Abstract
Platelet interaction with exposed adhesive ligands at sites of vascular injury is required to initiate a normal hemostatic response and may become a pathogenic factor in arterial diseases leading to thrombosis. We report a targeted disruption in a key receptor for collagen-induced platelet activation, glycoprotein (GP) VI. The breeding of mice with heterozygous GP VI alleles produced the expected frequency of wild-type, heterozygous, and homozygous genotypes, indicating that these animals had no reproductive problems and normal viability. GP VInull platelets failed to aggregate in response to type I fibrillar collagen or convulxin, a snake venom protein and known platelet agonist of GP VI. Nevertheless, tail bleeding time measurements revealed no severe bleeding tendency as a consequence of GP VI deficiency. Ex vivo platelet thrombus formation on type I collagen fibrils was abolished using blood from either GP VInull or FcR-γnull animals. Reflection interference contrast microscopy revealed that the lack of thrombus formation by GP VInull platelets could be linked to a defective platelet activation following normal initial tethering to the surface, visualized as lack of spreading and less stable adhesion. These results illustrate the role of GP VI in postadhesion events leading to the development of platelet thrombi on collagen fibrils.
Introduction
Platelet membrane receptors interact with surface-bound adhesive ligands and, as such, become essential for hemostasis and thrombosis.1 There are numerous unique receptors interacting with different adhesive ligands suggesting that a large opportunity exists for functional redundancy in platelet adhesion. However, an emerging theme of platelet biology is the relevance of different membrane receptors in different areas of the vasculature.2,3 A specific example is the exclusive role for the platelet glycoprotein (GP) Ib-IX-V complex and von Willebrand factor in areas of the vascular system where flow rates and high shear occur, such as in small arteries and arterioles.4 Thus, defining the physiologic relevance of an individual receptor and its ligand is an important aspect for understanding participation of the platelet in hemostasis and thrombosis.
Among adhesive ligands of the extravascular matrix, collagen is a significant component with a number of known collagen receptors on the platelet surface.5,6 One of the more recently characterized collagen receptors is GP VI.7 The molecular cloning of GP VI revealed it to be a member of the immunoglobulin superfamily of type I transmembrane proteins.8-10 The surface expression of GP VI requires the concomitant expression of the γ-subunit of the FcR receptor (FcR-γ) and their association is functionally relevant as collagen binding to GP VI results in platelet signaling via the immunoreceptor tyrosine–based activation motif (ITAM) located in the FcR-γ subunit.8,11-14 As with many of the platelet receptors, the in vivo relevance of GP VI was established prior to its description and recognition as a protein membrane receptor. In several clinical cases, patients lacking GP VI as a consequence of autoantibody inhibition or possible gene deletion have been described.15-19 Most commonly, these patients show a moderately prolonged bleeding time and mild bleeding diatheses like subcutaneous bleeding. No severe bleeding complication caused by the absent GP VI has been reported. Possible explanations for the moderate bleeding phenotype include the presence of multiple additional collagen receptors such as α2β1 or GP IV.14 It is also not clear in the clinical cases of autoantibody inhibition of GP VI whether such antibodies can produce a more global effect on platelet function. Indeed, the possibility of an antiplatelet antibody stimulating platelet activation pathways might mask the physiologic relevance of the receptor in these autoimmune cases. The possible cases of genetic deletion of human GP VI have yet to be defined at the molecular level, which makes the interpretation of data from these individuals less than definitive as a case of GP VI deficiency.
One experimental model to assess the physiologic relevance of individual platelet receptors is the use of targeted gene deletions in mice. This strategy generates an experimental model to directly assess the role of a membrane receptor in hemostasis and thrombosis and the contribution of the receptor to other biologic processes such as reproduction. Here, we report the characterization of the murine GP VI gene and its targeted disruption for the generation of a model of GP VI deficiency. Platelets from homozygous-deficient mice completely lack the ability to respond in an aggregometer to stimulation by type I fibrillar collagen. Yet, the in vivo tail bleeding times for GP VInull animals are within the range obtained for wild-type animals. In vitro models of thrombus formation demonstrated a direct role for GP VI in the activation events that lead to platelet spreading. These results define the in vivo relevance of an individual collagen receptor and its synergistic contribution to the initiation and amplification of platelet thrombus formation.
Materials and methods
Construction of mouse bone marrow cDNA library
Bone marrow was isolated from approximately 200 mouse femur bones and immediately frozen in liquid nitrogen. The tissue was treated in 4 M guanidinium isothiocyanate and total RNA purified by centrifugation through a CsCl cushion as described.20 Poly A+–enriched RNA was prepared by standard affinity purification using oligo-dT cellulose. The enriched poly A+ RNA was used to construct a cDNA library in the λZAP-CMV vector (Stratagene, La Jolla, CA). The isolation of a mouse GP VI cDNA from the bone marrow cDNA library was done using radiolabeled probes of a polymerase chain reaction (PCR) fragment representing the full-length human GP VI cDNA.8 Full-length mouse GP VI cDNAs corresponded to the recently reported sequence.9
Isolation of the mouse GP VI gene
A search of the high-throughput genomic sequence database using the mouse GP VI cDNA sequence identified a mouse clone (GenBank Accession no. AC087129.1) containing 8 GP VI exon sequences (Figure 1) similar to the exon/intron arrangement of the human GP VI gene. Oligonucleotides from mouse GP VI exon 4 were supplied to Incyte Genomics (St Louis, MO) for PCR screening to isolate a corresponding mouse bacterial artificial chromosome (BAC) plasmid. Three positive clones were characterized by restriction enzyme analysis and found to have a restriction fragment pattern predicted from the GenBank sequence found in Accession no. AC087129.1. Limited nucleotide sequence analysis was done on the BAC plasmid and no differences were noted with the GenBank deposited sequence.
