Abstract
We recently demonstrated that immunoglobulin E (IgE), in the absence of cross-linking agents, activates signaling pathways in healthy murine bone marrow–derived mast cells (BMMCs) and that this activation enhances BMMC survival, at least in part, via secretion of autocrine-acting cytokines. We report herein that IgE alone also triggers the adhesion of both BMMCs and connective tissue mast cells (CTMCs) to the connective tissue component, fibronectin (FN). This adhesion occurs to the same extent as that triggered by optimal levels of Steel factor (SF) or IgE + antigen (IgE + Ag) and is mediated by an increased avidity of the integrin very late antigen 5 (VLA-5). Moreover, this IgE-induced adhesion, which is prolonged compared with that elicited by SF or IgE + Ag, requires phosphatidylinositol 3-kinase (PI3K), phospholipase C γ (PLCγ), and extracellular calcium but not extracellular-regulated kinase (Erk) or p38. Interestingly, we found, using the calcium channel blocker, 2-APB (2-aminoethoxydiphenyl borate) and Lyn–/– BMMCs that both IgE- and IgE + Ag-induced adhesion to FN require extracellular calcium entry, whereas SF does not. Furthermore, our data suggest that FN acts synergistically with IgE to prolong intracellular phosphorylation events and to enhance IgE-induced inflammatory cytokine production and BMMC survival.
Introduction
Mature mast cells are located at the portals between self and nonself, and they serve as frontline defenders against invading bacteria and helminthic parasites.1 They do this by initiating an acute inflammatory response, which typically consists of a rapid degranulation that releases preformed inflammatory molecules (such as histamine, serotonin, and proteases) and a subsequent secretion of de novo synthesized arachidonic acid metabolites and inflammatory cytokines.1,2 Unfortunately, this inflammatory response can also be detrimental and lead to immediate-type allergic reactions1 and other inflammatory disorders like asthma,3 arthritis,4 and multiple sclerosis.5
Mature mast cells originate from hematopoietic stem cell–derived progenitors in the bone marrow that are recruited out of the circulation and into connective tissues.6,7 This recruitment, via adhesion of integrins on the surface of mast cell progenitors to components of connective tissue, like fibronectin (FN), is thought to play a critical role in mast cell retention and subsequent proliferation, differentiation, survival, priming, and activation.8,9 Various stimuli have been shown to induce the adhesion of bone marrow–derived mast cells (BMMCs) to FN, via the integrins α4 β1 and α5 β1 (very late antigen 4 and 5 [VLA-4 and -5], respectively).10 These include physiologically relevant stimuli, such as Steel factor (SF)11,12 and antigen–cross-linking (IgE + Ag [immunoglobulin E + antigen]) of the high-affinity IgE receptor, FcϵRI.13 Although the cell surface level of VLA-4 decreases with BMMC differentiation, that of VLA-5 remains high14 and appears to be the predominant integrin involved in mature mast cell adhesion to FN.15
We recently demonstrated that IgE, in the absence of cross-linking agents, stimulates multiple signaling pathways in healthy BMMCs grown in suspension cultures and that this leads to an increased survival of these cells in the absence of exogenous cytokines.16 We went on to show that this enhanced survival is due, at least in part, to IgE maintaining Bcl-XL levels and up-regulating the production of autocrine-acting cytokines.16 In the work reported herein we have explored the possibility that IgE alone might also affect biologic responses other than survival. Specifically, we asked whether IgE alone could enhance the adhesion of mast cells to FN.
Materials and methods
Generation of mast cells
BMMCs were derived from 4- to 8-week-old SHIP+/+ (Src-homology 2 domain-containing inositol phosphatase) and –/– C57B6 or Lyn+/+ and –/– C57B6 mice as described previously.17 By 8 weeks in culture, more than 98% of the cells were c-kit and FcϵRI positive, as assessed by fluorescein isothiocyanate (FITC)–labeled anti–c-kit antibodies (BD PharMingen, Mississauga, ON, Canada) and FITC-labeled IgE (anti–erythropoietin 26), respectively.17 Connective tissue mast cells (CTMCs) were derived from SHIP+/+ C57B6 BMMCs by coculturing with Swiss 3T3 fibroblasts as described.18 After 2 weeks of coculture more than 90% of the adherent mast cells were safranin positive.
IgEs
Unless indicated, mouse monoclonal SPE-7 anti-DNP (2,4-dinitrophenyl) IgE (Sigma, St Louis, MO) or monomeric SPE-7, prepared as described previously,16 was used throughout. Where indicated, trinitrophenyl (TNP)–lysine affinity purified, mouse monoclonal Liu anti-DNP (H1 26.82),19 or anti-kappa chain affinity purified, mouse monoclonal anti-Epo26 (anti–erythropoietin 26; StemCell Technologies., Vancouver, BC) was used. All IgEs were titered using an IgE enzyme-linked immunosorbent assay (ELISA; BD PharMingen, San Diego, CA).
Adhesion assay
The 96-well Nunc Maxisorp plates (NUNC, Naperville, IL) were coated with 50 μg/mL human FN (Sigma) in phosphate-buffered saline (PBS) for 2 hours at 37°C or 16 hours at 4°C, washed 3 times with PBS, blocked with 3% bovine serum albumin (BSA; Sigma) in HBSS (Hanks balanced salt solution-modified; StemCell Technologies.) for 1 hour at 37°C, then washed 3 times with HBSS + 0.03% BSA (assay medium). For stimulation with IgE or SF, BMMCs or CTMCs were washed with RPMI (without phenol red) + 0.1% BSA, resuspended at 1 × 106 cells/mL, and labeled with 3 μg/mL Calcein-am (Molecular Probes, Eugene, OR) for 20 minutes at 37°C. For stimulation with IgE + Ag, BMMCs or CTMCs were preloaded with 1 μg/mL IgE overnight at 37°C in the absence of FN, washed twice to remove unbound IgE, then resuspended and labeled as described earlier. After labeling, cells were washed and resuspended at 1 × 106 cells/mL in assay medium. Assay medium (50 μL) ± IgE, SF, or DNP-HSA (human serum albumin; Sigma) was added to each FN-coated well followed by 50 μL cell mixture and incubated at 37°C for the indicated times. The degree of adhesion was quantitated using a Cytofluor 2300 Microplate Reader (Millipore, Bedford, MA) and is expressed as the percentage of fluorescence remaining in the wells after washing away unbound cells. Treatment of cells with inhibitors or antibodies was carried out by incubating Calcein-am–labeled cells ± DMSO (dimethyl sulfoxide), LY294002, Compound 3 (bisindolylmaleimide I, HCl), PD98059, U0126, apigenin, U73122, SB203580, Gö6976 (all from Calbiochem, La Jolla, CA), 2-aminoethoxydiphenyl borate (2-APB; Sigma), or anti-CD49e (BD PharMingen) for 30 minutes at 37°C (inhibitors) or 23°C (antibodies) prior to the addition of the cells and inhibitors (or antibodies) to the assay plate. The peptides GRGDSP and GRGESP were from Invitrogen Life Technologies (Carlsbad, CA). For experiments without calcium, Calcein-am–loaded cells were washed 3 times with Dulbecco PBS, and the assays were carried out in this medium. A 2-tailed paired Student t test was used to determine statistical significance between adhesion values.
