Abstract
Integrin α2β1 is the principal adhesive receptor for collagen but platelets also adhere through glycoprotein VI (GPVI). Integrin αIIbβ3 may augment platelet adhesion. We have shown that disulfide exchange is necessary for platelet adhesion to fibrinogen, fibronectin, and collagen. However 2 questions remained: (1) Can activated αIIbβ3 explain the observed role of disulfide exchange in adhesion to collagen, or is this role common to other integrins? (2) Is disulfide dependence specific to the integrin receptors or shared with GPVI? To discriminate adhesive functions of α2β1 from those of αIIbβ3 we used Glanzmann platelets and αIIbβ3-specific antibodies applied to normal platelets. To resolve adhesive events mediated by α2β1 from those of GPVI we used synthetic peptides specific to each receptor. We addressed direct integrin ligation using purified α2β1 and recombinant I domain. We observed the following: adhesion to the α2β1-specific peptide was disulfide-exchange dependent and protein disulfide isomerase (PDI) mediated; membrane-impermeant thiol blockers inhibited α2β1, but not GPVI mediated, adhesion; direct blockade of PDI revealed that it is involved in adhesion through α2β1 but not GPVI; and purified α2β1, but not recombinant I domain, depended on free thiols for ligation. These data suggest that the enzymatically catalyzed adhesion-associated reorganization of disulfide bonds is common to members of the integrin family and specific to this family.
Introduction
The integrins, widely expressed adhesive receptors, undergo conformational changes subsequent to ligand binding.1 Recent reports document involvement of free sulfhydryls as well as disulfide exchange in integrin ligation, suggesting a role for thiol isomerization in integrin function. Thus, Yan et al2 have detected unpaired cysteines in the β3 subunit of αIIbβ3; disruption of disulfide bonds in the β3 subunit by site-directed mutagenesis or mild proteolysis increases the affinity of αIIbβ3 for its ligand, fibrinogen,3-7 and introduction of disulfide bonds into integrin αLβ2 affected its affinity.8,9 We have reported that extracellular sulfhydryls participate in conformational changes triggered by interaction of the platelet integrin α2β1 with its natural ligand collagen.10 We subsequently reported that disulfide exchange is required for platelet adhesion to collagen, fibronectin, and fibrinogen.11,12 Furthermore, involvement of disulfide exchange in adhesive receptor function is a feature specific to the integrin receptor family12 though other receptors may contribute to this process.13-15
This disulfide exchange appears to be controlled by protein-disulfide isomerase activity11,12 provided either by activity intrinsic to the integrin16 or by protein disulfide isomerase (PDI) present on the platelet surface.17-20
The observed involvement of disulfide exchange in platelet adhesion to collagen, fibronectin, and fibrinogen might be mediated by one of several pathways: a converging route, where stimulated αIIbβ3 contributes to adhesion to all 3 ligands and depends on exposure of free thiols; an integrin-specific route, where the ligand-specific integrins α2β1, α5β1, and αIIbβ3 each display thiol-dependent ligation; and a more general route where disulfide exchange is involved in ligation of receptors of different families.
The human platelet expresses several collagen receptors (recently reviewed by Clemetson,21 Jung and Moroi,22 and Siljander and Farndale23 ) 2 of which, integrin α2β1 and the immune receptor, glycoprotein VI (GPVI), are well characterized and particularly suitable to distinguish between these possible pathways. Integrin α2β1 exerts primarily adhesive function, recruiting platelets to collagen surfaces under arterial flow conditions.24,25 Deficiency of α2β1 leads to bleeding dysfunction,26-28 and its expression has been correlated with increased risk of coronary thrombosis and stroke.29-31 We have shown that increased collagen-binding affinity correlates with disulfide rearrangement within α2β1 on the intact platelet.10 In contrast, GPVI, recently cloned and sequenced,32,33 serves a key signaling role,34 which leads to aggregation through activation of αIIbβ3, which further augments platelet adhesion to collagen.35 Deficiency in GPVI also leads to bleeding diathesis.36
Synthetic peptide ligands specific for either GPVI or α2β1 have been developed in this laboratory. These adopt a collagen-like triple-helical conformation by virtue of their high Gly-Pro-Pro (GPP) or Gly-Pro-Hyp (GPO, O = hydroxyproline) content. The GPO polymer, collagen-related peptide (CRP), is specific for GPVI and, when cross-linked, is a potent platelet activator.37 The specific α2β1 ligand,38,39 GFOGER-GPP (GPC(GPP)5GFOGER(GPP)5GPC), comprises the recognition motif GFOGER from type I collagen40 within GPP repeats at the C- and N-termini, and it neither binds GPVI nor stimulates platelet aggregation. Both peptides support static platelet adhesion, allowing receptor-specific adhesive events to be discriminated.
The present work was designed to explore the involvement of disulfide exchange and PDI in specific receptor-ligand interactions. We studied platelet adhesion to immobilized CRP or GFOGERGPP or monomeric type I collagen in the presence of different blockers of free thiols or inhibitors of PDI to probe the specific competence of GPVI and α2β1. We also measured the effect of these agents on ligation of purified α2β1 and of its recombinant I domain. The following blocking agents were used: the membrane-impermeant thiol reagents DTNB (5,5′-dithio-bis(2-nitrobenzoic acid)) and pCMPS (p-chloromercuriphenyl-sulfonate); the antibiotic cyclic peptide bacitracin, in both crude and purified form; and 2 antibodies specific for PDI. To discriminate between possible activation-dependent platelet adhesion mediated by the fibrinogen receptor αIIbβ3 and direct adhesion mediated by collagen receptors, adhesion studies were performed using platelets from type I Glanzmann thrombasthenic patients, which lack αIIbβ3. In addition, normal platelets were used in the presence of fibrinogen receptor antagonists, the Arg-Gly-Asp (RGD) mimetic GR144053F and the αIIbβ3-specific monoclonal antibodies 7E3 and 10E5. Our data indicate that GPVI-mediated platelet adhesion to collagen is independent of disulfide exchange, that αIIbβ3 is not involved in thiol dependent adhesion to collagen, and that enzymaticallycatalyzed disulfide exchange is a necessary process in α2β1-mediated, but not I-domain–mediated, adhesion to collagen.
