staining was demonstrated by phalloidin staining.
SHIP-2 localizes to actin-rich regions in spreading platelets. (A) Platelets spread on a VWF matrix were fixed, permeabilized, stained with SHIP-2 antibodies, or costained with affinity-purified preimmune sera and phalloidin and visualized by confocal microscopy. Arrows indicate SHIP-2 staining at filopodia (ii), the central actin ring (iii), lamellipodia (iv) and diffuse central staining detected in some spread platelets (v). Bar, 10 μm. (B) Platelets spread on a VWF matrix were fixed, permeabilized, costained with affinity-purified SHIP-2 antibodies and phalloidin, and visualized by confocal microscopy. Arrows indicate colocalization of SHIP-2 and phalloidin at filopodia (i-ii), at the inner actin ring (iii) and at lamellipodia (iv) (merged images). The arrow showing SHIP-2 staining in subpanel iv indicates diffuse central staining detected in some spread platelets. Bar, 10 μm.
SHIP-2 localizes to actin-rich regions in spreading platelets. (A) Platelets spread on a VWF matrix were fixed, permeabilized, stained with SHIP-2 antibodies, or costained with affinity-purified preimmune sera and phalloidin and visualized by confocal microscopy. Arrows indicate SHIP-2 staining at filopodia (ii), the central actin ring (iii), lamellipodia (iv) and diffuse central staining detected in some spread platelets (v). Bar, 10 μm. (B) Platelets spread on a VWF matrix were fixed, permeabilized, costained with affinity-purified SHIP-2 antibodies and phalloidin, and visualized by confocal microscopy. Arrows indicate colocalization of SHIP-2 and phalloidin at filopodia (i-ii), at the inner actin ring (iii) and at lamellipodia (iv) (merged images). The arrow showing SHIP-2 staining in subpanel iv indicates diffuse central staining detected in some spread platelets. Bar, 10 μm.
To compare the localization of SHIP-2 with respect to actin in platelets spreading on a VWF matrix, we costained for SHIP-2 and polymerized actin; the latter was detected using phalloidin. In partially spread platelets, the staining of SHIP-2 at filopodia colocalized intensely with actin (Figure 2Bi-ii arrow) and at the central actin ring (also called the granulomere31 ; Figure 2Bi-ii). With increased platelet spreading, SHIP-2 colocalization with the central actin ring was predominantly on the inner aspect of the actin ring (Figure 2Biii arrow, merged images). In some platelets, as the actin ring enlarged, SHIP-2 staining was predominantly internal to the actin ring and was diffuse rather than “ringlike” at this site (Figure 2Biv arrow, SHIP-2 staining). Localization of SHIP-2 to lamellipodia was demonstrated in the merged images by the eccentric colocalization of SHIP-2 with actin staining at one side of the platelet (Figure 2Biv arrow, merged image). These studies demonstrate a novel localization of SHIP-2 in spreading platelets to filopodia and the central actin ring in addition to lamellipodia. The localization of SHIP-2 to these sites may serve to regulate PtdIns(3,4,5)P3 hydrolysis and thereby actin remodeling.
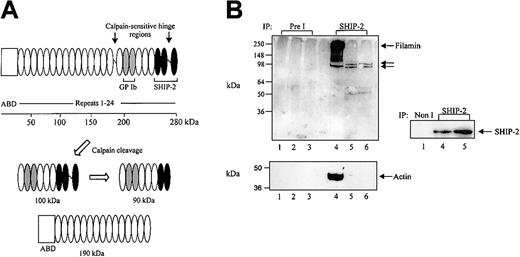
We have recently shown that the SHIP-2 proline-rich domain binds the actin-binding protein, filamin, at the C-terminal immunoglobulin repeats 22 to 24.18 In COS-7 cells, coimmunoprecipitation of SHIP-2 and filamin demonstrates that the two species form a constitutive complex in vivo, which is unaltered by EGF stimulation. We further pursued the investigation of this association in human platelets to reveal other potential components in this complex (ie, actin and GPIbα). We determined the complexes that SHIP-2 forms with filamin, actin, and GPIbα in both unstimulated and thrombin-or VWF-stimulated platelets. Filamin binds polymerized actin via its N-terminal actin-binding domain (Figure 3A). In platelets, the calcium-dependent protease calpain cleaves filamin into 2 fragments, one of approximately 190 kDa, which contains the actin-binding domain, and another of 100 kDa,8 with the latter further hydrolyzed to 90-kDa and 10-kDa polypeptides (Figure 3A). The SHIP-2 binding site on filamin is present in both the 100- and 90-kDa fragments but