Abstract
Oncostatin M (OM) transforms the lymph node (LN) into a “super lymphoid organ” with 2 striking features: massive thymus-independent T-cell development and major expansion of the memory T-cell pool. We report that T-cell development in the LckOM LN is regulated by a cyclooxygenase-2 (COX-2)–dependent neoangiogenesis involving high endothelial venules (HEVs). That LN HEVs are particularlyrich in OM-receptor β-chain provides aplausible explanation for the fact that extrathymic T-cell development in LckOM mice is limited to the LN. Moreover, we found that increased production of the CCL20 chemokine by LN stromal cells was instrumental in the expansion of the memory phenotype CD4 T-cell pool in LckOM mice. The generality of the latter finding was demonstrated by the fact that CCL20/CCR6 interactions increase the basal proliferation rate of CD62Llo CD4 T cells irrespective of their thymic (in non–OM-transgenic mice) or extrathymic (in LckOM mice) origin. To our knowledge, CCL20 is the first molecule found to increase the proliferation of memory phenotype CD4 T cells. These findings identify potential targets for the creation of thymic substitutes (LN HEVs) and for expansion of the CD4 memory T-cell compartment (CCL20).
Introduction
In all gnathostomes, the division of labor between primary and secondary lymphoid organs is instrumental in shaping the repertoire and regulating the homeostasis of T lymphocytes. The reason why the thymus unquestionably provides a unique environment for T-cell precursor differentiation remains largely unknown.1,2 Thymic epithelial cells (TECs) derived from the third pharyngeal pouch play a crucial role in supporting thymocyte maturation and positive selection.3,4 Indeed, ectopically grafted MTS24 epithelial progenitor cells are sufficient to generate a functional thymus in vivo.5,6 TECs provide 2 types of signals to thymocytes, T-cell receptor (TCR)–dependent and–independent.2,7,8 Indeed, the major histocompatibility complex (MHC)/peptide complexes displayed by TECs have a dominant influence on the nature of TCR clonotypes that are positively selected. Furthermore, TECs have a unique ability to provide TCR-independent interactions that are essential for several thymocyte developmental events but whose nature is still elusive.3,9,10 In addition, vascularization and mesenchyme-derived fibroblasts contribute to thymus development, probably by providing signals required for seeding of the thymic stroma by early T-cell precursors.11,12 Following emigration from the thymus, T lymphocytes home to secondary lymphoid organs. Under steady-state conditions, naive T cells require signals through their TCR and interleukin-7 receptor (IL-7R) for survival and homeostatic expansion.13,14 In contrast, memory T cells do not require TCR tickling by self–MHC-peptide complexes but need TCR-independent signals that have been defined for CD8 but not for CD4 memory cells. Thus, IL-7 and IL-15 jointly regulate homeostatic proliferation of memory CD8 but are not required for memory CD4 T cells.15-18 Furthermore, while CD8 T-cell memory is stably maintained for life, levels of specific CD4 memory T cells gradually decline.19
For T cells at various stages of maturation in the thymus or the periphery, development in the correct niche is regulated by chemokines.20-22 Developing thymocytes express multiple chemokine receptors and respond to chemokines produced by thymic stromal cells. Of particular relevance, functional evidence has been presented that colonization of the thymus by migrant precursors involves a chemokine-based mechanism.7,23 Consistent with this concept, indirect evidence suggests that CCL25 plays a redundant role in regulating the migration of prothymocytes into the thymus in concert with other elusive chemokines.24 Similarly, egress of mature T cells from the thymic medulla is chemokine dependent,25 involving CCL1926 and other elusive chemokines possibly including CXCL12.27 Furthermore, cognate interaction of chemokine receptor CCR7 on lymphocytes with its ligands CCL19 and CCL21 expressed on high endothelial venules (HEVs) is essential for effective migration of T cells across HEVs into secondary lymphoid organs.28-32
Strikingly, transgenic overexpression of 2 members of the IL-6 family, oncostatin M (OM) and leukemia inhibitory factor (LIF), transforms lymph nodes (LNs) into “super T-lymphoid organs” that display 2 striking features: an unforeseen ability to support massive extrathymic T-cell development and a major accumulation (∼7-fold increase) of mature T cells, of which the vast majority display a memory phenotype.33-35 OM-induced extrathymic T-cell development is found exclusively in the LNs, is totally thymus independent, and can be induced in athymic mice by repeated injection of OM over a few weeks.34,35 The effect of OM on extrathymic T-cell development in the LNs might be due to some amplification of a cryptic pathway recently characterized in nude mice.36 In sharp contrast to what is observed in other models of extrathymic T-cell development (for example, intraepithelial T cells in the gut37 ), the proportions of double-negative (DN), double-positive (DP), and single-positive (SP) T cells in the OM+ LN reproduce those found in a thymus.35 The mature T cells that accumulate in the OM+ LNs (and to a lesser extent in the spleen) show not only phenotypical but also functional attributes of memory T cells: a high basal proliferation rate35,38 and a rapid expansion following in vitro triggering with anti-CD3 or in vivo infection with viruses (M.-E. Blais, G. Gérard, and C.P., manuscript in preparation). Importantly, these extrathymic T cells are functional since they can reject allogeneic tumor cells.34 These considerations prompted us to investigate how OM modifies the LN microenvironment. Our goal was to discover what type of change(s) could enable the LN stroma to support T-cell development and to accommodate a massively increased memory T-cell compartment. Such knowledge should provide invaluable information about how stromal cells regulate crucial steps in T-cell development and homeostasis.