BMMC stimulation and Western blotting
To compare IgE-versus IgE + FN-induced signaling events, BMMCs were starved overnight in Iscoves modified Dulbecco medium (IMDM) + 10% fetal calf serum (FCS), resuspended at 1 × 106 cells/mL in IMDM + 0.1% BSA, divided into aliquots (2 mL/well) into either 6-well tissue culture plates (Falcon, Heidelberg, Germany) or BIOCOAT human FN-coated 6-well tissue culture plates (Falcon), and stimulated with 5 μg/mL IgE for the indicated times. The plates were then placed on ice, 4°C HBSS was added, and in the case of the standard tissue culture plates the cells (which were in suspension) were pelleted and lysed in phosphorylation stabilization buffer (PSB)20 containing 0.5% NP40. For the FN-coated plates, the nonadherent cells were washed away, and the adherent cells were lysed in PSB containing 0.5% Nonidet P40 (NP40). Protein (50 μg) was loaded per lane (based on bicinchoninic acid [BCA] assays; Pierce, Rockford, IL) and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).
To test the specificities and potencies of the inhibitors used in the adhesion assays, BMMCs were starved for 4 hours, resuspended at 1 × 106 cells/100 μL IMDM + 0.1% BSA, and incubated for 15 minutes at 37°C with the inhibitors at various concentrations before adding 5 μg/mL IgE or 100 ng/mL SF for 5 minutes. Cells were solubilized by boiling for 1 minute with SDS-sample buffer (using 1 × 106 BMMCs/sample for total cell lysates). The phospho-PKB (protein kinase B) (Ser473), phospho-Erk1/2 (extracellular-regulated kinase 1 and 2), and phospho-p38 antibodies were from Cell Signaling Technologies (Beverly, MA). The Erk1 and 4G10 antibodies were generous gifts from Drs. Steven Pelech (University of British Columbia, Vancouver, Canada) and Brian Druker (Howard Hughes Medical Institute, Portland, OR), respectively.
ELISAs
BMMCs were starved for 4 hours in IMDM + 10% FCS, resuspended at 1 × 106 cells/mL in IMDM + 0.1% BSA, and added to FN- or BSA-coated polystyrene beads (bead-to-cell ratio, 1:1) ± IgE and incubated at 37°C for the indicated times. To coat the beads, Polybead Polystyrene Microsphere 15 μ beads (Polysciences, Warrington, PA) were incubated for 1 hour at 23°C with 1 mg/mL human FN, then blocked with 3% BSA in HBSS for 30 minutes at 23°C or incubated with 3% BSA in HBSS for 1.5 hours at 23°C as described.21 Interleukin 6 (IL-6) and tumor necrosis factor α (TNFα) (BD PharMingen) ELISAs were performed with bead supernatants according to the manufacturer's instructions.
Fluorescence-activated cell sorter (FACS) analysis
BMMCs were washed and resuspended at 5 × 105 cells/mL in IMDM + 0.1% BSA ± 5 μg/mL IgE and incubated in 6-well tissue culture plates or BIOCOAT human FN-coated 6-well plates (1 mL/well) at 37°C for 1 hour or 4 hours. Cells were then harvested, washed once with HBSS + 2% FCS + 0.05% NaN3 (HFN), resuspended in 50 μL HFN + 1 μg anti-CD49e or an isotype control, and incubated for 30 minutes at 4°C. After washing, the cells were incubated with phycoerythrin (PE)–conjugated anti-rat IgG (Jackson Labs, West Grove, PA) for 30 minutes at 4°C, washed twice, resuspended in HFN, and analyzed using FACSCalibur (Becton Dickinson).
Calcium measurements
Calcium fluxes were measured as described previously.17 For stimulation with IgE or SF, BMMCs were incubated with 2 μM fura-2/am (Molecular Probes) in Tyrode buffer at 23°C for 45 minutes, washed twice, resuspended in Tyrode buffer at 5 × 105 cells/mL in a stirring cuvette, and stimulated with the indicated concentrations of IgE or SF. For stimulation with IgE + Ag, 5 μg/mL IgE was added during the fura-2 loading step, followed by stimulation with 20 ng/mL DNP-HSA. For the 2-APB studies, cells were labeled with fura-2 at 37°C in the presence of 50 μM 2-APB, then stimulated as described in “Adhesion assay.” For the EGTA (ethylene glycol tetraacetic acid) studies, 5 mM EGTA was added immediately prior to the addition of the stimulus.
Survival assay
Survival assays were carried out as described previously16 except that Falcon 1172 96-well flat-bottom plates were first treated for 1 hour at 37°C ± 50 μg/mL FN, and then all the plates treated for 1 hour at 37°C with 3% BSA. The wells were then washed with IMDM + 0.1% BSA, and the BMMCs, after washing with IMDM, were added at 5 × 105 cells/mL in IMDM + 0.1% BSA ± IgE. Viability was assessed by trypan blue exclusion.
Results
IgE alone stimulates the adhesion of both BMMCs and CTMCs to FN
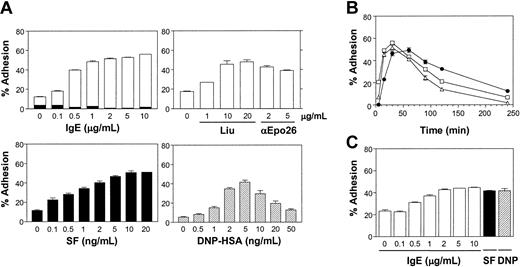
We recently demonstrated that monomeric IgE, in the absence of cross-linking agents or exogenous growth factors, was capable of enhancing the survival of BMMCs and did so, at least in part, by maintaining Bcl-XL levels and by producing autocrine-acting cytokines.16 To determine whether IgE alone had any other biologic effects on BMMCs we tested its ability to stimulate the adhesion of these cells to the connective tissue component, FN. As can be seen in Figure 1A (top left panel), IgE (SPE-7) alone stimulated the adhesion of Calcein-am–loaded healthy C57B6 mouse-derived BMMCs to FN in a dose-dependent fashion. With the use of a 60-minute adhesion assay, adhesion typically plateaued between 50% and 60% of input cells. The IgE concentration yielding half-maximal adhesion was found to be between 100 and 500 ng/mL IgE. When wells were coated with BSA instead of FN, no adhesion was observed in response to IgE (Figure 1A, top left panel). Similar results were obtained with monomeric IgE, derived by high-performance liquid chromatography (HPLC) fractionation of SPE-7 IgE,16 indicating that this was not due to low levels of IgE aggregates in the IgE preparation (data not shown). As well, similar results were obtained using the Liu anti-DNP IgE19 and an anti-erythropoietin IgE (anti-Epo 26),17 demonstrating that these results were not restricted to the SPE-7 anti-DNP IgE (Figure 1A; top right panel).