Materials and methods
Materials
Washed or gel-filtered platelets were used throughout within 3 hours of venipuncture. Blood was obtained either from healthy volunteers who had not ingested any medications for at least 12 days prior to venipuncture or from the apheresis clinic of the National Blood Service (Cambridge, United Kingdom). The project has approval from the local regional ethics committee, and is in accordance with the National Blood Service guidance for the use of donor blood for research. All donors gave written consent. Glanzmann type I platelets, obtained from informed donors, were from the Department of Haematology, Royal Hallamshire Hospital (Sheffield, United Kingdom). Bovine tendon type I collagen fibers, used for platelet aggregation, were a gift from Ethicon (Somerville, NJ) and were dialyzed against 0.01 M acetic acid. Monomeric type I collagen used for adhesion assays was purified from calfskin following limited pepsin digestion.41 For aggregation studies, polymerized collagen was used. The peptides CRP (GCO(GPO)10GCOG), GFOGDR-GPP (GPC(GPP)5GFOGDR(GPP)5GPC), GPP10 (GPC(GPP)10GPC), and GFOGER-GPP (GPC(GPP)5GFOGER(GPP)5GPC) were synthesized by standard Fmoc (9-fluorophenylmethoxycarbonyl) chemistry. Cys residues were not blocked, allowing cross-linking to produce crosslinked CRP (CRP-XL) for aggregation studies using the bifunctional SPDP (N-succinimidyl 3-(2-pyridyldithio) propionate) reagent (Sigma-Aldrich, Poole, United Kingdom).42
Human plasma fibrinogen type I, p-nitrophenyl phosphate, bovine serum albumin (BSA), prostaglandinE1 DTNB, pCMPS, and nonimmune mouse ascites were from Sigma-Aldrich. Bacitracin was from Calbiochem-Novabiochem (Nottingham, United Kingdom). Adhesion assays were performed either on 96-well plates (Immulon 2 HB; Dynex Technologies, Ashford, United Kingdom) or on 24-well plates (Suspension Culture Plates; Greiner Labortechnik, Kremsmunster, Austria). Antibodies 7E3, 10E5, and 6F1 were a kind gift from Dr B. S. Coller (The Rockefeller University, New York, NY). Mouse monoclonal anti-PDI, clone RL-90, (ascites fluid) was purchased from Affinity Bioreagents (Golden, CO). GR144053F was a gift from Glaxo Wellcome (Stevenage, United Kingdom). Fab fragments of rabbit anti-PDI and control Fab fragments were prepared as previously described.18,19 Bacitracin A, the predominant species in commercial bacitracin, was a generous gift of Dr Leo Kesner (Department of Biochemistry, SUNY-Downstate Medical Center, Brooklyn, NY). The bacitracin A was purified during the purification of the insulinase inhibitor fraction from commercial bacitracin (Sigma Chemical, St Louis, MO) using CMSepharose followed by Bio-Gel P4 chromatography as described in detail elsewhere.43 High-performance liquid chromatography (HPLC) analysis of each bacitracin fraction was performed as described and identified bacitracin A as the predominant species in the sixth peak off the P4 column.43
Methods
Platelet adhesion to protein- or peptide-covered plastic was measured using 2 methods that gave very similar results. In method A, 24-well suspension culture plates were coated with 20 μg/mL collagen or monomeric CRP or GFOGER-GPP in 0.01 M acetic acid for 2 hours at room temperature followed by washing in phosphate-buffered saline (PBS) and blocking with 3 mg/mL BSA for 1 hour as described.11 Coated surfaces were used on the day of preparation and not allowed to dry. Three hundred microliters of washed platelets44 or gel-filtered platelets10,45 were used per well at a final concentration of 2 × 107 platelets/mL in adhesion buffer (12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, 50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 2 mM MgCl2, 1 mM CaCl2, pH 7.3). Inhibitors in platelet-adhesion buffer were added to platelets 10 minutes before the adhesion process commenced. Monoclonal antibodies or isotype-matched ascites control were added 30 minutes prior to the assay. After 45 minutes of incubation, nonadherent platelets were removed by washing with PBS, and then adherent platelets were stained in May-Grünwald and counted microscopically. Protein-covered wells incubated with untreated platelets were always included as reference, showing adhesion of 250 to 1000 platelets/mm2 depending on the donor. Adhesion to BSA-covered surfaces was typically less than 1% of adhesion to blocked collagen or peptide-covered surfaces. Results were calculated as “% residual adhesion” (ie, the number of adherent platelets in the presence of the inhibitor relative to adherence to the same substratum without inhibitor).11,12 Each experiment was repeated at least 3 times.
In method B, 96-well plates were coated with 10 μg/mL collagen, monomeric CRP, or GFOGER-GPP dissolved in 0.01 M acetic acid for 1 hour at room temperature.46
Washed platelets were prepared by pelleting for 6 minutes at 1000g, resuspending in Jamieson buffer (5.5 mM dextrose, 128 mM NaCl, 4.26 mM Na2HPO4, 7.46 mM NaH2PO4, 4.77 mM trisodium citrate, 2.35 mM citric acid, and 0.35% BSA), pelleting again at 600g, and resuspending to 2 × 108/mL in adhesion buffer, pH 7.4 (Tris-buffered saline [TBS], containing 0.05 M Tris-HCl, 140 mM NaCl, 2 mM Mg2+, and 0.1% BSA). Washed platelets were incubated with inhibitors for 20 minutes then transferred to coated wells for 1 hour as described.47 After washing the adherent platelets, 150 μL lysis buffer (0.07 M trisodium citrate, 0.3 M citric acid, 5 mM P-nitrophenol phosphate, and 0.1% Triton X-100, vol/vol) were added. After 1 hour, the reaction was stopped by adding 100 μL 2 M NaOH and the product was quantitated by measuring A405.48 Results are expressed as % residual adhesion. Using this method, approximately 45% of platelets adhered to the collagen- or peptide-coated surface of the well under control conditions.