not the 190-kDa polypeptide8 (Figure 3A). To eliminate the possibility that actin may act as an intermediate in the association between SHIP-2 and filamin, SHIP-2 immunoprecipitations were performed in the presence or absence of DNase I, a potent inhibitor of actin polymerization. Platelet Triton-soluble lysates with or without EGTA (DNase I enzyme activity is dependent on the presence of divalent cations) were immunoprecipitated using affinity-purified antibodies specific for SHIP-2 and immunoblotted with filamin antibodies, which recognize both intact filamin as well as calpain-cleaved filamin polypeptides. In the presence of EGTA and without DNase-1, SHIP-2 immunoprecipitates, but not preimmune immunoprecipitates, demonstrated a 250-kDa polypeptide recognized by filamin antibodies (Figure 3B top, lanes 4 and 1, respectively), consistent with association between SHIP-2 and filamin in unstimulated platelets. Under these conditions, SHIP-2 immunoprecipitates also contained actin (Figure 3B bottom, lane 4). In the absence of EGTA and/or with DNase I, SHIP-2 immunoprecipitates immunoblotted with filamin antibodies demonstrated 100- and 90-kDa polypeptides consistent with calpain-cleaved filamin fragments that have lost the actin-binding domain (Figure 3B top, lanes 5-6). However, the level of association of the 100- and 90-kDa filamin fragments with SHIP-2 appeared decreased, compared with intact filamin. No actin was demonstrated in SHIP-2 immunoprecipitates under these conditions (Figure 3B bottom, lanes 5-6). In control studies we demonstrated equal immunoprecipitation of SHIP-2 in platelet lysates in the presence or absence of EGTA (Figure 3B). Collectively, these studies demonstrate a trimeric complex between SHIP-2, filamin, and actin in the Triton-soluble lysate from resting platelets. Under these conditions association between SHIP-2 and calpain-cleaved filamin appears independent of actin.
SHIP-2 associates with filamin independently of actin. (A) Schematic of the calpain-mediated cleavage profile of filamin A. The empty box represents the actin-binding domain (ABD) and the repeats 1-24 of filamin are shown as ovals. Gray and black filled ovals represent GPIb and SHIP-2 binding sites, respectively. The calpain-cleavage sites on intact filamin are shown. Calpain-mediated cleavage of filamin results in the production of 100-kDa and 90-kDa fragments, which retain the GPIb and partial SHIP-2 binding sites, and a 190-kDa fragment, which contains the actin-binding domain but lacks the SHIP-2 and GPIb binding site. (B) The Triton X-100–soluble fraction of platelets isolated in lysis buffer containing EGTA (lanes 1, 4), in the absence of EGTA (lanes 2, 5), or in the presence of DNase I without EGTA (lanes 3, 6) was immunoprecipitated with either preimmune (Pre I) sera or SHIP-2 affinity-purified antibodies and immunoblotted with filamin antibodies (top), actin antibodies (bottom), or SHIP-2 antibodies as shown. Arrows indicate the migration of intact filamin and calpain-cleaved filamin fragments.
SHIP-2 associates with filamin independently of actin. (A) Schematic of the calpain-mediated cleavage profile of filamin A. The empty box represents the actin-binding domain (ABD) and the repeats 1-24 of filamin are shown as ovals. Gray and black filled ovals represent GPIb and SHIP-2 binding sites, respectively. The calpain-cleavage sites on intact filamin are shown. Calpain-mediated cleavage of filamin results in the production of 100-kDa and 90-kDa fragments, which retain the GPIb and partial SHIP-2 binding sites, and a 190-kDa fragment, which contains the actin-binding domain but lacks the SHIP-2 and GPIb binding site. (B) The Triton X-100–soluble fraction of platelets isolated in lysis buffer containing EGTA (lanes 1, 4), in the absence of EGTA (lanes 2, 5), or in the presence of DNase I without EGTA (lanes 3, 6) was immunoprecipitated with either preimmune (Pre I) sera or SHIP-2 affinity-purified antibodies and immunoblotted with filamin antibodies (top), actin antibodies (bottom), or SHIP-2 antibodies as shown. Arrows indicate the migration of intact filamin and calpain-cleaved filamin fragments.
