Abstract
In Fanconi anemia (FA) C mice tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ) have key roles in the pathogenesis of bone marrow failure. In FA subjects TNF-α was found to be increased in the serum and overproduced by patient-derived B-cell lines. In acquired aplastic anemia, a disease in which, similarly to FA, marrow failure occurs, TNF-α and IFN-γ act as late mediators of the stem cell damage and are overexpressed in patient marrow lymphocytes. This study evaluated in marrow mononuclear cells (MNCs) of patients with FA, the expression of negative modulators of the hematopoiesis, such as TNF-α, IFN-γ, macrophage inflammatory protein 1α (MIP-1α), and surface Fas ligand, and the role of TNF-α on FA erythropoiesis in vitro. TNF-α and IFN-γ were significantly overexpressed in stimulated marrow MNCs of FA patients as compared to healthy controls. MIP-1α and Fas ligand were undetectable in patients and controls. In bone marrow cultures, the addition of anti–TNF-α increased the size and significantly increased the number of erythroid colony-forming units and erythroid burst-forming units grown from FA patients but not from healthy controls. This indicates that FA subjects have a marrow TNF-α activity that inhibits erythropoiesis in vitro. TNF-α has a relevant role in the pathogenesis of erythroid failure in FA patients.
Introduction
Fanconi anemia (FA) is a rare genetic disease characterized by bone marrow (BM) failure, somatic abnormalities, susceptibility to cancer, and chromosomal fragility. At least 8 complementation groups have been identified (A, B, C, D1, D2, E, F, G) and 7 genes (A, C, D2, E, F, G, and BRCA2, which has been reported to correspond to B and D1) have been cloned so far.1-3 FA gene products interact and form an intracellular complex that is mainly involved in DNA damage cell repair machinery.2
BM failure represents the major cause of morbidity and mortality in patients with FA. The currently available information on its pathogenesis is mostly derived from Fanconi anemia C (FANCC) mice models.4-6 These data indicate that the myelosuppressive cytokines interferon γ (IFN-γ) and tumor necrosis factor α (TNF-α) induce the death of hematopoietic progenitor cells through increased apoptosis, which occurs at very low cytokine concentrations4-6 and is, in part, mediated by Fas.6,7 The scanty information available in humans indicates that TNF-α is increased in the serum of FA patients8,9 and overproduced by lymphoblastoid cell lines derived from the patients themselves.9
TNF-α and IFN-γ are known to be the late effectors of the damage of the hematopoietic stem cell compartment that occurs in aplastic anemia (AA), an acquired disease that shares with FA the development of BM failure.10-12 The expression of myelosuppressive cytokines in BM cells from FA patients has not yet been investigated nor has the role of these cytokines in the pathogenesis of BM failure been addressed.
In the present study we assessed whether negative modulators of hematopoiesis, such as TNF-α, IFN-γ, macrophage inflammatory protein 1α (MIP-1α), and surface Fas ligand, were involved in the pathogenesis of BM failure in FA. To this end we tested, by flow cytometric analysis, the expression of TNF-α, IFN-γ, MIP-1α, and Fas ligand, in mononuclear cells (MNCs) isolated from the BM of 7 FA patients and 8 healthy controls. We also investigated the in vitro effects of TNF-α neutralization on the growth of erythroid progenitors from BM MNCs of FA patients and controls.
Patients, materials, and methods
Study subjects
Patients were recruited between December 1999 and February 2001. Patients were eligible if they had a confirmed diagnosis of FA, did not previously receive a BM transplant, and were infection free 4 weeks before and after the study. Controls were selected over the same time span among voluntary BM donors who had to match the same “infection-free” criteria as did the patients. The study protocol was approved by the Ethics Committee of the G. Gaslini Children's Hospital and by the committees of the other institutions who participated in the project.
BM sampling
BM cells were obtained from patients and controls after informed consent was given either by the parents or, whenever applicable, by the study subjects themselves in conformity with the Declaration of Helsinki principles.
BM samples from the patients were taken at routine diagnostic investigation or follow-up evaluation as required by the disease. In most cases marrow aspiration was performed with the patients under deep sedation. No marrow aspirations were ever performed for the purpose of this study.
Normal BM samples from voluntary healthy donors were obtained, under general anesthesia, during the procedure of marrow harvest for marrow donation. No marrow aspirations were ever performed for the purpose of this study.
BM cell collection and processing
BM MNCs from both patients and healthy controls were isolated on Ficoll-Hypaque density gradients (Pharmacia, Uppsala, Sweden). Approximately 2 × 106 cells/mL were obtained at the end of this procedure from FA patients.
After washing in phosphate-buffered saline (PBS; Sigma, St Louis, MO) supplemented with 1% fetal bovine serum (FBS; Sigma), MNCs were frozen in a solution containing 50% RPMI 1640 medium, 40% FBS, and 10% dimethyl sulfoxide (DMSO; Sigma), stored in liquid nitrogen, and then thawed.