IgE alone stimulates the adhesion of both BMMCs and CTMCs to FN. (A) Adhesion of normal BMMCs to FN following a 60-minute adhesion assay with increasing concentrations of IgE alone (SPE-7, top left panel; Liu anti-DNP or anti-Epo26, top right panel), SF (bottom left panel), or IgE + DNP-HSA (bottom right panel). The black bars in the top left panel indicate the level of adhesion to wells coated with BSA instead of FN. (B) A time course of BMMC adhesion to FN in the presence of 1 μg/mL IgE alone (•), 5 ng/mL SF (▵), or IgE + 2 ng/mL DNP-HSA (□). Background adhesion (2%-6%) was subtracted from each time point. (C) Adhesion of normal CTMCs to FN in response to IgE at the indicated concentrations, 5 ng/mL SF, or IgE + 5 ng/mL DNP-HSA for 60 minutes. Results shown are the mean ± SEM of triplicate determinations. Similar results were obtained in 5 (A), 3 (B), and 2 (C) separate experiments. Adhesion in response to all stimuli was significantly (P < .05) different from control values except for (C) 0.1 μg/mL.
IgE alone stimulates the adhesion of both BMMCs and CTMCs to FN. (A) Adhesion of normal BMMCs to FN following a 60-minute adhesion assay with increasing concentrations of IgE alone (SPE-7, top left panel; Liu anti-DNP or anti-Epo26, top right panel), SF (bottom left panel), or IgE + DNP-HSA (bottom right panel). The black bars in the top left panel indicate the level of adhesion to wells coated with BSA instead of FN. (B) A time course of BMMC adhesion to FN in the presence of 1 μg/mL IgE alone (•), 5 ng/mL SF (▵), or IgE + 2 ng/mL DNP-HSA (□). Background adhesion (2%-6%) was subtracted from each time point. (C) Adhesion of normal CTMCs to FN in response to IgE at the indicated concentrations, 5 ng/mL SF, or IgE + 5 ng/mL DNP-HSA for 60 minutes. Results shown are the mean ± SEM of triplicate determinations. Similar results were obtained in 5 (A), 3 (B), and 2 (C) separate experiments. Adhesion in response to all stimuli was significantly (P < .05) different from control values except for (C) 0.1 μg/mL.
For comparison, we carried out dose-response studies with SF (Figure 1A, bottom left panel), and a similar plateau was observed with optimal levels of SF, shown in previous studies to be highly effective at inducing adhesion of BMMCs to FN.12 We also found that IgE + Ag was capable of inducing BMMC adhesion to FN (Figure 1A, bottom right panel), in keeping with previous reports.13,22 For this study, BMMCs were presensitized overnight with 1 μg/mL IgE in the absence of FN, and then, after washing away unbound IgE, DNP-HSA was added at the indicated concentrations. Adhesion reached a plateau similar to that obtained with IgE alone or with SF. As reported previously, the concentrations of SF and antigen that gave half maximal adhesion (ie, approximately 0.5 ng/mL and 1.5 ng/mL, respectively) were far less (10- to 100-fold) than that required for half maximal stimulation of proliferation11 or degranulation,23,24 respectively. It is worthy to note that the background adhesion of BMMCs to FN varied from one experiment to another (from 4%-18%). This variation primarily reflects differences in the basal level of adhesion between different batches of BMMCs.
It is now well documented that SF and other agents that enhance adhesion of mast cells to FN do so in a transient way.11,23 We, therefore, carried out time course studies with IgE alone to determine whether the kinetics of adhesion and/or release were similar to that obtained with SF or IgE + Ag. As can be seen in Figure 1B, IgE-induced adhesion of BMMCs to FN was slower (ie, undetectable at 5 minutes) but remained at plateau levels significantly longer than with SF or IgE + Ag. This result may be due in part to the slow on rate of IgE25 and the slow internalization rate of uncross-linked IgE/FcϵRI,26 respectively.
We then asked whether IgE alone could also stimulate the adhesion of CTMCs to FN. As shown in Figure 1C, even though background adhesion was higher with these cells, IgE, as well as SF and IgE + Ag, triggered CTMC adhesion, and the concentration of IgE that gave half maximal adhesion was approximately 500 ng/mL. Thus, IgE-induced adhesion was not restricted to BMMCs.
IgE alone stimulates the adhesion of BMMCs to FN via an increase in the avidity of VLA-5
To gain some insight into the nature of the receptors on BMMCs that bind to FN in response to IgE alone, we carried out adhesion assays in the presence and absence of the peptide GRGDSP (which contains the RGD consensus sequence within FN that binds to a subset of integrins15 ) or a control peptide, GRGESP. As expected from previous reports,12 the RGD-containing peptide completely inhibited SF-induced adhesion, whereas the control RGE-containing peptide did not, and the same results were obtained with IgE (Figure 2A). To hone in on which integrin was involved in IgE-induced adhesion to FN, we next examined the effect of blocking VLA-5 with the anti-α5 integrin antibody MFR-5 (anti-CD49e) because it had been shown previously that this integrin was involved in SF- and IgE + Ag-induced adhesion of BMMCs to FN.11,22 As can be seen in Figure 2B, this antibody also inhibited IgE-induced adhesion to FN. However, this antibody did not completely block adhesion; thus, it is possible that another RGD-binding receptor also contributes to IgE- and SF-induced adhesion.