Materials and methods
Mice
C57BL/6 (B6) and B6.SJL-PtprcaPep3b/BoyJ (Ly5a) (B6.SJL; Ly 5.1+) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and cyclooxygenase-2 (COX-2)–deficient mice (B6;129P2-Ptgs2tm1)39 from Taconic (Germantown, NY). CCR6-deficient mice40 were kindly provided by Dr S. A. Lira (Schering-Plough Research Institute, Kenilworth, NJ). LckOM-transgenic mice on a C57BL/6 background have been previously described.34,35 Mice were bred and housed under specific pathogen-free conditions in sterile ventilated racks at the Guy-Bernier Research Center, Montreal, QC, Canada, according to the standards of the Canadian Council on Animal Care. Thymectomy and cell transplantation were performed as described.35,41
Immunohistochemistry
Acetone-fixed (5 μm) cryostat sections of lymphoid organs from 8-week-old mice were stained using a 3-step protocol: slides were incubated with purified primary antibody (one hour), biotinylated secondary antibody (30 minutes), followed by streptavidin-horseradish peroxidase conjugate (30 minutes). Staining was revealed with 3,3′-diaminobenzidine tetrahydrochloride (Ventana Medical Systems, Tucson, AZ). The number of CD62L ligand+ blood vessels per square micrometer was estimated as described.42 Primary antibodies against CD8α (clone 53-6.7), CD4 (clone RM4-5), CD11c (clone HL3), and peripheral node addressin (PNAd) carbohydrate epitope (CD62L ligand, clone MECA-79) were purchased from Pharmingen (Mississauga, ON, Canada); antibodies directed against B220, hyaluronic acid, reticular fibroblasts (clone ERTR7), pancytokeratin, fibronectin, and epiligrin (clone P3H9-2) were purchased from Cedarlane Laboratories (Hornby, ON, Canada), and the antibody directed against tenascin (clone MTn-12) was purchased from Sigma (St Louis, MO).
Flow cytometry and cell sorting
Cell surface staining and bromodeoxyuridine (BrdU) labeling were performed as previously described,35 and cells were analyzed on a FACScalibur (Becton Dickinson, Mountain View, CA) using CellQuest software. The following reagents were obtained from Pharmingen: peridinin chlorophyll protein (PerCP)– or allophycocyanin (APC)–conjugated anti-CD4, APC-conjugated anti-CD8, fluorescein isothiocyanate (FITC)–conjugated anti-BrdU and anti-CD11c, phycoerythrin (PE)–conjugated anti-CD19 and anti-IAb, biotin-conjugated anti–Ly 5.1 and anti–Ly 5.2 antibodies, and PE- or PerCP-conjugated streptavidin. Sorting was performed on a FACSVantage SE (Becton Dickinson).
mRNA expression using reverse transcriptase–polymerase chain reaction (RT-PCR)
For semiquantitative PCR analysis, total RNA was extracted from mouse organs with RNeasy kit for total RNA isolation (QIAGEN, Mississauga, ON, Canada), following the manufacturer's instructions. After treatment with Rnase-free Dnase I (Ambion, Austin, TX), total RNA was divided into 2 parts: one part was reverse transcribed into cDNA and the other was mock transcribed. First-strand cDNAs were generated using random hexamers and Moloney murine leukemia virus reverse transcriptase (Life Technologies, Burlington, ON, Canada). Sequences of interest were amplified by PCR in reactions containing the equivalent of 100 ng reverse-transcribed RNA. Trace labeling of PCR reactions was performed using 2.5 μCi (0.093 MBq) α-32P deoxycytidine-5′-triphosphate and products resolved on a 5% polyacrylamide gel. The gel was dried and the products quantified using a PhosphoImager (GS-525; Bio-Rad Laboratories, Mississauga, ON, Canada) and the Multi-Analyst software (Bio-Rad). PCR was performed in triplicate from at least 3 independent experiments. No specific PCR products were detected when the corresponding mock-transcribed mixture was used as a template. The PCR conditions were an initial melt at 94°C for 4 minutes, then 25 to 30 cycles of 94°C for 30 seconds, 55°C for 30 seconds, 72°C for 30 seconds, followed by a final extension cycle of 72°C for 10 minutes. The QuantumRNA alternate 18S rRNA internal standards (Ambion) with Ambion's Competimers were used as internal control. The optimal ratios of 18S primers–competimers were determined for each gene according to the manufacturer's instructions. 18S primers–competimers ratios used were 1:9, 1:10, 1:11, or 1:12. Sense and antisense primers used for RT-PCR were as follows: CCL2, 5′-CCC AAT GAG TAG GCT GGA GA-3′ and 5′-CCT TAG GGC AGA TGC AGT TT-3′ (202 bp); CCL4, 5′-TGT CTG CCC TCT CTC TCC TC-3′ and 5′-GTC TGC CTC TTT TGG TCA GG-3′ (201 bp); CCL17, 5′-CCA TCG TGT TTC TGA CTG TCC-3′ and 5′-TTG TGT TCG CCT GTA GTG CAT-3′ (250 bp); CCL19, 5′-CTC GGC CTC TCA GAT TCT TG-3′ and 5′-GTC ACA GAC AGG CAG CAG TC-3′ (189 bp); CCL20, 5′-CTT GCT TTG GCA TGG GTA CT-3′ and 5′-TCA GCG CAC ACA GAT TTT CT-3′ (200 bp); CCL21, 5′-TCC GAG GCT ATA GGA AGC AA-3′ and 5′-TTA GAG GTT CCC CGG TTC TT-3′ (199 bp); CCL22, 5′-GCC CTC TGG TCA TTA GAC ACC TG-3′ and 5′-TCG TTG GCA AGG CTC TTG CTG-3′ (413 bp); CCL25, 5′-GCA ACC TAC GTG CTG TGA GA-3′ and 5′-CGC TTG TAC TGT TGG GGT TC-3′ (201 bp); CXCL12, 5′-TCC TCT TGC TGT CCA GCT CT-3′ and 5′-GGC ACA GTT TGG AGT GTT GA-3′ (219 bp); XCL1, 5′-GCT GAT CCA GAA GCC AAA TG-3′ and 5′-CAA TGG GTT TGG GAA CTG AG-3′ (200 bp); CCR1, 5′-AGC CTG AAG CAG TGG AAG AG-3′ and 5′-CAG ATT GTA GGG GGT CCA GA-3′ (201 bp); CCR2, 5′-TGG CTG TGT TTG CCT CTC TA-3′ and 5′-CCT ACA GCG AAA CAG GGT GT-3′ (200 bp); CCR4, 5′-ATG GCG TTA ACA AGC TCC AC-3′ and 5′-CAC TCAAGG GCT CAT TGT CA-3′ (200 bp); CCR5, 5′-GTG TTT GCC TCT CTC CCA GA-3′ and 5′-CGA AAC AGG GTG TGG AGA AT-3′ (200 bp); CCR6, 5′-TAC GCT CCA GAA CAC TGA CG-3′ and 5′-CCC AAA GAA CAG CTC CAG TC-3′ (200 bp); CCR7, 5′-TGC TTC AAG AAG GAT GTG CGG-3′ and 5′-GAG GAA AAG GAT GTC TGC CAC G-3′ (151 bp); CCR9, 5′-CCA TGA TGC CCA CAG AAC TC-3′ and 5′-TGA CCT TCA GGA TCA AGA CAG C-3′ (660 bp); CXCR4, 5′-GTC TAT GTG GGC GTC TGG AT-3′ and 5′-ACA GGA GAG GAT GAC GAT GC-3′, (204 bp); and Duffy antigen receptor for chemokines (DARC), 5′-CAA GGG GCT GAA GAT AGC AC-3′ and 5′-GGC TTC TGT CAC ATT CAG CA-3′ (190 bp). IL-7 gene expression was determined using Ambion's mouse IL-7 Gene Specific Relative RT-PCR Kit (Ambion no. 5424).
