Abstract
Members of the RasGRP family of Ras activators have C1 domains that bind diacylglycerol (DAG) and DAG analogs such as the tumor-promoting phorbol esters. RasGRP members could be responsible for some of the DAG signaling processes that have previously been attributed to protein kinase C (PKC). We found that RasGRP3 is selectively expressed in B cells, suggesting that RasGRP3 might function downstream of the B-cell receptor (BCR). Indeed, stimulation of Ramos B cells with the DAG analog phorbol ester myristate (PMA) results in the association of RasGRP3 with the membrane fraction. However, we also made the unexpected observation that RasGRP3 is phosphorylated, coincident with Ras activation after stimulation. When inhibitors of PKC are present, Ras activation is attenuated, and this attenuation correlates with an inhibition of RasGRP3 phosphorylation. RasGRP3 is phosphorylated in vitro by PKC-θ and PKC-β2. When ectopically coexpressed in HEK-293 cells, a dominant-activated mutant of PKC-θ phosphorylates RasGRP3 and enhances Ras-Erk signaling. These results provide the first indication for a functional interaction between a RasGRP family member and a dissimilar DAG binding protein. A convergent DAG signaling system could be important in fine-tuning Ras signaling during B-cell development or during the humoral immune response.
Introduction
Diacylglycerol (DAG) acts as a messenger molecule in the plasma membrane, linking ligand receptor events to internal biochemical signaling events and biologic processes.1 DAG can be generated by receptor-mediated activation of phospholipase C and cleavage of phosphatidylinositol bis-phosphate (PIP2). Traditionally, DAG in the inner leaflet of the plasma membrane has been proposed to recruit and activate protein kinase C (PKC). Conventional forms of PKC (α, β1, β2, γ) are activated by DAG and calcium, whereas novel forms (δ, ϵ, η, θ) are activated by DAG but not by calcium.2 Both these families of PKC isozymes have C1 domains, protein modules containing specifically arranged cysteine, and histidine residues that coordinate zinc. C1 domains serve as membrane recruitment modules, and they bind to DAG with low nanomolar affinity, though this interaction also depends on the presence of acidic membrane lipids such as phosphatidylserine. The C1 domains of conventional and novel PKC forms also bind the phorbol ester class of DAG analog, which has been extensively studied in vivo as a tumor promoter and used in vitro as an artificial DAG stimulus in various experimental protocols. Atypical forms of PKC, such as ι and ζ, are not regulated by DAG or calcium.
Recently, it has been observed that other protein families have DAG and phorbol ester binding C1 domains, raising the question of whether some DAG and phorbol ester signaling processes might be mediated by these species.3 Unc-13 homologs are large proteins that have been linked to synaptic release in neurons.1,4,5 Protein kinase D is structurally distinct from any form of PKC.6 Chimerins have rac GAP activity and thus may attenuate signaling by this class of small GTPases.7,8 At least one form of DAG kinase has a DAG-binding C1 domain, suggesting that the DAG messenger might stimulate its own conversion to phosphatidic acid.9 It should be noted, however, that not all C1 domains bind DAG.
We recently discovered RasGRP1, a member of the CDC25 family of Ras guanyl nucleotide exchange factors.10 RasGRP1 is expressed in T cells, where it links the T-cell receptor (TCR) to Ras signaling.11,12 Stimulation of the TCR initiates a series of tyrosine kinase–mediated signaling processes that lead to phospholipase C-γ1 (PLC-γ1) activation and PIP2 breakdown. The resultant DAG serves to recruit RasGRP1 to the membrane, where it interacts with Ras. Ras activation stimulates various effector systems, leading to changes in gene transcription that are critical for T-cell development and activation during the cellular immune response. Importantly, the C1 domain of RasGRP1 binds DAG and is responsible for membrane recruitment.11,13
The existence of non-PKC proteins that respond to DAG argues that the DAG messenger might engage multiple, distinct biochemical responses, especially in cells that express multiple DAG-binding proteins. Additionally, because DAG may recruit several such proteins to the same membrane surfaces, the question arises whether these different classes of protein interact physically and functionally.
Using anti-RasGRP3 antibodies, we found that this family member was preferentially expressed in B cells. By analogy with our model for RasGRP1 function in TCR signaling, we hypothesized that RasGRP3 was activated downstream of B-cell–receptor (BCR) stimulation. Although we have not critically tested this hypothesis, our initial studies led to an unexpected observation: RasGRP3 is heavily phosphorylated after activation of the BCR. A number of our experiments support the idea that DAG signaling might engage RasGRP3 and a DAG-regulated protein kinase, perhaps PKC-θ or PKC-β2, and that RasGRP3 is regulated by protein phosphorylation.
Materials and methods
Reagents and antibodies
Okadaic acid was a gift from Charles Holmes (University of Alberta, Edmonton, Canada). The PKC inhibitors Go6976, Ro318220, and Bisindolylmaleimide I were purchased from Calbiochem (San Diego, CA). Phorbol 12-myristate 13-acetate (PMA) was purchased from SigmaAldrich (St Louis, MO). A polyclonal anti-RasGRP3 antibody was derived from rabbits immunized with bacterially expressed human RasGRP3, corresponding to the C-terminal residues 544 to 689. The antihuman immunoglobulin M (IgM) F(ab)2 fragment was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). The pan-Ras antibody clone RAS10 was supplied by Upstate Biotechnology (Lake Placid, NY). Anti–PKC-θ (P15120), anti–PKC-α (P16520), and anti–PKC-β (P17720) were purchased from Transduction Laboratories (Mississauga, ON, Canada), and the phospho p44/p42 ERK antibody (9101) was from Cell Signaling Technology (Beverly, MA). Antimouse IgM (μ-chain specific) and anti-mouse kappa (κ-chain specific) were purchased from Southern Biotechnology Associates (Birmingham, AL). Antimouse CD40 was supplied by BD Biosciences (Mississauga, ON, Canada).