Flow cytometric detection of intracellular cytokines
BM MNCs were cultured for 5 hours at 37°C in a 5% CO2 atmosphere in RPMI 1640 medium supplemented with 10% FBS in the presence of 50 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma), 250 ng/mL calcium ionophore (A23187; Sigma), and 5 μg/mL brefeldin A (Sigma), which inhibits intracellular transport of cytokines into the Golgi complex thus impeding the release of cytokines in the extracellular environment.
The choice of PMA and calcium ionophore for cell stimulation was dictated by the need to activate all cell types present in the BM MNC fraction, irrespective of the CD3+ or CD3– immunophenotype, to assess their contribution to the production/expression of tested hematopoietic modulators. After culture, cells were washed twice in PBS with 1% FBS and subsequently stained for 30 minutes at 4°C with the following murine monoclonal antibodies (mAbs): anti-CD3–tricolor (Caltag, Burlingame, CA) and unconjugated anti-CD178 (Fas ligand; BD Pharmingen, San Diego, CA). Fluorescein isothiocyanate (FITC)–conjugated antimouse IgG secondary mAb was purchased from BD Pharmingen. In all the experiments, to minimize background staining, Fc receptor blockade was achieved by incubation of cells with excess purified human immunoglobulin before staining. Stained cells were resuspended for 20 minutes at 4°C in Cytofix/Cytoperm fixation/permeabilization solution (Caltag), according to the manufacturer's instructions. Once permeabilized, cells were washed twice and stained for intracellular cytokines with the following mAbs: mouse anti-IFNγ–phycoerythrin (PE; Caltag), rat anti–TNF-α–PE (Caltag), and mouse anti–MIP-1α–PE (BD Pharmingen). Negative controls were PE-conjugated isotype-matched mAbs of irrelevant specificity and a ligand-blocking control in which fluorochrome-conjugated antibodies were preincubated with appropriate concentrations of matched recombinant cytokines, according to the manufacturer's instructions. In some experiments freshly isolated BM MNCs from patients and controls were tested for intracellular expression of TNF-α and IFN-γ omitting incubation with PMA, calcium ionophore, and brefeldin A.
Immunophenotypic dissection of BM MNCs according to the expression of the CD 3 marker was carried out because CD3+ T cells are known to elaborate the tested cytokines, in healthy individuals and in patients with other BM failure diseases, like AA.10-12 Flow cytometric analysis was performed using a FACScan cytometer (Becton Dickinson, San Jose, CA). Gates were selected on the basis of the CD3 staining. Results were expressed as: (1) percentage of CD3+ cells and CD3– cells and (2) mean ratio of relative fluorescence intensity (MRFI), calculated on the same cell fractions, as follows: mean fluorescence intensity (MFI) of cytokine staining/MFI of irrelevant isotype-matched mAb staining. MRFI was used to roughly quantitate the amount of target antigen expressed.
Clonogenic assay
The in vitro assay for the growth of erythroid colony- and burst-forming units (CFU-Es and BFU-Es, respectively) was performed using methyl cellulose media (MethoCult; StemCell Technologies, Vancouver, BC, Canada). In brief, 1 × 105 BM MNCs/dish were tested in triplicate (ie, a total of 3 × 105 seeded BM MNCs) in semisolid cultures containing 0.9% methyl cellulose, 30% FBS, 1% bovine serum albumin, 10–4 M 2-mercaptoethanol, and 2 mM l-glutamine. To support erythroid colony growth, 50 ng/mL recombinant human stem cell factor (rhSCF), 20 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF), 20 ng/mL recombinant human interleukin 3 (rhIL-3), 20 ng/mL rhIL-6, 20 ng/mL recombinant human granulocyte colony-stimulating factor (rhG-CSF), and 3 U/mL erythropoietin were added to the culture system (StemCell). Cultures were incubated for 7 to 9 days (CFU-Es) and 14 to 16 days (BFU-Es) under humidified conditions in a 5% CO2 atmosphere at 37°C, in the presence and in the absence of 1 or 10 μg/mL of the recombinant soluble human tumor necrosis factor (TNF) receptor-Fc fusion protein etanercept (Immunex, Seattle, WA). In this protein, the human p75 TNF receptor is fused with the Fc portion of human IgG1. Control cultures were carried out in the presence of the purified Fc fragment of human IgG1 (Alexis, Del Mar, CA).
CFU-Es and BFU-Es from both patients and controls were counted at the indicated times. The term CFU-E was used to identify progenitors that give rise to isolated single or paired clusters of 5 to approximately 100 sufficiently hemoglobinized erythroblasts. These colony numbers peak after 7 to 9 days of incubation and were scored at this time. The term BFU-E was used to identify more immature progenitors that give rise to larger, burst-configured erythroid colonies made up of 3 to 8 closely arranged clusters of hemoglobinized erythroblasts per colony, whose number peaks after 14 to 16 days of incubation.
