Abstract
Sphingosine 1-phosphate (S1P), a bioactive lipid, is produced and stored in platelets and is released from activated platelets during blood coagulation activation. Thrombin, which is also generated during blood coagulation, has been shown to induce tissue factor (TF), the initiator of blood coagulation, in endothelial cells (ECs); however, the effect of S1P on this process is not evaluated. Here we demonstrated that S1P strongly potentiated thrombin-induced TF expression in ECs and that S1P itself did not induce TF expression. Among signaling lipids, platelet-activating factor slightly enhanced thrombin-induced TF expression; other lipids, including lysophosphatidic acid, lysophosphatidylcholine, sphingosine, and C2-ceramide exert no effect on TF expression. S1P enhanced TF expression at the transcriptional level, possibly via promoting the activation of transcription factors nuclear factor–κB (NF-κB) and Egr-1. Thrombin weakly and S1P strongly activated extracellular signal–regulated kinase 1/2 (ERK1/2) mitogen-activated protein (MAP) kinase and, in the presence of both stimulants, enhanced and sustained activation of this kinase was observed. The ERK1/2-specific inhibitor PD98059 significantly inhibited enhanced TF expression induced by both stimulants but only weakly inhibited thrombin-induced TF expression, thus indicating the requirement of the ERK1/2 pathway in synergistic induction of TF expression. In addition, we found that thrombin and S1P rapidly up-regulated the expression of S1P receptors, endothelial differentiation gene-1 (EDG-1) and EDG-3, thereby suggesting that the effect of S1P on TF expression and other EC functions may be enhanced by thrombin and S1P itself. The present data reveal the synergistic effect of S1P on thrombin-induced TF expression in ECs, which may promote further thrombin and S1P generation, thus propagating a positive feedback reaction.
Introduction
Sphingosine 1-phosphate (S1P) is a naturally occurring, water-soluble lysophospholipid that exhibits strong hormone- and growth factor–like activities.1 S1P is produced by metabolism of the membrane phospholipid, sphingomyelin. Degradation of sphingomyelin by sphingomyelinase followed by sequential catalysis by ceramidase and sphingosine kinase results in the formation of S1P. S1P is abundantly stored in blood platelets, probably due to the presence of high activity of sphingosine kinase and to the lack of S1P lyase.2 Platelets release S1P extracellularly upon activation3 ; therefore, S1P, which is released during blood coagulation activation at sites of vascular injury, may stimulate endothelial cells (ECs) and thus play a role in the mechanisms of thrombosis, hemostasis, angiogenesis, and atherosclerosis. Recent studies have shown that S1P activates ECs by interacting with G protein–coupled receptors of the endothelial differentiation gene (EDG) family and induces proliferation, survival, and migration.4-10 So far, 8 EDG receptors have been cloned. EDG-1, -3, -5, and -8 show high sequence homology to each other and are considered to be the S1P receptors.11-14 A second cluster includes EDG-2, -4, and -7, which have been shown to act as receptors for a structurally related bioactive lipid, lysophosphatidic acid (LPA).15-17 EDG-6, which exhibits intermediate homology between the 2 clusters, has moderate to high affinity for S1P.18,19
Tissue factor (TF) functions as a high-affinity receptor for factor VIIa, with which it forms a complex that initiates the blood coagulation cascade by activating factors IX and X. Consistent with a protective role of TF in the hemostatic response, it is constitutively expressed in several extravascular cell types surrounding blood vessels and at boundaries of organs. Recent studies on transgenic and TF knock-out mice have demonstrated that TF, besides its important function in the clotting system, also plays critical roles in embryogenesis, angiogenesis, metastasis, and atherogenesis.20-25 In all of these processes, TF produced by vascular ECs plays a fundamental role. TF is not expressed in ECs but is rapidly induced in response to inflammatory stimuli including tumor necrosis factor-α (TNF-α), interleukin-1, lipopolysaccharide, and thrombin.
Thrombin, a multifunctional serine protease, is generated during activation of the blood coagulation cascade. Thrombin mediates the formation of fibrin from fibrinogen and strongly activates platelets via proteinase-activated receptors (PARs) that are also G protein–coupled receptors. The main receptor of thrombin in human platelets is PAR-1 and in mouse platelets PAR-4.26,27 The effect of thrombin on ECs is critical for the normal clotting and wound healing process.28,29 Thrombin stimulates ECs and promotes the release of von Willebrand factor, cell surface redistribution of P-selectin,30,31 and elevated expression of cytokines, growth factors, adhesion molecules, and TF.32-38 These actions facilitate the adherence of leukocytes and platelets to ECs at the sites of vessel damage and further promote the completion of the coagulation process. PAR-1 has been reported to be the predominant thrombin receptor expressed in human umbilical vein ECs (HUVECs) and to serve as a major thrombin receptor required for EC responses to thrombin.39 Receptor activation occurs following proteolytic cleavage of the N-terminus of PAR-1 by thrombin. A new N-terminus is exposed on the receptor that serves as a tethered peptide ligand, which in turn induces signaling across the cell membrane.
Thrombin is known to be a potent stimulator of TF expression in ECs.32-38 Thus, it is possible that thrombin participates in a positive feedback loop; thrombin stimulates ECs to express TF, which in turn via activating the coagulation system further increases thrombin generation. S1P is also known to be generated during activation of blood coagulation and to modulate EC function near sites of thrombin production.3-10 However, the effect of S1P on TF expression is unknown. In the present study we have investigated the role of S1P signaling in the regulation of TF expression in ECs.
Materials and methods
Materials
Blood coagulation factor VIIa, factor X, and prothrombin were kindly provided by Dr Tomohiro Nakagaki of the Chemo-Sero-Therapeutic Research Institute (Kumamoto, Japan). Thrombin was prepared from prothrombin and purified as described.37 All purified proteins were homogeneous with more than 95% purity, as judged by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis followed by silver staining; all of them were stored in small aliquots at –80°C until use. Recombinant human TNF-α was donated by the Asahi Chemical Industry (Fuji, Shizuoka, Japan). S1P, LPA, platelet-activating factor (PAF C16), and C2-ceramide were purchased from Biomol (Plymouth Meeting, PA); S1P from Cayman Chemical (Ann Arbor, MI) was also used in the assays. Lysophosphatidylcholine (LPC), sphingosine, Dulbecco phosphate-buffered saline (PBS), and heparin (174 U/mg) were from Sigma Chemical (St Louis, MO). The synthetic peptide substrate of factor Xa, N-benzoyl-L-isoleucyl-L-glutamyl-glycyl-L-arginine-p-nitroanilide hydrochloride (S2222), was purchased from Chromogenix (Mölndal, Sweden). Thrombin receptor agonist peptide consisting of S-F-L-L-R-N-P-N-D-K-Y-E-P-F (single-letter amino acid abbreviation) was from Kurabo Industry (Osaka, Japan). The MEK inhibitor PD98059 was obtained from Calbiochem (Carlsbad, CA). All other reagents were of highest grade and were purchased from Wako Pure Chemical (Osaka, Japan).