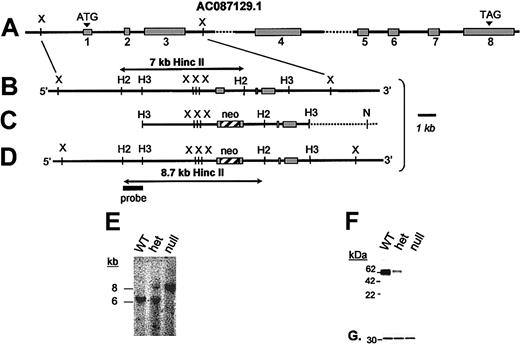
GP VI gene structure and targeted disruption. (A) The mouse GP VI gene is schematically presented as it spans a portion of GenBank Accession no. AC087129.1. Based on an alignment of the mouse GP VI cDNA sequence and genomic sequence, 8 exons (▦) and introns were identified. By sequence alignment with the human GP VI cDNA, a putative initiating Met codon (ATG) is shown in exon 1. (B) An expanded region of the wild-type mouse GP VI gene containing exons 1 to 3 is shown. (C) A 9-kb HindIII restriction fragment spanning exons 1 to 3 was subcloned into a pBS/KS-vector and altered via site-directed mutagenesis to place a stop codon (TGA) immediately 3′ to the initiating Met codon followed by an XhoI restriction site. Following successful mutagenesis a neocassette was cloned within the exon 1 sequence using the restriction site created during mutagenesis. (D) Successful homologous recombination in mouse ES cells results in the replacement of a 7-kb HincII restriction fragment with an 8.7-kb HincII fragment. (E) Germ line transmission of the ES cell genotype was obtained producing GP VIhet mice. The breeding of GP VIhet mice produced the expected 3 genotypes and shown is a representative Southern blot of each of the genotypes revealing the wild-type GP VI gene (7 kb) and the targeted allele (8.7 kb). (F) A mouse monoclonal antibody recognizing both human and mouse GP VI proteins was used to verify the lack of the GP VI polypeptide in platelet lysates from GP VInull mice. Shown is a Western blot of platelet lysates from each of the 3 genotypes. A gene dosage effect of GP VI levels is seen as a consequence of the altered GP VI allele. (G) The same filter was reprobed with an anti–14-3-3ζ polyclonal antibody to confirm the presence of platelet proteins in each lane.
GP VI gene structure and targeted disruption. (A) The mouse GP VI gene is schematically presented as it spans a portion of GenBank Accession no. AC087129.1. Based on an alignment of the mouse GP VI cDNA sequence and genomic sequence, 8 exons (▦) and introns were identified. By sequence alignment with the human GP VI cDNA, a putative initiating Met codon (ATG) is shown in exon 1. (B) An expanded region of the wild-type mouse GP VI gene containing exons 1 to 3 is shown. (C) A 9-kb HindIII restriction fragment spanning exons 1 to 3 was subcloned into a pBS/KS-vector and altered via site-directed mutagenesis to place a stop codon (TGA) immediately 3′ to the initiating Met codon followed by an XhoI restriction site. Following successful mutagenesis a neocassette was cloned within the exon 1 sequence using the restriction site created during mutagenesis. (D) Successful homologous recombination in mouse ES cells results in the replacement of a 7-kb HincII restriction fragment with an 8.7-kb HincII fragment. (E) Germ line transmission of the ES cell genotype was obtained producing GP VIhet mice. The breeding of GP VIhet mice produced the expected 3 genotypes and shown is a representative Southern blot of each of the genotypes revealing the wild-type GP VI gene (7 kb) and the targeted allele (8.7 kb). (F) A mouse monoclonal antibody recognizing both human and mouse GP VI proteins was used to verify the lack of the GP VI polypeptide in platelet lysates from GP VInull mice. Shown is a Western blot of platelet lysates from each of the 3 genotypes. A gene dosage effect of GP VI levels is seen as a consequence of the altered GP VI allele. (G) The same filter was reprobed with an anti–14-3-3ζ polyclonal antibody to confirm the presence of platelet proteins in each lane.
Generation of targeting vector for disruption of mouse GP VI synthesis
As schematically illustrated in Figure 1, the mouse GP VI gene contains an initiating Met codon within the putative exon 1 sequence. A 9-kb HindIII restriction fragment spanning exons 1 to 3 was cloned into pBS/KS-(Figure 1A). Within this subcloned fragment a premature stop codon was created immediately 3′ to the initiation codon by double-stranded site-directed mutagenesis using Platinum Pfx DNA polymerase (Stratagene). A unique restriction site was also added 3′ to the premature stop codon to allow insertion of a phosphoglycerate-kinase neor cassette (kindly provided by Dr Richard Hynes, Massachusetts Institute of Technology, Cambridge) within exon 1 (Figure 1). The final targeting construct contained 4.5-kb arms, both 5′ and 3′, and the 1.7-kb neor selectable marker immediately downstream to the initiating Met codon (Figure 1C).