Statistical analysis
Because of the small sample size of the groups of FA patients and of healthy controls, the nonparametric Mann-Whitney U test was used to compare the 2 groups for BM intracytoplasmic cytokine expression. The same test was also used to compare cases and controls for the increase of the CFU-Es and BFU-Es (expressed as percentage of increase with respect to baseline) after the addition of anti–TNF-α fusion protein to the cultures. Furthermore, the Wilcoxon test was used to evaluate the effect of the addition of anti–TNF-α on the growth of CFU-Es and BFU-Es expressed as number of colonies after 7 and 14 days, both in cases and controls.
For each subject the percentage of increment of CFU-E and BFU-E growth with respect to baseline conditions was calculated by subtracting the baseline number of colonies from the number obtained after the addition of TNF-α fusion protein. This figure was subsequently divided by the baseline number of colonies and then multiplied by 100. The median value for patients and controls was calculated and reported.
The Cohen K coefficient was used to evaluate the degree of concordance between intracytoplasmic TNF-α expression and colony growth after addition of anti–TNF-α.13,14
Statistical significance was defined as P < .05. All the statistical tests were 2-sided. The Statistica Version 6.0 software was used to perform all the analyses (StatSoft, Statistica, Tulsa, OK).
Results
Eight FA patients entered the study; one of them developed an infection a week after the sampling and was then excluded from the analysis. This left 1 boy and 6 girls with FA eligible and evaluable for the study. Their median age was 8 years (mean, 9 years; range, 2-16 years) and their complementation group was A in 4 patients (UPN 1, 3, 5, 6), C in 1 (UPN 7) and unknown in the remaining 2 (UPN 2 and 4). Table 1 reports on their most relevant hematologic characteristics at the time of the study.
During the same period, 8 voluntary BM donors (3 boys, 5 girls) agreed to be included in the study. At study entry their median age was 3 years (mean, 6.3 years; minimum, 1 year; maximum, 16 years). Their median hematologic values were within the normal range for their age (white blood cell [WBC] count, 9.150 × 103/μL; polymorphonuclear [PMN] cell count, 3.058 × 103/μL; hemoglobin [Hb] level, 13.2 g/dL; platelet count, 293 × 103/μL). The 2 groups were comparable for age at study entry (P = .29).
TNF-α and IFN-γ intracytoplasmic expression
Table 2 reports on TNF-α and IFN-γ expression in stimulated marrow MNCs from FA patients and controls. Data are presented both as percentage of positive cells and as MRFI in all analyzed subsets of marrow MNCs (“Flow cytometric detection of intracellular cytokines”).
In the FA patients the TNF-α content of all tested marrow MNC subsets, both if expressed as percentage of positive cells and as MRFI, were increased as compared to the control group in whom almost no cytokine was detectable. The difference was statistically significant in all the combinations (P = .006 for TNF-α+/CD3+ cells both as percentage of positive cells and as MRFI; P = .016 for TNF-α+/CD3– cells as percentage of positive cells; P = .045 for TNF-α+/CD3– cells as MRFI; Table 2).
The expression of IFN-γ in the patient group was higher than that found in the controls in all tested marrow cellular subsets. Statistical significance was achieved in 3 of the 4 assessed combinations: IFN-γ+/CD3+ cells/percent of positive cells (P = .013); IFN-γ+/CD3+ cells/MRFI (P = .021); IFN-γ+/CD3– cells/percent of positive cells (P = .014; Table 2).
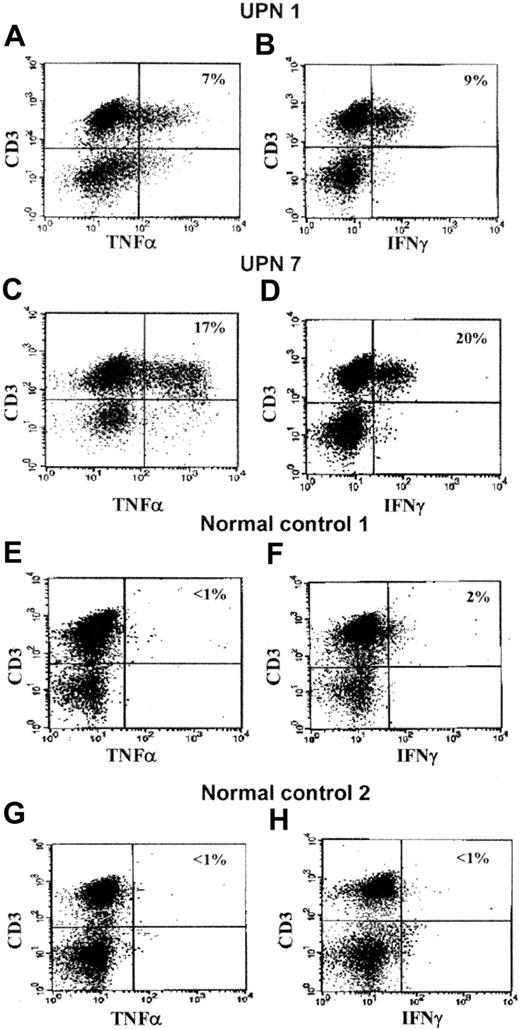
Representative scattergrams of TNF-α and IFN-γ expression in CD3+ BM MNCs obtained from 2 patients and 2 controls are shown in Figure 1. Detailed results of percentages of positive cells and MRFI values of individual patients are provided in Figure 2.