Plasmids
Full-length human RasGRP3 complementary DNA (cDNA) was cloned as a BamH1/SalI fragment into BamH1/XhoI-cut pBKCMV (Stratagene, Cedar Creek, TX) and into BamH1/SalI-cut pMAL-c2 (New England Biolabs, Beverly, MA). The cDNAs encoding the constitutively active mutants of PKC-α (A25E) and PKC-θ (A148E) in pEFneo were a generous gift from Gottfried Baier (University of Innsbruck, Austria).
Cell culture and transfection
Human Burkitt lymphoma cell line Ramos (mIgM+) (American Type Culture Collection, Manassas, VA) was grown in RPMI supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, and antibiotics at 37°C in a 10% CO2 atmosphere. Human Burkitt lymphoma cell lines Daudi and Raji and the human pre–B-cell leukemia, Hoon, were provided by Andrew Shaw (University of Alberta, Edmonton, Canada). Human T-cell leukemias Molt-4, PEER, and CEM were provided by Chris Bleackley (University of Alberta, Edmonton, Canada), as was the human natural killer (NK)–like T-cell leukemia, YTINDY. Human acute T-cell leukemia, Jurkat, and 2 mouse myeloma cell lines, NS1 and SP/20, were generous gifts from Hanne Ostergaard (University of Alberta, Edmonton, Canada).
Human embryonic kidney 293 (HEK-293) cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and antibiotics. Cells were grown in 60-mm dishes and transfected with plasmid DNA by calcium-phosphate–mediated precipitation.14 Briefly, 10 μg CMVRasGRP3 and 10 μg pEF-PKC were mixed with 250 μL of 0.25 M CaCl2 before the dropwise addition of 250 μL of 2 × BES buffer (50 mM BES, 280 mM NaCl, 1.5 mM Na2HPO4 · 2H2O, pH 6.96). After 10 to 20 minutes, the DNA-calcium phosphate complex was added to 60-mm dishes containing 5 × 106 HEK-293 cells. Cultures were incubated at 37°C overnight in 5% CO2. After 14 hours, cells were rinsed twice with medium before the addition of fresh medium. Transiently transfected cells were harvested at 24 hours after transfection, following 10-minute treatment with either dimethyl sulfoxide (DMSO) or 10 nM PMA. Baculoviruses expressing PKC-θ or PKC-β2 were constructed using the Bac-to-Bac system, and proteins were expressed in Sf9 cells, according to the manufacturer's instructions (Gibco BRL, Grand Island, NY).
Subcellular fractionation and electrophoretic mobility shift assays
Ramos B cells were resuspended in hypotonic buffer (20 mM Tris, pH 7.5, 5 mM EGTA [ethyleneglycotetraacetic acid], 2 mM EDTA [ethylenediaminetetraacetic acid] plus protease inhibitors) and were disrupted in a glass homogenizer, as previously described.11 After low-speed centrifugation to remove unbroken cells and nuclei, the lysates were centrifuged at 100 000g to prepare P100 (particulate) and S100 (soluble) fractions. Proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5% gels run for extensive periods of time to optimize RasGRP3 electrophoretic mobility shifts. After electrophoresis, gels were transferred to nitrocellulose (Bio-Rad, Mississauga, ON, Canada) and were analyzed by immunoblotting using anti-RasGRP3 and anti-PKC antibodies. Splenocytes isolated from C57 BL/6J females at age 6 to 8 weeks were stimulated with PMA (100 nM), soluble anti-IgM (50 μg/mL), anti-kappa (30 μg/mL), or anti-CD40 (2 μg/mL) for 10 minutes at 37°C. Cells were immediately centrifuged at 1400g for 5 minutes at 4°C. Cell pellets were then lysed with lysis buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 10 mM sodium fluoride, 10% glycerol, 40 mM β-glycerophosphate, 1% NP-40 plus protease inhibitors). After centrifugation to remove nuclei, the resultant lysates were analyzed by immunoblotting using the anti-RasGRP3 antibody.
Ras and Erk activation assays
Ras activation was assayed by comparing the amount of Ras-GTP and the amount of total Ras in cell lysates, as described previously.11 Ramos cells were concentrated by centrifugation and resuspended in 1 mL original medium in an Eppendorf tube (5 × 106 cells/assay) and incubated for 5 minutes at 37°C. Cells were then pretreated with the PKC inhibitors Go6976 (20 μM), Ro318220 (10 μM), or Bisindolylmaleimide I (5 μM) for 15 minutes, followed by 10-minute stimulation with either 100 nM PMA or 10 μg/mL anti-IgM. Cells were immediately centrifuged at 1400g for 5 minutes at 4°C. Cell pellets were then lysed in Ras activation buffer (25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.5, 150 mM NaCl, 1% NP-40, 10% glycerol, 25 mM NaF, 10 mM MgCl2, 1 mM EDTA, 1 mM sodium orthovanadate, and protease inhibitors). After centrifugation to remove nuclei, 80% of each supernatant was incubated with GST-Raf (Ras binding domain) to collect Ras-GTP, and the remaining 20% was used to detect total Ras, phospho p44/p42 Erk, and RasGRP3. Immunoreactive species were detected using the enhanced chemiluminescence (ECL; Pierce, Rockford, IL) system.