Generation of GP VInull mice
The targeting vector was linearized with NotI and electroporated into DS2A embryonic stem (ES) cells at the Dartmouth Transgenic Facility (Lebanon, NH). Transfected cells were selected for geneticin (G418) resistance and approximately 180 clones were expanded for analysis by Southern blotting. A HincII/HindIII restriction fragment outside the targeting vector sequence was used to probe HincII digests of ES cell DNA. Correct homologous recombination was further confirmed by Southern blot analysis of an XbaI-digested ES cell DNA hybridized with a 1.1-kb fragment containing exons 2 and 3. All probes were labeled by [α32P]dATP (deoxyadenosine triphosphate) using Prime-It II Random Primer Labeling kit from Stratagene. Three clones with the predicted homologous recombination event were confirmed with additional probes and PCR analysis to validate the correct generation of a GP VI–targeted allele (Figure 1D). The positive ES cells were microinjected into C57BL/6J mouse blastocysts and were implanted into pseudopregnant females. Chimeric mice were bred to Swiss Black mice to test for germ line transmission. Two chimeric mice from different ES cells produced germ line offspring as assessed by coat color transmission. Heterozygous mice were crossed to produce the 3 genotypes: wild type (GP VIWT), heterozygous (GP VIhet), and homozygous deficient (GP VInull). Studies comparing the 3 genotypes, such as tail bleeding times and aggregation assays, were performed using littermates from GP VIhet × GP VIhet crosses prior to genotype analysis. Mice deficient in the FcR-γ subunit were kindly provided by Prof Toshiyuki Takai (Tohoku University, Sendai, Japan) and have been previously described.21
Chemicals, immunologic reagents, and flow cytometry
An anti–human GP VI monoclonal antibody was generated using recombinant GP VI immunogen produced by expression using a baculovirus system in Hi 5 cells. Primary hybridomas were screened by enzyme-linked immunosorbent assay (ELISA) for reactivity of supernatants with recombinant human GP VI. Upon characterization the monoclonal antibody was found to cross-react, albeit less strongly, with mouse GP VI antigen. Convulxin was purified from Crotalus durissus terrificus venom (Miami Serpentarium Laboratories, Miami, FL) as described.22 A rabbit anti–FcR-γ chain polyclonal antibody was purchased from Upstate Biotechnology (Lake Placid, NY). Horseradish peroxidase (HRP)–conjugated rabbit antimouse IgG and HRP-conjugated goat antirabbit IgG were obtained from Zymed Laboratories (South San Francisco, CA). A rabbit anti–14-3-3ζ polyclonal antibody was purchased from Santa Cruz Technologies (Santa Cruz, CA).
Mouse platelet preparation
Murine blood was withdrawn from the retro-orbital plexus using heparin-coated micro-hematocrit capillaries (Fisher Scientific, Pittsburgh, PA) and transferred to tubes with heparin at final concentration of 30 units/mL (Sigma, St Louis, MO). Platelet-rich plasma (PRP) was obtained by centrifugation of whole blood at 500g for 6 minutes. To obtain platelet lysates, platelets were washed one time in a modified Tyrode buffer (5 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] [pH 6.5]; 137 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 2.8 mM Dextrose) with 5 U/mL apyrase and 10 μM prostaglandin E1 and then centrifuged at 1500g for 5 minutes. The platelet pellet was resuspended in 100 μL Tyrode buffer (pH 7.4) and lysed with an equal volume of 50 mM Tris (tris(hydroxymethyl)aminomethane; pH 7.5) and 10% sodium dodecyl sulfate (SDS) and boiled for 5 minutes. Platelet poor plasma (PPP) was obtained by centrifuging at 2000g for 7 minutes.
Platelet aggregation
Following the preparation of PRP (see “Mouse platelet preparation”) the platelet counts were normalized to 240 × 109/L with PPP. Four hundred microliters of PRP was prewarmed at 37°C. Convulxin, bovine fibrillar collagen (type I), and 50 μM phorbol 12-myristate 13-acetate (PMA; Sigma) were added to PRP as agonists. Aggregation profiles were generated in a Chrono-Log aggregometer (Havertown, PA). PPP was used as a blank for aggregation study.
Immunoblotting
Proteins were separated by 4% to 20% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (Invitrogen, Carlsbad, CA). Membranes were blocked with TBS-T (20 mM Tris [pH 7.5]; 150 mM NaCl, 0.05% Tween 20) containing 5% skim milk (30 minutes, room temperature [RT]). Antibodies were incubated with the membranes for 1 hour at RT. Afterward, the membranes were washed 3 times with TBS-T and the membranes were incubated with either HRP rabbit antimouse IgG or HRP goat antirabbit IgG (30 minutes, RT). Membranes were developed using an enhanced chemiluminescence detection system (Amersham-Pharmacia Biotech UK Limited, Little Chalfont, United Kingdom).
Bleeding time assays
Mouse tail bleeding times were determined as described.23 Briefly, 1 to 3 mm of distal tail was removed and immediately immersed in isotonic saline (37°C). A complete cession of bleeding was defined as the bleeding time.