Examples of intracytoplasmic expression of TNF-α and IFN-γ in CD3+BM MNCs from 2 representative FA patients (UPN 1 and 7) and 2 representative healthy control subjects. As shown in Table 2 and Figure 2, TNF-α and IFN-γ were also expressed in CD3– cells. In the top right corner of each scattergram the percentage of positive BM cells is represented. UPN 1: (A) CD3+/TNF-α+ cells, 7%; (B) CD3+/IFN-γ+ cells, 9%. UPN 7: (C) CD3+/TNF-α+ cells, 17%; (D) CD3+/IFN-γ+ cells, 20%. Healthy control 1: (E) CD3+/TNF-α+ cells, less than 1%; (F) CD3+/IFN-γ+ cells, 2%. Healthy control 2: (G) CD3+/TNF-α+ cells, less than 1%; (H) CD3+/IFN-γ+ cells, less than 1%.
Examples of intracytoplasmic expression of TNF-α and IFN-γ in CD3+BM MNCs from 2 representative FA patients (UPN 1 and 7) and 2 representative healthy control subjects. As shown in Table 2 and Figure 2, TNF-α and IFN-γ were also expressed in CD3– cells. In the top right corner of each scattergram the percentage of positive BM cells is represented. UPN 1: (A) CD3+/TNF-α+ cells, 7%; (B) CD3+/IFN-γ+ cells, 9%. UPN 7: (C) CD3+/TNF-α+ cells, 17%; (D) CD3+/IFN-γ+ cells, 20%. Healthy control 1: (E) CD3+/TNF-α+ cells, less than 1%; (F) CD3+/IFN-γ+ cells, 2%. Healthy control 2: (G) CD3+/TNF-α+ cells, less than 1%; (H) CD3+/IFN-γ+ cells, less than 1%.
TNF-α and IFN-γ intracytoplasmic expression in BM MNCs in individual FA patients. CD3 phenotypically defined MNCs were chosen because they contain T cells that in healthy individuals and in other marrow failure diseases such as AA10-12 are known to elaborate the tested cytokines. TNF-α and IFN-γ expression are provided for each patient, both as percentage of positive cells and as MRFI, in each analyzed marrow cellular subset (▪ CD3+ cells, ▨ CD3– cells). (A) TNF-α intracytoplasmic expression shown as percentage of cells in the cellular subsets (CD3+, CD3– cells) expressing this cytokine. (B) TNF-α intracytoplasmic expression shown as MRFI of cells in the cellular subsets (CD3+, CD3– cells) expressing this cytokine. (C) IFN-γ intracytoplasmic expression shown as percentage of cells in the cellular subsets (CD3+, CD3– cells) expressing this cytokine. (D) IFN-γ intracytoplasmic expression shown as MRFI of cells in the cellular subsets (CD3+, CD3– cells) expressing this cytokine.
TNF-α and IFN-γ intracytoplasmic expression in BM MNCs in individual FA patients. CD3 phenotypically defined MNCs were chosen because they contain T cells that in healthy individuals and in other marrow failure diseases such as AA10-12 are known to elaborate the tested cytokines. TNF-α and IFN-γ expression are provided for each patient, both as percentage of positive cells and as MRFI, in each analyzed marrow cellular subset (▪ CD3+ cells, ▨ CD3– cells). (A) TNF-α intracytoplasmic expression shown as percentage of cells in the cellular subsets (CD3+, CD3– cells) expressing this cytokine. (B) TNF-α intracytoplasmic expression shown as MRFI of cells in the cellular subsets (CD3+, CD3– cells) expressing this cytokine. (C) IFN-γ intracytoplasmic expression shown as percentage of cells in the cellular subsets (CD3+, CD3– cells) expressing this cytokine. (D) IFN-γ intracytoplasmic expression shown as MRFI of cells in the cellular subsets (CD3+, CD3– cells) expressing this cytokine.