IgE alone stimulates the adhesion of BMMCs to FN via an increase in the avidity of VLA-5. (A) Adhesion of normal BMMCs to FN in response to assay medium alone (C), 1 μg/mL IgE, or 5 ng/mL SF in the absence (□) or presence of 400 μg/mL RGD-containing peptide (▪)or400 μg/mL control RGE-containing peptide (▨) for 15 minutes. Adhesion in response to IgE and IgE + RGE peptide, but not IgE + RGD peptide, was significantly (P < .05) different from control values. (B) Adhesion of BMMCs to FN in response to assay medium alone (C), 0.5 μg/mL IgE, or 5 ng/mL SF for 15 minutes in the absence (□) or presence (▪)of40 μg/mL anti-CD49e added 30 minutes prior to stimulation. Adhesion was significantly (P < .05) different between control (C) and all stimulated samples as well as between stimulated and stimulated + anti-CD49e–treated samples. Results shown are the mean ± SEM of triplicate determinations and similar results were obtained in 3 (A) and 3 (B) separate experiments. BMMCs were incubated on (C) BSA for 1 hour or (D) BSA for 4 hours (top panel) or on FN for 4 hours (bottom panel) in the absence (solid line) or presence (dashed line) of 2 μg/mL IgE. The cells were then stained with anti-CD49e antibody (1 μg/5 × 105 cells) for 30 minutes at 4°C and analyzed by FACS. The blackened area profiles were obtained with isotype control antibody.
IgE alone stimulates the adhesion of BMMCs to FN via an increase in the avidity of VLA-5. (A) Adhesion of normal BMMCs to FN in response to assay medium alone (C), 1 μg/mL IgE, or 5 ng/mL SF in the absence (□) or presence of 400 μg/mL RGD-containing peptide (▪)or400 μg/mL control RGE-containing peptide (▨) for 15 minutes. Adhesion in response to IgE and IgE + RGE peptide, but not IgE + RGD peptide, was significantly (P < .05) different from control values. (B) Adhesion of BMMCs to FN in response to assay medium alone (C), 0.5 μg/mL IgE, or 5 ng/mL SF for 15 minutes in the absence (□) or presence (▪)of40 μg/mL anti-CD49e added 30 minutes prior to stimulation. Adhesion was significantly (P < .05) different between control (C) and all stimulated samples as well as between stimulated and stimulated + anti-CD49e–treated samples. Results shown are the mean ± SEM of triplicate determinations and similar results were obtained in 3 (A) and 3 (B) separate experiments. BMMCs were incubated on (C) BSA for 1 hour or (D) BSA for 4 hours (top panel) or on FN for 4 hours (bottom panel) in the absence (solid line) or presence (dashed line) of 2 μg/mL IgE. The cells were then stained with anti-CD49e antibody (1 μg/5 × 105 cells) for 30 minutes at 4°C and analyzed by FACS. The blackened area profiles were obtained with isotype control antibody.
To determine whether IgE, in the absence of Ag, was enhancing BMMC adhesion to FN by up-regulating the cell surface level of VLA-5, we carried out flow cytometry using anti-CD49e. As can be seen in Figure 2C, there was no increase in the cell surface level of α5 integrin following a 1-hour treatment with IgE (when binding to FN is maximal [Figure 1B]). Thus, IgE alone, similar to what has been reported previously with SF and IgE + Ag,22 appeared to enhance adhesion not by up-regulating integrin receptors but by increasing the avidity of VLA-5 via “inside-out” signaling. Also, there was no change in the cell surface level of α5 integrin following a 4-hour treatment with IgE in the presence of either BSA (Figure 2D, top panel) or FN (Figure 2D, bottom panel). This observation demonstrates that the detachment of IgE-stimulated BMMCs from FN at this time was not due simply to a reduction in the level of cell surface VLA-5.
IgE-induced adhesion of BMMCs to FN requires PI3K but not Erk or p38
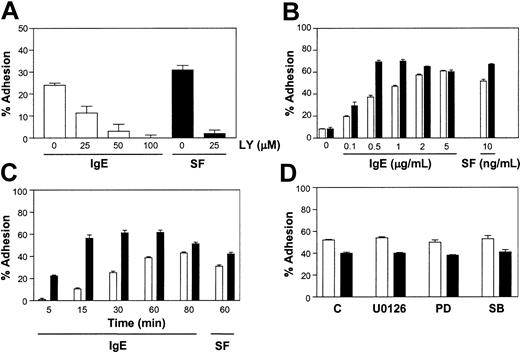
To gain some insight into the intracellular pathways through which IgE alone triggered adhesion to FN we first explored the role of the PI3K pathway because it had been shown previously to be involved in SF- and IgE + Ag-induced BMMC adhesion to FN.22,27,28 To do this, we tested the PI3K inhibitor, LY294002, and found that it inhibited both SF- and IgE-induced adhesion of BMMCs to FN (Figure 3A). Similar results were obtained with wortmannin (data not shown). We carried out these and subsequent inhibitor studies at times and concentrations of IgE and SF that were suboptimal for adhesion to FN to maximize the sensitivity of the assay to potential inhibitors and minimize exposure of the cells to inhibitors. As well, we titrated the various inhibitors used via Western analyses of BMMCs stimulated for 5 minutes with 5 μg/mL IgE or 10 ng/mL SF, so that we used the lowest concentration that completely inhibited the target pathway (data not shown).
IgE-induced adhesion of BMMCs to FN requires PI3K but not Erk or p38. (A) Adhesion of normal BMMCs to FN in response to 1 μg/mL IgE or 5 ng/mL SF in the presence of the indicated concentrations of LY294002 (LY) following a 30-minute adhesion assay. All wells contained the same level of DMSO (vehicle for LY). Adhesion was significantly (P < .05) different between control stimulated and stimulated + LY294002-treated samples. (B) Adhesion of SHIP+/+ (□) and –/– (▪) BMMCs to FN following a 60-minute exposure to the indicated concentrations of IgE or SF. Adhesion was significantly (P < .05) different between SHIP+/+ and –/– levels except for the control, 0.1 and 5 μg/mL values. (C) A time course of the adhesion of SHIP+/+ (□) and –/– (▪) BMMCs to FN in the presence of 1 μg/mL IgE and, for comparison, a 60-minute exposure to 0.5 ng/mL SF. Adhesion was significantly (P < .05) different between SHIP+/+ and –/– values. (D) Adhesion of normal BMMCs to FN for 30 minutes in the presence of 1 μg/mL IgE (□) or 2 ng/mL SF (▪) in the absence (C) or presence of 1 μM U0126, 20 μM PD98059 (PD), or 10 μM SB203580 (SB). All wells contained the same level of DMSO (vehicle for the inhibitors). Adhesion was not significantly different between IgE- and IgE + U0126-, PD98059-, or SB203 580-stimulated samples. Results shown are the mean ± SEM of triplicate determinations. Background adhesion was subtracted from the values graphed in (A, 14%), (C, 4%-11%), and (D, 5%-11%). Similar results were obtained in 4 (A), 5 (B), 3 (C), and 3 (D) separate experiments.