SHIP-2, filamin, and GPIb form a complex in the Triton-soluble compartment of resting platelets but not stimulated platelets
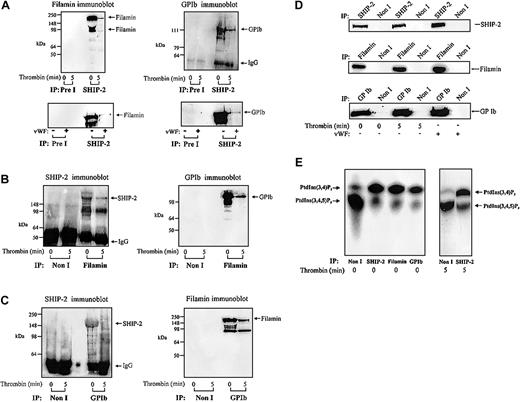
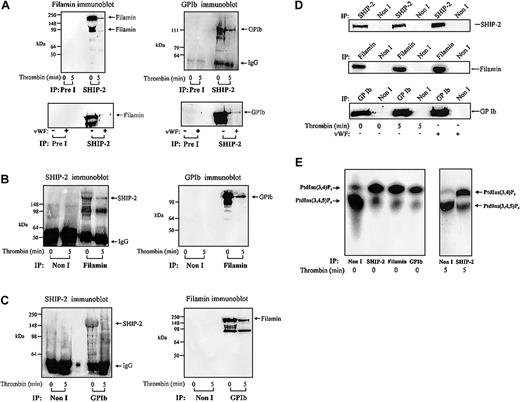
The intracellular domain of GPIbα associates with filamin, which in turn directly binds actin.5,8 In resting platelets, this interaction stabilizes the platelet membrane. In addition, under conditions of high shear the interaction between GPIbα and filamin is essential for receptor anchorage to VWF matrix.4,8 As we have shown, SHIP-2 associates with filamin and actin, we determined, if GPIbα was also present in this complex. This was also of interest as we have previously shown that PI 3-kinase binds to GPIbα via an interaction with 14-3-3, and therefore may generate PtdIns(3,4,5)P3, the substrate for SHIP-2 in close association with the receptor.6 Triton-soluble platelet lysates were isolated in the presence of EGTA (to minimize calpain cleavage of filamin) from resting or thrombin-activated platelets and immunoprecipitated with SHIP-2–specific antibodies and immunoblotted with antibodies to filamin or glycocalicin (Figure 4A). The latter antibody recognizes the extracellular domain of GPIbα.6 Both intact filamin and cleaved filamin were detected in SHIP-2 immunoprecipitates from the Triton-soluble fraction of unstimulated platelets. Following platelet activation, the level of filamin detected in SHIP-2 immunoprecipitates significantly decreased (Figure 4A). SHIP-2 immunoprecipitates were probed with anti-GPIb and detected a 125-kDa polypeptide, consistent with association of GPIb and SHIP-2, in the Triton-soluble fraction of unstimulated platelets. Detection of the GPIb–SHIP-2 complex reduced significantly in this fraction following thrombin activation. These results are in contrast to the constitutive association of SHIP-2 and filamin we have previously reported in the Triton-soluble fraction of EGF-stimulated COS-7 cells.18
Filamin and GPIb coimmunoprecipitate with SHIP-2. (A) The Triton X-100–soluble fraction of resting, thrombin-stimulated (1 U/mL, final concentration for 5 minutes), or VWF-stimulated platelets (10 μg/mL, and botrocetin, 3 μg/mL; final concentration for 5 minutes) was isolated. The Triton X-100–soluble fraction was immunoprecipitated with either preimmune (Pre I) sera or SHIP-2 antibodies and immunoblotted with filamin or GPIb antibodies. (B) The Triton X-100–soluble fraction of resting and thrombin-stimulated platelets was isolated as described in panel A, immunoprecipitated with either nonimmune (Non I) sera or filamin antibodies and immunoblotted with either affinity-purified SHIP-2 antibodies or GPIb antibodies. (C) The Triton X-100–soluble fraction of resting and thrombin-stimulated platelets was isolated as described in panel A, immunoprecipitated with either nonimmune (Non I) sera or GPIb antibodies and immunoblotted with affinity-purified SHIP-2 antibodies or filamin antibodies. (D) The Triton X-100–soluble fraction of resting, thrombin-stimulated (1 U/mL, final concentration for 5 minutes), or VWF-stimulated platelets (10 μg/mL, and botrocetin, 3 μg/mL; final concentration for 5 minutes) was isolated. The Triton X-100–soluble fraction was immunoprecipitated with either nonimmune (Non I) sera, SHIP-2, filamin, or GPIb antibodies and immunoblotted with the immunoprecipitating antibody. (E) Triton X-100–soluble fractions were isolated from untreated platelets and immunoprecipitated with either nonimmune (Non I) sera, affinity-purified SHIP-2 antibodies, filamin antibodies, or GPIb antibodies. The Triton X-100–soluble fraction was also isolated from thrombin-stimulated (1 U/mL, final concentration for 5 minutes) platelets and immunoprecipitated with either nonimmune (Non I) sera or affinity-purified SHIP-2 antibodies. Immunoprecipitates captured on protein-A–Sepharose were subjected to PtdIns(3,4,5)P3 5-phosphatase assays and the lipid products of the enzyme assay were examined by TLC. The migration of the phospholipids was compared with known standards PtdIns(3,4,5)P3 and PtdIns(3,4)P2.