Metabolic labeling
Exponentially growing Ramos cells were labeled for 4 hours in phosphate-free DMEM that had been supplemented with 0.05 vol regular DMEM plus 1.0 mCi (37 MBq) per milliliter 32Pi (ICN Biomedicals, Irvine, CA). Cells were unstimulated or stimulated with 10 μg/mL anti-IgM for the last 10 minutes of labeling. Cells were then lysed with lysis buffer. After centrifugation to remove nuclei, the resultant supernatant was precleared with protein A Sepharose (Amersham Biosciences, Freiburg, Germany) for 30 minutes at 4°C. The cleared lysates were incubated with anti-RasGRP3 prebound to protein A Sepharose for 2 hours at 4°C. Immunoprecipitates were washed once with lysis buffer, twice with high-salt buffer (0.5 M NaCl, 0.02 M Tris, pH 8.0), and once with lysis buffer. Proteins were resolved by 7.5% SDS-PAGE using conditions that were not optimal for RasGRP3 shifting and were visualized by autoradiography.
Phosphatase assays
For in vivo phosphatase inhibition, Ramos cells were pretreated with 1 μM okadaic acid for 60 minutes before stimulation with various concentrations of PMA. After stimulation, cells were lysed in Ras activation buffer and assayed for Ras-GTP levels, total Ras, and RasGRP3. For in vitro phosphatase treatment, cells were either unstimulated or were treated with 100 nM PMA or 10 μg/mL anti-IgM for 10 minutes. After stimulation, cells were lysed with lysis buffer, and the postnuclear supernatants were precleared with protein A Sepharose before the addition of anti-RasGRP3 coupled to protein A Sepharose. Phosphatase reactions were carried out by incubating immunoprecipitates with calf intestinal alkaline phosphatase (New England Biolabs) in a buffer containing 50 mM Tris, pH 8.0, 20 mM KCl, and 10 mM MgCl2 for 15 minutes at 37°C. Phosphatase treatments were performed in the presence and absence of 0.5 mM sodium orthovanadate (Sigma). Samples were resolved by 7.5% SDS-PAGE, transferred to Immobilon-P (Millipore, Nepean, ON, Canada), and analyzed by immunoblotting using an anti-RasGRP3 antibody.
Immune complex kinase assay
Sf9 cells were infected with either PKC-θ– or PKC-β2–expressing baculovirus or they were mock infected for 48 hours. Before harvesting, cells were treated with 100 nM PMA for 15 minutes. Cells were lysed in lysis buffer, and postnuclear supernatants were incubated with or without anti–PKC-θ or anti–PKC-β antibody. After collection on protein A Sepharose beads, immune complexes were washed 6 times with lysis buffer and once with 20 mM Tris-HCl, pH 7.5, 20 mM MgCl2. To each reaction (25 μL packed-bead volume) was added 25 μL reaction mixture containing equal volumes of lipid (0.2 mg/mL diolein, 1 mg/mL phosphatidylserine; Avanti Polar Lipids, Alabaster, AL), 30 mM dithiothreitol (DTT), 12 mM Ca(CH3COO)2, and 1 mg/mL protein substrate. Lipids were prepared by mixing equal volumes of diolein and phosphatidylserine (dissolved in chloroform), dried under N2, and resuspended in 50 mM Tris-HCl, pH 7.5. To each reaction, 1 μL [γ32P]ATP (3000 Ci/mmol [11 100 × 1010 Bq/mmol]; Amersham Biosciences, Baie d'Urfa, QC, Canada) was added before incubation for 15 minutes at 30°C. Reactions were stopped by the addition of an equal volume of 2 × SDS sample buffer. Proteins were resolved by 10% SDS-PAGE and visualized by autoradiography.
Results
RasGRP3 is expressed in B cells but not T cells
Using an antibody that recognizes the predicted 84-kDa RasGRP3 polypeptide in an immunoblotting protocol, we determined the expression pattern of this Ras activator in extracts of various cell lines and mouse tissues (Figure 1). As controls, we examined rat2 cells expressing the full-length human cDNA (R2/RasGRP3) or an empty vector (R2/Puro). We observed obvious expression in human and mouse B-cell lines but not in T cells. As would be expected from the above result, normal mouse spleen expressed RasGRP3 but normal mouse thymus did not. RasGRP1 mice do not express detectable levels of RasGRP1, and their spleens contain few T cells but normal numbers of B cells.12 As expected, these spleens expressed RasGRP3. Finally, Rag1 mice do not develop T or B cells,15 and spleens of these mice had little RasGRP3. Thus, RasGRP3 is expressed in B cells, leading to the suggestion that RasGRP3 may function downstream of the BCR, much as RasGRP1 does downstream of the TCR in T cells. We also probed these lysates with an antibody that recognizes PKC-θ. This protein has been touted as relatively specific for the T-cell lineage among hemopoietic cell types.16,17 As expected, we observed expression in T-cell lines and thymus (Figure 1). However, we also observed expression in Ramos B cells, the B-cell line with the highest expression of RasGRP3.