Results
Study design
The fact that massive thymo-independent T-cell development and preferential homing of recirculating T cells take place specifically in the LckOM LN strongly suggests that chronic exposure to OM induces one or several changes that uniquely affect the LN stroma, which is composed of fibroblasts, and endothelial and dendritic cells. We therefore conducted a multidimensional comparison of the LN microenvironment in LckOM and nontransgenic control mice. When a notable change was found in the LckOM LN, we questioned its presence in the LckOM spleen and the control thymus. The LckOM spleen served as an informative negative control, that is, a secondary lymphoid organ exposed to OM but devoid of the peculiar features found in the LckOM LN. Comparison with control thymus evaluated whether and to what extent changes in the LckOM LN rendered this organ more “thymuslike.”
Architecture of the LckOM LN
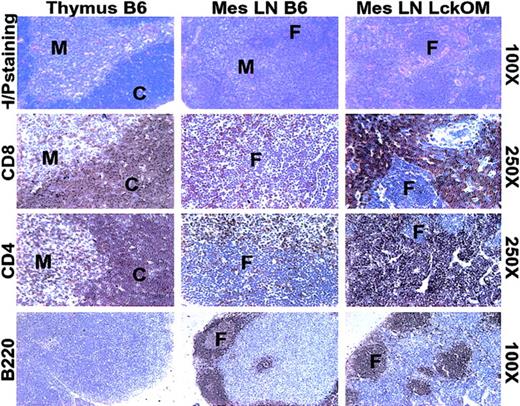
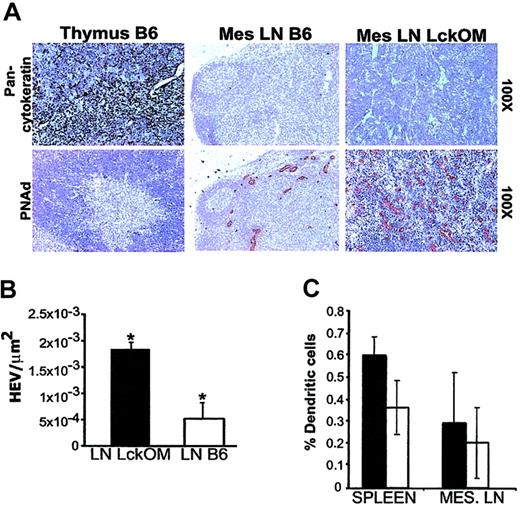
The composition and structure of the LckOM LN were compared with those of a B6 thymus and LN following staining with hematoxylin-phloxin and labeling with selected antibodies against antigens expressed by lymphoid (Figure 1) and stromal (Figure 2) cells. As in a normal LN, a clear segregation exists between B-cell zones (follicles) and T-cell zones in the LckOM LN. In the thymus, the darkly stained cortex is composed mainly of DP T cells, while the pale medulla is composed essentially of SP T cells. No corticomedullary demarcation is present in the LckOM LN T-cell zone whose appearance is similar to a thymic cortex, since most lymphoid cells were stained by anti-CD4 and anti-CD8 antibodies (Figure 1). As expected, and in striking contrast with a thymic cortex, no epithelial cells (pancytokeratin+) were found in the LckOM LN (Figure 2A). The following parameters were similar in the B6 and LckOM LNs: the proportion of MHC class II+CD11c+ dendritic cells (DCs; Figure 2C), the content of extracellular matrix components (epiligrin, fibronectin, hyaluronic acid, and tenascin), and the content of ERTR7 fibroblasts (data not shown). The key difference between the stroma of B6 and LckOM LNs was the number of HEVs per square micrometer, which was increased 3.5-fold in the LckOM LN (P < .0001) as determined with anti-CD62L ligand (PNAd) staining (Figure 2A-B). As in controls, no HEVs (PNAd+ vessels) were found in the LckOM spleen (data not shown). HEVs are present in Peyer patches. However, we found neither extrathymic T-cell development nor increased HEV numbers in the Peyer patches of LckOM mice (data not shown). The latter observation indicates that OM-induced extrathymic T-cell development and HEV angiogenesis are geographically linked events. In addition, the fact that HEVs in the LNs versus Peyer patches display significant functional differences43 led us to speculate that Peyer patch HEVs may lack one (or several) crucial feature for the development of the LckOM phenotype.
T- and B-cell zones in the LckOM LN. Sections from B6 thymus and LN and from LckOM LN were stained with hematoxylin-phloxin or labeled with antibodies specific for CD4, CD8, and B220. C indicates thymic cortex; F, B-cell follicle; and M, thymic medulla. The text to the right of the panels indicates the magnification for each row.
T- and B-cell zones in the LckOM LN. Sections from B6 thymus and LN and from LckOM LN were stained with hematoxylin-phloxin or labeled with antibodies specific for CD4, CD8, and B220. C indicates thymic cortex; F, B-cell follicle; and M, thymic medulla. The text to the right of the panels indicates the magnification for each row.
Structure and composition of the LckOM LN stroma. (A) Sections from B6 thymus and LN and from LckOM LN were stained with hematoxylin and labeled with antibodies specific for cytokeratin and CD62L ligand (PNAd carbohydrate epitope). The text to the right of the panels indicates the magnification for each row. (B) The number of CD62L ligand+ blood vessels per square micrometer was significantly increased in the LckOM relative to the LN (*P < .0001, Student t test). (C) Flow cytometry analyses revealed no significant difference in the proportion of DCs (MHC class II+CD11c+) between B6 (□) and LckOM (▪) secondary lymphoid organs.