Intracellular cytokine expression in uncultured BM MNCs from 5 patients (UPN 1, 4-7) and 5 healthy controls was also investigated. The results of these experiments were consistently negative.
MIP-1α intracytoplasmic and Fas ligand surface expression
Neither the patients nor the controls expressed intracytoplasmic MIP-1α or surface Fas ligand in stimulated CD3+ and CD3– marrow cells. This occurred both when cytokine expression was evaluated as percentage of positive cells and as MRFI.
Colony studies
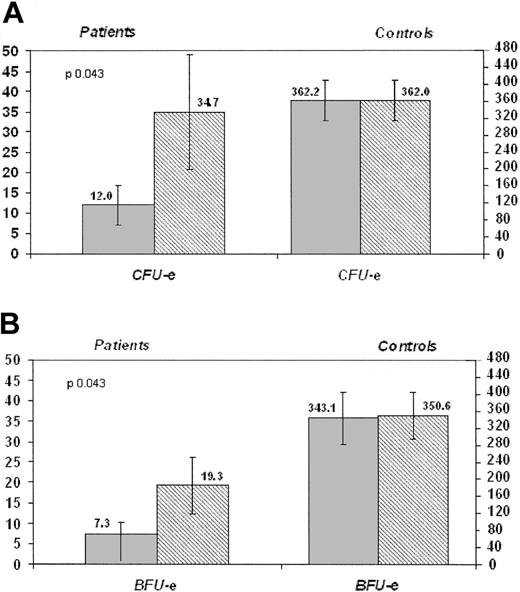
CFU-Es. In the patients, the mean number of CFU-Es (colonies/3 × 105 seeded BM MNCs) grown in the absence of anti–TNF-α was 12.0 (median, 9.0; minimum, 0; maximum, 37; SE, 4.8), whereas in the normal control group it was 362.2 (median, 353.5; minimum, 102; maximum, 522; SE, 47.3). In both groups no differences were seen when colonies were grown in the presence of purified Fc fragment of human IgG1 (data not shown).
When low-dose (LD = 1μg/mL) and high-dose (HD = 10μg/mL) anti–TNF-α fusion protein was added to the culture samples from the FA patients, the mean number of CFU-Es significantly increased to 34.7 (median, 19.0; minimum, 0; maximum, 100; SE, 14.1; P = .043) (Figure 3A) and 38.1 (median, 15.0; minimum, 0; maximum, 96; SE, 15.0; P = .028), respectively. On the other hand, in the control group, the addition of anti–TNF-α fusion protein did not increase the mean number of colonies as compared to baseline values (362.2) both at LD (362.0; median, 349.0; minimum, 96; maximum, 525; SE, 48.0; Figure 3A) and at HD (340.0; median, 346.0; minimum, 81; maximum, 528; SE, 62.7; P = .944 and P = .075, respectively).
Effect of anti–TNF-α on the growth of erythroid committed progenitors in FA patients and controls. (A) Each column represents mean values; error bars indicate the SE of the estimate. We also reported in brackets median, minimum, and maximum values. In the patients, CFU-Es (mean, 34.7; median, 19.0; minimum, 0; maximum, 100) grown after the addition of LD anti–TNF-α (1 μg/mL) significantly outnumbered (P = .043) that grown in the absence of anti–TNF-α (mean, 12.0; median, 9.0; minimum, 0; maximum, 37; left side). A similar significant difference was obtained with HD anti-TNF-α (10 μg/mL; P =.028; diagram not shown). The addition of anti–TNF-α had no effect on the growth of CFU-Es in healthy controls (mean number of colonies without LD anti–TNF-α, 362.2; median, 353.5; minimum, 102; maximum, 522; mean number of colonies with LD anti–TNF-α, 362.0; median, 349.0; minimum, 96; maximum, 525; right side). (B) Each column represents mean values; error bars indicate the SE of the estimate. We also reported in brackets median, minimum, and maximum values. In the patients, BFU-Es grown after the addition of LD anti–TNF-α (mean, 19.3; median, 12.0; minimum, 0; maximum, 45) significantly outnumbered (P =.043) that grown in the absence of anti–TNF-α (mean, 7.3; median, 6.0; minimum, 0; maximum, 24; left side). The same held true, with the identical significance (P = .043), also for HD anti–TNF-α (diagram not shown). The addition of anti–TNF-α had no effect on the growth of BFU-Es in healthy controls (mean number of colonies without LD anti–TNF-α, 343.1; median, 336.5; minimum, 66; maximum, 691; mean number of colonies with LD anti–TNF-α, 350.6; median, 332.5; minimum, 142; maximum, 695; right side).  indicates no anti–TNF-α; ▦ with anti–TNF-α (1 μg/mL).
indicates no anti–TNF-α; ▦ with anti–TNF-α (1 μg/mL).