Significance of the effect of inhibitors on platelet adhesion was tested by one-way analysis of variance (ANOVA), using mean values from at least 3 separate experiments and identified using the Student-Newman-Keuls multiple comparison test.
Integrin α2β1 was purified from solubilized membranes of human platelets by affinity chromatography on collagen-sepharose,40,46 biotinylated as described, used in solid-phase binding assay,40,46 and detected using horseradish peroxidase (HRP)–coupled streptavidin. Purified α2β1 contained no detectable PDI as demonstrated by Western blotting using the anti-PDI antibody RL-90 (data not shown).
Recombinant α2 I domain was produced as a glutathione S-transferase (GST) fusion protein and used in solid-phase binding assays as described previously.38,46 It was detected using a goat anti-GST antibody (Pharmacia Biotech, Bucks, United Kingdom).
Adhesion of either α2β1 or recombinant I domain (0.1 μg in 100 μL adhesion buffer) was measured after incubating for 1 hour at 20°C on GFOGER-coated surfaces. To estimate relative adhesion of purified α2β1 and I domain to GFOGER-GPP, 100 μL adhesion buffer containing the indicated concentrations of α2β1 or α2 I domain in the presence of either 2 mM MgCl2 or EDTA (ethylenediaminetetraacetic acid; 2 mM) were incubated in peptide-covered wells for 1 hour at 20°C. Wells were washed 5 times with 200 μL adhesion buffer containing 2 mM MgCl2 or EDTA (2 mM) before adding 100 μL adhesion buffer containing monoclonal anti–α2 I-domain antibody (Serotec MCA743) at 1:1000 dilution and incubated at room temperature for 45 minutes. Wells were washed 5 times before adding antimouse HRP conjugate (Dako, Ely, United Kingdom) at 1:2000 dilution and incubating at room temperature for 30 minutes. After washing, color was developed using an ImmunoPure TMB Substrate Kit (Pierce, Chester, United Kingdom) according to the manufacturer's instructions.
Platelet aggregation studies were performed by turbidimetric aggregometry, as described.49 Briefly, platelets were suspended in Tyrode buffer (138 mM NaCl, 5.6 mM glucose, 1 mM MgCl2, 0.4 mM NaH2PO4, 12 mM NaHCO3, 2.7 mM KCl, 10 mM HEPES, 1.5 mM CaCl2, and 0.35% BSA),50 which was supplemented with 1.5 mg/mL fibrinogen. The assay was carried out with native type I collagen fibers from bovine tendon, CRP-XL, or thrombin as agonists. In the latter case fibrinogen was omitted from the platelet suspension.
Results
Inhibitory effect of bacitracin on collagen- but not CRP-XL–induced platelet aggregation
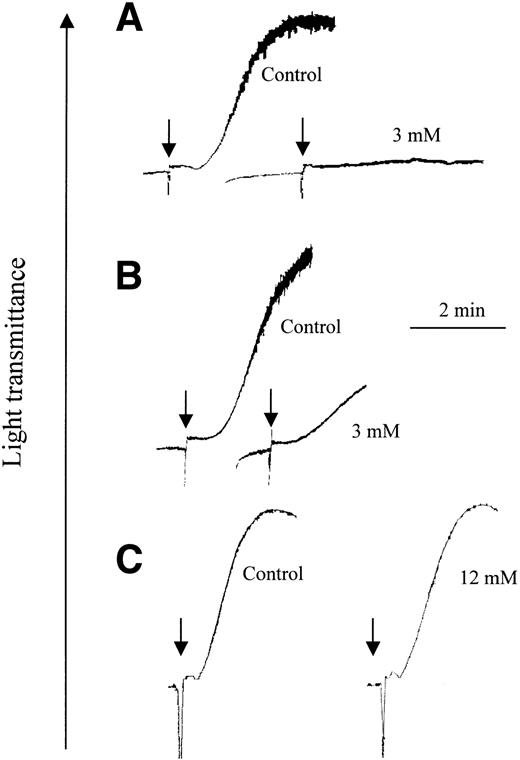
Platelet aggregation was performed with washed platelets, allowing comparison with previous reports.18,19 Type I collagen fibers and CRP-XL were used at the minimum concentration that caused full aggregation (0.5 μg/mL and ∼0.1 μg/mL, respectively). Preincubation of platelets with 3 mM bacitracin was sufficient to block collagen-induced platelet aggregation completely (Figure 1A), whereas CRP-XL still triggered some platelet aggregation (Figure 1B).
Bacitracin causes reversible inhibition of collagen- but not CRP-XL–induced platelet aggregation. Platelets were preincubated for 10 minutes with the indicated concentration of commercial bacitracin, then agonist was applied at the points shown by arrows: (A) collagen fibers at 0.5 μg/mL and (B) CRP-XL at 0.1 μg/mL. In panel C, platelets were preincubated with or without bacitracin (12 mM or control, respectively) for 1 hour, washed and resuspended in HEPES-Tyrode buffer without bacitracin, and then aggregation was stimulated with collagen (1 μg/mL). Aggregation, measured as described in “Methods,” is shown as an increase in light transmittance, a representative experiment of 3.
Bacitracin causes reversible inhibition of collagen- but not CRP-XL–induced platelet aggregation. Platelets were preincubated for 10 minutes with the indicated concentration of commercial bacitracin, then agonist was applied at the points shown by arrows: (A) collagen fibers at 0.5 μg/mL and (B) CRP-XL at 0.1 μg/mL. In panel C, platelets were preincubated with or without bacitracin (12 mM or control, respectively) for 1 hour, washed and resuspended in HEPES-Tyrode buffer without bacitracin, and then aggregation was stimulated with collagen (1 μg/mL). Aggregation, measured as described in “Methods,” is shown as an increase in light transmittance, a representative experiment of 3.
To ascertain whether bacitracin impaired the viability of platelets or invoked its effect by proteolytic activity, aggregation tests were performed using platelets that had been preincubated with 12 mM bacitracin, more than sufficient to block aggregation completely. Platelets were then centrifuged and resuspended in buffer without bacitracin. Normal aggregation was induced by collagen fibers (Figure 1C) in the resuspended platelets, indicating that the inhibitory effect of bacitracin was reversible and not caused by general toxic or lytic activity. Similar data were obtained for aggregation stimulated by CRP-XL (not shown).