IgE-induced adhesion of BMMCs to FN requires PI3K but not Erk or p38. (A) Adhesion of normal BMMCs to FN in response to 1 μg/mL IgE or 5 ng/mL SF in the presence of the indicated concentrations of LY294002 (LY) following a 30-minute adhesion assay. All wells contained the same level of DMSO (vehicle for LY). Adhesion was significantly (P < .05) different between control stimulated and stimulated + LY294002-treated samples. (B) Adhesion of SHIP+/+ (□) and –/– (▪) BMMCs to FN following a 60-minute exposure to the indicated concentrations of IgE or SF. Adhesion was significantly (P < .05) different between SHIP+/+ and –/– levels except for the control, 0.1 and 5 μg/mL values. (C) A time course of the adhesion of SHIP+/+ (□) and –/– (▪) BMMCs to FN in the presence of 1 μg/mL IgE and, for comparison, a 60-minute exposure to 0.5 ng/mL SF. Adhesion was significantly (P < .05) different between SHIP+/+ and –/– values. (D) Adhesion of normal BMMCs to FN for 30 minutes in the presence of 1 μg/mL IgE (□) or 2 ng/mL SF (▪) in the absence (C) or presence of 1 μM U0126, 20 μM PD98059 (PD), or 10 μM SB203580 (SB). All wells contained the same level of DMSO (vehicle for the inhibitors). Adhesion was not significantly different between IgE- and IgE + U0126-, PD98059-, or SB203 580-stimulated samples. Results shown are the mean ± SEM of triplicate determinations. Background adhesion was subtracted from the values graphed in (A, 14%), (C, 4%-11%), and (D, 5%-11%). Similar results were obtained in 4 (A), 5 (B), 3 (C), and 3 (D) separate experiments.
To explore the role of the PI3K pathway further we compared the IgE-induced adhesion of SHIP+/+ and –/– BMMCs to FN because SHIP–/– BMMCs have been shown to have elevated phosphatidylinositol-3,4,5-trisphosphate (PIP3) levels.29 Specifically, we carried out dose-response (Figure 3B) and time-course (Figure 3C) studies using SHIP+/+ and –/– BMMCs and found that the SHIP–/– BMMCs displayed an increased IgE-mediated adhesion at suboptimal IgE concentrations and a more rapid IgE-mediated adhesion than the SHIP+/+ cells. This observation was also seen when these 2 cell types were stimulated with SF (Figures 3B-C). Consistent with this observation we found that low concentrations of LY294002 delayed IgE-induced adhesion of SHIP+/+ BMMCs to FN (data not shown). Because the cell surface expression of VLA-5 was comparable on SHIP+/+ and –/– BMMCs (data not shown), the increased adhesion of SHIP–/– BMMCs suggests that SHIP plays a role in restraining the increase in VLA-5 avidity.
We then asked whether the Erk or p38 pathways were playing a role in IgE-induced adhesion to FN because they are more active in IgE + Ag-induced SHIP–/– BMMCs.30 Specifically, we tested the MEK (MAP kinase/Erk kinase) inhibitors, U0126 and PD98059, and the p38 inhibitor, SB203580, using concentrations that totally blocked IgE- or SF-induced Erk and p38 phosphorylation.30 As can be seen in Figure 3D, these inhibitors had no effect on either IgE- or SF-induced adhesion of BMMCs to FN.
IgE, but not SF, requires entry of extracellular calcium for BMMC adhesion to FN
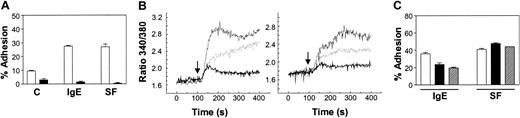
Because the binding of calcium to the extracellular domain of the VLA-5 dimer has been shown to be essential for integrin-mediated adhesion induced via inside-out signaling,24,31 we next asked whether extracellular calcium (
IgE, but not SF, requires the entry of extracellular calcium to trigger BMMC adhesion to FN. (A) Adhesion of normal BMMCs to FN in response to medium alone (C), 1 μg/mL IgE, or 5 ng/mL SF for 30 minutes with (□) or without (▪) the addition of 1.8 mM CaCl2. (B) Intracellular calcium measurements in BMMCs stimulated with 5 μg/mL IgE (thin solid line; left panel) or 50 ng/mL SF (thin solid line; right panel) alone, or in the presence of 50 μM 2-APB (dashed line) or 5 mM EGTA (thick solid line). The 2-APB was pre-incubated with the cells for 30 minutes, whereas the EGTA was added immediately prior to the addition of IgE or SF at 100 seconds (↓). (C) Adhesion of BMMCs to FN in response to 1 μg/mL IgE or 5 ng/mL SF for 30 minutes in the presence of vehicle control (□), 25 μM (▪), or 50 μM (▨) 2-APB added 30 minutes prior to stimulation. The background adhesion (6%) was subtracted. Adhesion was significantly (P < .05) different between IgE- and IgE + 2-APB–stimulated, but not SF- and SF + 2-APB–stimulated samples. Results shown are the mean ± SEM of triplicate determinations. Similar results were obtained in 3 (A), 3 (B), and 3 (C) separate experiments.
IgE, but not SF, requires the entry of extracellular calcium to trigger BMMC adhesion to FN. (A) Adhesion of normal BMMCs to FN in response to medium alone (C), 1 μg/mL IgE, or 5 ng/mL SF for 30 minutes with (□) or without (▪) the addition of 1.8 mM CaCl2. (B) Intracellular calcium measurements in BMMCs stimulated with 5 μg/mL IgE (thin solid line; left panel) or 50 ng/mL SF (thin solid line; right panel) alone, or in the presence of 50 μM 2-APB (dashed line) or 5 mM EGTA (thick solid line). The 2-APB was pre-incubated with the cells for 30 minutes, whereas the EGTA was added immediately prior to the addition of IgE or SF at 100 seconds (↓). (C) Adhesion of BMMCs to FN in response to 1 μg/mL IgE or 5 ng/mL SF for 30 minutes in the presence of vehicle control (□), 25 μM (▪), or 50 μM (▨) 2-APB added 30 minutes prior to stimulation. The background adhesion (6%) was subtracted. Adhesion was significantly (P < .05) different between IgE- and IgE + 2-APB–stimulated, but not SF- and SF + 2-APB–stimulated samples. Results shown are the mean ± SEM of triplicate determinations. Similar results were obtained in 3 (A), 3 (B), and 3 (C) separate experiments.