Effect of anti–TNF-α on the growth of erythroid committed progenitors in FA patients and controls. (A) Each column represents mean values; error bars indicate the SE of the estimate. We also reported in brackets median, minimum, and maximum values. In the patients, CFU-Es (mean, 34.7; median, 19.0; minimum, 0; maximum, 100) grown after the addition of LD anti–TNF-α (1 μg/mL) significantly outnumbered (P = .043) that grown in the absence of anti–TNF-α (mean, 12.0; median, 9.0; minimum, 0; maximum, 37; left side). A similar significant difference was obtained with HD anti-TNF-α (10 μg/mL; P =.028; diagram not shown). The addition of anti–TNF-α had no effect on the growth of CFU-Es in healthy controls (mean number of colonies without LD anti–TNF-α, 362.2; median, 353.5; minimum, 102; maximum, 522; mean number of colonies with LD anti–TNF-α, 362.0; median, 349.0; minimum, 96; maximum, 525; right side). (B) Each column represents mean values; error bars indicate the SE of the estimate. We also reported in brackets median, minimum, and maximum values. In the patients, BFU-Es grown after the addition of LD anti–TNF-α (mean, 19.3; median, 12.0; minimum, 0; maximum, 45) significantly outnumbered (P =.043) that grown in the absence of anti–TNF-α (mean, 7.3; median, 6.0; minimum, 0; maximum, 24; left side). The same held true, with the identical significance (P = .043), also for HD anti–TNF-α (diagram not shown). The addition of anti–TNF-α had no effect on the growth of BFU-Es in healthy controls (mean number of colonies without LD anti–TNF-α, 343.1; median, 336.5; minimum, 66; maximum, 691; mean number of colonies with LD anti–TNF-α, 350.6; median, 332.5; minimum, 142; maximum, 695; right side).  indicates no anti–TNF-α; ▦ with anti–TNF-α (1 μg/mL).
indicates no anti–TNF-α; ▦ with anti–TNF-α (1 μg/mL).
The mean percentage of increment with respect to baseline observed in the patients after the addition of both LD (206%; median, 89.2%) and HD (257.7%; median, 66.7%) anti–TNF-α fusion protein was significantly greater than the no increment (–0.60%; median, –0.51% with LD; –4.41%; median, –1.85% with HD) seen in the healthy controls (P = .011 and P = .0043 for LD and HD anti–TNF-α, respectively).
Noteworthy, the addition of LD and HD anti–TNF-α also resulted in an increase of the size of the erythroid colonies grown at day 7 (Figure 4A), whereas this effect was not seen in healthy controls.
Effect of anti–TNF-α on erythroid-committed progenitor growth in FA patients. (A) CFU-Es grown in the absence of anti–TNF-α are shown (i,ii; medium alone). The size of CFU-Es cultured in the absence of anti–TNF-α but in the presence of purified Fc fragment of human IgG1 were not different (not shown). The addition of LD (1 μg/mL) anti–TNF-α (iii,iv) increased the size of CFU-Es grown from marrow MNCs of FA patients. A similar effect was seen with HD (10μg/mL) anti–TNF-α (not shown). (B) BFU-Es grown in the absence of anti–TNF-α are shown (i,ii; medium alone). The size of BFU-Es cultured in the absence of anti–TNF-α but in the presence of purified Fc fragment of human IgG1 were not different (not shown). Panel Bii shows a minor nonhemoglobinized component at the periphery. The addition of LD anti–TNF-α (iii,iv) increased the size of BFU-Es grown from BM MNCs of FA patients. A similar effect was seen with HD anti–TNF-α (not shown).
Effect of anti–TNF-α on erythroid-committed progenitor growth in FA patients. (A) CFU-Es grown in the absence of anti–TNF-α are shown (i,ii; medium alone). The size of CFU-Es cultured in the absence of anti–TNF-α but in the presence of purified Fc fragment of human IgG1 were not different (not shown). The addition of LD (1 μg/mL) anti–TNF-α (iii,iv) increased the size of CFU-Es grown from marrow MNCs of FA patients. A similar effect was seen with HD (10μg/mL) anti–TNF-α (not shown). (B) BFU-Es grown in the absence of anti–TNF-α are shown (i,ii; medium alone). The size of BFU-Es cultured in the absence of anti–TNF-α but in the presence of purified Fc fragment of human IgG1 were not different (not shown). Panel Bii shows a minor nonhemoglobinized component at the periphery. The addition of LD anti–TNF-α (iii,iv) increased the size of BFU-Es grown from BM MNCs of FA patients. A similar effect was seen with HD anti–TNF-α (not shown).
BFU-Es. In the patients, the mean number of BFU-Es (colonies/3 × 105 seeded BM MNCs) grown in the absence of anti–TNF-α was 7.3 (median, 6.0; minimum, 0; maximum, 24; SE, 3.1), whereas in the healthy controls the mean number of colonies was 343.1 (median, 336.5; minimum, 66; maximum, 691; SE, 60.9). In both groups no differences were seen when colonies were grown in the presence of purified Fc fragment of human IgG1.