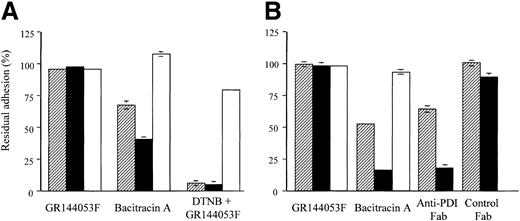
Effect of GR144053F, bacitracin, and anti-PDI on thrombasthenic platelet adhesion to collagen, CRP, and GFOGER-GPP
To provide definitive resolution of the role of αIIbβ3 from that of α2β1 or GPVI in disulfide-exchange–dependent platelet response to collagen, adhesion of Glanzmann platelets from 2 separate donors to surfaces coated with monomeric collagen, GFOGER-GPP, or CRP was examined. GR144053F had no effect on adhesion of thrombasthenic platelets to any of the collagenous substrates (Figure 2) confirming it as a specific αIIbβ3 inhibitor. Bacitracin A (3 mM) blocked adhesion of Glanzmann platelets to collagen by 35% and 50% and to GFOGER-GPP by 60% and 85% in platelets from the 2 donors, while having no effect on adhesion to CRP, confirming bacitracin as α2β1 specific (Figure 2A-B). The thiol-exchange blocker, DTNB, used in one experiment, caused near-complete blockade of adhesion to both monomeric collagen and GFOGER-GPP, having only a slight effect on adhesion to CRP (Figure 2A). In the second experiment (Figure 2B), Fab fragments of anti-PDI, previously shown to fully inhibit collagen-induced platelet aggregation,18 inhibited Glanzmann platelet adhesion to monomeric collagen and to GFOGER-GPP by 36% and 82%, respectively. Control Fab fragments had no effect on adhesion to either substrate.
Inhibition of thrombasthenic platelet adhesion to collagen and GFOGER-GPP. Thrombasthenic platelets were incubated with GR144053F (2 μM), bacitracin A (3 mM), DTNB (2.5 mM), or anti-PDI Fab fragments (400 μg/mL) as indicated, then allowed to adhere to the indicated substrates for 1 hour using adhesion method B. A single experiment performed in triplicate (mean ± SD) was performed with platelets from each of 2 donors, A and B. Residual adhesion is expressed relative to untreated controls. ▨ indicates collagen; ▪, GFOGER; and □, CRP.
Inhibition of thrombasthenic platelet adhesion to collagen and GFOGER-GPP. Thrombasthenic platelets were incubated with GR144053F (2 μM), bacitracin A (3 mM), DTNB (2.5 mM), or anti-PDI Fab fragments (400 μg/mL) as indicated, then allowed to adhere to the indicated substrates for 1 hour using adhesion method B. A single experiment performed in triplicate (mean ± SD) was performed with platelets from each of 2 donors, A and B. Residual adhesion is expressed relative to untreated controls. ▨ indicates collagen; ▪, GFOGER; and □, CRP.
Distinguishing mediation by integrin αIIbβ3 from mediation by α2β1 and GPVI in disulfide-exchange–dependent adhesion of normal platelets
To assess the possible contribution of activated αIIbβ3 versus that of α2β1 in normal platelets, the effect of 6 mM bacitracin and of the blocking monoclonal antibody 6F1 against α2β1 was compared with that of the blocking antibodies against αIIbβ3, 10E5, and 7E3, as well as the RGD-mimetic GR144053F, using adhesion method A. Collagen, GFOGER, and CRP were used as adhesive substrates and isotype-matched mouse ascites was used as control. The results, shown in Table 1, demonstrate that adhesion to collagen was 75% inhibited by 6 mM bacitracin and not at all by GR144053F, 10E5, or 7E3, indicating lack of involvement of αIIbβ3 in disulfide-exchange–mediated adhesion to collagen. Adhesion to CRP was not inhibited by either anti-α2β1 or anti-αIIbβ3, nor was it inhibited by bacitracin, stressing lack of involvement of either disulfide exchange or αIIbβ3 in platelet interaction with the GPVI-specific peptide. Adhesion to GFOGER was not inhibited by anti-αIIbβ3, but totally inhibited by both anti-α2β1 and bacitracin, indicating direct involvement of the collagen receptor α2β1 in disulfide-exchange–mediated adhesion. It should be noted that monomeric collagen does not activate platelets51 despite possessing GPVI recognition sites (D.J. Onley, C.G.K., R.W.F., unpublished data, 2001).
Dose-dependent inhibitory effect of bacitracin on platelet adhesion to GFOGER but not CRP
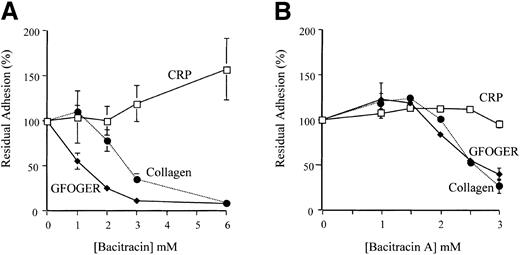
Adhesion of platelets to surfaces coated with monomeric collagen, GFOGER-GPP, or CRP was tested as a function of bacitracin concentration. Platelet adhesion to CRP was not inhibited by up to 6 mM bacitracin, whereas adhesion to GFOGER-GPP was substantially inhibited at less than 3 mM (Figure 3A). Adhesion to collagen was also inhibited, in agreement with previous data.11
Bacitracin and bacitracin A inhibit platelet adhesion to monomeric type I collagen and GFOGER-GPP but not CRP. (A) Platelets were preincubated with up to 6 mM bacitracin for 10 minutes before adding to wells coated with the indicated substrate. Adhesion was measured after 45 minutes at room temperature. This graph contains data (mean ± SD) from 3 separate experiments, each performed in triplicate, using adhesion method A. (B) Platelets were preincubated with up to 3 mM bacitracin A for 20 minutes before adding to wells coated as indicated. Samples were measured after 1 hour at room temperature. The data (mean ± SD) are compiled from 3 experiments each performed in triplicate, using adhesion method B. Where error bars are absent, they were too small to show.