Structure and composition of the LckOM LN stroma. (A) Sections from B6 thymus and LN and from LckOM LN were stained with hematoxylin and labeled with antibodies specific for cytokeratin and CD62L ligand (PNAd carbohydrate epitope). The text to the right of the panels indicates the magnification for each row. (B) The number of CD62L ligand+ blood vessels per square micrometer was significantly increased in the LckOM relative to the LN (*P < .0001, Student t test). (C) Flow cytometry analyses revealed no significant difference in the proportion of DCs (MHC class II+CD11c+) between B6 (□) and LckOM (▪) secondary lymphoid organs.
T-cell development and angiogenesis in the LckOM LN
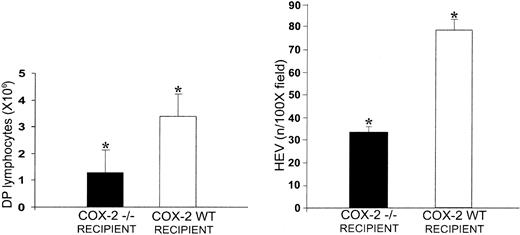
The increase in the number of HEVs in the LckOM LN is interesting, namely for 2 reasons. First, the 2 cytokines of the IL-6 family that can induce T-cell development in the LN, OM, and LIF are precisely those that have an in vitro angiogenic effect (IL-6 is ineffective in both cases).33,34,44,45 Second, the OM-receptor β-chain in the LNs is expressed only by endothelial cells,46 and it is overexpressed by LN HEV endothelial cells relative to flat endothelial cells.47 In vitro studies have shown that OM-induced proliferation of microvascular endothelial cells requires induction of COX-2.48 We took advantage of the latter finding to determine whether the OM-induced HEV angiogenesis was important for the occurrence of extrathymic T-cell development. Thus, we injected OM+Ly5.1+ fetal liver cells in adult-thymectomized irradiated COX-2–/– mice and COX-2+ littermates, and assessed extrathymic T-cell development on day 90 after transplantation. COX-2–/– mice that did not undergo transplantation have normal lymphoid organs39,49 and HEV density in their LNs is similar to COX-2+ littermates (data not shown). Strikingly, the number of DP T cells in the LNs of COX-2–/– recipients of LckOM fetal liver cells was decreased 3-fold and HEV density per square micrometer was decreased 2.5-fold relative to COX-2+ littermates (Figure 3). These data strongly suggest that COX-2–dependent angiogenesis is instrumental in supporting extrathymic T-cell development in the OM+ LN.
COX-2–dependent angiogenesis is instrumental in supporting extrathymic T-cell development in the OM+ LN. Fetal liver cells from OM+ Ly5.1+ mice were injected in adult thymectomized irradiated COX-2–/– mice and COX-2+ littermates. The number of CD4+CD8+ T cells per mesenteric LN (left panel) and the mean number of CD62L ligand+ HEVs per 100 × field (right panel) were assessed on day 90 after transplantation. There were 3 mice per group. *P < .001, COX-2–/– mice versus COX-2+ littermates.
COX-2–dependent angiogenesis is instrumental in supporting extrathymic T-cell development in the OM+ LN. Fetal liver cells from OM+ Ly5.1+ mice were injected in adult thymectomized irradiated COX-2–/– mice and COX-2+ littermates. The number of CD4+CD8+ T cells per mesenteric LN (left panel) and the mean number of CD62L ligand+ HEVs per 100 × field (right panel) were assessed on day 90 after transplantation. There were 3 mice per group. *P < .001, COX-2–/– mice versus COX-2+ littermates.
Expression of chemokine and chemokine receptor genes in the LckOM LN
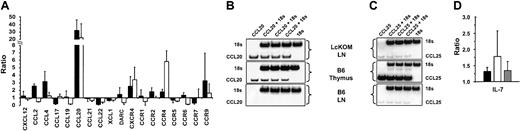
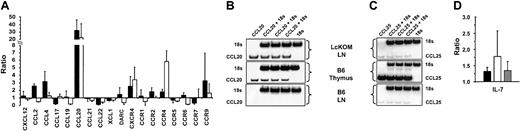
Chemokines play a key role in shaping central and peripheral T-cell compartments. We therefore sought to determine whether chronic exposure to OM modified the expression profile of chemokines and chemokine receptors in the LckOM LN, and whether such changes would make the LckOM LN more “thymuslike.” We assessed, by semiquantitative PCR, the expression of 19 chemokine and chemokine receptor genes known to be expressed in lymphoid organs (Figure 4). Emerged from these experiments were 2 key points. First, expression of one chemokine, CCL20, was dramatically increased in both the LckOM LN and the B6 thymus compared with the B6 LN (Figure 4A-B). Relative to the B6 LN, expression of CCL20 was increased approximately 20-fold in the B6 thymus and approximately 30-fold in the LckOM LN. In LckOM mice, increased levels of CCL20 transcripts were found exclusively in the LNs (Figure 4) and not in other sites such as the spleen, Peyer patches, intestines, lung, liver, and skin (Figure 5A). The specificity of CCL20 primer pairs used herein was confirmed by direct sequencing of PCR products (data not shown). Second, CCL25 expression was not increased in the LckOM LN relative to the B6 LN (Figure 4C). This is noteworthy because abundance of CCL25 transcripts is more than 100-fold greater in the B6 thymus than the LNs. This expression profile of CCL25 is consistent with the notion that thymus epithelial cells are the predominant source of CCL2550 and suggests that CCL25 is not involved in the behavior of the LckOM LN as a primary T-lymphoid organ. Besides, comparison of the B6 and LckOM LNs showed a 2- to 3-fold increase in CCL2, CCL4, CXCR4, and CCR9 transcripts and a 6- to 7-fold decrease in CCL17 and CCL22 (Figure 4A). While these lesser changes might be biologically relevant, their significance will not be pursued here. Nevertheless, we surmise that at least some of these differences between the B6 and LckOM LNs are probably caused by the greater amount of blood vessels (CCL2, CCL4, CXCR4) and of immature thymocytes (CXCR4, CCR9) in the LckOM LN.51-54 CCL17 and CCL22 are produced mainly by macrophages and DCs and attract T helper 2 (Th2) T cells. Local production of OM, which is a Th1-type cytokine,55,56 could be responsible for the low levels of CCL17 and CCL22 in the LckOM LN. Indeed, it has been shown that CCL22 is down-regulated by Th1-type cytokines.57
Expression of chemokines, chemokine receptors, and IL-7 in the LckOM LN. Expression of 10 chemokines, 9 chemokine receptors, and IL-7 was assessed by semiquantitative RT-PCR done in triplicate in at least 3 independent experiments. The level of cDNA of interest relative to the level of 18S was estimated in the thymus and mesenteric LNs of B6 mice and in the mesenteric LNs of LckOM mice (12 weeks of age). (A) For 9 chemokine and 9 chemokine receptor genes, results are depicted in a histogram as the mean ± SD. Expression level in B6 LNs was arbitrarily defined as 1 and the relative expression in B6 thymus and LckOM LN is depicted as B6 thymus/B6 LN and LckOM LN/B6 LN ratios, respectively. Phosphoimagery of (B) CCL20 and (C) CCL25 expression. (D) Expression of IL-7. In panels A and D, ▪ indicates LckOM LN/B6 LN; □, B6 thymus/B6 LN; and ▦, LckOM spleen/B6 spleen.