To confirm the role of
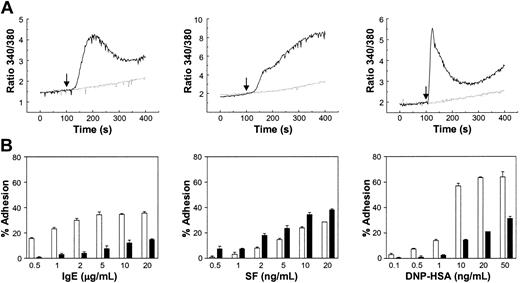
Lyn –/– BMMCs, which do not show increased intracellular calcium with IgE, SF, or IgE + Ag, display impaired adhesion to FN in response to IgE or IgE + Ag but not to SF. (A) Intracellular calcium measurements in Lyn+/+ (solid line) and –/– (dashed line) BMMCs in response to 10 μg/mL IgE (left panel), 100 ng/mL SF (middle panel), or IgE + 20 ng/mL DNP-HSA (right panel) injected at 100 seconds (↓). (B) Adhesion of Lyn+/+ (□) and –/– (▪) BMMCs to FN following a 60-minute exposure of the cells to the indicated concentrations of IgE, SF, or IgE + DNP-HSA. The background adhesion (8%-15%) was subtracted. Adhesion was significantly (P < .05) different between Lyn+/+ and –/– BMMCs except for 0.5, 1 ng/mL SF and 0.1, 0.5, 1 ng/mL DNP-HSA. Results shown are the mean ± SEM of triplicate determinations. Similar results were obtained in 3 (A) and 3 (B) separate experiments.
Lyn –/– BMMCs, which do not show increased intracellular calcium with IgE, SF, or IgE + Ag, display impaired adhesion to FN in response to IgE or IgE + Ag but not to SF. (A) Intracellular calcium measurements in Lyn+/+ (solid line) and –/– (dashed line) BMMCs in response to 10 μg/mL IgE (left panel), 100 ng/mL SF (middle panel), or IgE + 20 ng/mL DNP-HSA (right panel) injected at 100 seconds (↓). (B) Adhesion of Lyn+/+ (□) and –/– (▪) BMMCs to FN following a 60-minute exposure of the cells to the indicated concentrations of IgE, SF, or IgE + DNP-HSA. The background adhesion (8%-15%) was subtracted. Adhesion was significantly (P < .05) different between Lyn+/+ and –/– BMMCs except for 0.5, 1 ng/mL SF and 0.1, 0.5, 1 ng/mL DNP-HSA. Results shown are the mean ± SEM of triplicate determinations. Similar results were obtained in 3 (A) and 3 (B) separate experiments.
To explore the signaling requirements for IgE-induced adhesion further we tested the phospholipase Cγ (PLCγ) inhibitor, U73122. PLCγ cleaves PI-4,5-P2 to generate 2 second messengers: diacylglycerol (DAG), which binds and activates a subset of PKC family members,36 and inositol 1,4,5-triphosphate (IP3), which binds to IP3 receptors on endoplasmic reticula and mitochondria to release intracellular stores of calcium.33 This release in turn triggers the entry of
IgE, but not SF, may require a calcium-dependent PKC to trigger BMMC adhesion to FN. (A) Adhesion of normal BMMCs to FN in response to 1 μg/mL IgE or 5 ng/mL SF in the presence of vehicle control (□), 1 μM U73122 (▪), or 1 μM U73343 (▨). Adhesion was significantly (P < .05) different between stimulated samples in the presence and absence of U73122 but not U73343. (B) Adhesion of BMMCs to FN in response to 1 μg/mL IgE or 5 ng/mL SF in the presence of the indicated concentrations of Compound 3 (C3). Adhesion was significantly (P < .05) different between stimulated samples in the presence and absence of C3 at 25 and 50 μM C3. (C) Adhesion of BMMCs to FN in response to 1 μg/mL IgE or 5 ng/mL SF in the presence of the indicated concentrations of Gö6976 (Gö). Adhesion was significantly (P < .05) different between IgE- but not SF-stimulated samples in the presence and absence of Gö. All adhesion assays were 30 minutes, and the results shown are the mean ± SEM of triplicate determinations. Background adhesions (A, 4%; B, 13%; and C, 4%) were subtracted. Similar results were obtained in 4 (A), 4 (B), and 3 (C) separate experiments.
IgE, but not SF, may require a calcium-dependent PKC to trigger BMMC adhesion to FN. (A) Adhesion of normal BMMCs to FN in response to 1 μg/mL IgE or 5 ng/mL SF in the presence of vehicle control (□), 1 μM U73122 (▪), or 1 μM U73343 (▨). Adhesion was significantly (P < .05) different between stimulated samples in the presence and absence of U73122 but not U73343. (B) Adhesion of BMMCs to FN in response to 1 μg/mL IgE or 5 ng/mL SF in the presence of the indicated concentrations of Compound 3 (C3). Adhesion was significantly (P < .05) different between stimulated samples in the presence and absence of C3 at 25 and 50 μM C3. (C) Adhesion of BMMCs to FN in response to 1 μg/mL IgE or 5 ng/mL SF in the presence of the indicated concentrations of Gö6976 (Gö). Adhesion was significantly (P < .05) different between IgE- but not SF-stimulated samples in the presence and absence of Gö. All adhesion assays were 30 minutes, and the results shown are the mean ± SEM of triplicate determinations. Background adhesions (A, 4%; B, 13%; and C, 4%) were subtracted. Similar results were obtained in 4 (A), 4 (B), and 3 (C) separate experiments.
To probe the role of DAG and calcium further we asked whether one or more of the 12 isoforms of PKC might be involved because both calcium and DAG are known activators of certain PKCs.36 To test this we first added the pan-specific PKC inhibitor, Compound 3 (bisindolylmaleimide I) to our adhesion assay. As shown in Figure 6B, no inhibition was observed with various concentrations of Compound 3, including 25 μM, which completely blocked IgE + Ag-induced phosphorylation of IkB (inhibitor of NF-κB [nuclear factor-κB]) at Ser32.30 In fact, a modest stimulation was consistently observed, both in the absence and presence of IgE alone or SF. This observation is consistent with a previous report demonstrating that Compound 3 did not inhibit SF-induced adhesion of BMMCs.37 Thus, it is likely that one or more isoforms of PKC may actually play a negative role in regulating the avidity of VLA-5 on BMMCs for FN. We then tested the classic PKC inhibitor, Gö6976, at various concentrations and found, consistent with the 2-APB results described earlier, that it potently inhibited IgE-induced (and IgE + Ag-induced) but not SF-induced adhesion to FN (Figure 6C). This observation suggested that a calcium-dependent PKC may positively regulate IgE- but not SF-induced adhesion. Alternatively, Gö6976 may be acting through a different kinase to inhibit IgE-induced adhesion.