Filamin and GPIb coimmunoprecipitate with SHIP-2. (A) The Triton X-100–soluble fraction of resting, thrombin-stimulated (1 U/mL, final concentration for 5 minutes), or VWF-stimulated platelets (10 μg/mL, and botrocetin, 3 μg/mL; final concentration for 5 minutes) was isolated. The Triton X-100–soluble fraction was immunoprecipitated with either preimmune (Pre I) sera or SHIP-2 antibodies and immunoblotted with filamin or GPIb antibodies. (B) The Triton X-100–soluble fraction of resting and thrombin-stimulated platelets was isolated as described in panel A, immunoprecipitated with either nonimmune (Non I) sera or filamin antibodies and immunoblotted with either affinity-purified SHIP-2 antibodies or GPIb antibodies. (C) The Triton X-100–soluble fraction of resting and thrombin-stimulated platelets was isolated as described in panel A, immunoprecipitated with either nonimmune (Non I) sera or GPIb antibodies and immunoblotted with affinity-purified SHIP-2 antibodies or filamin antibodies. (D) The Triton X-100–soluble fraction of resting, thrombin-stimulated (1 U/mL, final concentration for 5 minutes), or VWF-stimulated platelets (10 μg/mL, and botrocetin, 3 μg/mL; final concentration for 5 minutes) was isolated. The Triton X-100–soluble fraction was immunoprecipitated with either nonimmune (Non I) sera, SHIP-2, filamin, or GPIb antibodies and immunoblotted with the immunoprecipitating antibody. (E) Triton X-100–soluble fractions were isolated from untreated platelets and immunoprecipitated with either nonimmune (Non I) sera, affinity-purified SHIP-2 antibodies, filamin antibodies, or GPIb antibodies. The Triton X-100–soluble fraction was also isolated from thrombin-stimulated (1 U/mL, final concentration for 5 minutes) platelets and immunoprecipitated with either nonimmune (Non I) sera or affinity-purified SHIP-2 antibodies. Immunoprecipitates captured on protein-A–Sepharose were subjected to PtdIns(3,4,5)P3 5-phosphatase assays and the lipid products of the enzyme assay were examined by TLC. The migration of the phospholipids was compared with known standards PtdIns(3,4,5)P3 and PtdIns(3,4)P2.
VWF mediates platelet adhesion through the binding of matrix-bound VWF to GPIb-IX-V, which then transmits signals leading to platelet activation. We investigated whether the association of SHIP-2 and filamin was also influenced by signaling, specifically mediated through the GPIb-IX-V complex. Anti–SHIP-2 immunoprecipitates from the Triton-soluble fraction of untreated or VWF-stimulated platelets were immunoblotted with filamin or GPIb antibodies (Figure 4A bottom). These studies demonstrated that SHIP-2 immunoprecipitates contained both filamin and GPIb. However, following VWF treatment, the level of the SHIP-2/filamin/GPIb complex detected in the Triton-soluble fraction decreased significantly.
To confirm interactions between SHIP-2, filamin, and GPIb, reciprocal immunoprecipitation experiments were performed in which filamin was immunoprecipitated from the Triton-soluble fraction, derived from resting or thrombin-stimulated platelets, and probed using affinity-purified polyclonal antibodies to SHIP-2 or GPIb (Figure 4B). SHIP-2 was present in antifilamin immunoprecipitates from the Triton-soluble fraction of resting or thrombin-stimulated platelets (Figure 4B) but decreased significantly following thrombin stimulation. We also noted some proteolysis of SHIP-2 in filamin immunoprecipitates. Filamin immunoprecipitates demonstrated GPIb in complex in the Triton-soluble fraction of unstimulated platelets. However, the level of GPIb detected in filamin immunoprecipitates decreased following thrombin activation.
We also performed anti-GPIb immunoprecipitations of the Triton-soluble fraction of resting or thrombin-stimulated platelets and probed the immunoprecipitates with polyclonal antibodies to SHIP-2 or monoclonal antibodies to filamin (Figure 4C). SHIP-2 was present in anti-GPIb immunoprecipitates of the Triton-soluble platelet fraction of unstimulated platelets and decreased in this fraction following thrombin stimulation. Association between GPIb and filamin was detected in the Triton-soluble fraction of unstimulated platelets. We noted, however, that the detection of this complex decreased upon thrombin activation. In control studies we immunoprecipitated SHIP-2, filamin, or GPIb from the Triton-soluble fraction of resting, thrombin-, or VWF-activated platelets and immunoblotted with the immunoprecipitating antibody. The level of SHIP-2, filamin, and GPIb immunoprecipitated did not change following platelet activation, demonstrating that the relative amount of immunoprecipitated antigen did not change as a result of platelet activation, although the level of the SHIP-2, filamin, and/or GPIb detected in complex decreased (Figure 4D).