The addition of anti–TNF-α fusion protein to the culture of the patients resulted in a significant increase of the mean number of BFU-Es to 19.3 (median, 12.0; minimum, 0; maximum, 45; SE, 6.9) with LD (Figure 3B) and to 16.1 with HD (median, 12.0; minimum, 0; maximum, 49; SE, 6.7) anti–TNF-α fusion protein (P = .043). On the other hand, in the healthy controls the addition of anti–TNF-α fusion protein did not significantly increase the mean number of the colonies as compared to baseline values (343.1) both at LD (mean, 350.6; median, 332.5; minimum, 142; maximum, 695; SE, 55.7; Figure 3B) and at HD (mean, 369.0; median, 326.5; minimum, 199; maximum, 689; SE, 68.6).
In the patients, the mean percentage of increment observed after the addition of both LD (175.8%; median, 71.4%) and HD (92.4%; median, 100%) anti–TNF-α was significantly greater for both LD (P = .028) and HD (P = .045) than that seen in the healthy controls (13.7%; median, –1.17% for LD; 32.2%; median, –1.06% for HD).
Moreover, anti–TNF-α was also able to induce a remarkable increase in the size of BFU-Es (Figure 4B), an effect not seen in healthy controls.
In the patient group the growth of granulocyte-macrophage colony-forming units (CFU-GMs) in baseline conditions was notably reduced in comparison to that of erythroid-committed progenitors. This impeded the use of CFU-GM data for appropriate comparisons with anti–TNF-α conditions and with healthy controls.
When looking at patients individually, the observed degree of agreement between the intracytoplasmic TNF-α expression and the colony growth with anti–TNF-α was 71.4% (5 of 7 concordant and 2 of 7 nonconcordant), corresponding to a fair degree of concordance (k = 0.30). In particular, in 4 patients (UPN 1, 2, 3, 7) the increase of intracytoplasmic TNF-α was associated with an enhancement in the number of erythroid colonies grown in the presence of either LD or HD anti–TNF-α. In another patient (UPN 4) with undetectable intracytoplasmic TNF-α in BM MNCs, the addition of anti–TNF-α had no effect on erythroid colony growth. Finally there were 2 nonconcordant patients. In one patient (UPN 6) the increase of intracellular TNF-α was not accompanied by a rise in the colony growth with anti–TNF-α. This patient had advanced marrow failure and severely reduced hematopoiesis that led him to marrow transplantation shortly after the test. In the remaining nonconcordant patient (UPN 5), no intracytoplasmic TNF-α was detected, but a rise of colony growth with anti–TNF-α was observed.
Discussion
Our study provides the first evidence that in vitro-stimulated BM MNCs from FA patients overexpress TNF-α and IFN-γ and that in marrow cultures of FA patients there is a TNF-α activity that inhibits erythropoiesis in vitro. We have also found that other negative modulators of hematopoiesis, such as MIP-1α and surface Fas ligand, are not overexpressed in stimulated BM MNC fractions from FA patients.
TNF-α and IFN-γ were not detected in uncultured and unstimulated BM MNCs. This finding may indicate that an additional stress/stimulus is required to detect increased intracellular cytokines. Another possible explanation might be that uncultured BM MNCs, differently from stimulated cells, were not treated with brefeldin A, whose function is that of blocking the release of cytokines outside the cells. Thus it cannot be excluded that, in the absence of brefeldin A, newly synthesized TNF-α and IFN-γ were rapidly secreted in the extracellular milieu and were not detectable intracellularly.
Because all analyzed patients had not had transfusions and were infection free at least 4 weeks before and after the study, the increased expression of IFN-γ and TNF-α after PMA stimulation in the BM cannot be linked to infections and transfusion and should therefore be considered as a disease-related characteristic.
A previous study demonstrated that the IFN-γ mRNA was not increased in unstimulated BM MNCs from FA patients.12 It is possible that the short-term stimulation that cells underwent in our experiments may account for the IFN-γ overexpression we detected in our study. It has to be noted, however, that normal BM MNCs stimulated under the same conditions did not accumulate IFN-γ in their cytoplasm.
The marrow overexpression of TNF-α we demonstrated following stimulation agrees with previous data showing that this cytokine is increased in the serum of FA patients8,9 and overproduced by lymphoblastoid cells derived from patients themselves.9 It is unknown whether this increased TNF-α BM content reflects the condition of increased apoptosis of FA cells or is a consequence of the genetic defect.