Analysis of platelet adhesion and thrombus formation by real-time videomicroscopy
The blood of mice anesthetized by inhalation of methoxyflurane (Medical Developments, Springvale, Australia) was drawn from the retro-orbital venous plexus through a heparinized glass capillary tube and collected into plastic tubes containing a solution of unfractionated heparin (sodium salt) from porcine intestinal mucosa (grade III; Sigma) to give a final concentration of 40 U/mL. Apyrase (grade III; Sigma) was then added at a final concentration of 1.5 ATPase U/mL in order to prevent desensitization of platelet purinergic receptors. Epifluorescence and confocal videomicroscopy were performed as described in detail elsewhere.3,24 In brief, purified fibrillar type I collagen from bovine tendon (acid insoluble; Sigma) was coated onto a glass coverslip that was subsequently assembled into a parallel plate rectangular flow chamber. Mouse platelets were labeled in whole blood by addition of the fluorescent dye mepacrine (quinacrine dihydrochloride; final concentration 10 μM) before perfusion through the chamber by aspiration with a syringe pump (Harvard Apparatus, Holliston, MA) at the desired flow rate. The perfusion chamber was mounted on the stage of an inverted microscope (Axiovert 135M; Carl Zeiss, Thornwood, NY) for real-time visualization of platelet adhesion and aggregation in flowing blood, and the process was recorded continuously on videotape with a VCR (SVO-9500MD; Sony, Inchinomiya, Japan) at the acquisition rate of 30 frames/second. Thrombus volume was measured in real time by confocal videomicroscopy (LSM 410; Carl Zeiss).3 We also performed experiments to visualize in real time the interface between platelets and immobilized collagen using reflection interference contrast microscopy (RICM). In this technique, which does not require labeling of the blood cells, interference colors indicate the distance between 2 surfaces.25,26 In our studies, the interference was caused by the glass surface onto which the substrate was coated and by the membrane of cells flowing in its proximity or interacting with it. Since we used a black-and-white video camera, information on the separation between the 2 surfaces was obtained on a gray scale, in which zero-order black corresponds to a distance of 4 to 12 nm and white corresponds to a distance greater than 20 to 30 nm.25,26 Structures that are separated by a distance greater than 30 nm appear as out of focus. All these experiments were recorded on S-VHS videotape. Image analysis was performed off-line using the Metamorph software package (Universal Imaging, West Chester, PA). The video clips available for viewing on the Blood website (see the Supplemental Videos link at the top of the online article) were prepared by digitizing and editing the recorded analog tapes with Adobe Premiere (Adobe Systems, San Jose, CA).
Results
The mouse GP VI gene is composed of 8 exons schematically represented in Figure 1A. The intron/exon arrangement was deduced from a comparison of the mouse GP VI cDNA sequence and a genomic clone present in GenBank, accession no. AC087129.1. A targeting vector to promote homologous recombination in murine ES cells was constructed in which a stop codon and a phosphoglycerate kinase (PGK)–neomycin resistance (neor) cassette were inserted immediately 3′ to the putative Met codon present in exon 1 (Figure 1C). Accordingly, successful homologous recombination after transfection of the targeting vector would generate ES cellular DNA in which a 7-kb HincII DNA fragment is replaced with an 8.7-kb HincII fragment (Figures 1B,D).
A total of 184 G418-resistant ES colonies were screened for homologous recombination and the recombination event depicted in Figure 1D was confirmed in 2 different colonies using additional restriction enzymes, Southern blotting, and PCR analysis. Both clones with an altered GP VI gene were chosen for microinjection into mouse blastocysts and chimeric males derived from 2 different cell lines produced germ line offspring. Mice containing heterozygous GP VI loci (GP VIhet) were bred (GP VIhet × GP VIhet) and DNA analysis of the resultant offspring revealed all 3 expected GP VI genotypes: wild-type (GPVIWT), heterozygous (GP VIhet), and homozygous-deficient (GP VInull) animals (Figure 1E). To date, we have observed no unusual ratios in the generation of expected genotypes. Thus, it appears the absence of GP VI has no impact on the fertility of mice, nor do we have any evidence the absence of GP VI impairs development or viability of the animal. As a platelet-expressed receptor, hematologic parameters were also determined and no striking differences were observed as a consequence of GP VI deficiency (data not shown).
A murine monoclonal antibody was prepared against a recombinant fragment of human GP VI and found to have cross-reactivity, albeit more weakly, with a 60-kDa protein found in mouse platelet lysates. A characterization of platelet lysates from each of the GP VI genotypes revealed a complete absence of GP VI polypeptide sequence in GP VInull platelets (Figure 1F). Similarly, a gene dosage effect was observed for the presence of GP VI alleles, as evidenced by the GP VIhet genotype containing less GP VI antigen compared with the GP VIWT (Figure 1F). An internal platelet antigen, 14-3-3ζ, was expressed at similar levels in each platelet sample and used to verify platelet proteins in each lane (Figure 1G).
The platelet GP VI polypeptide requires the presence of FcR-γ for efficient surface expression. Indeed, others have shown the inability of GP VI to be membrane expressed without a concomitant expression of FcR-γ.8,11,12 Figure 2 demonstrates that the expressed levels of FcR-γ do not change in the absence of GP VI. Whether the expressed FcR-γ reaches the membrane surface or is associated with another receptor in the absence of GP VI was not determined because of the lack of an immunologic reagent that specifically recognizes the short extracytoplasmic domain of the FcR-γ subunit.