Cell culture
HUVECs were purchased from Sanko Junyaku (Tokyo, Japan) and routinely grown in EC culture medium CHL-MCDB 131 (Chlorella Industry, Tokyo, Japan) supplemented with 2% fetal bovine serum (Life Technologies, Grand Island, NY), 50 μg/mL gentamicin (Sigma), 0.01 μg/mL epithelial growth factor (Becton Dickinson, San Jose, CA), 10 μg/mL endothelial cell growth factor (Becton Dickinson), and 10 μg/mL heparin under an atmosphere consisting of 95% air and 5% CO2 using 100-mm tissue-culture dishes coated with collagen (Becton Dickinson). Cells were used between passage numbers 5 and 8.
Preparation of peripheral blood mononuclear cells (PBMCs)
Blood was obtained from healthy donors by vein puncture using EDTA (ethylenediaminetetraacetic acid) as anticoagulant. PBMCs were isolated by Histopaque-1077 (Sigma), according to the manufacturer's instructions. The PBMC phase comprising monocytes and lymphocytes was harvested, washed twice with PBS, and resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 μg/mL streptomycin, and 100 μg/mL penicillin. This suspension contained 20% monocytes, as determined by flow cytometric analysis employing a fluorescein isothiocyanate (FITC)–labeled murine monoclonal antibody to human CD14 (Beckman Coulter, Fullerton, CA). These studies were approved by the institutional review board of the University of Occupational and Environmental Health, Kitakyushu, Japan. Informed consent was provided according to the Declaration of Helsinki.
Measurement of TF activity on the EC surface
TF activity was measured as factor Xa generation induced by TF–factor VIIa complex on confluent ECs in collagen-coated 24-well plates (2 × 105 cells per well). ECs were cultured in human endothelial serum-free medium (HESFM; Life Technologies) for 3 hours before stimulation. After culturing ECs in the presence and absence of lipids (S1P, LPA, LPC, PAF, sphingosine, or C2-ceramide) for 10 minutes, they were incubated in the presence and absence of thrombin for various times up to 24 hours. To assess the effect of transient exposure to the stimulants, ECs were stimulated with thrombin, S1P, or thrombin plus S1P for 30 minutes, washed with PBS, and then incubated in HESFM for the indicated times. At the end of the incubation time, ECs were washed twice with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered saline (50 mM HEPES [pH 7.5], 150 mM NaCl, 5 mM CaCl2) and then incubated at room temperature for 30 minutes in a reaction mixture (250 μL) containing factor VIIa (3.76 nM) and factor X (980 nM). After stopping the reaction by adding 10 μL of 100 mM EDTA, the chromogenic substrate S2222 was added and incubated for 20 minutes at room temperature. The reaction was stopped with 10 μL of 30% acetic acid, and color development was determined by measuring the absorbance at 405 nm with a microplate reader (Bio-Rad model 550, Hercules, CA). The increase in absorbance in this assay was converted to TF activity (milliunits per well) using a commercial thromboplastin preparation (Thromborel S, Dade Behring Marburg, Marburg, Germany) in which 1 μg thromboplastin was equivalent to 100 mU TF activity.
Determination of procoagulant activity (PCA)
PCA of ECs and PBMCs was determined by one-stage clotting assay.40 Monolayers of ECs were incubated with or without thrombin (4.8 nM) and S1P (4 μM) for 5 hours and harvested by treating with PBS containing 10 mM EDTA for 10 minutes at 37°C. PBMCs suspended in RPMI medium were incubated with TNF-α (1000 U/mL) for 5 hours. After these ECs and PBMCs were washed and resuspended in PBS, 50 μL cell suspension was mixed with 50 μL of 25 mM CaCl2. Clotting was initiated by the addition of 50 μL normal human plasma, and the clotting time at 37°C was recorded. The PCA was extrapolated from a standard curve drawn using Thromborel S, as described.40 PCA of ECs and PBMCs induced by the stimulants was confirmed to depend on TF using polyclonal goat anti-TF antibody (American Diagnostica, Greenwich, CT).
Flow cytometric analysis of EC surface TF antigen
Monolayers of ECs were incubated with or without thrombin and S1P for 5 hours and harvested by treating with Dulbecco PBS containing 10 mM EDTA for 10 minutes at 37°C. The cells were then washed with cold Dulbecco PBS and resuspended (2 × 106 cells) in 100 μL PBS. The cells were incubated with mouse monoclonal antihuman TF immunoglobulin G (IgG) (5 μg/mL)37 for 30 minutes at 4°C and subsequently washed 3 times with PBS. The cells were then incubated with the F(ab′)2 fraction of an FITC-labeled antimouse IgG goat antibody (1:20 dilution) for 30 minutes at 4°C. The cells were then washed 3 times with PBS and fixed in fluorescence-activated cell sorter (FACS) lysing solution (Becton Dickinson) according to the manufacturer's instructions. Flow cytometric analysis was performed using a FACScan analyzer (Becton Dickinson).
Analysis of mRNA in ECs by reverse transcription–polymerase chain reaction (RT-PCR) and Northern blotting
All solutions used for RNA isolation were treated with 0.1% diethyl pyrocarbonate and sterilized or prepared with diethyl pyrocarbonate–treated water. Total RNA was prepared from treated and control ECs by the guanidine isothiocyanate procedure using TRIzol reagent (Life Technologies), and the mRNA was isolated with Oligotex-dT30-Super (Takara Bio, Shiga, Japan), according to the manufacturer's instructions. The isolated mRNA was reverse transcribed using SuperScript Preamplification System (Life Technologies). Reverse-transcribed complementary DNA (cDNA) was amplified in a PC-800 thermal cycler (ASTEC, Fukuoka, Japan) using recombinant Taq DNA polymerase (Roche, Indianapolis, IN). PCR was carried out with 33 cycles of 30 seconds of denaturation at 94°C, 30 seconds of hybridization at 62°C, and 2.5 minutes of elongation at 72°C. An aliquot of each sample was loaded onto a 1.2% agarose gel. DNA bands were visualized with an ultraviolet transilluminator after ethidium bromide staining. To evaluate EDG-1 and EDG-3 expression, we performed quantitative RT-PCR using ABI PRISM 7700 (Perkin Elmer Applied Biosystems, Foster City, CA) with the following reaction conditions: 40 cycles of a 2-step PCR (95°C for 15 seconds and 60°C for 1 minutes) after initial denaturation (95°C for 10 minutes) with 0.1 μg cDNA and 1 × SYBR Green PCR Master Mix. Serial dilution of cDNA obtained from the cells treated with the combination of thrombin and S1P was used as a standard. Products were visualized by electrophoretic separation on 2% agarose gels and ethidium bromide staining. The sequence of oligonucleotide primer pairs used (Amersham Biosciences, Tokyo, Japan) were as follows: 5′-GATGAAGATGGTTTGGAGGTGTAA-3′ (sense) and 5′-AAACGGATCCCGCTTACATG-3′ (antisense) for EDG-1; 5′-ATGGCTGCCATCTCTACTTCCAT-3′ (sense) and 5′-CCACTACCCGCCGGTTGCTCA-3′ (antisense) for EDG-2; 5′-GCAGCCACTCTCCGAAGGT-3′ (sense) and 5′-GCGTTCTTGTCCATGATGCA-3′ (antisense) for EDG-3; 5′-CTGGCGGCCCAAGGATGTGGT-3′ (sense) and 5′-CCAGACGGCCAAATAGGAGCG-3′ (antisense) for EDG-4; 5′-AGTACCTGAACCCCAACAAGG-3′ (sense) and 5′-GTCACCACGCACAGCACATAA-3′ (antisense) for EDG-5; 5′-CTTCTCCACAATGAATGAGTGTC-3′ (sense) and 5′-CAGGCAGAGATGTTGCAGAG-3′ (antisense) for EDG-7; and 5′-TTGCCAGGAGCGATGAACGCAAG-3′ (sense) and 5′-GCTGTCATGTCCGAAAGCCCTG-3′ (antisense) for Egr-1.