Bacitracin and bacitracin A inhibit platelet adhesion to monomeric type I collagen and GFOGER-GPP but not CRP. (A) Platelets were preincubated with up to 6 mM bacitracin for 10 minutes before adding to wells coated with the indicated substrate. Adhesion was measured after 45 minutes at room temperature. This graph contains data (mean ± SD) from 3 separate experiments, each performed in triplicate, using adhesion method A. (B) Platelets were preincubated with up to 3 mM bacitracin A for 20 minutes before adding to wells coated as indicated. Samples were measured after 1 hour at room temperature. The data (mean ± SD) are compiled from 3 experiments each performed in triplicate, using adhesion method B. Where error bars are absent, they were too small to show.
Commercially available bacitracin may contain impurities that have nonspecific effects on platelet function.52 Therefore, as for aggregation, we determined whether exposure to bacitracin had irreversible (nonspecific) effects on platelet adhesion. After preincubation with either 12 mM or 30 mM bacitracin, the platelets were centrifuged once and resuspended in adhesion buffer. After the removal of bacitracin, residual adhesion of platelets to monomeric collagen was 119% and 76%, respectively, where adhesion in the presence of these levels of bacitracin was reduced to 31% and 7% (P < .001, data not shown). Thus, platelets substantially regained their ability to adhere to monomeric collagen after incubation with high levels of bacitracin, in agreement with our previous report.11
In addition to the reversibility tests described above, we tested the effect of purified bacitracin A on platelet adhesion to the same coated surfaces. Again, inhibition of platelet adhesion to monomeric collagen and to GFOGER-GPP was near-complete using 3 mM bacitracin A, with EC50 about 2.5 mM, whereas adhesion to CRP was not affected (Figure 3B).
Effects of anti-PDI antibodies on adhesion to receptor-specific peptides
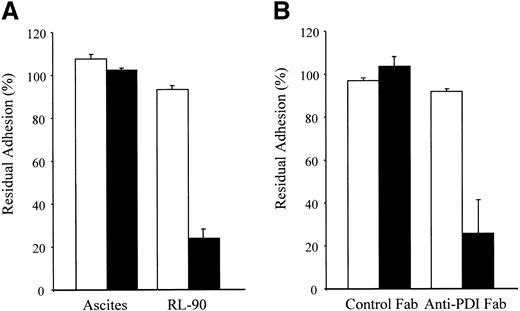
We have reported that monoclonal anti-PDI antibody RL-90 inhibits platelet adhesion to collagen,11 and that Fab fragments of polyclonal anti-PDI antibody inhibit collagen-induced platelet aggregation.18 We therefore tested both antibodies for their effect on platelet adhesion to CRP and to GFOGER-GPP. RL-90 inhibited platelet adhesion to the α2β1-specific ligand, GFOGER-GPP, by more than 70% but had no effect on adhesion to the GPVI-specific peptide CRP (Figure 4A). RL-90 also inhibited platelet adhesion to monomeric collagen (data not shown) as previously reported.11 Moreover, Fab fragments of the polyclonal anti-PDI inhibited platelet adhesion to GFOGER-GPP by a similar extent but had no effect on adhesion to CRP (Figure 4B). In these experiments both ascites and Ig Fab fragment controls were without effect.
Anti-PDI antibodies inhibit adhesion of platelets to GFOGER-GPP but not CRP. (A) Washed platelets were incubated with 0.5% dilution of monoclonal antibody RL-90 or control ascites, as indicated, then allowed to adhere to GFOGERGPP (▪) or CRP (□). Residual adhesion after 45 minutes was measured as described in “Methods,” using adhesion method A. Data represent mean ± SD of 2 separate experiments, each measured in duplicate. (B) Platelets were incubated for 20 minutes with Fab fragments of either polyclonal anti-PDI or control IgG (400 μg/mL), then allowed to adhere to GFOGER-GPP (▪) or CRP (□). Residual adhesion after 1 hour was measured as described in “Methods,” using adhesion method B. Data are mean ± SD of 3 experiments, each measured in triplicate (P < .01).
Anti-PDI antibodies inhibit adhesion of platelets to GFOGER-GPP but not CRP. (A) Washed platelets were incubated with 0.5% dilution of monoclonal antibody RL-90 or control ascites, as indicated, then allowed to adhere to GFOGERGPP (▪) or CRP (□). Residual adhesion after 45 minutes was measured as described in “Methods,” using adhesion method A. Data represent mean ± SD of 2 separate experiments, each measured in duplicate. (B) Platelets were incubated for 20 minutes with Fab fragments of either polyclonal anti-PDI or control IgG (400 μg/mL), then allowed to adhere to GFOGER-GPP (▪) or CRP (□). Residual adhesion after 1 hour was measured as described in “Methods,” using adhesion method B. Data are mean ± SD of 3 experiments, each measured in triplicate (P < .01).
Effects of DTNB and pCMPS on adhesion to receptor-specific peptides
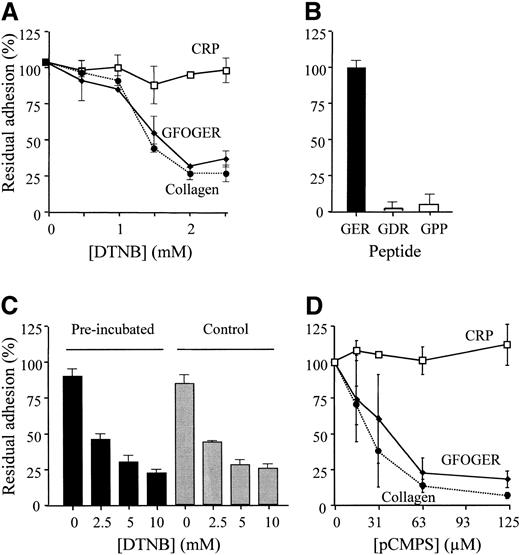
DTNB, an irreversible inhibitor of disulfide exchange, blocked platelet adhesion to monomeric collagen and to GFOGER-GPP in a dose-dependent manner, inhibition being near-complete at 2.5 mM. Adhesion to CRP was not affected (Figure 5A).