FcR-γ levels in the absence of GP VI. Mouse platelet lysates were separated on a 4% to 20% SDS-PAGE gel under reducing conditions and immunoblotted with anti–FcR-γ chain antiserum. The same membrane was subsequently reacted with an anti–14-3-3ζ polyclonal antibody to document the approximate protein load for each lane. Both antibodies were detected by HRP goat antirabbit IgG and an enhanced chemiluminescence detection system. Also shown for comparison is the platelet lysate from FcR-γnull platelet lysates. The 14-3-3ζ is seen at 32 kDa and FcR-γ is seen at 7 kDa.
FcR-γ levels in the absence of GP VI. Mouse platelet lysates were separated on a 4% to 20% SDS-PAGE gel under reducing conditions and immunoblotted with anti–FcR-γ chain antiserum. The same membrane was subsequently reacted with an anti–14-3-3ζ polyclonal antibody to document the approximate protein load for each lane. Both antibodies were detected by HRP goat antirabbit IgG and an enhanced chemiluminescence detection system. Also shown for comparison is the platelet lysate from FcR-γnull platelet lysates. The 14-3-3ζ is seen at 32 kDa and FcR-γ is seen at 7 kDa.
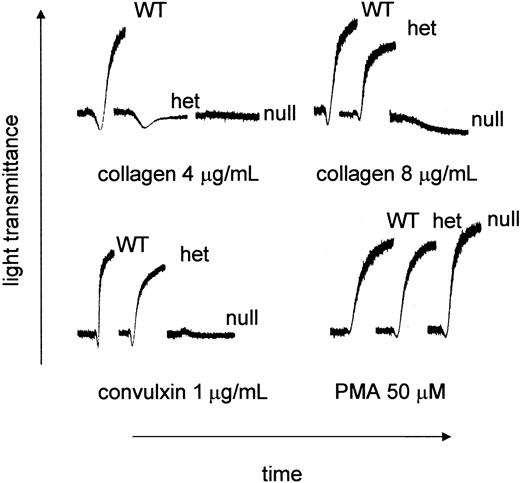
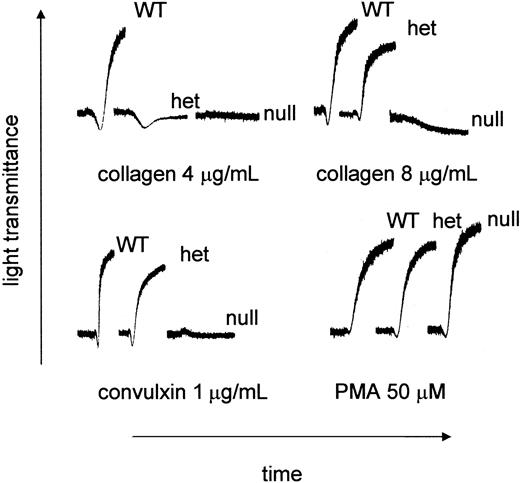
PRP was prepared from littermates produced from GP VIhet × GP VIhet crosses. The ability of type I fibrillar collagen to support aggregation was characterized using PRP from each of the genotypes. As shown in Figure 3, there was no aggregation using platelets from GP VInull animals. Platelets from the GP VIhet animals failed to aggregate using 4 μg/mL collagen, unlike their WT counterpart, yet a downward shift in the aggregation profile suggested a platelet shape change. Higher concentrations of collagen, such as 8 μg/mL, did produce aggregation using GP VIhet platelets. At the highest collagen concentration tested (100 μg/mL) no platelet aggregation was observed using GP VInull platelets (data not shown). Another property of GP VI is its ability to aggregate platelets in the presence of the snake venom protein, convulxin. Convulxin was unable to support platelet aggregation using GP VInull PRP (Figure 3). Using a nonspecific platelet agonist, PMA, platelet aggregation was indistinguishable among GP VIWT, GP VIhet, and GP VInull samples (Figure 3).
Aberrant collagen and convulxin-induced aggregation in GP VInull mice. Blood was withdrawn from GP VIWT, GP VIhet, and GP VInull mice. Mouse platelet-rich plasma was obtained by pooling blood from animals with the same genotype. The platelet number in each sample was normalized to 240 × 109/L with platelet-poor plasma. Indicated concentrations of acid-insoluble fibrillar collagen (type I), convulxin, and PMA were added to stirred platelets and the aggregation profiles are presented.
Aberrant collagen and convulxin-induced aggregation in GP VInull mice. Blood was withdrawn from GP VIWT, GP VIhet, and GP VInull mice. Mouse platelet-rich plasma was obtained by pooling blood from animals with the same genotype. The platelet number in each sample was normalized to 240 × 109/L with platelet-poor plasma. Indicated concentrations of acid-insoluble fibrillar collagen (type I), convulxin, and PMA were added to stirred platelets and the aggregation profiles are presented.
Although the platelet aggregometer identified an abnormality in GP VI–deficient mice, the extent to which that abnormality would translate to an effect on hemostasis and thrombosis is less clear. Shown in Figure 4 are tail bleeding time assays done on littermates from GP VIhet × GP VIhet crosses prior to their genotyping. The absence of GP VI does not have a major impact on the tail bleeding time.