SHIP-2 in the SHIP-2/filamin/GPIb complex in resting platelets is catalytically active
SHIP-2 hydrolyzes the 5-position phosphate from PtdIns(3,4,5)P3 generating PtdIns(3,4)P2 and thereby regulates PtdIns(3,4,5)P3-induced actin remodeling. We investigated in unstimulated platelets whether SHIP-2 bound to filamin/actin and GPIb was enzymatically active. Anti–SHIP-2, antifilamin, and anti-GPIb immunoprecipitates from the Triton-soluble fraction of unstimulated or thrombin-stimulated platelets were bound to protein-A–Sepharose and washed extensively. The protein-A–Sepharose pellets were incubated with PtdIns(32P-3,4,5)P3 and standard 5-phosphatase enzyme assays were performed as described in “Materials and methods.” PtdIns(3,4,5)P3 and the lipid products of the 5-phosphatase assays were extracted and analyzed by TLC (Figure 4E). Incubation of preimmune immunoprecipitates with PtdIns(3,4,5)P3 resulted in little formation of PtdIns(3,4)P2. In contrast, the SHIP-2, filamin, and GPIb immunoprecipitates all demonstrated evidence of significant PtdIns(3,4,5)P3 5-phosphatase activity. In these immunoprecipitates, but not preimmune immunoprecipitates, the addition of PtdIns(3,4,5)P3 resulted in the formation of PtdIns(3,4)P2, consistent with expression of functionally active SHIP-2 enzyme in complex with both filamin/actin and GPIb in unstimulated platelets. There was no change in the relative amount of SHIP-2 PtdIns(3,4,5)P3 5-phosphatase activity immunoprecipitated following thrombin stimulation (Figure 4E).
Localization of SHIP-2, filamin, and GPIb with actin in spreading platelets
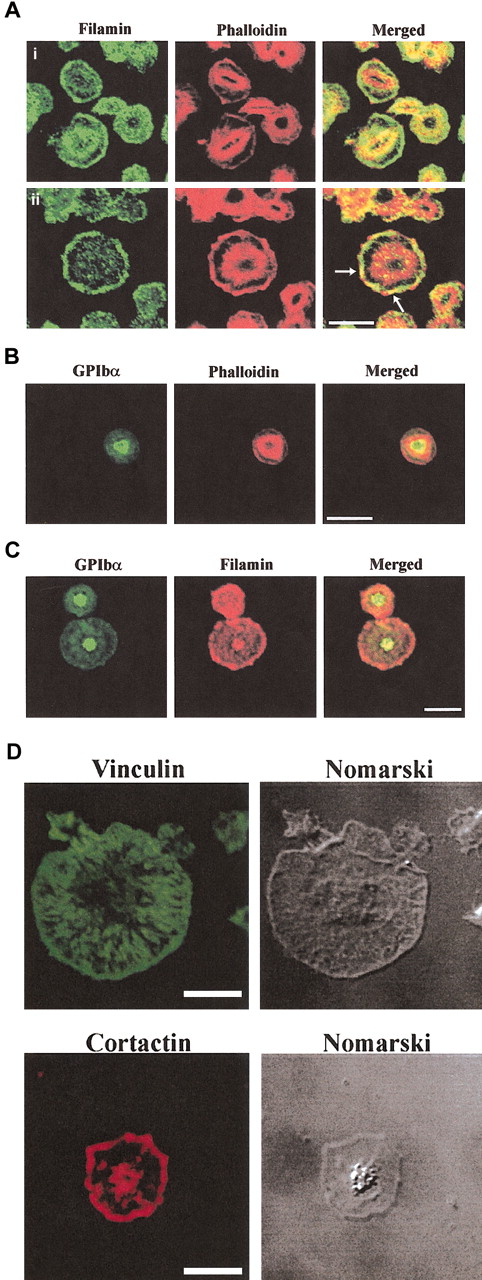
The results of the immunoprecipitation analysis demonstrate SHIP-2 in a complex with filamin, actin, and GPIbα. Following thrombin-stimulation the level of the SHIP-2 and filamin, and filamin and GPIb complex significantly decreased in the Triton-soluble fraction. We investigated the association between SHIP-2, filamin, actin, and GPIb-IX-V in the RIPA-extracted cytoskeletal fraction using immunoprecipitation of each species and immunoblot analysis. A complex was not reproducibly detected between SHIP-2 and filamin and/or GPIb-IX-V in the RIPA-extracted cytoskeletal fraction from either unstimulated or activated platelets (data not shown). There are several possible interpretations of this result. The SHIP-2/GPIb/filamin complex may disassociate following platelet activation, or the complex may incorporate into the activated actin cytoskeleton and due to the technical constraints of the harsh extraction conditions using RIPA extraction buffer, which contains 1% Nonidet P40 (NP40) and 0.1% SDS, we cannot demonstrate this complex in the activated actin cytoskeleton. Therefore, to investigate these species in the activated cytoskeleton, the intracellular localization of SHIP-2, filamin, and GPIb (detected using specific antibodies) was colocalized with actin (detected by phalloidin staining, which stains polymerized actin) in platelets spreading on a VWF matrix (Figure 5). The colocalization of filamin and GPIb with actin in a functional model of spreading platelets, to our knowledge, has not been previously reported.