Thiol reagents differentially inhibit platelet adhesion to GFOGERGPP, collagen, and CRP. (A) Platelet adhesion to the 3 substrates was measured, as described, in the presence of increasing levels of DTNB. Data (mean ± SD) are from 3 different experiments performed in triplicate using adhesion method B. (B) Platelet adhesion to GFOGDR-GPP and to GPP10 was compared with adhesion to GFOGER using method B in a single experiment repeated in triplicate (mean ± SD) confirming previously published data.38,42 (C) Surfaces were coated with GFOGER, as for panel A, then pretreated for 30 minutes with 10 mM DTNB (▪) or the vehicle for DTNB, phosphate buffer (▦). After washing the surfaces with phosphate buffer, platelets were added in the presence of the indicated level of DTNB and adhesion was measured as for panel A. (D) Platelet adhesion to the 3 substrates was measured, as described, in the presence of increasing levels of pCMPS. Data are from 3 separate experiments, each performed in triplicate (mean ± SD), using adhesion method A. Where error bars are absent, they were too small to show.
Thiol reagents differentially inhibit platelet adhesion to GFOGERGPP, collagen, and CRP. (A) Platelet adhesion to the 3 substrates was measured, as described, in the presence of increasing levels of DTNB. Data (mean ± SD) are from 3 different experiments performed in triplicate using adhesion method B. (B) Platelet adhesion to GFOGDR-GPP and to GPP10 was compared with adhesion to GFOGER using method B in a single experiment repeated in triplicate (mean ± SD) confirming previously published data.38,42 (C) Surfaces were coated with GFOGER, as for panel A, then pretreated for 30 minutes with 10 mM DTNB (▪) or the vehicle for DTNB, phosphate buffer (▦). After washing the surfaces with phosphate buffer, platelets were added in the presence of the indicated level of DTNB and adhesion was measured as for panel A. (D) Platelet adhesion to the 3 substrates was measured, as described, in the presence of increasing levels of pCMPS. Data are from 3 separate experiments, each performed in triplicate (mean ± SD), using adhesion method A. Where error bars are absent, they were too small to show.
To test the possibility that platelet surface proteins became disulfide bridged directly to the immobilized peptide, 2 sets of experiments were performed. In the first, an analog of GFOGER, the control peptide GFOGDR, which does not recognize α2β1,38 and the control peptide GPP10, which does not recognize GPVI,42 were used. As seen in Figure 5B, platelets did not adhere to the control peptides, whereas they adhered well to the α2β1-specific peptide GFOGER, in agreement with previous reports.38,42 In a second experimental design, GFOGER-coated surfaces were pretreated with DTNB or with buffer control. DTNB was removed by washing and adhesion of platelets to the surface was measured in the presence of increasing concentrations of DTNB as done before. We observed that pretreatment of the peptide-covered surface with DTNB led to no loss of platelet adhesion, and the dose-dependent effect of added DTNB was identical whether the surface peptide had been pretreated with DTNB or not (Figure 5C).
The action of pCMPS, another irreversible blocker of thiols and inhibitor of disulfide exchange, proved to be similar to DTNB, with significant inhibition of adhesion to both monomeric collagen and GFOGER-GPP at levels of pCMPS, which had no effect on adhesion to CRP (Figure 5D). Data for collagen are consistent with our previous report.11
The role of thiols and disulfide exchange in ligation of purified α2β1 and of its recombinant I domain
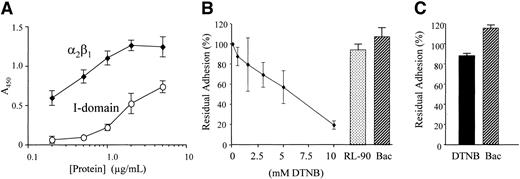
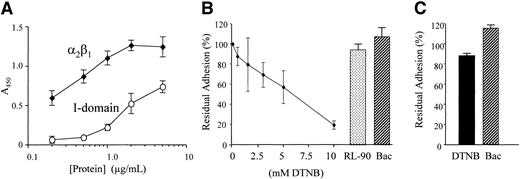
To compare the kinetics of binding of α2β1 with that of its I domain, the adhesion of purified integrin and recombinant I domain (which is the collagen-binding region of α2β1 and the specific binding domain of GFOGER) to GFOGER-GPP–coated surfaces was examined as a function of their concentration. Adhesion was detected using an anti-α2 I-domain antibody. Background adhesion (the minor component remaining in the presence of EDTA) was subtracted from that in the presence of 2 mM Mg2+. We observed a much higher binding of α2β1 than of the I domain for their common ligand (Figure 6A), indicating higher affinity of the intact α2β1 to GFOGER. Integrin binding became saturated at 2 μg/mL, whereas I-domain binding did not yet reach saturation at 5 μg/mL. In order to assess the role of disulfide exchange in direct interaction of α2β1 with its ligand, ligation of purified α2β1 (1 μg/mL) to its specific ligand, GFOGER-GPP, was measured in the presence of increasing concentrations of the thiol blocker DTNB, the disulfide-exchange inhibitor bacitracin, and anti-PDI RL-90. Three different experiments were performed, each in triplicate. As can be seen (Figure 6B), DTNB inhibits binding of α2β1 to GFOGER-GPP in a concentration-dependent manner indicating that free thiols are necessary for adhesion of the purified receptor to its ligand. However, blocking disulfide exchange with bacitracin, or inhibiting PDI with RL-90, did not affect this ligation. We next tested ligation of the recombinant I domain. In 3 experiments we observed that neither DTNB nor bacitracin had a significant effect on ligation (Figure 6C).