Tail bleeding time assays. Littermates from GP VIhet × GP VIhet crosses were subjected to tail bleeding assays. A 2- to 3-mm portion of distal tail was removed from 5-week-old animals and the cessation of bleeding time was recorded. Following a determination of the bleeding time, genotype analysis was performed and the data are presented correlating the bleeding time with the genotype. Data obtained from individual animals are shown.
Tail bleeding time assays. Littermates from GP VIhet × GP VIhet crosses were subjected to tail bleeding assays. A 2- to 3-mm portion of distal tail was removed from 5-week-old animals and the cessation of bleeding time was recorded. Following a determination of the bleeding time, genotype analysis was performed and the data are presented correlating the bleeding time with the genotype. Data obtained from individual animals are shown.
To evaluate directly the role of platelet GP VI in thrombus formation, perfusion experiments were performed using whole blood obtained from GP VIWT, GP VIhet, GP VInull, and FcR-γnull animals. Platelets were labeled with fluorescent mepacrine in whole blood containing heparin and apyrase. Type I collagen (acid-insoluble) was coated on a glass surface and the blood was perfused over the surface. Representative images are shown in Figure 5 after 2.5 minutes of blood flow (Video 1, available online, provides a more detailed representation of these results). Blood from GP VIWT animals displayed large thrombi throughout the perfusion chamber reflecting the generation of platelet aggregates at all flow rates examined. Quantitation of the thrombus formed at a shear rate of 1500 s–1 revealed a volume of 3.4 × 105 μm3. Platelet adhesion for both GP VInull and FcR-γnull animals was significantly reduced to values corresponding to a background monolayer of platelets visible as individual fluorescent particles (Figure 5).
In vitro thrombus formation on surface-bound collagen. Blood was collected from anesthetized mice via a retro-orbital puncture using 40 U/mL heparin as anticoagulant. Apyrase was added to a final concentration of 1.5 U/mL. Glass coverslips were coated with insoluble fibrillar type I collagen (2.5 mg/mL) and placed in a parallel plate flow chamber. Mouse blood was treated with mepacrine for platelet visualization and perfused through the chamber at 1500/second wall shear rate. (A) Single frames taken from a continuous recording show the collagen-coated surface after 2.5 minutes of blood perfusion. In the left panel, thrombi formed by normal platelets are seen at a relatively low magnification (original magnification, × 10). The center and right panels show the surfaces exposed to GP VInull and FcR-γnull platelets, respectively, at a relatively higher magnification (original magnification, × 40); in either case, a complete surface coverage by single platelets is apparent, with formation of small clusters particularly in the case of FcR-γnull platelets but absence of thrombus formation. See Video 1 for a clearer representation of these results. (B) Postperfusion thrombus volume was determined from serial z-sections. The results presented are the mean from 3 independent experiments.
In vitro thrombus formation on surface-bound collagen. Blood was collected from anesthetized mice via a retro-orbital puncture using 40 U/mL heparin as anticoagulant. Apyrase was added to a final concentration of 1.5 U/mL. Glass coverslips were coated with insoluble fibrillar type I collagen (2.5 mg/mL) and placed in a parallel plate flow chamber. Mouse blood was treated with mepacrine for platelet visualization and perfused through the chamber at 1500/second wall shear rate. (A) Single frames taken from a continuous recording show the collagen-coated surface after 2.5 minutes of blood perfusion. In the left panel, thrombi formed by normal platelets are seen at a relatively low magnification (original magnification, × 10). The center and right panels show the surfaces exposed to GP VInull and FcR-γnull platelets, respectively, at a relatively higher magnification (original magnification, × 40); in either case, a complete surface coverage by single platelets is apparent, with formation of small clusters particularly in the case of FcR-γnull platelets but absence of thrombus formation. See Video 1 for a clearer representation of these results. (B) Postperfusion thrombus volume was determined from serial z-sections. The results presented are the mean from 3 independent experiments.
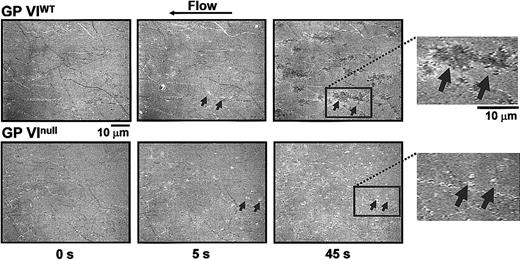
After observing a lack of thrombus formation in GP VInull platelets, we investigated the dynamics of platelet adhesion to surface-bound collagen using reflection interference contrast microscopy. This analysis allowed us to visualize the surface membrane interactions occurring during platelet adhesion. As shown in Figure 6, single platelets are visibly adhering to fibrillar collagen as early as 5 seconds after the initiation of flow (Video 2, available online). By 45 seconds, platelets from control animals have spread and several of them in close proximity form the surface-contacting base of the thrombi illustrated in Figure 5. In contrast, platelets from the GP VInull animals interacting with the collagen surface for the same duration remain round and isolated and never show the spreading that is indicative of activation. Thus, the defect in a GP VInull platelet is an inability to activate following the initial adhesion event. The inability to activate produces the platelet monolayer seen in Figure 5 and precludes platelet accumulation and formation of a thrombus.