Expression of RasGRP3 in the B-cell lineage and coexpression with PKC-θ in Ramos B cells. Cellular proteins in postnuclear supernatants (100 μg per sample) were immunoblotted with the anti-RasGRP3 antibody (top panel). The uppermost arrow indicates a nonspecific band. Lysates were also probed with an anti–PKC-θ antibody (bottom panel).
Expression of RasGRP3 in the B-cell lineage and coexpression with PKC-θ in Ramos B cells. Cellular proteins in postnuclear supernatants (100 μg per sample) were immunoblotted with the anti-RasGRP3 antibody (top panel). The uppermost arrow indicates a nonspecific band. Lysates were also probed with an anti–PKC-θ antibody (bottom panel).
RasGRP3 translocates to the particulate fraction and assumes reduced electrophoretic mobility after stimulation of Ramos B cells
To determine whether RasGRP3 is recruited to the plasma membrane in response to DAG signaling, we stimulated Ramos B cells with PMA. Control and treated cell populations were homogenized in hypotonic buffer, fractionated into S100 and P100 fractions by ultracentrifugation, and probed with RasGRP3 antibodies using the immunoblotting protocol. In resting cells, most of the RasGRP3 is present in the soluble fraction. In contrast, after brief stimulation with the DAG analog, RasGRP3 is preferentially recovered in the particulate fraction (Figure 2A). Unexpectedly, we noted an obvious shift in the electrophoretic mobility of RasGRP3 in the stimulated cells, suggesting that the protein was subject to rapid phosphorylation.
BCR-stimulated Ras activation in Ramos B cells is associated with a shift in the electrophoretic mobility of RasGRP3. (A) Homogenates of Ramos B cells from untreated cells or from cells stimulated with 100 nM PMA for 10 minutes were separated into particulate (Pellet) and soluble (Sup) fractions and assayed for RasGRP3 by immunoblotting. (B) Ramos B cells were stimulated with anti-IgM for various times, and aliquots were assayed for RasGRP3, phospho Erk1/2, Ras-GTP, and total Ras. (C) Total splenocytes isolated from C57BL/6J mice were stimulated with PMA, anti-IgM, anti-kappa, or anti-CD40 for 10 minutes and then assayed for RasGRP3 by immunoblotting. — indicates untreated control.
BCR-stimulated Ras activation in Ramos B cells is associated with a shift in the electrophoretic mobility of RasGRP3. (A) Homogenates of Ramos B cells from untreated cells or from cells stimulated with 100 nM PMA for 10 minutes were separated into particulate (Pellet) and soluble (Sup) fractions and assayed for RasGRP3 by immunoblotting. (B) Ramos B cells were stimulated with anti-IgM for various times, and aliquots were assayed for RasGRP3, phospho Erk1/2, Ras-GTP, and total Ras. (C) Total splenocytes isolated from C57BL/6J mice were stimulated with PMA, anti-IgM, anti-kappa, or anti-CD40 for 10 minutes and then assayed for RasGRP3 by immunoblotting. — indicates untreated control.
To examine the RasGRP3 mobility reduction under conditions that might more closely reflect a normal B-cell signaling response, we stimulated Ramos B cells with anti-BCR antibodies. In this case, we performed a time-course experiment to determine the kinetics and reversibility of the phenomenon. We also examined the status of Ras and Erk, molecules that respond to RasGRP3 activity. RasGRP3 was converted to the slower mobility form within 10 minutes of physiological stimulation, and this process was at least partially reversed between 2 and 3 hours (Figure 2B). Strikingly, the level of Ras-GTP and the level of phospho-Erk followed a similar pattern, consistent with the idea that posttranslational modification of RasGRP3 might be related to its functional activity.
To verify that RasGRP3 undergoes stimulus-induced electrophoretic mobility reduction in other B cells, total splenocytes from C57BL/6J mice were studied. As expected, stimulation with either PMA, anti-IgM, anti-kappa, or anti-CD40 IgG led to a reduction in the electrophoretic mobility of RasGRP3 relative to untreated cells (Figure 2C).
RasGRP3 is phosphorylated
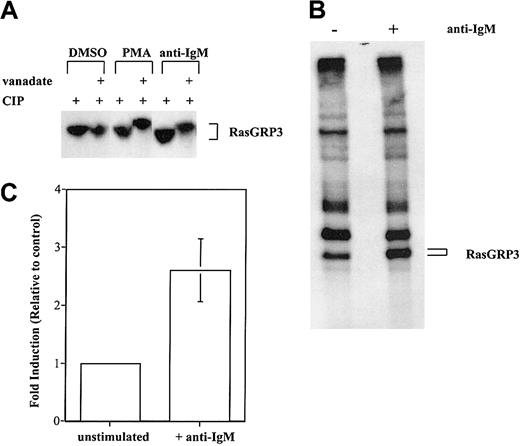
To confirm that phosphorylation is the basis for reduced RasGRP3 electrophoretic mobility, we examined whether the protein could be converted to a faster migrating form by treatment with a phosphatase. RasGRP3 was separately immunoprecipitated from control or stimulated Ramos cells. One half of each immunoprecipitate was then treated with phosphatase. As a control, the other half was incubated with the same phosphatase; additionally, the phosphatase inhibitor sodium orthovanadate was included. Clearly, RasGRP3 from stimulated cells is converted to a faster migrating form by active phosphatase. No such change was observed with RasGRP3 from unstimulated cells (Figure 3A).