Whatever the mechanism responsible for TNF-α and IFN-γ excess in stimulated FA marrow cells, it is of note that these cytokines are overexpressed in marrow lymphocytes from acquired AA,10 a disease that shares with FA the occurrence of BM failure. In acquired AA, autoreactive T cells are activated by an antigenic stimulus and release, among other cytokines, TNF-α and IFN-γ that act as the late effectors of the damage on the stem cell compartment.11 No evidence so far indicates that in FA an immune activation stimulates marrow MNCs to hyperproduce IFN-γ and TNF-α. Nevertheless, their presence in the marrow seems consistent with a possible role for these cytokines in the pathogenesis of marrow failure also in FA.
To further investigate this issue, we grew marrow-committed progenitor cells in semisolid assay in the presence and in the absence of etanercept, an anti–TNF-α fusion protein used in the treatment of autoimmune diseases.15 The shortage of cells allowed us to grow only erythroid-committed progenitors and only with an anti–TNF-α but not with an anti-IFN-γ antagonist.
In the patients, at variance from controls, we found that etanercept provoked a significant rise of erythropoiesis in vitro, as shown both by the increase of the number and of the size of CFU-Es and BFU-Es with respect to values detected in the absence of anti–TNF-α. FA subjects had many fewer baseline colonies than healthy individuals, but the increase after TNF-α neutralization indicated that in FA marrow cells there was a TNF-α effect, lacking in healthy controls, that significantly inhibited erythropoiesis in vitro. We did not measure TNF-α production from FA marrow cultures; therefore whether this effect reflects an overproduction or an increased sensitivity to TNF-α of FA versus normal marrow progenitor cells still remains an open question.
The fair concordance between TNF-α intracellular expression and recovery of erythroid colony growth following etanercept may suggest that the TNF-α activity is, to some extent, attributable to the TNF-α production by marrow MNCs. In the single patient (UPN 5) in whom the rise of BFU-Es and CFU-Es with anti–TNF-α was not associated with an increase of intracytoplasmic TNF-α in the BM cells, we can hypothesize that etanercept blocked TNF-β, which binds to the same receptors as TNF-α.16 Another plausible explanation for UPN 5's responsiveness to anti–TNF-α resides in the differences between the intracytoplasmic assay (5 hours of treatment with PMA and heterogeneous cell population read-out) versus progenitor assay (7-14 days of stimulatory cytokines and progenitor read-out).
All together, the TNF-α results indicate that this cytokine has a relevant role in the pathogenesis of erythropoietic failure of FA. We did not test the growth of myeloid- and megakaryocytic-committed progenitors, but it is possible that TNF-α has a similar suppressive effect on these marrow lineages as well as on the stem cell compartment. We could not test whether there was a similar IFN-γ–mediated myelosuppressive effect, which seems logical to hypothesize given that IFN-γ was also overexpressed in stimulated marrow MNCs.
This marrow TNF-α activity may have some clinical implications. Indeed the demonstration of the TNF-α myelosuppressive effect in vitro may identify those FA subjects with marrow failure who, through treatment with anti–TNF-α, may have a positive effect on erythroid and possibly other hematopoietic lineages. However, the potential value of anti–TNF-α needs to be confirmed in other studies before entering the established care of FA patients.
As for the other negative modulators of the hematopoiesis we have assessed, MIP-1α and surface Fas ligand were not detectable in patients or healthy controls. Very low concentrations of MIP-1α exerted a myelosuppressive effect in FANCC mice.6 Although this cytokine was not expressed in stimulated BM MNCs from patients, a role for MIP-1α in the origin of BM failure in FA subjects cannot be excluded, because it is still possible that FA cells are hypersensitive to amounts of MIP-1α below the threshold of detection of our experimental setting.
The lack of expression of surface Fas ligand on marrow MNCs does not exclude the existence of a Fas-mediated apoptosis in FA cells4,7 because CD 34+ cells from FA patients have been shown to express constitutively Fas ligand.4 In our study, failure to detect surface Fas ligand plus MNCs is most likely due to the paucity of CD34+ cells in patients with FA.
In summary, our study shows that in stimulated BM MNCs of patients with FA there is an overexpression of the myelosuppressive cytokines TNF-α and IFN-γ and that marrow cultures from FA subjects produce a TNF-α activity that suppresses erythropoiesis in vitro. These data provide further insight into the pathogenesis of marrow failure in FA and may represent an experimental support for future clinical trials investigating the possibility of treating selected FA patients with anti–TNF-α.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2003-01-0114.
Supported in part by Associazione Italiana per la Ricerca sull'Anemia di Fanconi (AIRFA), Associazione Bambino Emopatico ed Oncologico (ABEO), Barncancerfonden, Sweden, Stiftelsen Samariten Stockholm, Sweden, and SAAR depositi oleari portuali Genova, Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Ms Sara Calmanti and Ms Francesca Garaventa are acknowledged for secretarial assistance in the preparation of the tables and figures of this manuscript.