Expression of chemokines, chemokine receptors, and IL-7 in the LckOM LN. Expression of 10 chemokines, 9 chemokine receptors, and IL-7 was assessed by semiquantitative RT-PCR done in triplicate in at least 3 independent experiments. The level of cDNA of interest relative to the level of 18S was estimated in the thymus and mesenteric LNs of B6 mice and in the mesenteric LNs of LckOM mice (12 weeks of age). (A) For 9 chemokine and 9 chemokine receptor genes, results are depicted in a histogram as the mean ± SD. Expression level in B6 LNs was arbitrarily defined as 1 and the relative expression in B6 thymus and LckOM LN is depicted as B6 thymus/B6 LN and LckOM LN/B6 LN ratios, respectively. Phosphoimagery of (B) CCL20 and (C) CCL25 expression. (D) Expression of IL-7. In panels A and D, ▪ indicates LckOM LN/B6 LN; □, B6 thymus/B6 LN; and ▦, LckOM spleen/B6 spleen.
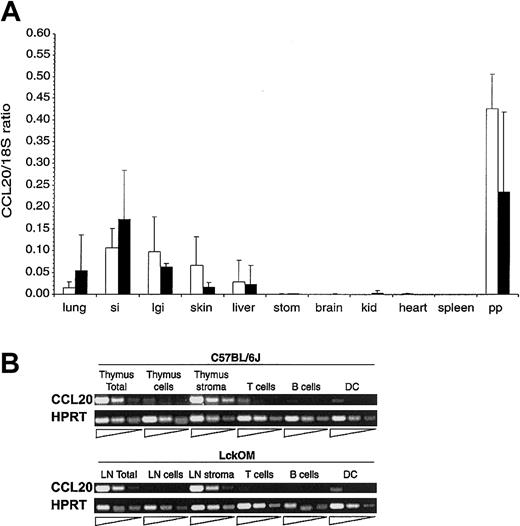
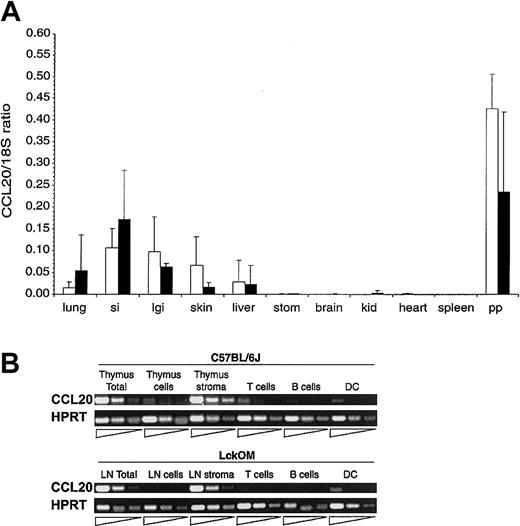
Expression of CCL20 transcripts in lymphoid and nonlymphoid organs. (A) CCL20 mRNA expression was estimated in various organs of B6 (□) and LckOM (▪) mice by semiquantitative RT-PCR. Results are shown as the ratio of CCL20 expression over internal control (18S) and represent the mean ± SDof3to9 mice per organ. si indicates small intestine; lgi, large intestine; stom, stomach; kid, kidney; and pp, Peyer patches. No significant difference was found between B6 versus LckOM organs (Mann-Whitney U test and Student t test). (B) CCL20 is produced by nonlymphoid stromal cells in the B6 thymus and LckOM LN. RNA was extracted from the B6 thymus and LckOM LN as follows: total organ, a cell suspension (cells) obtained after mechanical disruption of the thymus or LN through a cell strainer, and the remaining nonsuspendable fraction (stroma). Cell suspensions from collagenase-treated organs were sorted into T cells (Thy1.2+), B cells (CD19+), and DCs (CD11c+MHC II+). Serial 1:5 dilutions of each cDNA were used as template in semiquantitative RT-PCR. Representative results of 3 experiments are shown.
Expression of CCL20 transcripts in lymphoid and nonlymphoid organs. (A) CCL20 mRNA expression was estimated in various organs of B6 (□) and LckOM (▪) mice by semiquantitative RT-PCR. Results are shown as the ratio of CCL20 expression over internal control (18S) and represent the mean ± SDof3to9 mice per organ. si indicates small intestine; lgi, large intestine; stom, stomach; kid, kidney; and pp, Peyer patches. No significant difference was found between B6 versus LckOM organs (Mann-Whitney U test and Student t test). (B) CCL20 is produced by nonlymphoid stromal cells in the B6 thymus and LckOM LN. RNA was extracted from the B6 thymus and LckOM LN as follows: total organ, a cell suspension (cells) obtained after mechanical disruption of the thymus or LN through a cell strainer, and the remaining nonsuspendable fraction (stroma). Cell suspensions from collagenase-treated organs were sorted into T cells (Thy1.2+), B cells (CD19+), and DCs (CD11c+MHC II+). Serial 1:5 dilutions of each cDNA were used as template in semiquantitative RT-PCR. Representative results of 3 experiments are shown.