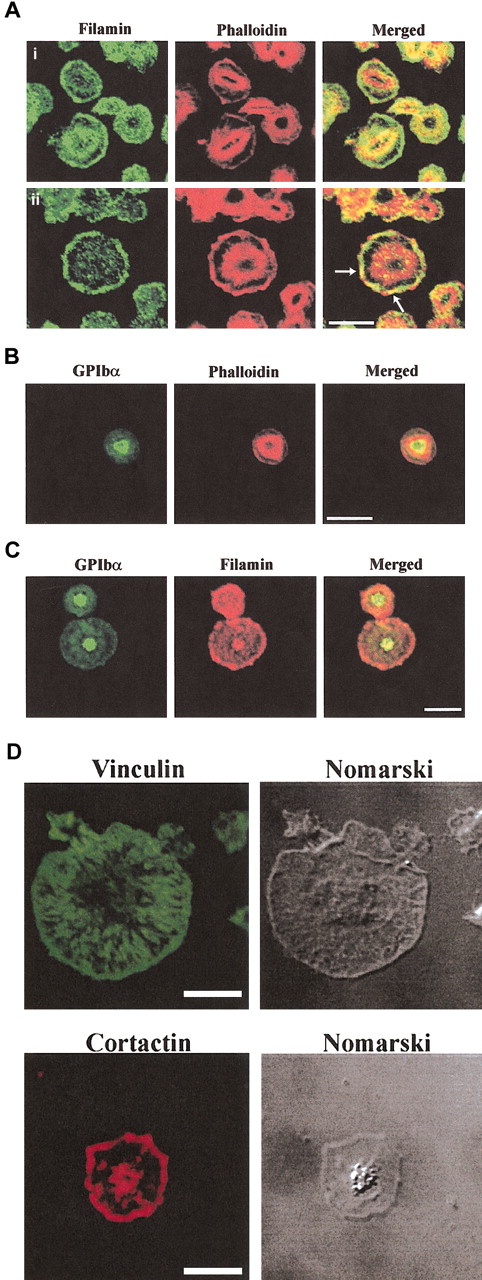
Localization of filamin, GPIb and actin in spreading platelets. Platelets spread on a VWF matrix were fixed, permeabilized, and costained with filamin antibodies and phalloidin (Ai-ii), GPIbα antibodies and phalloidin (B), GPIbα and filamin antibodies (C), vinculin antibodies (D), and cortactin antibodies (D). Arrows in merged images in (5Aii) indicate colocalization of filamin and submembraneous actin at lamellipodia. Cells were visualized by confocal microscopy. Bars, 10 μm.
Localization of filamin, GPIb and actin in spreading platelets. Platelets spread on a VWF matrix were fixed, permeabilized, and costained with filamin antibodies and phalloidin (Ai-ii), GPIbα antibodies and phalloidin (B), GPIbα and filamin antibodies (C), vinculin antibodies (D), and cortactin antibodies (D). Arrows in merged images in (5Aii) indicate colocalization of filamin and submembraneous actin at lamellipodia. Cells were visualized by confocal microscopy. Bars, 10 μm.
In partially spread platelets, filamin localized in the cytosol and plasma membrane staining was prominent (Figure 5Ai). Patchy colocalization with actin was demonstrated in between filamin and actin at the central actin ring (Figure 5Ai, merged image). With increased platelet spreading, intense filamin staining was demonstrated at the plasma membrane with little cytosolic staining (Figure 5Aii). Filamin staining colocalized with submembraneous actin at the plasma membrane lamellipodia (Figure 5Aii arrows, merged images). In spread platelets there was little colocalization between filamin and actin at the central actin ring.