As control, the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was also amplified by PCR. The sequences of the primers used for GAPDH amplification were 5′-CCACCCATGGCAAATTCCATGGCA-3′ (sense) and 5′-TCTAGACGGCAGGTCAGGTCCACC-3′ (antisense).
Northern blotting was carried out according to standard protocols. Briefly, denatured total RNA (10 μg) was separated on agarose/formaldehyde gels and transferred to Hybond N+ nylon membrane (Amersham Pharmacia Biotech, Freiburg, Germany). Filters were UV cross-linked and subsequently hybridized with a human TF complementary DNA probe labeled with [α32P]deoxycytidine triphosphate ([α32P]dCTP, 3000 Ci/mmol [111 000 GBq/mmol]) by means of a random-primed DNA-labeling kit BcaBest (Takara). The membrane was exposed to imaging plates and analyzed using a BAS-2000 Image Analyzer (Fuji Photo Film, Tokyo, Japan).
Electrophoretic mobility shift assay (EMSA)
Preparation of nuclear extracts and analysis of nuclear protein binding to radiolabeled oligonucleotides were carried out as previously described38 with minor modifications. ECs (5 × 105 cells) were washed twice with cold PBS and scraped with a rubber policeman in 0.4 mL cold lysis buffer containing 10 mM HEPES (pH 7.5), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA (ethylene glycol tetraacetic acid), 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 0.1 mg/mL aprotinin, 2 μg/mL leupeptin, and 0.5 mg/mL benzamidine. After 5 minutes of incubation on ice, the nuclei were collected by centrifugation at 15 000g for 1 minute. The nuclear pellet was resuspended in high-salt extraction buffer containing 0.5 M KCl and 20% glycerol, and the nuclear extract was obtained by pelleting for 30 minutes at 105 000g in an Optima TL ultracentrifuge (Beckman Coulter). Protein concentration in the extracts was determined by the Bradford method using a protein assay kit (Bio-Rad). After incubation with 3 μg poly(dIdC) (Amersham Biosciences), a nonspecific competitor DNA, for 20 minutes on ice, 15 μg of crude extracts was incubated with double-stranded oligonucleotide probes (Amersham Biosciences); these were 5′ end-labeled with [γ32P]adenosine triphosphate ([γ32P]ATP) using a DNA labeling kit MegaLabel (Takara). The sequence of the probe containing prototypic nuclear factor–κB (NF-κB) binding site was 5′-CAGAGGGACTTTCCGAGA-3′ and that of the Egr-1–specific probe was 5′-GAGCGGCGGGGGCGGGCGCCGG-3′. Reactions were performed in 20 μL binding buffer (10 mM HEPES [pH 7.5], 100 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, and 4% glycerol) containing 100 000 cpm of the 32P-labeled probe. After 30 minutes of incubation at room temperature, samples were separated on a 4% nondenaturating polyacrylamide gel run in 0.5 × Tris-borate-EDTA (TBE) buffer. After electrophoresis, the gel was dried and subjected to image analysis using the BAS-2000 Image Analyzer.
Western blotting analysis
For analysis of extracellular signal–regulated kinase 1/2 (ERK1/2) activation, ECs were treated with thrombin and/or S1P for 5, 10, and 30 minutes. The cells were washed with ice-cold PBS and lysed immediately in cold lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane] [pH 7.4], 4 mM EDTA, 2 mM EGTA, 1 mM PMSF, 100 μg/mL aprotinin, 200 μg/mL leupeptin, 50 mM NaF, 5 mM Na4P2O7, 1 mM Na3VO4, and 1% Nonidet P-40). For analysis of EDG-1 and -3 protein expression, ECs were treated with thrombin and/or S1P for 3 hours and the membrane fraction was obtained as follows. The cells were washed with ice-cold PBS before being scraped and pelleted in PBS at 1000 rpm. The cells were resuspended in a small volume of hypotonic buffer (20 mM Tris [pH 7.4], 4 mM EDTA, 2 mM EGTA, 1 mM PMSF, 100 μg/mL aprotinin, 200 μg/mL leupeptin, 0.5 mM dithiothreitol [DTT]) and homogenized 20 times using a Wheaton tissue grinder. The nuclear fraction was pelleted by centrifugation at 15 000 rpm for 10 seconds. The supernatant was centrifuged at 160 000g to pellet the membrane fraction. Proteins from the membrane fraction were extracted using Nonidet P-40 lysis buffer.
Extracts (60 μg per lane) were suspended with an equal volume of 2 × loading buffer (0.1 M Tris-HCl [pH 6.8], 4% SDS, 0.005% bromophenol blue, 20% glycerol, and 0.7 M β-mercaptoethanol) and subjected to 7.5% to 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Gels were then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA), which were immersed overnight in Tris-buffered saline/Tween 20 containing 5% nonfat dry skim milk. Activated ERK1/2, EDG-1, and EDG-3 were detected using a rabbit polyclonal antibody against phosphorylated ERK1/2 (New England Biolabs, Beverly, MA), a goat polyclonal antibody against human EDG-1 (Santa Cruz Biotechnology, Santa Cruz, CA), and a mouse monoclonal antibody against human EDG-3 (Oncogene Research Products, San Diego, CA), respectively. After washing, the membranes were incubated with horseradish peroxidase (HRP)–linked secondary antibodies for 1 hour. After washing, membrane-bound secondary antibodies were visualized with an enhanced chemiluminescence detection system (Amersham, Arlington Heights, IL).
Statistics
Data were expressed as mean ± SD. Statistical difference between samples was calculated using Student t test. A P value of less than .05 was considered as significantly different.
Results
Synergistic potentiation of thrombin-induced TF expression by S1P on the surface of ECs
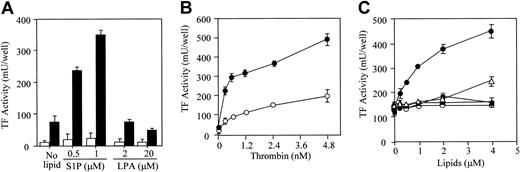
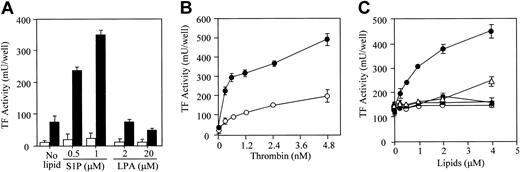
S1P and thrombin synergistically induced TF expression on the surface of ECs (Figure 1). However, S1P alone (in the absence of thrombin) did not induce the expression of TF (Figure 1A; Figure 2, open circle; and Figure 3B, open circle). LPA, a structurally related lysophospholipid, did not affect thrombin-induced TF expression. Dose-dependent induction of TF activity by thrombin was potentiated in the presence of S1P (Figure 1B). Among other bioactive lipids tested, only S1P enhanced the thrombin-induced TF expression in a dose-dependent manner (Figure 1C). PAF, at high concentration (4 μM), slightly enhanced thrombin-induced TF activity.