IL-7 gene expression in the LckOM LN
Experiments in which LckOM mice were bred with IL-7 receptor–deficient mice showed that, as observed in a normal thymus, extrathymic T-cell development in LckOM mice is IL-7Rα dependent.58 Furthermore, indirect evidence suggests that IL-7 overexpression might promote extrathymic T-cell development.59 This prompted us to determine whether IL-7 gene expression was significantly increased in OM-transgenic mice. The amount of IL-7 transcripts was minimally increased, and at similar levels in the LNs and spleen of LckOM mice when compared with B6 mice (Figure 4D). These results argue against the hypothesis that the LN phenotype of LckOM mice is due to IL-7 overexpression and show that IL-7 levels could not account for the selective development of the LckOM phenotype solely in the LNs.
Source of CCL20 in the LckOM LN
To determine the source of CCL20 in the B6 thymus and the LckOM LN, CCL20 transcript levels were assessed by RT-PCR analysis in total organs, stromal cells, teased nonadherent cells, and sorted T cells (Thy1.2+), B cells (CD19+), and DCs (CD11c+MHC II+). CCL20 transcripts were abundant in the stromal fraction but barely detectable in the nonadherent cell fraction (Figure 5B). The stromal fraction contains nonhematopoietic stromal cells and a proportion of the DC population.29 CCL20 transcripts were significantly less abundant in sorted DCs than in the stromal fraction. We therefore concluded that, although DCs also may contribute, nonhematopoietic stromal cells were the main source of CCL20 in the normal thymus and the LckOM LN.
CCL20 influence on T-cell development or homeostasis
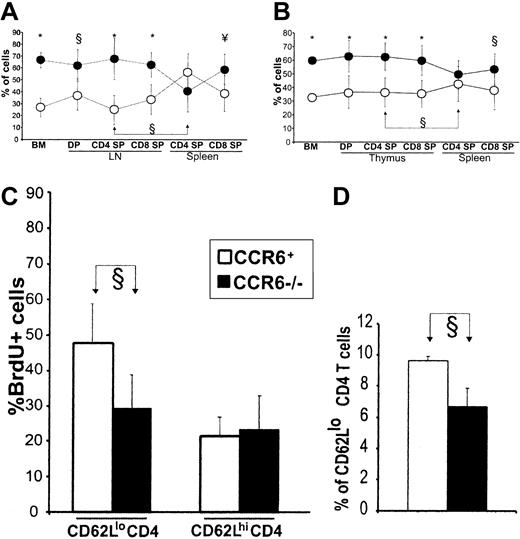
CCR6 is the only known receptor for CCL20.40,60 To determine whether CCL20 had a role in OM-induced extrathymic T-cell development, we reconstituted thymectomized/irradiated recipients with a 50:50 mixture of T-cell–depleted bone marrow from CCR6+OM+ (Ly5.1+) and CCR6–/–OM– (Ly5.1–) donors. Competition assays represent the most sensitive approach to evaluate the influence of a molecule on cell development.61,62 Furthermore, we reported that under competition conditions, OM+ and OM– cells contribute to the same extent to extrathymic T-cell development (ie, to the reconstitution of the DP and SP T-cell compartments).35 This means that OM exerts its key effect on T-cell development in a paracrine fashion and that the repopulation potential of OM+ and OM– cells can be compared in the same host. A likely explanation is that the key effect of OM is on the stromal cells and thereby influences T-lineage cells irrespective of their OM+ or OM– genotype. Among bone marrow cells and LN T cells (DP, CD4 SP, and CD8 SP), the proportion of CCR6–/– cells was significantly greater than that of CCR6+ cells (Figure 6A). Thus, CCR6–/– hematopoietic progenitors have some elusive advantage over CCR6+ cells for bone marrow seeding and/or proliferation. More relevant to the present work, these data signify that CCL20 interactions with CCR6 are not essential for initiation of extrathymic T-cell development in the OM+ LN.
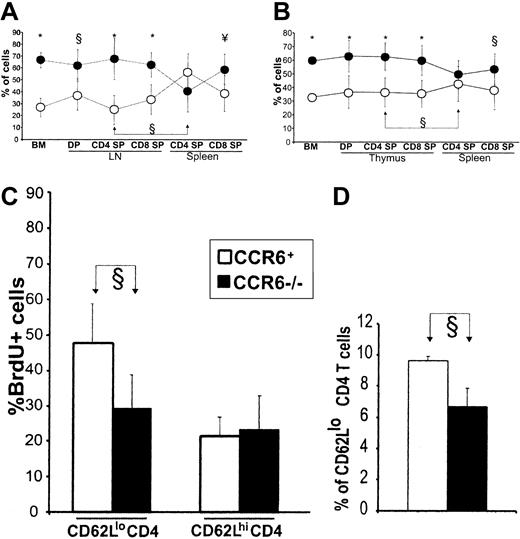
CCL20 regulates accumulation of CD4 T cells in secondary lymphoid organs. (A) Thymectomized irradiated B6 recipients were reconstituted with a 50:50 mixture of T-cell–depleted bone marrow from CCR6+ (OM+Ly5.1+, ○) and CCR6–/– (OM–Ly5.1–, •) donors. (B) Euthymic irradiated B6 recipients were reconstituted with a 50:50 mixture of T-cell–depleted bone marrow from non–OM-transgenic CCR6+ (Ly5.1+, ○) and CCR6–/– (Ly5.1–, •) donors. Panels A and B show the proportion (mean ± SD from 2 experiments involving a total of 6 mice per group) of CCR6+ and CCR6– cells in the recipients' bone marrow and lymphoid organs. Symbols of statistical significance (§, ¥, *) shown at the top of panels refer to the proportion of CCR6+ versus CCR6– cells at various stages of differentiation. Also indicated (joined arrows) is the significant increase in the proportion of CCR6+ CD4 T cells present in the secondary (spleen) relative to the “primary” T-lymphoid organ (B6 thymus and LckOM LN). (C) Non–OM-transgenic CCR6–/– (▪) and CCR6+ (□) mice were given BrdU-supplemented water for 10 days. The proportion of BrdU-labeled T cells among CD62Llo and CD62Lhi CD4 spleen T cells is depicted. (D) Numbers of CD4 CD62Llo T cells in non–OM-transgenic CCR6–/– (▪) and CCR6+ (□) mice; gated on LN CD4 T cells. Data in panels C and D represent the mean ± SD from one experiment with 4 mice per group. §P < .05; ¥P < .01; *P < .001.