VLA-5 activation acts together with IgE to prolong intracellular signaling and to enhance cytokine production and BMMC survival
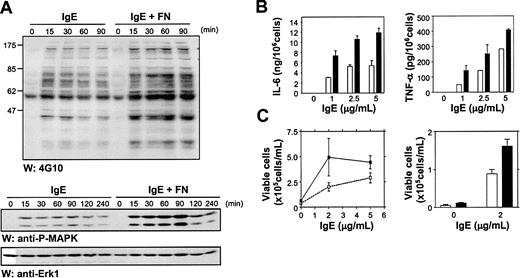
To investigate the downstream ramifications of IgE-induced adhesion of BMMCs to FN we first asked whether there were any apparent differences in intracellular signaling events when cells were stimulated with IgE alone versus IgE + FN (ie, do IgE-induced adherent BMMCs display a different signaling pattern because of input from the activated VLA-5?). To test this question, we first compared the overall tyrosine phosphorylation pattern of BMMCs in suspension versus attached to FN, at different exposure times to IgE. As can be seen in Figure 7A (top panel), tyrosine phosphorylation was substantially prolonged with FN-adherent cells. We then carried out similar time-course studies to specifically examine the effect of IgE alone versus IgE + FN on Erk1 and Erk2 phosphorylation and found that phosphorylation was both prolonged and more intense when the cells were attached to FN (Figure 7A, middle panel). A reprobe with anti-Erk antibodies demonstrated equal loading (Figure 7A, bottom panel).
FN binding enhances IgE-induced intracellular signaling events, cytokine production, and survival. (A) Total cell lysates (50 μg as assessed by BCA assays) from IgE-stimulated suspension and FN-adhered BMMCs were subjected to Western analysis using anti-phosphotyrosine antibodies (4G10) (top panel) or antiphospho-specific Erk antibodies (middle panel) and reprobed with anti-Erk antibodies (bottom panel) to demonstrate equal loading. (B) BMMCs were stimulated for 3 hours at the indicated concentrations of IgE in the presence of 15 μ polystyrene beads coated with BSA (□) or FN (▪). IL-6 (left panel) and TNF-α (right panel) levels in the supernatants were detected by ELISA. (C) BMMCs were plated at 5 × 105 cells/mL in IMDM + 0.1% BSA ± IgE at 2 and 5 μg/mL in FN (▪) or BSA (□) coated wells. On day 4, viable cells were counted by trypan blue exclusion (left panel). In the right panel, BMMCs were set up as above ± 2 μg/mL IgE and viable cells counted on day 7. Data points are the mean ± SEM of 6 (B), 2 (C, left) and 3 (C, right) determinations. Similar results were obtained in 3 (A), 4 (B), and 3 (C) separate experiments.
FN binding enhances IgE-induced intracellular signaling events, cytokine production, and survival. (A) Total cell lysates (50 μg as assessed by BCA assays) from IgE-stimulated suspension and FN-adhered BMMCs were subjected to Western analysis using anti-phosphotyrosine antibodies (4G10) (top panel) or antiphospho-specific Erk antibodies (middle panel) and reprobed with anti-Erk antibodies (bottom panel) to demonstrate equal loading. (B) BMMCs were stimulated for 3 hours at the indicated concentrations of IgE in the presence of 15 μ polystyrene beads coated with BSA (□) or FN (▪). IL-6 (left panel) and TNF-α (right panel) levels in the supernatants were detected by ELISA. (C) BMMCs were plated at 5 × 105 cells/mL in IMDM + 0.1% BSA ± IgE at 2 and 5 μg/mL in FN (▪) or BSA (□) coated wells. On day 4, viable cells were counted by trypan blue exclusion (left panel). In the right panel, BMMCs were set up as above ± 2 μg/mL IgE and viable cells counted on day 7. Data points are the mean ± SEM of 6 (B), 2 (C, left) and 3 (C, right) determinations. Similar results were obtained in 3 (A), 4 (B), and 3 (C) separate experiments.
To look at the biologic ramifications of IgE-induced adhesion to FN we asked whether IgE alone, which does not trigger detectable degranulation in suspension cultures of BMMCs,16 might now trigger degranulation of FN-adhered cells. However, no significant degranulation was observed (data not shown). We then compared the levels of pro-inflammatory cytokines secreted into the medium from nonadherent versus FN-adherent IgE-treated BMMCs. For these experiments, BMMCs were stimulated with 5 μg/mL IgE for 3 hours in the presence of 15 μ polystyrene beads previously coated with BSA or FN.21 IL-6 and TNFα ELISAs revealed that IgE-induced activation of VLA-5 substantially increased the levels of these cytokines (Figure 7B).
Because we had previously shown that the production of autocrine-acting cytokines may contribute to IgE-mediated enhancement of BMMC survival,16 we next performed survival studies with IgE in the presence and absence of FN. Two concentrations of IgE were initially tested, 2 μg/mL and 5 μg/mL, and viable cells were counted after 4 days. We observed, as seen in Figure 7C (left panel), that adhesion to FN significantly enhanced this IgE-mediated survival, and this enhancement appeared to be greatest at suboptimal concentrations of IgE. Even after 1 week in the presence of 2 μg/mL IgE, for example, IgE-induced binding to FN consistently enhanced BMMC survival approximately 2-fold (Figure 7C, right panel).
Discussion
Mast cells congregate in connective tissue and are especially numerous beneath the epithelial surfaces of the skin and in the respiratory, gastrointestinal, and genitourinary tracts. This tissue localization, which plays a critical role in enabling mast cells to respond rapidly to invading parasites, bacteria, and environmental antigens, is thought to involve the binding of mast cell progenitors via their integrins to connective tissue components. We demonstrate herein that IgE alone (at approximately 2 μg/mL) is capable of maximally triggering adhesion of both BMMCs and CTMCs to FN and does so to the same extent as optimal levels of SF (approximately 10 ng/mL) or IgE + Ag (5 ng/mL Ag). Interestingly, these levels of SF and Ag are substantially lower than those required to induce BMMC proliferation and degranulation, respectively, confirming earlier reports11,12,23 and may suggest that, in vivo, low levels of these stimuli play an important role in mast cell recruitment. Relevant to this, the concentration of SF in normal human serum is approximately 3 ng/mL,38 whereas that of IgE ranges from less than 0.1 μg/mL in healthy individuals to more than 30 μg/mL in highly atopic individuals.39 Thus, it is possible that IgE alone only plays a significant role in mediating recruitment, adhesion, and subsequent cytokine production and survival of mast cells during infections or allergic reactions.
