Myeloid metaplasia with myelofibrosis (MMM) is a rare chronic myeloproliferative disorder characterized by myelofibrosis, extramedullary hematopoiesis, and absence of BCR-ABL rearrangement.1,2
Myeloproliferation is considered clonal and fibrosis, reactive.2 Hierarchic level, primary mechanism, and/or gene alteration responsible for the malignant clone remain unknown. Here, we analyzed the clonality of CD34+ cells (CD34+), and questioned the hierarchic level of the disease and the origin of karyotypically normal CD34+.
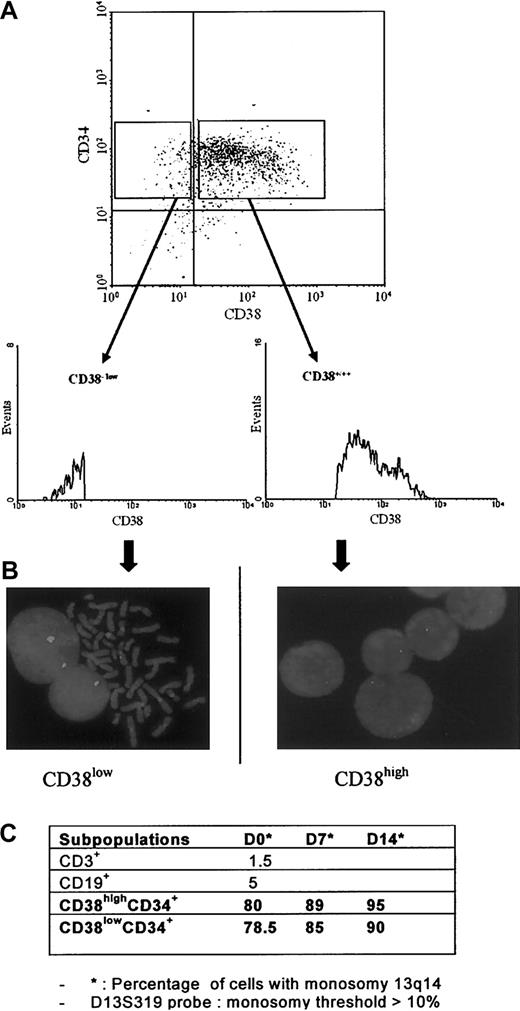
Karyotypes were performed on white blood cells (WBCs) and on immunomagnetically selected circulating CD34+ (purity, ≥ 97%) from 23 patients as described.3-5 According to previous reports,1,6 34.8% (8/23) of patients exhibited a high proportion of cytogenetic abnormal WBCs (nearly 100%). CD34+ carried the same cytogenetic aberrations as WBCs in patients 13, 17, 19, and 33 (Table 1), but CD34+ abnormal cell percentages were heterogeneous: 100% abnormal metaphases in patients 13, 17, and 19; 33% in patient 33; and 0% in patient 57.
This mosaicism could be due to normal residual CD34+ whose proliferation is inhibited by unknown mechanism(s).4 Normal WBC and CD34+ karyotypes in the other 14 patients strengthen the alternative hypothesis that the primitive genetic lesion remains cryptic and that karyotypic alterations occur secondarily. Mosaicism present in several CD34+ karyotypes and absent in WBCs also raises the question of the hierarchic level of the clonal event in MMM. The 6-fold higher proportion of CD34highCD38low cells in MMM than in normal blood (25% vs 4%) suggested that the clonal myeloproliferation derives from primitive hematopoietic progenitors.3 Therefore, cytogenetic and fluorescence in situ hybridization (FISH) studies were performed on CD34+CD38low, CD34+CD38high, CD3+, and CD19+ sorted cells (99% pure) in patient 19. FISH confirmed the reciprocal translocation with monoallelic 13q14 deletion (13q14–) (lsi D13S319 probe; Vysis, Downers Grove, IL).
Interestingly, in patient 19, 13q14–(FISH) was detected only in about 80% of freshly CD34+CD38low- and CD34+CD38high-sorted subpopulations (Figure 1A-B). This percentage increased after 7-day and 14-day cultures in the progeny of CD34+CD38low and CD38highCD34+, suggesting a growth advantage of 13q14–cells. CD3+ (T lymphocytes) and CD19+ (B lymphocytes) compartments did not show the genetic marker (Figure 1C). These results contrast with those of Reeder et al8 showing a heterogeneous clonal involvement (13q–,20q–) of immunomagnetically selected T and B lymphocytes from 4 patients. Spleen fibroblast karyotypes performed in patients 19 and 33 were normal, confirming that, in MMM, fibroblasts are polyclonal reactive cells.
Patient 19: CD34+CD38high and CD34+CD38low subpopulation analyses. (A) Flow cytometry profiles; (B) FISH (lsi D13S319 Vysis probe); and (C) 13q14 deletion distribution.
Patient 19: CD34+CD38high and CD34+CD38low subpopulation analyses. (A) Flow cytometry profiles; (B) FISH (lsi D13S319 Vysis probe); and (C) 13q14 deletion distribution.
In conclusion, we demonstrate that circulating primitive CD34+CD38low are clonal in MMM, at least in some patients. The absence of a cytogenetic marker in a variable percentage of CD34+, T and B cells could reflect residual normal hematopoiesis. Alternatively, karyotypically normal cells could derive from primitive progenitors/stem cells whose gene alteration(s) is cryptic.
Investigating these not mutually exclusive hypotheses would allow a better understanding of MMM pathogenesis and would improve its therapy.
C.B.-N. and C.B. contributed equally to this work.
Members of the French INSERM Network on Myeloid Metaplasia with Myelofibrosis: Dr Jean-Loup Demory, St Vincent Hospital, Lille, France; Dr Brigitte Dupriez, Dr Schaffner Hospital, Lens, France; Dr Dominique Bordessoule, Dupuytren Hospital, Limoges, France; Dr Jean Brière, Beaujon Hospital, Clichy, France; Dr Jean-François Emile, Paul Brousse Hospital, Villejuif, France; and Dr Jean-François Viallard, Victor Segalen University, Bordeaux, France.