CCL20 regulates accumulation of CD4 T cells in secondary lymphoid organs. (A) Thymectomized irradiated B6 recipients were reconstituted with a 50:50 mixture of T-cell–depleted bone marrow from CCR6+ (OM+Ly5.1+, ○) and CCR6–/– (OM–Ly5.1–, •) donors. (B) Euthymic irradiated B6 recipients were reconstituted with a 50:50 mixture of T-cell–depleted bone marrow from non–OM-transgenic CCR6+ (Ly5.1+, ○) and CCR6–/– (Ly5.1–, •) donors. Panels A and B show the proportion (mean ± SD from 2 experiments involving a total of 6 mice per group) of CCR6+ and CCR6– cells in the recipients' bone marrow and lymphoid organs. Symbols of statistical significance (§, ¥, *) shown at the top of panels refer to the proportion of CCR6+ versus CCR6– cells at various stages of differentiation. Also indicated (joined arrows) is the significant increase in the proportion of CCR6+ CD4 T cells present in the secondary (spleen) relative to the “primary” T-lymphoid organ (B6 thymus and LckOM LN). (C) Non–OM-transgenic CCR6–/– (▪) and CCR6+ (□) mice were given BrdU-supplemented water for 10 days. The proportion of BrdU-labeled T cells among CD62Llo and CD62Lhi CD4 spleen T cells is depicted. (D) Numbers of CD4 CD62Llo T cells in non–OM-transgenic CCR6–/– (▪) and CCR6+ (□) mice; gated on LN CD4 T cells. Data in panels C and D represent the mean ± SD from one experiment with 4 mice per group. §P < .05; ¥P < .01; *P < .001.
Of special interest, CCR6+ cells were specifically enriched among spleen SP CD4 but not CD8 T cells (Figure 6A). Thus, CCL20/CCR6 interactions regulate the size of the spleen CD4 T-cell pool in OM+ mice. It is important to remember that in OM+ mice, the spleen remains a pure secondary organ, while the LN is a dual primary and secondary lymphoid organ containing both T cells produced in situ and recirculating T cells. This prompted us to evaluate whether CCR6 would influence the number of CD4 T cells in the absence of OM, that is, in secondary lymphoid organs of euthymic non–OM-transgenic mice. Euthymic irradiated wild-type mice were reconstituted with a 50:50 mixture of T-cell–depleted bone marrow from CCR6+ (Ly5.1+) and CCR6–/– (Ly5.1–) donors. The results agreed with those in OM+ mice: CCR6–/– were more abundant than CCR6+ cells in the bone marrow and thymus (Figure 6B); and there was a selective enrichment in CCR6+ T cells among CD4 SP T cells in the spleen (Figure 6B) and LNs (data not shown). Thus, whether CD4 T cells originate from the OM+ LN or the wild-type thymus, CCR6 interactions regulate their abundance in the secondary lymphoid organs. This means that interactions between CCL20 (produced by stromal cells) and CCR6 on CD4 SP T cells promote emigration from the primary lymphoid organ and/or survival/expansion in the periphery. The fact that CCR6–CD4 T cells did not accumulate in the primary lymphoid organ strongly argues against the emigration hypothesis.26
To elucidate how CCR6 might regulate the size of the peripheral CD4 T-cell pool, we compared the rate of division (BrdU labeling) and of apoptosis (Annexin V staining) in CCR6–/– mice and B6 controls. Mice were given BrdU-supplemented drinking water for 10 days, and CD62Lhi and CD62Llo CD4 cell subsets were analyzed separately because they have different proliferation rates.63 The proportion of apoptotic cells among CD62Lhi and CD62Llo CD4 T cells was similar in CCR6–/– mice and B6 controls (data not shown). Here, the key finding is that CCR6–/– mice showed a decreased proportion of BrdU-labeled cells among CD62Llo CD4 T splenocytes (Figure 6C). Furthermore, the number of CD62Llo CD4 T cells was lower in CCR6–/– than in wild-type mice (Figure 6D). Together, these results provide conclusive evidence that CCR6 interactions with CCL20 regulate the accumulation of CD62Llo CD4 T cells in the periphery by increasing their proliferation rate. We cannot formally exclude the possibility that CCR6 also influences the distribution of memory phenotype CD4 T cells. Nonetheless, we found that the proportion of CLA+ and α4β7+ elements among LN and spleen CD4CD62Llo T cells was similar in CCR6-deficient and wild-type mice (S.C., unpublished observations, December 2002). This suggests that CCR6 does not specifically influences skin homing (CLA+) or gut homing (α4β7+) CD4+CD62Llo T-cell subsets.
Discussion
The thymus appeared in Chondrichthyes, the first vertebrates known to elicit adaptive immune responses, and constitutes the primary T-lymphoid organ in all jawed vertebrates.64 Moreover, no other organ can compensate for defective thymic function.8,65 Although commonly taken for granted, the conservation of the thymus over 450 million years of evolution and the lack of any thymus equivalent or substitute in the animal kingdom is remarkable. This can be illustrated by a comparison with hematopoietic organs.64 Indeed, the bone marrow appeared as a site of hematopoiesis for the first time only in the most evolved urodelans. In more primitive vertebrates, hematopoietic stem cells house in many distinct organs as diverse as the heart, gonads, kidney, intestines, liver, and meninges. Furthermore, in subjects with bone marrow fibrosis, compensatory extramedullary hematopoiesis is observed in several organs.66 Given the remarkable degree of conservation of the thymus, it is notable that a functional and diverse T-cell repertoire develops in the OM+ LN that lacks at least 3 canonical thymic features: epithelial cells, cortex-medulla segregation, and high levels of the CCL25 chemokine. This means that a primary T-lymphoid organ does not have to share all the attributes of the thymus.