S1P synergistically potentiates thrombin-induced TF activity expression. (A) Confluent EC monolayers were treated with S1P and LPA in the presence (▪) and absence (□) of 2.4 nM thrombin. (B) The monolayers were treated with varying concentrations of thrombin in the presence (•) and absence (○) of 1 μM S1P. (C) In the presence of 2.4 nM thrombin, ECs were treated with varying concentrations of S1P (•), C2-ceramide (○), sphingosine (▴), PAF (▵), and LPC (▪). TF activity on the EC surface was determined after 5 hours of stimulation as factor Xa generation induced by TF–factor VIIa complex using purified factors VIIa and X as described in “Materials and methods.” These data represent the means ± SDs of 4 points per condition. Similar results were observed in 2 independent experiments using ECs of different lots.
S1P synergistically potentiates thrombin-induced TF activity expression. (A) Confluent EC monolayers were treated with S1P and LPA in the presence (▪) and absence (□) of 2.4 nM thrombin. (B) The monolayers were treated with varying concentrations of thrombin in the presence (•) and absence (○) of 1 μM S1P. (C) In the presence of 2.4 nM thrombin, ECs were treated with varying concentrations of S1P (•), C2-ceramide (○), sphingosine (▴), PAF (▵), and LPC (▪). TF activity on the EC surface was determined after 5 hours of stimulation as factor Xa generation induced by TF–factor VIIa complex using purified factors VIIa and X as described in “Materials and methods.” These data represent the means ± SDs of 4 points per condition. Similar results were observed in 2 independent experiments using ECs of different lots.
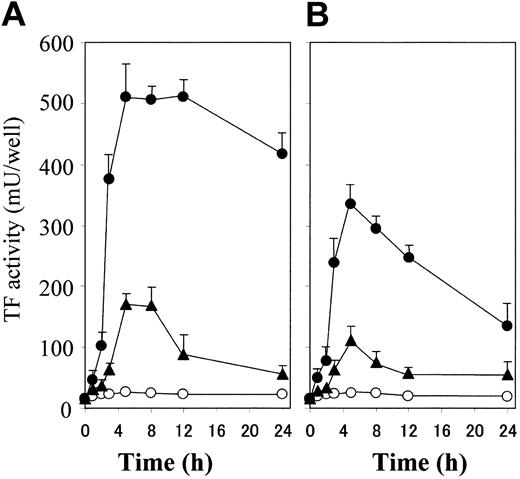
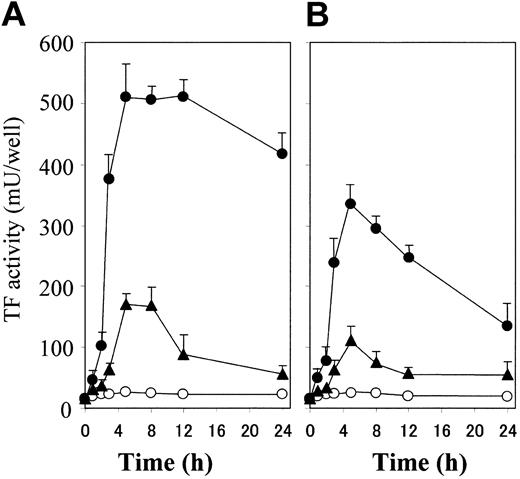
Thrombin plus S1P induces a rapid, synergistic, and enduring TF activity expression on ECs. (A) Confluent monolayers of ECs were incubated with 4.8 nM thrombin (▴) and 2 μM S1P (○) used alone or in combination (•). TF activity on the EC surface was determined at 0, 1, 2, 3, 5, 8, 12, and 24 hours after incubation as described in “Materials and methods.” (B) The monolayers were treated briefly (30 minutes) with 4.8 nM thrombin (▴) and 2 μM S1P (○) used alone or in combination (•). TF activity on the EC surface was determined after 0, 1, 2, 3, 5, 8, 12, and 24 hours of stimulation as described in “Materials and methods.” These data represent the means ± SDs of 4 points per condition. Similar results were observed in 2 independent experiments using ECs of different lots.
Thrombin plus S1P induces a rapid, synergistic, and enduring TF activity expression on ECs. (A) Confluent monolayers of ECs were incubated with 4.8 nM thrombin (▴) and 2 μM S1P (○) used alone or in combination (•). TF activity on the EC surface was determined at 0, 1, 2, 3, 5, 8, 12, and 24 hours after incubation as described in “Materials and methods.” (B) The monolayers were treated briefly (30 minutes) with 4.8 nM thrombin (▴) and 2 μM S1P (○) used alone or in combination (•). TF activity on the EC surface was determined after 0, 1, 2, 3, 5, 8, 12, and 24 hours of stimulation as described in “Materials and methods.” These data represent the means ± SDs of 4 points per condition. Similar results were observed in 2 independent experiments using ECs of different lots.
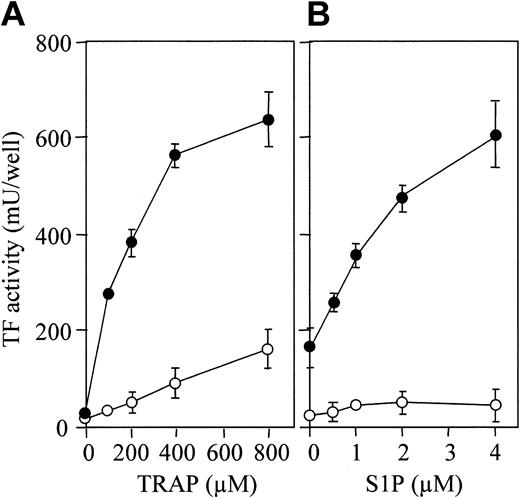
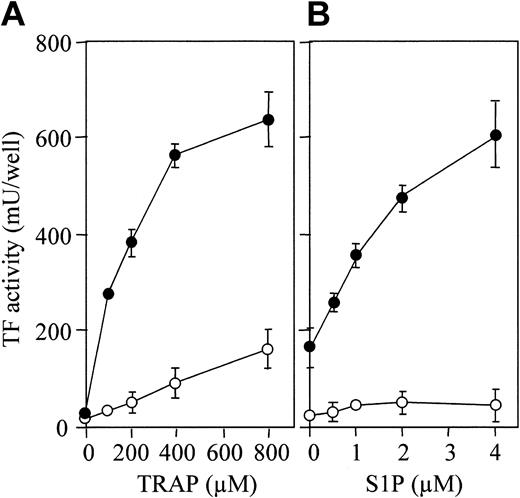
S1P enhances TRAP-induced TF activity expression. (A) Confluent monolayers of ECs were treated with varying concentrations of TRAP in the presence (•) and absence (○) of 4 μM S1P. (B) The monolayers were treated with varying concentrations of S1P in the presence (•) or absence (○) of 800 μM TRAP. TF activity on the EC surface was determined after 5 hours of stimulation as described in “Materials and methods.” These data represent the means ± SDs of 4 points per condition. Similar results were observed in 2 independent experiments using ECs of different lots.