Time course analysis of the contact interface between platelets and collagen fibrils. Blood was obtained as described in the legend to Figure 5, with the exception that no mepacrine was added. The technique of RICM, described in “Materials and methods,” allows visualization of the larger collagen fibrils immobilized on the glass bottom of the flow chamber before the beginning of blood perfusion (time 0 s). After 5 seconds of perfusion with either normal or GP VInull blood, comparable numbers of single platelets (arrows) are seen interacting with the surface. Their shape is round indicating that spreading following activation has not yet taken place. After 45 seconds, in the case of normal blood perfusion the adherent platelets have become spread and occupy a larger portion of the surface; in contrast, in the case of GP VInull blood perfusion, platelets have the same morphology as after 5 seconds, indicating that they have not become activated and, thus, have not spread. The insets to the right present a larger magnification of the surface after 45 seconds of perfusion. Note that the individual boundaries of spread platelets tend to be lost. In this technique, the darker color of spread platelets compared with those that have not spread indicates a closer proximity to the collagen fibrils. Video 2 presents a more detailed view of these events and demonstrates that the spread platelets represent the base of large thrombi attached to the collagen fibrils and protruding into the flow path.
Time course analysis of the contact interface between platelets and collagen fibrils. Blood was obtained as described in the legend to Figure 5, with the exception that no mepacrine was added. The technique of RICM, described in “Materials and methods,” allows visualization of the larger collagen fibrils immobilized on the glass bottom of the flow chamber before the beginning of blood perfusion (time 0 s). After 5 seconds of perfusion with either normal or GP VInull blood, comparable numbers of single platelets (arrows) are seen interacting with the surface. Their shape is round indicating that spreading following activation has not yet taken place. After 45 seconds, in the case of normal blood perfusion the adherent platelets have become spread and occupy a larger portion of the surface; in contrast, in the case of GP VInull blood perfusion, platelets have the same morphology as after 5 seconds, indicating that they have not become activated and, thus, have not spread. The insets to the right present a larger magnification of the surface after 45 seconds of perfusion. Note that the individual boundaries of spread platelets tend to be lost. In this technique, the darker color of spread platelets compared with those that have not spread indicates a closer proximity to the collagen fibrils. Video 2 presents a more detailed view of these events and demonstrates that the spread platelets represent the base of large thrombi attached to the collagen fibrils and protruding into the flow path.
Discussion
Platelet GP VI is a relatively recent addition to the repertoire of receptors supporting adhesion and aggregation at a wound site. The recent focus to GP VI was greatly aided by the molecular cloning of the human GP VI cDNA and gene.8-10 However, clinical cases with a presumed defect in GP VI or autoantibodies directed against GP VI were described several years earlier.15-19 To date, the molecular defects associated with these rare forms of GP VI–deficient platelets have not been defined. Thus, interpreting the mild bleeding phenotype typical of most of these patients or the potential relevance of GP VI in thrombus formation has been difficult since the molecular basis of the dysfunctional human GP VI remains unknown.
Our study was undertaken to make a predefined genetic lesion in the mouse GP VI gene and abolish synthesis of the GP VI polypeptide. The chosen strategy would place a stop codon immediately 3′ to the initiating Met codon. Upon generation of homozygous-deficient mice the absence of GP VI polypeptide was confirmed by negative results by Western blot analysis of GP VInull platelets (Figure 1F) and a complete absence of platelet aggregation induced by the snake venom protein, convulxin (Figure 3). Our preliminary expansion of the GP VInull colony has not identified any obvious phenotypic consequences of GP VI deficiency other than its role as a platelet adhesion and activation receptor. The absence of type I fibrillar collagen–induced platelet aggregation was taken as further evidence that GP VI synthesis was abolished.
Previous studies have documented the requirement of the adapter protein, FcR-γ, for efficient surface expression of GP VI.11,12 Thus, the normal levels of FcR-γ that we observe in the absence of mouse GP VI (Figure 2) might be considered surprising. Indeed, studies of human GP VI–deficient platelets have demonstrated reduced or undetectable levels of FcR-γ.11 A number of possibilities could explain the presence of FcR-γ in GP VI–deficient platelet lysates. First, it should be recognized that the GP VI–deficient platelets cited in the human study were from a patient with a novel antiplatelet antibody, presumably directed against GP VI.15 Thus, the previous study on human GP VI–deficient platelets and our mouse model represent 2 very different situations. In the GP VInull mouse model, the FcR-γ never associates with a GP VI polypeptide, whereas in the case of an immune-induced model of GP VI deficiency, an assembled GP VI–FcR-γ complex is most likely removed in its entirety from the platelet surface. The FcR-γ polypeptide can be expressed independently as confirmed by different groups using heterologous cell transfection but even this result might be misleading for conclusions on stability since the FcR-γ is overexpressed.27,28 It is interesting to see the expanding number of polypeptide chains in addition to GP VI for which FcR-γ can associate, such as members of the leukocyte inhibitory receptor (ILT) family, their murine homologues (PIRA), and the high-affinity FcγRI receptor for IgE.29 These results illustrate the utility of FcR-γ as an adapter protein for intracellular signaling whose role goes beyond GP VI and even raises the possibility that in the genetic absence of mouse GP VI, the FcR-γ polypeptide might associate with another platelet protein.