The fact that epithelial cells are not required for T-cell development and positive selection in the OM+ LN defines the following: (1) under the influence of OM, LN stromal cells can provide some elusive TCR-independent signal(s) normally provided only by TECs, and (2) the unique influence of epithelial cells on positive selection of TCR clonotypes (ie, provision of the TCR-dependent signal) may be largely contingent upon their anatomic location.8,67 Moreover, since the OM+ LN is very effective in deleting autoreactive T cells bearing the 2C and the anti–H-Y TCR,38 we propose that an anatomically segregated medulla is not necessary for negative selection of some if not all T cells. Finally, consistent with reports on CCR9-deficient mice,24,68 our data show that the high levels of CCL25 found in the thymus are not necessary for T-cell development irrespective of whether the primary lymphoid organ is the thymus or the OM+ LN. This suggests that the putative regulatory role of CCL25 on the homing of blood T-cell precursors can be played by other redundant chemokines.
Interestingly, the wild-type LN has a unique ability to support many stages of T-cell development. Thus, when injected intravenously, DP or even DN thymocytes from the thymus of fetal or adult donors differentiate into mature SP T cells in the LN but not the spleen of athymic mice.69,70 However, no T-cell development is found in the LN when fetal liver cells rather than thymocytes are injected.69 This means that normal LNs are unable to attract T-cell progenitors or to support some early postseeding event occurring at the CD4–CD8– stage. Entry of prothymocytes into the thymus is regulated by microvascular gates that are located within the postcapillary venules at the corticomedullary junction.71,72 Considering that the most dramatic feature of the LckOM LN is precisely a neoangiogenesis of postcapillary venules (ie, HEV) and that blocking of HEV angiogenesis by COX-2 deficiency impairs extrathymic T-cell development, we infer that OM-induced changes in HEVs are critical for allowing the LN to support T-cell development. Since HEVs dictate which blood-borne cells are recruited to LNs,73 we speculate that HEV changes induced by OM allow the LN to attract T-cell progenitors. This we presume to be the limiting step that prevents the wild-type LN from supporting T-cell development.69 It is interesting to note that postcapillary venules at the thymus corticomedullary junction (that is, the site of entry of T-cell progenitors) share several distinctive features with HEVs. Indeed, the endothelial cells of thymic venules have been described as “intermediately high” and were found to express MadCAM-1 and HECA-452 homing receptors.74-76 Now the imperative is to discover critical molecular changes in HEVs, downstream of OM signals, that are responsible for attraction and seeding of T-cell progenitors. Indeed, the possibility of transforming LNs into thymus substitutes, by modulating expression of discrete genes in HEVs, might have a tremendous impact in the management of senescence- or disease-related thymic hypoplasia.77
The notable change emerging from our studies of chemokines and chemokine receptors in the LckOM LN was the dramatic increase in the levels of CCL20 transcripts. Taken together, the following data strongly suggest that HEV endothelial cells represent the primary source of CCL20 in the LckOM LN: the OM-receptor β-chain in the LNs is expressed only by endothelial cells46 ; it is overexpressed by LN HEV endothelial cells relative to flat endothelial cells47 ; HEV endothelial cells exhibit intense biosynthetic and secretory activity78 ; and the number of HEVs is increased in the LckOM LN (Figure 2) where the high levels of CCL20 transcripts are essentially produced by nonhematopoietic stromal cells (Figure 5B). We cannot however formally discard fibroblasts as a source of CCL20 in the LckOM LN. Could CCL20, produced by endothelial cells or acquired by transcytosis, be presented in the lumen of HEVs and thereby attract T-cell progenitors? Data shown in Figure 6 indicate that CCL20 interactions with CCR6 are not essential for initiation of extrathymic T-cell development in the OM+ LN. Thus, further studies are required to determine whether (1) CCL20 has no influence on early steps of T development, or (2) CCL20 has a redundant effect that can be mediated by other molecules in the OM+ LN, or (3) CCL20's effect on T-cell development is mediated by another receptor than CCR6.
One key finding of this work is that CCL20/CCR6 interactions regulate the accumulation of CD62Llo CD4 T cells in the periphery by increasing their proliferation rate. This effect was observed in CD4 T cells derived from the OM+ LN as well as from the wild-type thymus. Selective involvement of memory phenotype CD4 cells is consistent with the fact that CCR6 expression by T-lineage cells is limited to memory cells, particularly of the CD4 type.60,79 Of note, proliferation of CD62Llo T cells is deficient but not absent in CCR6-deficient mice (Figure 6C). This could be explained by 2 nonmutually exclusive hypotheses: in keeping with the fact that CCR6 is expressed essentially on interferon-γ–producing CD4 memory T cells,79-82 CCL20/CCR6 interactions may specifically regulate the proliferation of Th1 as opposed to Th2 memory CD4 T cells; and, other redundant ligand-receptor(s) can compensate for the absence of CCR6. Notably, overexpression of CCL20 cannot be held solely responsible for the expansion of the memory phenotype T-cell pool in LckOM mice. Indeed, though we found no evidence that CCR6 regulates CD8 T-cell homeostasis, LckOM mice present a dramatic accumulation of memory phenotype CD8 T cells. Thus, other unidentified mechanisms must be instrumental in the expansion of memory phenotype extrathymic T cells in LckOM mice. Further studies will be needed to accurately estimate the contribution of CCL20 in this process. Nevertheless, CCL20 is, to our knowledge, the first molecule found to increase the proliferation of CD4 memory T cells. This could be of significant interest considering evidence that CD4 memory is commonly limiting under physiologic conditions: compared with CD8 T cells, CD4 memory T cells expand less extensively and have a limited life span.19 Further studies are warranted to understand the mechanism of this effect and, following vaccination, to evaluate its role in modulating the size of the CD4 memory T-cell compartment.
Prepublished online as Blood First Edition Paper, April 17, 2003; DOI 10.1182/blood-2003-01-0316.
Supported by a grant from the Canadian Institutes of Health Research (CIHR) to C.P. N.L. is supported by a CIHR new-investigator scholarship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr S. Lira for kindly providing CCR6–/– breeder mice, I. Patenaude for technical help, and J. A. Kashul for editorial assistance.