S1P enhances TRAP-induced TF activity expression. (A) Confluent monolayers of ECs were treated with varying concentrations of TRAP in the presence (•) and absence (○) of 4 μM S1P. (B) The monolayers were treated with varying concentrations of S1P in the presence (•) or absence (○) of 800 μM TRAP. TF activity on the EC surface was determined after 5 hours of stimulation as described in “Materials and methods.” These data represent the means ± SDs of 4 points per condition. Similar results were observed in 2 independent experiments using ECs of different lots.
To estimate whether the level of TF expressed by stimulated ECs is sufficient to promote blood coagulation, PCA of ECs was evaluated by one-stage clotting assay. Confluent 2 × 105 ECs expressed 12.3 ± 6.4 PCA units. Thrombin (4.8 nM) in the absence and presence of S1P (4 μM) increased 8-fold (95.3 ± 8.2 PCA units) and 34-fold (408.4 ± 20.2 PCA units) the PCA of ECs, respectively. Because monocytes are known to participate in coagulation processes by expressing TF markedly after being stimulated with inflammatory mediators,41,42 PCA of TNF-stimulated monocytes was measured and compared with that of stimulated ECs. PCA of intact and TNF-α (1000 U/mL)–stimulated monocytes (2 × 105 cells) was 30.4 ± 8.4 PCA units per 106 PBMCs and 420.2 ± 15.4 PCA units per 106 PBMCs, respectively. PCA of ECs and PBMCs induced by the stimulants was confirmed to depend on TF, because it was specifically inhibited by anti-TF antibody. These results suggest that TF expressed by stimulated ECs could promote blood coagulation, and the PCA level of ECs induced by the combination of thrombin and S1P is similar to that of TNF-stimulated monocytes.
The combination of thrombin and S1P induced TF activity on ECs more rapidly and enduringly than did thrombin alone (Figure 2A). The TF activity induced by thrombin alone or thrombin plus S1P reached the peak at 5 hours. However, the activity induced by thrombin plus S1P was already detected at 2 hours, reached 80% of the peak at 3 hours, peaked between 5 hours and 12 hours, and remained 85% of the peak at 24 hours, while that induced by thrombin alone was only 30% of the peak at 3 hours, peaked between 5 hours and 8 hours, and was reduced by half at 12 hours. As shown in Figure 2B, we also examined the effect of transient exposure to the stimulants, because it is conceivable that the actions of thrombin and S1P generated during blood coagulation are rapidly suppressed by plasma inhibitors and degradation enzymes in vivo.1,2 Although the levels of TF induction were lower than those in the continuous exposure condition, thrombin could induce TF activity, and S1P provoked a rapid and prolonged enhancement of thrombin-induced TF activity.
PAR-1–activating peptide, also termed thrombin receptor agonist peptide (TRAP), is a 14–amino acid peptide that has the sequence of the functional N-terminal region of PAR-1 and that mimics the effect of thrombin.43 TRAP induced TF production by ECs in a dose-dependent fashion (Figure 3A, open circle), S1P markedly enhancing this TF production (Figure 3A, closed circle). TF expression enhancement by S1P was dose dependent (Figure 3B).
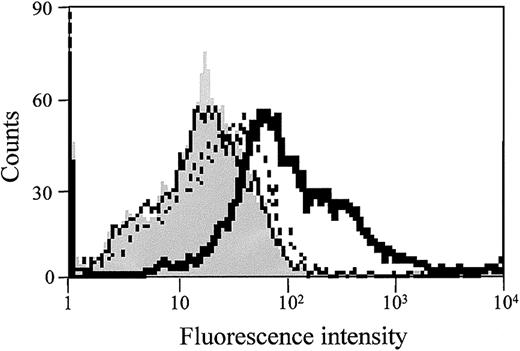
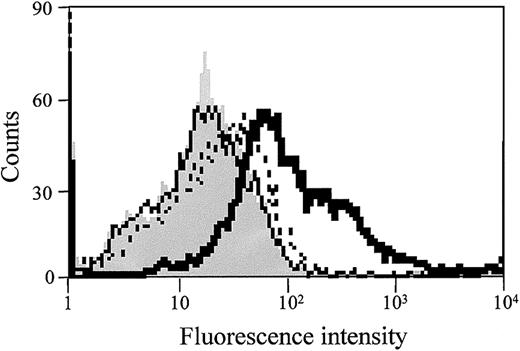
FACScan analysis was performed to determine the effect of both S1P and thrombin on TF protein expression on the EC surface. As shown in Figure 4, S1P alone did not affect TF antigen expression on ECs, but TF expression was markedly increased when the cells were stimulated with both S1P and thrombin. Thus, the enhancement of TF activity by S1P is probably due to the increased expression of TF protein on the EC surface.
S1P enhances thrombin-induced EC surface TF antigen expression. Confluent monolayers of ECs were treated with 1 nM thrombin (dotted line), 4 μM S1P (solid line), or 1 nM thrombin plus 4 μM S1P (bold solid line) for 5 hours. The cells were then harvested and incubated with murine monoclonal anti-TF IgG. FITC-labeled goat antimouse IgG antibody was used as the second antibody. Fluorescence intensity was determined by FACScan as described in “Materials and methods.” Gray histograms depict untreated cells. Similar results were observed in 2 independent experiments.
S1P enhances thrombin-induced EC surface TF antigen expression. Confluent monolayers of ECs were treated with 1 nM thrombin (dotted line), 4 μM S1P (solid line), or 1 nM thrombin plus 4 μM S1P (bold solid line) for 5 hours. The cells were then harvested and incubated with murine monoclonal anti-TF IgG. FITC-labeled goat antimouse IgG antibody was used as the second antibody. Fluorescence intensity was determined by FACScan as described in “Materials and methods.” Gray histograms depict untreated cells. Similar results were observed in 2 independent experiments.
Synergistic effect of S1P on thrombin-induced TF mRNA expression in ECs
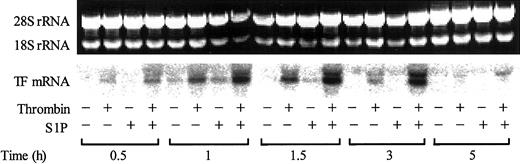
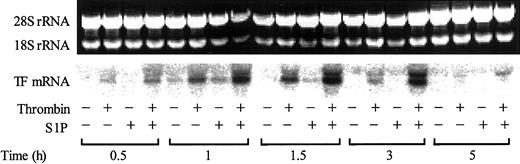
The increase in cell surface expression of TF was paralleled by an increase in TF mRNA expression (Figure 5). While S1P itself only slightly induced TF mRNA expression, it markedly enhanced the thrombin-induced TF mRNA expression.
S1P enhances thrombin-induced TF mRNA expression. Confluent monolayers of ECs were treated with or without 2.5 nM thrombin, 4 μM S1P, or 2.5 nM thrombin plus 4 μM S1P for 0.5 to 5 hours. The cells were then harvested and processed for detection of TF mRNA by Northern blot analysis. The upper panel shows the total RNA samples as detected by ethidium bromide staining; intact 18S and 28S rRNA bands served as control of equal RNA loading for Northern blot analysis. The results shown are representative of 3 independent experiments.