Binding of α2β1 and I domain to GFOGER: effect of thiol blocking and of inhibition of disulfide exchange and PDI. (A) Adhesion to GFOGER-GPP of purified α2β1 or recombinant I domain was measured using a monoclonal antibody to α2 integrin I domain as described in “Methods.” Data are mean absorbance (± SD) of 4 experiments, each performed in triplicate. (B) The α2β1 from human platelets was biotinylated then incubated with increasing concentrations of DTNB, 30 mM bacitracin (Bac), or RL-90, as in Figure 4, and allowed to adhere to immobilized GFOGER-GPP as described in “Methods.” Adhesion was measured using HRP-linked streptavidin and data shown are mean ± SD from 3 experiments, each performed in triplicate. Residual adhesion is expressed relative to untreated controls. (C) Recombinant α2 I domain was incubated with 10 mM DTNB or 30 mM bacitracin (Bac), allowed to adhere to immobilized GFOGER-GPP, and estimated using anti-GST as described in “Methods.” Data are mean ± SD from 3 experiments each performed in triplicate concomitantly with panel A. Residual adhesion is expressed relative to untreated controls.
Binding of α2β1 and I domain to GFOGER: effect of thiol blocking and of inhibition of disulfide exchange and PDI. (A) Adhesion to GFOGER-GPP of purified α2β1 or recombinant I domain was measured using a monoclonal antibody to α2 integrin I domain as described in “Methods.” Data are mean absorbance (± SD) of 4 experiments, each performed in triplicate. (B) The α2β1 from human platelets was biotinylated then incubated with increasing concentrations of DTNB, 30 mM bacitracin (Bac), or RL-90, as in Figure 4, and allowed to adhere to immobilized GFOGER-GPP as described in “Methods.” Adhesion was measured using HRP-linked streptavidin and data shown are mean ± SD from 3 experiments, each performed in triplicate. Residual adhesion is expressed relative to untreated controls. (C) Recombinant α2 I domain was incubated with 10 mM DTNB or 30 mM bacitracin (Bac), allowed to adhere to immobilized GFOGER-GPP, and estimated using anti-GST as described in “Methods.” Data are mean ± SD from 3 experiments each performed in triplicate concomitantly with panel A. Residual adhesion is expressed relative to untreated controls.
Discussion
Recently, we have suggested that disulfide exchange has a role in the affinity-modulation of the collagen receptor, integrin α2β1,10,11 as well as of integrins αIIbβ311,12,18,20 and α5β1.11 Together, these studies suggested that disulfide exchange is a general requirement for outside-in signaling by integrins. However, the blood platelet expresses 2 different receptors for collagen: integrin α2β1 and the immunoglobulin superfamily receptor GPVI. The activated fibrinogen receptor, integrin αIIbβ3, may also mediate indirect adhesion to collagenous ligands following platelet activation.35,46 Therefore, disulfide-exchange–dependent interaction with collagen could in principle be mediated by either of the collagen receptors or by the activated fibrinogen receptor. This study aimed to probe the receptor specificity of disulfide exchange and PDI in platelet adhesion to collagen. Bacitracin, an inhibitor of disulfide exchange,53,54 inhibits platelet aggregation triggered by collagen.12,18,20 To investigate the possible involvement of disulfide exchange in the function of GPVI, the effect of bacitracin was first examined on platelet aggregation stimulated by cross-linked CRP, which acts specifically through this receptor.42,55 Although bacitracin reduced platelet aggregation triggered by CRP-XL, some aggregation persisted despite the presence of bacitracin at concentrations that totally inhibited collagen-induced aggregation (Figure 1). As expected, higher levels of bacitracin inhibited aggregation by CRP-XL as well as by thrombin, because the aggregation process mediated by sustained ligation of integrin αIIbβ3 is itself sensitive to blockade of disulfide exchange.12 Bacitracin was not toxic or proteolytic because the ability of platelets to aggregate was restored by washing it from the suspension. These observations suggested greater dependence on disulfide exchange of the activation pathway stimulated by native collagen fibers, where receptors other than GPVI, such as integrin α2β1 or activated αIIbβ3, may contribute.
Platelets from 2 Glanzmann thrombasthenic patients, shown by flow cytometry to lack αIIbβ3 on their surface (M.M., unpublished data, 1997), allowed us to resolve direct dependence of the collagen receptor(s) on disulfide exchange from αIIbβ3 mediation in disulfide exchange. We made use of the 2 peptides specific to the collagen receptors and selective for them: GFOGER, the α2 I-domain recognition motif from type I collagen40 in a triple-helical synthetic peptide that is specific for integrin α2β138,39 ; and CRP, the GPVI-specific peptide.42,55 This also allowed resolution of the possible selectivity of disulfide exchange in ligation of the 2 collagen receptors. First, the functional defect in these platelets was confirmed by their inability to aggregate when treated with a normal level of CRP-XL (not shown). We then used these Glanzmann platelets in adhesion studies where we observed that the RGD-mimetic, GR144053F, had no effect on their adhesion to either GFOGER-GPP or CRP (Figure 2). Therefore, adhesion of thrombasthenic platelets to GFOGER-GPP and CRP is completely independent of αIIbβ3 but dependent on integrin α2β1 and GPVI, respectively. Inhibition of adhesion of these platelets to GFOGER-GPP by DTNB, bacitracin, or anti-PDI Fab fragments (Figure 2) show that free thiols and PDI-mediated disulfide exchange are directly involved in the function of α2β1. In contrast, lack of effect on adhesion to CRP (Figure 2) shows that GPVI-mediated adhesion is independent of disulfide exchange.
To probe possible involvement of αIIbβ3 in thiol-mediated adhesion of normal platelets to the collagenous peptides, integrin αIIbβ3 was blocked using either antibodies 10E5 and 7E356 or the RGD-mimetic GR144053F.37,42 Under these conditions, adhesion to GFOGER-GPP or CRP was not affected (Table 1). As expected,37,46 monoclonal antibody 6F1, directed at α2β1,57 totally blocked adhesion to GFOGER-GPP and to monomeric collagen but not to CRP. Similarly, 3 mM bacitracin inhibited platelet adhesion to GFOGER-GPP and to collagen but not to CRP (Table 1). These data suggest that among those receptors mediating adhesion of the normal platelet to collagen, it is α2β1, but not αIIbβ3 or GPVI, that is an important target for disulfide exchange.