Stimulation-induced phosphorylation of RasGRP3. (A) Lysates from unlabeled Ramos B cells were unstimulated or treated with 100 nM PMA or 10 μg/mL anti-IgM. RasGRP3 was immunoprecipitated before incubation with a protein phosphatase in the absence or presence of the phosphatase inhibitor vanadate. The resultant immune complexes were separated by 7.5% SDS-PAGE followed by immunoblotting. (B) Ramos B cells were metabolically labeled for 4 hours with 32Pi and were either unstimulated or stimulated with 10 μg/mL anti-IgM for the last 10 minutes of incubation. Cells were lysed, and RasGRP3 was immunoprecipitated from postnuclear supernatants. Proteins were separated in 7.5% SDS-PAGE and visualized by autoradiography. (C) Densitometric analysis was used to determine the amount of 32Pi incorporated. The average value obtained with stimulated cells is shown normalized to the control value (n = 3; error bar represents standard deviation of the mean).
Stimulation-induced phosphorylation of RasGRP3. (A) Lysates from unlabeled Ramos B cells were unstimulated or treated with 100 nM PMA or 10 μg/mL anti-IgM. RasGRP3 was immunoprecipitated before incubation with a protein phosphatase in the absence or presence of the phosphatase inhibitor vanadate. The resultant immune complexes were separated by 7.5% SDS-PAGE followed by immunoblotting. (B) Ramos B cells were metabolically labeled for 4 hours with 32Pi and were either unstimulated or stimulated with 10 μg/mL anti-IgM for the last 10 minutes of incubation. Cells were lysed, and RasGRP3 was immunoprecipitated from postnuclear supernatants. Proteins were separated in 7.5% SDS-PAGE and visualized by autoradiography. (C) Densitometric analysis was used to determine the amount of 32Pi incorporated. The average value obtained with stimulated cells is shown normalized to the control value (n = 3; error bar represents standard deviation of the mean).
As a more direct test of whether RasGRP3 is phosphorylated in stimulated Ramos B cells, we used an in vivo phosphate-labeling method. After prelabeling Ramos B cells with 32Pi for 4 hours, isotope was consistently found in immunoprecipitated RasGRP3. The amount of radioactivity increased 2.8-fold (n = 3) on average, after stimulation with anti-BCR antibodies for 10 minutes (Figure 3B-C).
PKC inhibitors coordinately influence RasGRP3 phosphorylation and Ras-Erk signaling
To explore the possible functional consequence of RasGRP3 phosphorylation, we investigated the effects of PKC inhibitors on RasGRP3 electrophoretic mobility shifting and on Ras-Erk signaling. Although treatment of Ramos B cells with PMA resulted in strong Ras-Erk signaling and a pronounced RasGRP3 mobility shift, pretreatment with Ro318220 virtually blocked these responses (Figure 4A). Bisindolylmaleimide appeared to be less effective at blocking the mobility shift, and it was also less effective at blocking Ras activation. By contrast, Go6976, which is selective for conventional PKC isozymes, was ineffective at blocking the Ras-Erk signaling and the RasGRP3 mobility shift. When cells were stimulated with anti-BCR antibodies, a radically dissimilar pattern of drug activity was observed (Figure 4B). Pretreatment with Go6976 was effective at blocking RasGRP3 mobility shift and Ras-Erk signaling. By contrast Ro318220 and Bisindolylmaleimide were ineffective. These observations were reproducible over 3 experiments. Thus, despite these differences in the effects of the 3 PKC inhibitors in the 2 stimulation protocols, there was a very good correspondence between the RasGRP3 mobility shift and Ras-Erk signaling. In principle, such an effect might arise from a feedback loop such that Erk or some downstream kinase phosphorylates RasGRP3. To rule out this idea, we showed that the Mek inhibitor PD098059 did not block the stimulus-induced RasGRP3 mobility shift. As expected, this drug blocked the activation of Erk but not that of Ras.
RasGRP3 phosphorylation and Ras-Erk signaling are correlated despite distinct conditions of cell stimulation and PKC inhibition. Ramos B cells were preincubated with various PKC inhibitors for 15 minutes before stimulation with either (A) 100 nM PMA or (B) 10 μg/mL anti-IgM for 10 minutes. Cell lysates were assayed for RasGRP3, Ras-GTP, phospho-Erk1/2, and total Ras. Data are representative of 3 separate experiments. — indicates untreated control.
RasGRP3 phosphorylation and Ras-Erk signaling are correlated despite distinct conditions of cell stimulation and PKC inhibition. Ramos B cells were preincubated with various PKC inhibitors for 15 minutes before stimulation with either (A) 100 nM PMA or (B) 10 μg/mL anti-IgM for 10 minutes. Cell lysates were assayed for RasGRP3, Ras-GTP, phospho-Erk1/2, and total Ras. Data are representative of 3 separate experiments. — indicates untreated control.