The ability of IgE alone to enhance BMMC survival was recently published back to back by our group16 and Asai et al.40 However, although Asai et al40 contend that this IgE-induced survival does not involve intracellular signaling, our data strongly implicate signaling. In fact, more recent studies in our laboratory reveal that all IgEs studied to date (including SPE-7, anti-Epo26, and the Liu anti-DNP IgE) can trigger signaling, albeit to different degrees, in normal BMMCs, and the ability of a particular IgE to induce signaling correlates with its ability to promote survival (J.K. et al, unpublished observation, 2003). Importantly, we have found in the current study that, regardless of the IgE used, adhesion can be blocked using intracellular signaling inhibitors (data not shown).
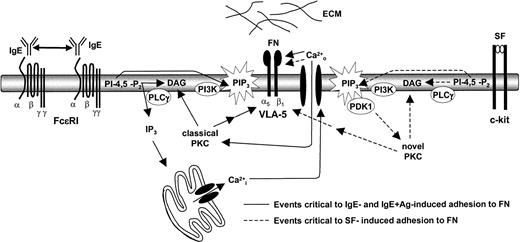
Taken together, our inside-out signaling studies suggest that the activation of both the PI3K pathway and the PLCγ-generated IP3 pathway (and subsequent draining of intracellular calcium stores,
A model of IgE-induced adhesion to FN. IgE binding to the FcϵR1 on BMMCs allows them to aggregate at a low frequency. This results in a relatively low (compared with IgE + Ag) but prolonged signal that activates, among other pathways, the PI3K and PLCγ pathways. These 2 pathways play a critical role in IgE- and IgE + Ag-induced adhesion to FN by inducing the entry of extracellular calcium and the activation of a classical PKC. SF-induced adhesion of BMMCS to FN, however, appears to require the stimulation of the PI3K and PLCγ pathways and the subsequent critical activation of a novel (DAG-dependent, calcium-independent) PKC.
A model of IgE-induced adhesion to FN. IgE binding to the FcϵR1 on BMMCs allows them to aggregate at a low frequency. This results in a relatively low (compared with IgE + Ag) but prolonged signal that activates, among other pathways, the PI3K and PLCγ pathways. These 2 pathways play a critical role in IgE- and IgE + Ag-induced adhesion to FN by inducing the entry of extracellular calcium and the activation of a classical PKC. SF-induced adhesion of BMMCS to FN, however, appears to require the stimulation of the PI3K and PLCγ pathways and the subsequent critical activation of a novel (DAG-dependent, calcium-independent) PKC.
Our results are consistent with earlier studies showing that the PI3K pathway plays a critical role in SF- and IgE + Ag-induced adhesion of BMMCs to FN.22,27,37 Intriguingly, Kinashi et al22 found that BMMC adhesion to FN could be triggered via a constitutively active PI3K but not a constitutively active Akt/PKB, suggesting that elevated PIP3 but not the downstream Akt pathway is critical to inside-out signaling. Although Vosseller et al28 concluded that a calcium-independent, PIP3-dependent PKC might be involved in SF-mediated adhesion to FN, they did not observe calcium entry in response to SF. In keeping with our results, however, Ueda et al35 recently reported that SF does induce calcium entry in c-kit–transfected Ba/F3 cells and that Lyn is a key mediator of this influx. Also relevant to our model, Lorentz et al42 very recently reported that SF-stimulated adhesion of human intestinal mucosa–derived mast cells to FN is blocked with 100 nM wortmannin or 20 μM apigenin but not with 2 μM Gö6976. Apart from their results with apigenin, a putative Erk pathway inhibitor, this is in agreement with our SF findings. Our results with the MEK inhibitors, PD98059 and U0126, suggest that IgE-, SF- and IgE + Ag-induced adhesion does not require the Erk pathway. Moreover, we found that apigenin is not a specific Erk pathway inhibitor. In fact, it inhibits many pathways at 20 μM, including the SF-induced tyrosine phosphorylation of c-kit, whereas at 5 μM it inhibits neither Erk phosphorylation nor SF-induced adhesion (data not shown).
The role that
Engagement of integrins by their ligands is known to activate various signaling pathways and this is referred to as “outside-in” signaling. These outside-in signals have been shown to modulate signals coming in from other receptors and to alter biologic responses triggered by these other receptors.43 Our observation that tyrosine phosphorylation events are prolonged and of greater intensity when VLA-5 activation acts in concert with IgE stimulation is reminiscent of earlier studies by Hamawy et al44 who showed that FcϵRI aggregation–induced adhesion of RBL-2H3 cells to FN increased the tyrosine phosphorylation of focal adhesion kinase (FAK). Furthermore, the enhanced inflammatory cytokine production that we observe with IgE-induced adhesion is somewhat consistent with earlier studies showing that adhesion of mast cells to FN via VLA-5 enhances IgE + Ag-induced degranulation13,23 and release of inflammatory cytokines.13 Interestingly, the enhanced IgE-induced survival we observe with FN-bound BMMCs may explain, at least in part, the elevated mast cell numbers seen in atopic individuals with elevated plasma IgE levels. Related to this, Ra et al13 found that the IgE + Ag-induced adhesion of rat- and mouse-cultured mast cells to FN led to greater survival (perhaps via the autocrine action of IL-3) than with IgE + Ag alone. In addition, it is conceivable that the ability of IgE to induce cytokine production could play a role in IgE-induced adhesion. Coward et al,45 for example, recently showed that TNFα acts as a positive autocrine signal to augment NFκB activity in human lung mast cells, making it a good candidate for the induction of adhesion in a feedback loop. However, Lorentz et al42 found that TNFα, nerve growth factor (NGF), interferon γ (IFNγ), transforming growth factor β (TGFβ), IL-6, IL-8, IL-10, or IL-13 did not stimulate the adhesion of human intestinal mast cells to FN. As well, apart from preformed TNFα, it is unlikely that IgE-induced cytokine production is rapid enough to contribute significantly to the initial increase in adhesion triggered by IgE. However, it is conceivable that IgE-induced cytokines play a role in IgE-induced adhesion at later time points, and we are currently testing this possibility.
Our findings increase the repertoire of biologic effects elicited by IgE alone and raise the possibility that IgE, in the absence of Ag, may help in vivo to concentrate mast cells at sites of inflammation and promote, in concert with FN, inflammatory cytokine release and mast cell survival.
Prepublished online as Blood First Edition Paper, April 17, 2003; DOI 10.1182/blood-2002-10-3176.
Supported by the National Cancer Institute of Canada (NCI-C) with core support from the BC Cancer Foundation and the BC Cancer Agency and by a grant from the National Institutes of Health (grant GM-49814). J.K. holds a Michael Smith Foundation for Health Research Trainee Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Christine Kelly for typing the manuscript. Lyn –/– mice were a generous gift from Dr Cliff Lowell, University of California San Francisco.