The genetic removal of GP VI from the platelet surface directly confirms the importance of GP VI in collagen-induced platelet aggregation (Figure 3). Our data demonstrate using fibrillar type I collagen that other collagen receptors do not participate in this process or this type of aggregation requires an initial interaction with GP VI that must precede the engagement of other collagen receptors. Indeed, the ablation of the integrin receptor, α2β1, in mouse platelets did not prevent aggregation in response to fibrillar collagen but did result in a lag time for the aggregation response.30,31 Perhaps the delay in aggregation seen with α2β1-deficient platelets reflects a more global change in the platelet membrane that occurs and prevents an immediate engagement of the GP VI–FcR-γ complex. This statement is supported by our result demonstrating that the aggregation observed with type I fibrillar collagen is completely dependent upon a functional GP VI receptor. The issue is different with soluble collagen, as blockage of α2β1 function with a monoclonal antibody prevents platelet thrombus formation in flowing blood exposed to immobilized pepsin-digested type I collagen but not to the same collagen renatured to form fibrils32 ; and α2β1-deficient platelets do not aggregate in the presence of enzymatically digested collagen.30 However, the physiologic relevance of soluble collagen to hemostasis and thrombosis has yet to be defined.
Antibody inhibition of mouse GP VI has previously been shown to moderately increase the tail bleeding time,33 unlike our data from the GP VInull platelet. However, this antibody was not completely effective in inhibiting aggregation using higher concentrations of fibrillar collagen,34 leading the investigators to conclude the presence of a second collagen receptor or second collagen binding site on mouse GP VI important for aggregation. Our data do not support this conclusion as we observed a complete absence of aggregation at concentrations exceeding those used by investigators using inhibitory antibodies.34 Moreover, in more recent work,35 the attainment of an antibody-induced platelet GP VI deficiency produced a lack of platelet adhesion and aggregation on a damaged vessel wall. Our results do not support a direct participation of GP VI in the initial tethering of platelets to a surface presenting collagen type I fibrils (Figures 5, 6; Videos 1-2), in agreement with the concept that essential for this process under high flow conditions is the interaction of GP Ibα with collagen-bound von Willebrand factor.3 The absence of GP VI severely impaired the transition from a transient interaction to irreversible adhesion. The latter is required for subsequent aggregation and the absence of GP VI resulted in a complete blockade of platelet spreading indicative of defective activation. Our results, therefore, are consistent with the characterization of GP Ib-IX-V as a critical platelet adhesion receptor and underlines how distinct adhesion and activation pathways provide their unique synergistic contribution to achieve an efficient progression from initial platelet tethering to stable adhesion and aggregation.
Perhaps a more intriguing problem posed by the present study is how to reconcile the required function of GP VI in platelet aggregation (Figure 3) and ex vivo thrombus growth (Figure 5) with the nonessential role for GP VI in supporting the hemostasis associated with a tail bleeding assay (Figure 4). Indeed, conclusions from each experimental assay must be carefully considered when trying to understand the physiologic impact of GPVI on normal hemostasis and thrombosis. The aggregometer can be appreciated as an experimental tool that has provided much of the fundamental knowledge on platelet function. However, the aggregometer's ability to mimic the complex processes occurring at a wound site or the site of pathologic thrombosis is limited. In this regard the aggregometer might be better viewed as a unique binding assay between platelets and their ligand with the outcome or readout being the ability of the platelets to agglutinate and aggregate. In contrast, the mechanisms controlling tail bleeding time assays are more likely global with contributions from blood coagulation, as hemophilic mice have a prolonged bleeding time,36,37 and platelet components, as absent platelet receptors GP Ib-IX or αIIbβ3 also produce prolonged bleeding times23,38 coupled to a potentially heterogeneous subendothelium. Thus, the results and conclusions from experiments performed in the aggregometer compared with the conclusions derived from the tail bleeding assay may represent 2 extreme experimental conditions examining platelet function.
Our results would suggest that GP VI is an essential receptor for thrombus growth yet its absence does not prolong the bleeding time. As an adhesive ligand of extravascular matrix, collagen has long been thought of as an essential component of thrombus formation.39 Indeed, our mouse in vivo bleeding times seem to be consistent with the apparent minor bleeding tendencies associated with the few human patients that have been characterized as GP VI deficient. Again, although the genetic defects in this minor group of individuals remain to be defined, similar results and conclusions were recently made by Goto et al40 using human blood from one of these individuals. However, we are faced with the intriguing possibility that GP VI may be more relevant for pathologic thrombus formation and less essential for normal hemostasis, an exciting possibility when considering potential targets for antithrombotic therapies.
Prepublished online as Blood First Edition Paper, May 8, 2003; DOI 10.1182/blood-2003-03-0717.
Supported by grants from the Heart, Lung, and Blood Institute of the National Institutes of Health, HL50545 (J.W.); HL46979 (T.J.K.); HL42846, HL48728, HL31950 (Z.M.R.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors acknowledge the Sam and Rose Stein Charitable Trust for the establishment of the DNA Core Facility within the Department of Molecular and Experimental Medicine at The Scripps Research Institute. The laboratory of Dr Steven Fiering (Dartmouth Medical School, Lebanon, NH) is acknowledged for the transfection and injection of mouse ES cells. The authors also acknowledge help by Rolf Habermann in the editing and generation of videos.