S1P enhances thrombin-induced TF mRNA expression. Confluent monolayers of ECs were treated with or without 2.5 nM thrombin, 4 μM S1P, or 2.5 nM thrombin plus 4 μM S1P for 0.5 to 5 hours. The cells were then harvested and processed for detection of TF mRNA by Northern blot analysis. The upper panel shows the total RNA samples as detected by ethidium bromide staining; intact 18S and 28S rRNA bands served as control of equal RNA loading for Northern blot analysis. The results shown are representative of 3 independent experiments.
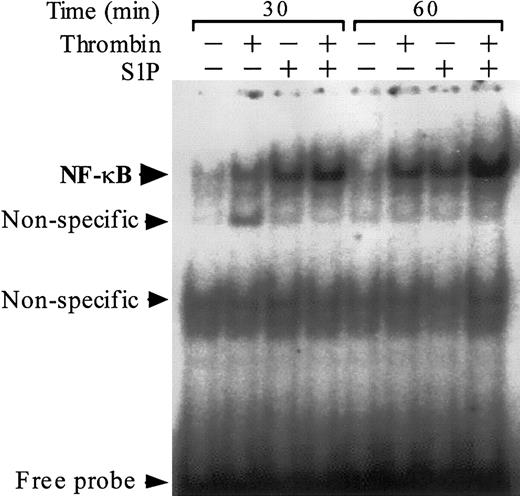
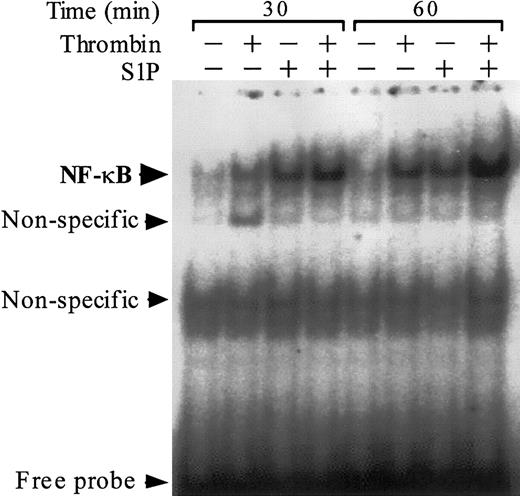
Previous work suggests that the NF-κB/Rel family of transcriptional factors plays a major role in thrombin-induced TF gene transcription in ECs.38,44 We assessed the activation and nuclear translocation of NF-κB by EMSA using an oligonucleotide probe that contains a prototypic κB site (Figure 6). As previously reported,38 thrombin induced activation and nuclear translocation of NF-κB. S1P induced NF-κB activation more rapidly than thrombin did, and the combination of S1P and thrombin exerted a synergistic effect on NF-κB activation.
Thrombin and S1P synergistically induce NF-κB binding activity. NF-κB DNA binding activity was measured by EMSA in nuclear extracts of ECs that were exposed to 10 nM thrombin, 4 μM S1P, or 10 nM thrombin plus 4 μM S1P for 30 or 60 minutes. Similar results were observed in 2 independent experiments.
Thrombin and S1P synergistically induce NF-κB binding activity. NF-κB DNA binding activity was measured by EMSA in nuclear extracts of ECs that were exposed to 10 nM thrombin, 4 μM S1P, or 10 nM thrombin plus 4 μM S1P for 30 or 60 minutes. Similar results were observed in 2 independent experiments.
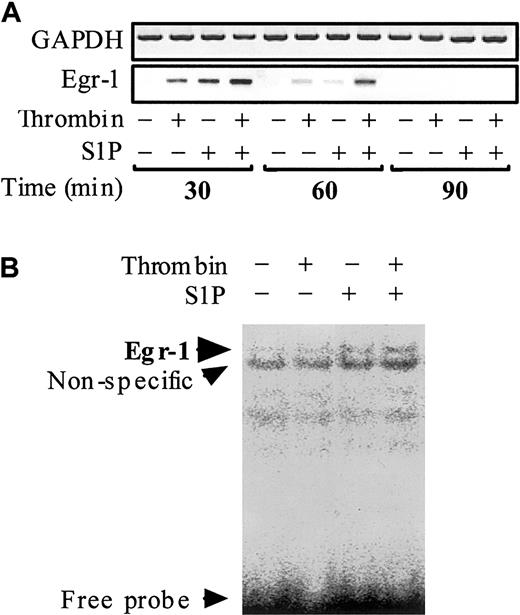
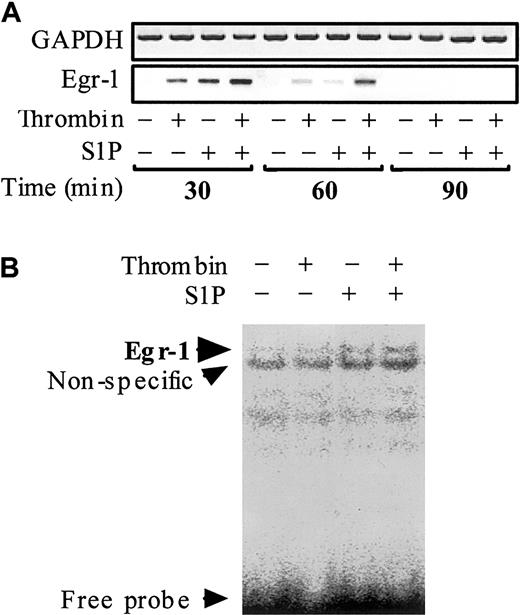
Egr-1, which belongs to the immediate-early gene products, is also required for the inducible transcription of TF gene in ECs.45-50 However, to the best of our knowledge it is not known whether thrombin and S1P can affect Egr-1 mRNA expression in ECs. To clarify this point, we examined the effect of thrombin and S1P on Egr-1 mRNA transcription and Egr-1 DNA binding activity (Figure 7). No Egr-1 mRNA was detected in nonstimulated ECs. Both thrombin and S1P induced Egr-1 mRNA expression rapidly (within 30 minutes) and transiently (back to basal level within 90 minutes). The effect of thrombin and S1P was synergistic (Figure 7A). EMSA disclosed Egr-1 binding to a prototypic Egr-1 DNA binding site only when the nuclear extracts from ECs treated simultaneously with thrombin and S1P were used (Figure 7B).
Thrombin and S1P synergistically induce Egr-1 mRNA expression and Egr-1 DNA binding activity. (A) Confluent monolayers of ECs were treated with or without 10 nM thrombin, 4 μM S1P, or 10 nM thrombin plus 4 μM S1P for 30, 60, and 90 minutes. The cells were then harvested and processed for determination of Egr-1 mRNA levels by semiquantitative RT-PCR. GAPDH mRNA levels were also measured as control. (B) Treated and untreated ECs for 30 minutes were subjected to EMSA using the Egr-1–specific probe.
Thrombin and S1P synergistically induce Egr-1 mRNA expression and Egr-1 DNA binding activity. (A) Confluent monolayers of ECs were treated with or without 10 nM thrombin, 4 μM S1P, or 10 nM thrombin plus 4 μM S1P for 30, 60, and 90 minutes. The cells were then harvested and processed for determination of Egr-1 mRNA levels by semiquantitative RT-PCR. GAPDH mRNA levels were also measured as control. (B) Treated and untreated ECs for 30 minutes were subjected to EMSA using the Egr-1–specific probe.