Treatment with okadaic acid causes a RasGRP3 mobility shift associated with inhibition of Ras signaling
We reasoned that conditions that inhibit RasGRP3 dephosphorylation might be functionally equivalent to those that increase the rate of RasGRP3 phosphorylation. Accordingly, we treated Ramos cells with okadaic acid at a concentration (1 μM) that inhibits protein phosphatase 2A (PP2A), a major cellular phosphatase involved in dephosphorylating many cellular proteins. As predicted, this treatment caused RasGRP3 to migrate with a dramatically reduced electrophoretic mobility (Figure 5). However, the levels of basal Ras-GTP actually decreased, contrary to our prediction. When cells were pretreated with okadaic acid followed by treatment with PMA, no additional electrophoretic mobility shift was apparent. With increasing dosages of PMA, Ras-GTP levels were higher. However, at each dose of PMA, pretreatment with okadaic acid actually decreased the observed level of PMA-induced Ras activation. We also confirmed the observation that Erk is independently activated in okadaic acid–treated cells18,19 and thus is uncoupled from Ras (data not shown).
Effect of in vivo phosphatase inhibition on RasGRP3 electrophoretic mobility and Ras activation. Control and okadaic acid–pretreated Ramos B cells were stimulated with the indicated concentrations of PMA and analyzed for RasGRP3, Ras-GTP, and total Ras.
Effect of in vivo phosphatase inhibition on RasGRP3 electrophoretic mobility and Ras activation. Control and okadaic acid–pretreated Ramos B cells were stimulated with the indicated concentrations of PMA and analyzed for RasGRP3, Ras-GTP, and total Ras.
RasGRP3 is positively regulated by PKC-θ and directly phosphorylated by PKC-θ and PKC-β2
As an alternative approach to investigating the relationship between RasGRP3 phosphorylation and activity, we asked whether RasGRP3 and candidate forms of PKC might interact in a cotransfection assay. HEK-293 cells, which do not express RasGRP family members, were transfected with plasmids expressing RasGRP3 and with constitutively active versions of PKC-α and -θ, alone and in various combinations (Figure 6A). In HEK-293 cells transiently transfected with RasGRP3, PMA-induced Ras and Erk activation was more pronounced than in mock-transfected cells, in agreement with published results.20,21 We also observed a pronounced RasGRP3 electrophoretic mobility shift. When RasGRP3 was cotransfected with a plasmid encoding PKC-θ, we noted 2 reproducible effects. First, although RasGRP3 mobility was still affected by PMA treatment, a partial electrophoretic mobility shift was also observed even in the absence of PMA stimulation. Second, we observed that the basal Ras-Erk signaling level was elevated. Neither of these effects was seen when the PKC-α cDNA plasmid was cotransfected with RasGRP3.
RasGRP3 is positively regulated by PKC-θ and directly phosphorylated by PKC-θ and PKC-β2. (A) HEK-293 cells were cotransfected with 10 μg RasGRP3 and 10 μg constitutively active mutants of PKC-α (A25E) or PKC-θ (A148E). At 24 hours after transfection, cells were stimulated with either 10 nM PMA or DMSO (vehicle control) for 10 minutes. Cell lysates were assayed for PKC-θ, PKC-α, RasGRP3, Ras-GTP, phospho-Erk1/2, and total Ras. (B) Sf9 cells were mock infected or infected with PKC-θ or PKC-β2 expressing baculovirus. Lysates were either immunoprecipitated with anti–PKC-θ, anti–PKC-β, or primary antibody was omitted, as a control. An immunocomplex kinase assay was conducted using either a 43-kDa MBP or a 127-kDa MBP-RasGRP3 fusion protein followed by resolution by SDS-PAGE and autoradiography. The positions of MBP-RasGRP3, autophosphorylated PKC-θ, PKC-β2, and MBP are shown. Autoradiographic exposure was for 20 minutes.
RasGRP3 is positively regulated by PKC-θ and directly phosphorylated by PKC-θ and PKC-β2. (A) HEK-293 cells were cotransfected with 10 μg RasGRP3 and 10 μg constitutively active mutants of PKC-α (A25E) or PKC-θ (A148E). At 24 hours after transfection, cells were stimulated with either 10 nM PMA or DMSO (vehicle control) for 10 minutes. Cell lysates were assayed for PKC-θ, PKC-α, RasGRP3, Ras-GTP, phospho-Erk1/2, and total Ras. (B) Sf9 cells were mock infected or infected with PKC-θ or PKC-β2 expressing baculovirus. Lysates were either immunoprecipitated with anti–PKC-θ, anti–PKC-β, or primary antibody was omitted, as a control. An immunocomplex kinase assay was conducted using either a 43-kDa MBP or a 127-kDa MBP-RasGRP3 fusion protein followed by resolution by SDS-PAGE and autoradiography. The positions of MBP-RasGRP3, autophosphorylated PKC-θ, PKC-β2, and MBP are shown. Autoradiographic exposure was for 20 minutes.
To confirm that PKC-θ could directly phosphorylate RasGRP3, we used immune complex kinase assays. These experiments used kinase expressed in Sf9 cells infected with a PKC-θ–encoding baculovirus and RasGRP3 expressed in Escherichia coli fused to maltose-binding protein (MBP-RasGRP3). Immunoprecipitated PKC-θ phosphorylated MBP-RasGRP3 but not parental MBP (Figure 6B). The present study and other ongoing studies have confirmed that RasGRP3 can serve as an in vitro substrate of PKC-θ. We also showed that an additional form of PKC, PKC-β2, could phosphorylate RasGRP3 (Figure 6B).