To determine the intracellular localization of GPIb we used 2 distinct antibodies: polyclonal antibodies to GPIbβp, which recognizes cytoplasmic sequence within the GPIbβ chain (data not shown), and polyclonal antibodies to the extracellular domain of GPIbα (Figure 5B). Results with both antibodies were comparable. GPIbβp antibodies intensely stained a central region of the platelet, which was localized predominantly internal to the central actin ring. Only very faint plasma membrane staining was detected using this antibody (data not shown). Antibodies to the extracellular region of GPIbα demonstrated intense staining at the center of the spreading platelet, which localized internal to the central actin ring and demonstrated patchy colocalization with actin at this site. No plasma membrane staining was demonstrated using this antibody (Figure 5B). We also examined whether GPIb colocalized with filamin (Figure 5C). Consistent with the immunoprecipitation data demonstrating decreased detection of the filamin and GPIb complex with platelet activation in the Triton-soluble fraction, patchy colocalization between these species was observed. In spread platelets, filamin localized predominantly at the plasma membrane, while the receptor localized to the center of the platelet. Patchy colocalization was detected at the center of the spread platelet with no colocalization detected at the plasma membrane (Figure 5C). In control studies, to demonstrate specificity of the antibodies we examined the intracellular localization of vinculin and cortactin. Vinculin localized to the platelet membrane and diffusely in the cytosol with no staining detected at the central actin ring, whereas cortactin localized to lamellipodia at the plasma membrane as has recently been reported in platelets (Figure 5D).32
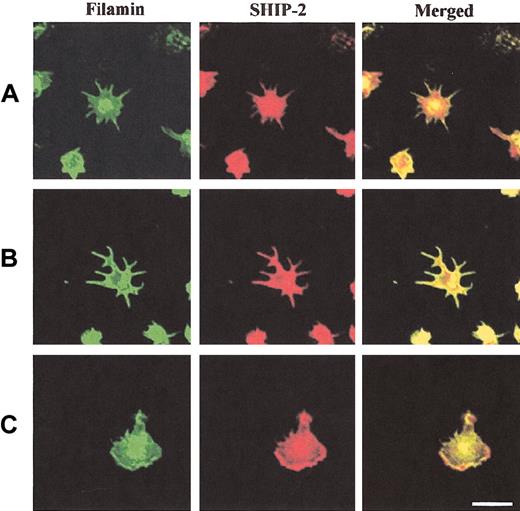
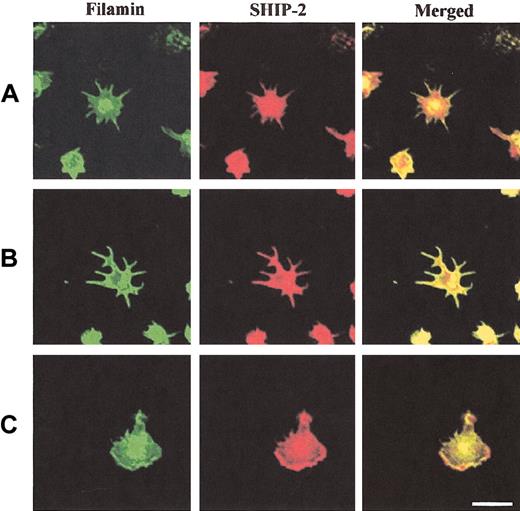
We also determined the colocalization of SHIP-2 with filamin in spreading platelets (Figure 6). SHIP-2 and filamin colocalized intensely at filopodia and also demonstrated colocalization at the central actin ring (Figure 6A-B). In spread platelets, filamin localized most intensely at the plasma membrane (Figure 6C). Colocalization between filamin and SHIP-2 was demonstrated at plasma membrane lamellipodia (Figure 6C, merged images). In the cytosol SHIP-2 staining was detected, but filamin staining was less intense and the 2 species demonstrated only patchy colocalization at this site. These studies suggest the SHIP-2–filamin complex may be incorporated into the submembraneous actin cytoskeleton, as platelets are activated by spreading on a VWF matrix.
SHIP-2 colocalizes with filamin in spreading platelets at lamellipodia. Platelets spread on a VWF matrix were fixed, permeabilized, costained with filamin antibodies and affinity-purified SHIP-2 antibodies, and visualized by confocal microscopy. Bar, 10 μm.
SHIP-2 colocalizes with filamin in spreading platelets at lamellipodia. Platelets spread on a VWF matrix were fixed, permeabilized, costained with filamin antibodies and affinity-purified SHIP-2 antibodies, and visualized by confocal microscopy. Bar, 10 μm.
Discussion
SHIP-2 is a PtdIns(3,4,5)P3 5-phosphatase that has recently been shown to regulate submembraneous actin, lamellipodia formation, and integrin-dependent cell adhesion. The function of this signal-terminating enzyme, however, has not been characterized in highly motile cells. The results of this study demonstrate a number of significant novel findings. First, in human platelets, SHIP-2 forms an indirect complex with actin via interaction with the actin-binding protein, filamin. In spreading platelets, SHIP-2 colocalizes with actin at filopodia, lamellipodia, and the central actin ring. The enzyme also dynamically relocates in spread platelets internal to the central actin ring. Second, SHIP-2 forms a functionally active complex with filamin, and the level of this complex decreases in the Triton-soluble fraction following platelet adhesion and activation. This is in contrast to adherent COS-7 cells in which the complex between these proteins is constitutive in the Triton-soluble fraction following EGF stimulation. Third, SHIP-2 also complexes with GPIb-IX-V and following platelet activation less complex is detected in the Triton-soluble fraction. Fourth, we have also investigated the relationship between SHIP-2/filamin/actin and GPIb-IX-V and colocalized these species by imaging platelets spreading on a VWF matrix. These studies indicate GPIb-IX-V is localized primarily to the center of the platelet, while filamin localizes with SHIP-2 at lamellipodia at the submembrane actin cytoskeleton, suggesting the complex between SHIP-2 and filamin may relocalize to this site rather than disassociate upon platelet activation.