Effect of S1P and thrombin on ERK1/2 MAP kinase activation
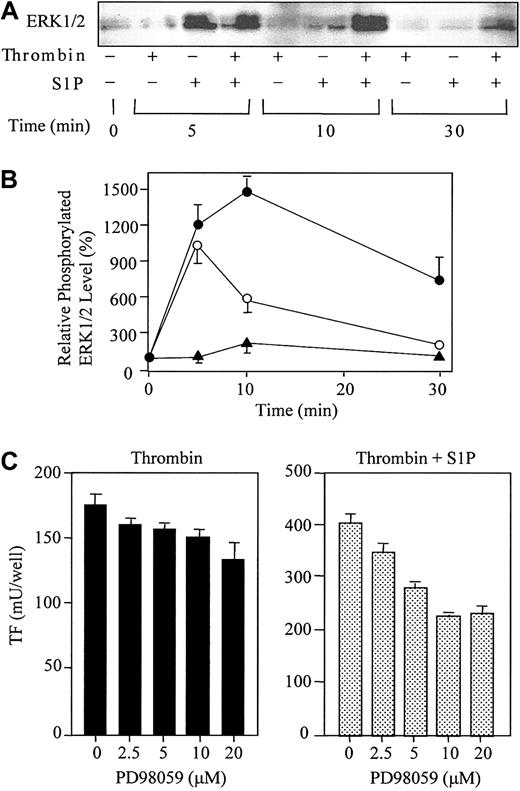
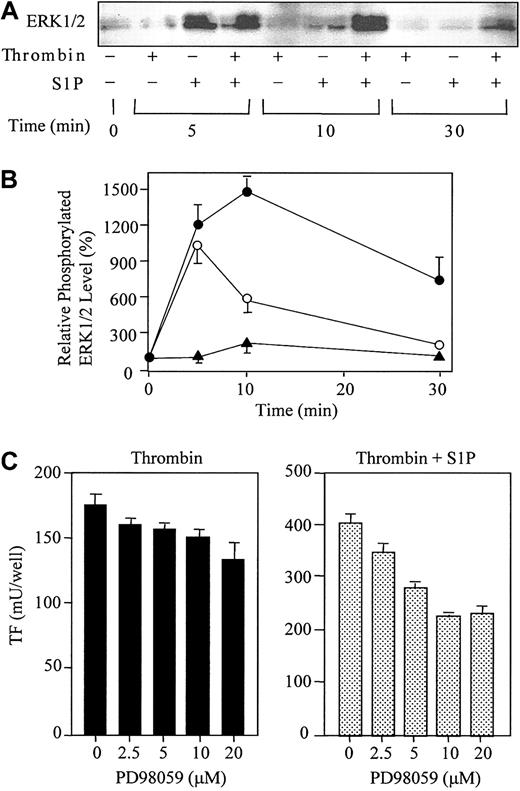
S1P ligation of S1P receptors (EDG-1, -3, and -5) has been shown to activate the ERK1/2 signaling pathway in human kidney 293, Cos-1, and HTC4 rat hepatoma cell expression systems.11,51,52 Thus, we next examined whether S1P could also activate ERKs in HUVECs, which endogenously possess EDG-1 and -3 5-8 (Figure 8). The levels of the active forms of ERKs were measured by Western blot analysis using antibodies against phosphorylated ERK1/2 (Figure 9A-B). Consistent with previous observations in cells expressing exogenous EDG receptors, S1P rapidly activated ERK1/2 (peaked within 5 minutes). S1P-induced ERK1/2 activation was transient and returned to basal level within 10 minutes. Thrombin also induced ERK1/2 activation at 10 minutes, but this effect was much weaker than that of S1P. A greater activation of ERK1/2 occurred over 10 minutes when ECs were simultaneously stimulated with thrombin and S1P, which was still apparent at 30 minutes.
Thrombin and S1P synergistically induce ERK1/2 phosphorylation, and PD98059 reduces the synergistic induction of TF. (A) ECs were treated with or without 10 nM thrombin, 4 μM S1P, or 10 nM thrombin plus 4 μM S1P for 5, 10, and 30 minutes. The cells were then harvested and processed for Western blotting with antibodies to phospho-ERK1/2. (B) The data in panel A were densitometrically quantified and are expressed as the percentage of the control (basal phospho-ERK1/2 in the absence of thrombin and S1P). The values represent the means ± SDs of 3 independent experiments. (C) ECs were pretreated for 20 minutes with indicated concentrations of PD98059 prior to incubation with thrombin (4 nM) or thrombin (1 nM) plus S1P (4 μM). These data represent the means ± SDs of 4 independent experiments with 2 points per condition.
Thrombin and S1P synergistically induce ERK1/2 phosphorylation, and PD98059 reduces the synergistic induction of TF. (A) ECs were treated with or without 10 nM thrombin, 4 μM S1P, or 10 nM thrombin plus 4 μM S1P for 5, 10, and 30 minutes. The cells were then harvested and processed for Western blotting with antibodies to phospho-ERK1/2. (B) The data in panel A were densitometrically quantified and are expressed as the percentage of the control (basal phospho-ERK1/2 in the absence of thrombin and S1P). The values represent the means ± SDs of 3 independent experiments. (C) ECs were pretreated for 20 minutes with indicated concentrations of PD98059 prior to incubation with thrombin (4 nM) or thrombin (1 nM) plus S1P (4 μM). These data represent the means ± SDs of 4 independent experiments with 2 points per condition.
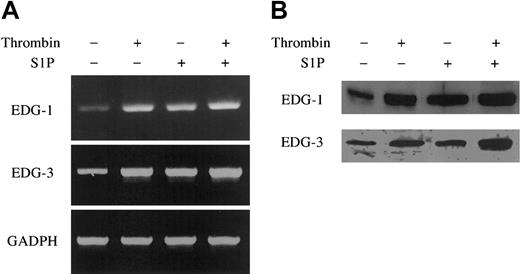
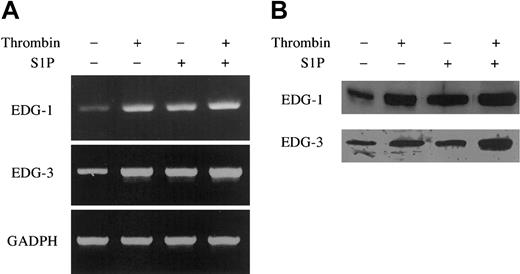
Thrombin and S1P up-regulate EDG-1 and -3 mRNA and protein expression. ECs were treated with or without 10 nM thrombin and 4 μM S1P for 30 minutes (A) and 3 hours (B). The cells were then harvested and processed for determination of EDG-1 and -3 mRNA and protein level by quantitative RT-PCR (A) and by Western blot analysis (B), respectively. As control, GAPDH was also analyzed by quantitative RT-PCR (A). Similar results were observed in 2 independent experiments.