Discussion
The present study demonstrates that RasGRP3 is expressed in B cells but not in T cells, leading to the hypothesis that this RasGRP family member may function downstream of the BCR. By RNA expression analysis, RasGRP3 was also expressed in brain and kidney.20 RasGRP1, RasGRP2, and RasGRP3 are all expressed in brain, kidney, and various blood lineages,10 though the extent to which they are expressed within the same cells has not been explored in detail.22,23 The more recently described RasGRP4 is expressed in mast cells and the myeloid lineage, but expression outside the blood system has not been reported.24,25 Our observation that PKC-θ expression partially overlaps RasGRP3 is not entirely unexpected given that other investigators have reported that this kinase may be expressed in some B cells.26,27 Our data also suggest that the conventional isoform PKC-β2 may be involved in regulating RasGRP3 activity in B cells. This idea may provide insight into the phenotype of the PKCβ–/– mouse, which exhibits a defect in B-cell development.28
We have provided preliminary evidence that RasGRP3 associates with the plasma membrane in stimulated B cells, as does RasGRP1 in T cells. Previously, it was shown that a green fluorescent protein-tagged version of RasGRP3 exhibited membrane recruitment in HEK-293 cells in response to DAG analog treatment.21 Furthermore, the C1 domain of RasGRP1 and RasGRP3 have been shown to bind DAG analogs in vitro,10,21,29 an important point that has not been verified with RasGRP2 and RasGRP4. In situ imaging methods will be needed to demonstrate convincingly the physiological recruitment of RasGRP3 to the plasma membrane in B cells.
An unexpected observation, and the focus of this report, is the reduced electrophoretic mobility displayed by RasGRP3 extracted from stimulated B cells. Several lines of evidence support the conclusion that reversible protein phosphorylation underlies this mobility shift. RasGRP3 mobility shift is observed under conditions known to activate kinases and conditions that inhibit phosphatases. Coexpression of RasGRP3 and at least one form of PKC, PKC-θ A148E, results in a partial electrophoretic mobility shift. This form of PKC, as well as PKC-β2, was also able to phosphorylate RasGRP3 in vitro. The low-mobility form of RasGRP3 extracted from activated cells is converted to a high-mobility form by phosphatase treatment. Finally, direct analysis of RasGRP3 from phosphate-labeled Ramos B cells confirms that RasGRP3 is subject to stimulus-induced phosphorylation. Several points about the phosphorylation deserve note. First, because nearly all the RasGRP3 molecules assume a slow migrating form after full stimulation, the reaction must occur at high stoichiometry. Second, under conditions in which stimulation is submaximal, including conditions that involve partial inhibition by PKC inhibitors, the RasGRP3 populations migrate uniformly with an intermediate electrophoretic mobility. These observations, and the magnitude of the full shift, argue that RasGRP3 is phosphorylated on multiple sites, a point confirmed by our ongoing in vitro analysis. Because RasGRP3 from stimulated cells does not react with antiphosphotyrosine antibodies, it likely contains phosphothreonine or phosphoserine, further supporting its direct activation by PKC.
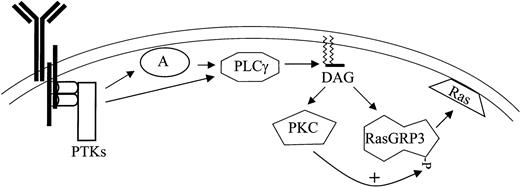
The phosphorylation of RasGRP3 occurs under conditions expected to generate a DAG signal, and it closely parallels Ras-Erk signaling in several different settings. The time course of reversible phosphorylation mirrors the rise and fall in Ras-GTP and phospho-Erk in Ramos B cells stimulated with anti-BCR antibodies. PKC inhibitors that block RasGRP3 phosphorylation also block Ras-Erk signaling, whether Ramos B cells are stimulated with a DAG analog or with anti-BCR antibodies. Importantly, our experiments with the Mek inhibitor PD098059 have shown that RasGRP3 phosphorylation is not solely a result of feedback phosphorylation by Erk or downstream kinases, in contrast to the situation with other Ras activators.30 We also observed that in splenocytes from C57BL/6J mice, PMA, BCR, and CD40 stimulation all induced a shift in RasGRP3 mobility (Figure 2C), indicating that this response is not restricted to transformed B-cell lines. Finally, cotransfection of an activated version of PKC-θ with RasGRP3 consistently leads to a partial mobility shift and a modest level of PMA-independent RasGRP3-Ras-Erk signaling. These results, along with our demonstration that RasGRP3 can serve as a substrate of PKC-θ and PKC-β2 in vitro, led us to propose a provisional hypothesis of B-cell signaling (Figure 7). This model incorporates the conventional models of BCR signaling,31,32 our new view of immune receptor signaling derived from T cells,12,33 and the data presented here. According to our proposal, BCR stimulation leads to the activation of protein tyrosine kinases, including members of the src, syk, and tec families. Tyrosine phosphorylation of adaptors such as BLNK and of PLC-γ2 leads to recruitment of the latter enzyme to the plasma membrane, where PLC-γ2 can generate a DAG signal. DAG signals directly activate RasGRP3 and various forms of PKC through their C1 domains; additionally, those PKC forms can positively regulate RasGRP3 (Figure 7).