PtdIns(3,4,5)P3 is a critical regulator of many intracellular signaling pathways. Recent studies have highlighted the role this lipid signaling cascade, in particular PtdIns(3,4,5)P3, plays in instructing actin remodeling.14,15,17 PtdIns(3,4,5)P3 localizes to lamellipodia and regulates Rac and Rho activation. The metabolism of PtdIns(3,4,5)P3 has emerged as molecular mechanism regulating cell migration.33-35 SHIP-2 localizes to lamellipodia and inhibits membrane ruffling via regulation of PtdIns(3,4,5)P3. In this study, we have shown SHIP-2 indirectly binds actin via an association with filamin. The signaling adapter protein, 14-3-3ζ, directly binds the cytoplasmic domain of GPIbα and also PI 3-kinase,6 indicating PtdIns(3,4,5)P3 is generated in close proximity to the receptor. The demonstration of functionally active SHIP-2, a specific PtdIns(3,4,5)P3 5-phosphatase associated with GPIb-IX-V, provides evidence for the localized regulation of this signaling molecule in unstimulated platelets.
The results from the studies reported here showing decreased SHIP-2/filamin complex in the detergent-soluble fraction of thrombin- or VWF-stimulated platelets is different from the constitutive association of SHIP-2 and filamin in adherent EGF-stimulated COS-7 cells. There are several possible interpretations of these results. In platelets, the SHIP-2/filamin/GPIb complex may disassociate upon thrombin or VWF stimulation, or alternatively the complex may become incorporated into the activated platelet cytoskeleton. The failure to detect the SHIP-2/filamin complex in the actin cytoskeletal fraction may be a consequence of the complex existing below detectable levels, if it represents only a minor proportion of each species, or the complex may be highly sensitive to the RIPA extraction buffer used to extract the actin cytoskeletal fraction.
In human platelets that express high levels of calpain, the decreased association of SHIP-2 with filamin in activated platelets may result from calpain-mediated cleavage of filamin at the hinge region, thereby disrupting the SHIP-2 binding site. Previously, we used yeast two-hybrid analysis to show that the association between SHIP-2 and filamin is maximal when mediated by filamin repeats 22 to 24, which includes the calpain-cleaved hinge II region located between repeats 23 and 24 (Figure 3A).18 Repeats 22 and 23 in combination, with or without the hinge region II, interacted with SHIP-2; however, this was weaker than with intact repeats 22 to 24. However, we cannot exclude the possibility that the decreased detection of the complex between SHIP-2 and filamin/GPIb-IX-V reflects the incorporation of this complex from the Triton-soluble fraction into the cytoskeletal fraction. In support of this contention, we have used immunoconfocal microscopy to show that SHIP-2 and filamin, but not GPIb-IX-V, colocalize with each other and submembraneous actin at both filopodia and lamellipodia.
Filamin plays a complex role in regulating platelet activation. In addition to binding GPIb-IX-V, filamin binds various other macro-molecules, including engaging the β-chain of β-3 integrin,36 the small GTPases, Cdc42 and Rac, and their guanine nucleotide exchange factor, Trio.7 To date, more than 20 binding partners have been identified and many of these binding partners facilitate the activation of localized signaling pathways involving actin polymerization. The GPIbα chain binds repeats 17 to 19 of filamin.37 The sequences of the GPIbα receptor that mediate this interaction have recently been shown to encompass residues 570 to 590, which are also essential for membrane anchorage of the receptor complex under conditions of high shear stress.10 The results of the study reported here demonstrate filamin may also provide a scaffold for the assembly of signaling proteins, such as SHIP-2, to facilitate signaling via the GPIb-IX-V receptor. We also demonstrated in spread platelets that the filamin and the GPIb-IX-V complex are localized in distinct cytoskeletal compartments with filamin at the submembraneous cytoskeleton and the GPIb-IX-V receptor centralized to the inner central actin ring. This receptor localization is consistent with previous studies using electron microscopy that GPIb-IX-V is located centrally in the platelet, and although receptor localization with respect to polymerized actin or colocalization with filamin was not demonstrated, receptor centralization was shown to be dependent on an intact actin cytoskeleton.38 However, in contrast to our findings that show a decrease in the filamin/GPIb-IX-V complex detected in the Triton-soluble fraction of activated platelets, Kovacsovics and Hartwig38 showed constitutive association between these species by coimmunoprecipitation analysis. The reason for these differences may relate to technical differences in that we did not routinely include DNase I, which potently inhibits actin polymerization, or phallacidin, which stabilizes existing actin filamints in either the lysis or the immunoprecipitation buffer. The results of our analysis are consistent with the contention that as platelets spread and actin polymerizes, GPIb remains internal to the central actin ring, and filamin localizes with SHIP-2 to submembraneous actin, rather than incorporating with a centralized/internalized receptor complex.
Prepublished online as Blood First Edition Paper, April 3, 2003; DOI 10.1182/blood-2002-09-2897.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Robert Andrews, Susan Brown, and Lisa Ooms for helpful discussions. Confocal images were obtained at the Biomedical Confocal Imaging Facility of Monash University.