Thrombin and S1P up-regulate EDG-1 and -3 mRNA and protein expression. ECs were treated with or without 10 nM thrombin and 4 μM S1P for 30 minutes (A) and 3 hours (B). The cells were then harvested and processed for determination of EDG-1 and -3 mRNA and protein level by quantitative RT-PCR (A) and by Western blot analysis (B), respectively. As control, GAPDH was also analyzed by quantitative RT-PCR (A). Similar results were observed in 2 independent experiments.
Effect of PD98059 on S1P- and thrombin-induced TF expression
To evaluate the potential role of ERKs in TF expression in ECs, we stimulated these cells with thrombin or with thrombin plus S1P in the presence of various concentrations of the specific inhibitor of ERK, PD98059. The inhibitor acts upstream of ERK by selectively inhibiting mitogen-activated protein kinase (MAPK) extracellular signal–regulated kinase kinase-1 (MEK-1). As shown in Figure 9C, PD98059 significantly reduced TF expression synergistically induced by thrombin and S1P (45% inhibition by 10 μM PD98059). On the other hand, PD98059 minimally affected thrombin-induced TF expression by ECs (13% inhibition by 10 μM PD98059).
Increased expression of S1P receptors EDG-1 and EDG-3 by thrombin and S1P
Quantitative RT-PCR showed that thrombin and S1P up-regulated the mRNA levels of EDG-1 and -3 in ECs (Figure 8A). The mRNA levels of EDG-1 and EDG-3 were increased, respectively, 100-fold and 50-fold in ECs treated with thrombin, 100-fold and 50-fold in ECs treated with S1P, and 200-fold and 100-fold in ECs treated with thrombin plus S1P, compared with those in ECs without treatment. Among other S1P receptors, mRNA of EDG-5 was detectable by RT-PCR (data not shown) in ECs, and this is consistent with a previous report,6,53 but neither thrombin nor S1P up-regulated the expression of EDG-5 in ECs. Western blotting analysis confirmed EDG-1 and EDG-3 up-regulation by thrombin and S1P at the protein level (Figure 8B).
Discussion
Thrombin and S1P are generated during blood coagulation and platelet activation at sites of vascular injury; both are known to modulate several EC functions.26,28,29,54 However, the concomitant effect of thrombin and S1P on EC functions has not yet been evaluated. We have shown in the present study that S1P synergistically potentiates thrombin-induced TF expression in ECs, S1P alone exerting no effect on TF expression.
Up-regulation of TF activity and protein expression was associated with enhanced mRNA steady-state levels of TF. Stimulation of HUVECs with thrombin resulted in the activation of NF-κB, which is believed to play a major role in regulation of thrombin-induced TF mRNA synthesis.38,44 We showed here for the first time that S1P stimulation of ECs also induces NF-κB activation and that it markedly enhances the thrombin-induced NF-κB activation; this effect may explain the synergistic activation of TF gene transcription in the presence of both thrombin and S1P. Notably, however, S1P-induced NF-κB activation is not sufficient to induce TF mRNA synthesis (Figure 5).
Apart from NF-κB, Egr-1 is also known to promote TF gene transcription in ECs.45-50 Egr-1 expression can be induced in ECs by diverse stimuli including growth factors, TNF-α, hypoxia, fluid shear stress, and oxidized phospholipids.49,50,55,56 Vascular endothelial growth factor and oxidized phospholipids up-regulate TF in ECs by activating Egr-1 but not NF-κB.49,50 Furthermore, induction of Egr-1 expression is required in addition to NF-κB for maximal induction of TF by lipopolysaccharide in monocytic cells.57 In this report, we have demonstrated for the first time that S1P and thrombin induce Egr-1 expression and that both stimulants synergistically induce Egr-1 expression. Although Egr-1 DNA binding activity was not detected in ECs treated with thrombin alone or weak in cells treated with S1P alone, Egr-1 DNA binding was strong in ECs treated with both stimulants. Thus, Egr-1 expression and activation may contribute to the synergy between thrombin and S1P on TF up-regulation.
Previous reports have shown that ECs possess EDG-1 and -3 subtypes as S1P receptor,5-8 and in this report we confirmed EDG-1 and -3 mRNA expressions in HUVECs by RT-PCR and Northern blotting. It is known that EDG-1 and -3 are coupled to ERK1/2 MAP kinases in Chinese hamster ovary (CHO) cells13,54 and that the ERK1/2 pathway is involved in Egr-1 expression in ECs49,50,55,56 ; thus, we evaluated S1P-mediated activation of ERK1/2 in ECs. S1P induced rapid and transient activation of ERK1/2. Thrombin alone only weakly activated ERK1/2, but thrombin plus S1P induced a strong and sustained activation of ERK1/2, which was correlated with both Egr-1 and TF expression. The inhibition of TF expression by PD98059 suggests that the ERK1/2 pathway is involved in the enhanced TF expression induced by thrombin plus S1P. Taken together, these results support the relationship between the synergistic activation of ERK1/2 and the synergistic induction of Egr-1 and TF expression.
LPA is a molecule structurally related to S1P and, like S1P, this serum-borne lysophospholipid is released extracellularly following thrombin-induced platelet activation, and it can stimulate ECs and induce their shape changes.58 However, recent studies suggest that both the production of LPA in platelets and the effect of LPA on ECs are much less than those of S1P.3,6 In our experiments, LPA did not affect TF expression. The LPA receptors, EDG-2, -4, and -7, are all expressed in HUVECs, as detected by RT-PCR (data not shown) and, like EDG-1 and -3, are coupled to Gi. Thus, the explanation for the minor effects of LPA on ECs remains unclear.
Up-regulation of TF induced by the interplay between thrombin and S1P may be critical for the control of hemostasis and thrombogenesis. Expression of TF after thrombin and S1P stimulation initiates the activation of coagulation cascade leading to further generation of thrombin and S1P, thus causing propagation of thrombus formation at sites of EC injury. In addition, thrombin and S1P up-regulate the expression of EDG-1 and -3 in ECs and thus may amplify the effect of S1P in this system.
This positive feedback loop may also play a critical role in other physiological and pathological conditions. Binding of S1P to its receptors EDG-1 and -3 on ECs modulates a variety of EC functions such as chemotaxis,9 morphogenic differentiation,5,43 and angiogenesis.6 On the other hand, thrombin is a growth factor for a variety of cell types including ECs, smooth muscle cells, and fibroblasts, and by this effect thrombin may regulate processes such as embryogenesis, wound healing, tissue remodeling, vasculogenesis, angiogenesis, and atherogenesis.28,29 Furthermore, TF itself, apart from its role in coagulation, may also have a direct effect on the several processes regulated by both thrombin and S1P.21-23 Overall, the results of this study suggest the importance of the interplay between TF, thrombin, and S1P in diverse physiological and pathological processes. Blockade of this pathway of TF production may be critical for preventing the occurrence of pathological processes associated with abnormal activation of the blood coagulation system.
Prepublished online as Blood First Edition Paper, May 1, 2003; DOI 10.1182/blood-2002-11-3607.
Supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology (H.T., E.C.G., and K.S.), the Mie Medical Research Foundation (H.T.), the Welfide Medicinal Research Foundation (H.T.), and a new project research fund from Mie University (H.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Tomohiro Nakagaki of the Chemo-Sero-Therapeutic Research Institute (Kumamoto, Japan) for providing coagulation proteins and Ms Taemi Hibi for her excellent technical assistance.