Proposed model for BCR signaling to Ras based on convergent diacylyglycerol signaling through PKC and RasGRP3. BCR ligation activates protein tyrosine kinases such as Btk, Lyn, and Syk (PTK), leading to the phosphorylation of adaptor proteins such as BLNK (A). Phospholipase C-γ2 (PLC) is also tyrosine phosphorylated and is recruited to the membrane by association with adaptors, where it can stimulate hydrolysis of PIP2 (not shown) releasing DAG. DAG messenger recruits and activates RasGRP3 and some forms of PKC; this is followed by regulatory phosphorylation of the former by the latter.
Proposed model for BCR signaling to Ras based on convergent diacylyglycerol signaling through PKC and RasGRP3. BCR ligation activates protein tyrosine kinases such as Btk, Lyn, and Syk (PTK), leading to the phosphorylation of adaptor proteins such as BLNK (A). Phospholipase C-γ2 (PLC) is also tyrosine phosphorylated and is recruited to the membrane by association with adaptors, where it can stimulate hydrolysis of PIP2 (not shown) releasing DAG. DAG messenger recruits and activates RasGRP3 and some forms of PKC; this is followed by regulatory phosphorylation of the former by the latter.
This hypothesis will likely have to be revised to accommodate several observations. First, the result obtained with okadaic acid, while confirming that RasGRP3 phosphorylation can cause a mobility shift, actually points to a negative role of protein phosphorylation in Ras activation. Possibly the okadaic acid allows a different class of kinase to phosphorylate RasGRP3, or possibly Ras signaling is influenced in some other fashion unrelated to RasGRP3 activity. Another problem concerns the normal sources of RasGRP3 phosphorylation in cells other than Ramos B cells. Because PKC-θ is preferentially expressed in T cells, whereas RasGRP3 is found in most B cell lines, we consider it likely that additional DAG-activated forms of PKC, for example PKC-β2, perform the role proposed in Figure 7. Conversely, we are also pursuing the idea that PKC-θ normally phosphorylates RasGRP1 in thymocytes and T cells. Another aspect of the data that is unexplained concerns the radically different behavior of the PKC inhibitors in the 2 different stimulation protocols. It is particularly puzzling that the relatively specific Go6976, which is expected to inhibit the conventional forms but not the novel forms of PKC, inhibits more than the pan-specific inhibitors when anti-BCR antibodies are used as the stimulus. It should be kept in mind that in this stimulation protocol, the inhibitors might affect the interaction between PKC and other cellular enzymes that function downstream of BCR. Nonetheless, complex models involving multiple kinases, multiple phosphorylation sites, and positive and negative regulation of RasGRP3 might ultimately be warranted. Finally, the underlying biochemical mechanism(s) whereby RasGRP3 phosphorylation could influence its activity must be uncovered.
In spite of these uncertainties, the discovery of stimulus-induced RasGRP3 phosphorylation and the derived preliminary model for RasGRP3 regulation are interesting and potentially important in a number of respects. The initial linear view that DAG and phorbol ester signalings exclusively involve PKC has been gradually replaced by the concept of divergent DAG signaling. First, it was recognized that multiple forms of PKC responded to DAG, followed by the discoveries of non-PKC DAG-responsive systems. Multiple DAG-binding proteins might independently relay membrane DAG messenger signals into distinct and parallel signaling systems. The present work, however, highlights the likelihood that these pathways can be highly interdependent, in what might be described as convergent DAG signaling. Precedents for this idea have been presented in the case of PKC phosphorylation of PKD.34
Quantitatively appropriate signaling through BCR and TCR are required to ensure the development of a functional immune system and to mount a proper immune response. DAG is a critically important messenger downstream of the BCR and the TCR. We have argued that DAG analogs such as bryostatin-1, which is currently being tested in clinical trials as an anticancer agent, might be used to manipulate RasGRP function.11,13 Other DAG analogs have been extensively studied in vivo for their tumor-promoter properties and as a means of artificially controlling PKC activity in signaling experiments in vitro. Clearly, it is critically important in all these areas to identify all the DAG-responsive elements within the cell and to begin to study their possible functional interrelations. Finally, our model is formally similar to that proposed for the regulation of protein kinase B (PKB). Membrane-bound phosphoinositides, including phosphatidylinositol (PI), PI4P, and PI4,5P2, are phosphorylated by phosphatidylinositol 3-kinases (PI3Ks) to produce 3′ phosphorylated derivatives. These lipid products recruit PKB through its pleckstrin homology domain.35 However, phosphoinositide-dependent kinases also have pleckstrin homology domains and are similarly recruited to the membrane where they can phosphorylate PKB for its full activation.36,37 How these complicated, nonlinear, lipid-signaling systems contribute to cellular function remains to be determined.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2002-11-3621.
Supported by grants from the Alberta Cancer Board (J.C.S.), the National Cancer Institute of Canada (J.C.S.), and the Canadian Institutes of Health Research (J.C.S.). J.C.S. is an Alberta Heritage Foundation for Medical Research Senior Scientist. C.T. is a postdoctoral fellow supported by the Canadian Institutes of Health Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank G. Baier for PKC plasmids and useful discussions and C. Holmes for okadaic acid. We also thank A. Shaw and C. Bleackley for their generous gifts of cell lines used in this study and H. Ostergaard and P. Blumberg for their helpful discussions.