Bacitracin inhibited adhesion to GFOGER-GPP in a dose-dependent manner while having no inhibitory effect on adhesion to CRP (Figure 3A). Adhesion to monomeric collagen, a process also essentially dependent on α2β1, was inhibited by bacitracin, in agreement with our previous observations.11 The effect of bacitracin is reversible, as adhesion is fully restored if it is washed away, confirmed here (not shown) and as reported previously,11 ruling out any possible proteolytic or other toxic effect. The purified active fraction of bacitracin, bacitracin A, showed similar selectivity for α2β1 (Figure 3B), further ruling out possible effects of impurities. Thus, it is the integrin-mediated adhesion that depends on disulfide-exchange activity.
Bacitracin was recently shown to inhibit disulfide-exchange activity endogenously expressed in β3 and possibly β1 integrins16 as well as that of PDI. Therefore, the direct involvement of PDI in collagen-receptor ligation was examined by exploring the effect of 2 antibodies specificto this enzyme on platelet adhesion to the 2 peptides. Monoclonal antibody RL-90, raised against purified PDI,58 blocks PDI activity59 and platelet adhesion to monomeric collagen.11 This antibody inhibited normal platelet adhesion to GFOGER-GPP but not to CRP (Figure 4A). Fab fragments of polyclonal anti-PDI, previously shown to inhibit agonistinduced platelet aggregation,18 also inhibited adhesion to GFOGERGPP and not to CRP (Figure 4B). Thus, notwithstanding possible involvement of endogenous disulfide-exchange activity in α2β1, these data suggest that PDI is also involved in α2β1-mediated adhesion.
Direct blocking of free sulfhydryls inhibits platelet adhesion to collagen in a manner very similar to that of bacitracin or anti-PDI.11 Here 2 thiol blockers, DTNB and pCMPS, inhibited adhesion to GFOGER-GPP in a concentration-dependent manner, whereas adhesion to CRP was not affected (Figures 5A-B). The doses used for these reagents, as for bacitracin, were consistent with previous reports.11,12,20,60 To control for the possibility that platelets formed disulfide bonds nonspecifically with the cysteine-containing peptides, GFOGER-GPP–coated surfaces were derivatized with supramaximal levels of DTNB. This affected neither platelet adhesion after washing the surfaces free of soluble DTNB nor the inhibitory properties of DTNB on platelet adhesion (Figure 5C). Two lines of evidence further support specificity of free thiols for α2β1-mediated adhesion: (1) in confirmation of previous reports,38,42 nonspecific contact with homologous cysteine-containing peptides, GFOGDR-GPP and GPP10, to which neither α2β1 nor GPVI will bind,38,42 did not support platelet adhesion (Figure 5B); and (2) specific platelet adhesion, mediated by GPVI, to CRP was completely insensitive to the presence of DTNB (Figure 2A). Thus, free thiols are necessary specifically for α2β1-mediated adhesion.
Bacitracin-induced inhibition of adhesion of lymphoid cells to collagen has been reported by Mou et al.60 However, these authors suggested that because both their anti-PDI and DTNB had little effect on cell adhesion to collagen, the action of bacitracin was nonspecific. Our results differ, since DTNB had a very marked inhibitory action on platelet adhesion (Figures 2A and 5A) as did anti-PDI (Figures 2B and 4). Further, we obtained similar data using a second thiol reagent, pCMPS (Figure 5D). Possibly the antibody and Fab fragments used in our study were more specific for the catalytic activity of PDI than that used by Mou et al.60 It may also be, however, that the regulation of α2β1 in lymphocytes differs from that in platelets.
Up to this point this study shows that disulfide exchange is a necessary step in adhesion mediated by integrin α2β1 and implies specific involvement of surface-expressed PDI activity in this process. It still does not address possible involvement of endogenous disulfide-exchange activity of the integrin as recently suggested by work on αIIbβ3.16 It also does not exclude possible involvement of other integrin-associated proteins in this thiol-dependent process. We therefore used purified α2β1 and its I domain in adhesion studies.
To compare the kinetics of binding of purified intact α2β1 with that of its isolated I domain to GFOGER, which is the collagen-binding region of α2β1 specifically recognized by the I domain,38 we exposed GFOGER-coated surfaces to increasing levels of these proteins, estimating adhesion of each using the same monoclonal antibody. These experiments showed that on a molar basis the apparent affinity of intact α2β1 was almost 2 orders of magnitude higher than that of the free α2 I domain, the structure within α2β1 that binds type I collagen. This suggests that components of the integrin other than the I domain are responsible for the regulation of its affinity.
We therefore probed ligation of α2β1 to GFOGER-GPP in the presence of DTNB, bacitracin, and RL-90. The α2β1, purified from normal human platelets and free of PDI, was increasingly inhibited from binding to GFOGER-GPP by increasing concentrations of DTNB (Figure 6B). Thus, free thiols are necessary for direct interaction of α2β1 with its ligand. Presentation of free thiols by a purified integrin has been previously demonstrated for αIIbβ3.2,61 This receptor was also shown to carry endogenous disulfide-exchange activity as does αvβ3.16 Thus, endogenous disulfide-exchange activity of α2β1 could explain thiol involvement in the absence of PDI. However, inhibiting disulfide exchange activity with bacitracin had no effect on the direct ligation of α2β1 (Figure 6B). The reasons for this difference between the integrins will have to be further investigated. As expected, blocking PDI activity with RL-90 had no effect on ligation of purified α2β1 (Figure 6B).
We further probed thiol dependence of the interaction of recombinant I domain with immobilized GFOGER. We found that ligation of the I domain was independent of free thiols as well as of disulfide exchange (Figure 6C). This observation agrees with the previous report showing that no cysteine in the I domain plays a role in its ligation.62 Yet our data allow us to define integrin α2β1 as the site of disulfide exchange. We therefore suggest that the initial interaction of the I domain in α2β1 with its ligand causes allosteric conformational changes that expose free thiols in another domain on α2β1 needed for sustained ligation of α2β1. We have recently demonstrated such sequence of processes, whereby initial integrin ligation is thiol independent, followed by thiol dependence for sustained ligation in αIIbβ3.12 This sequence is also supported by our previous report showing that following adhesion to collagen, integrin α2β1 undergoes affinity modulation involving disulfide-bond rearrangement.10 Further, a regulatory mechanism whereby ligation of the I domain in α2β1 involves regulation through conformational changes in the β1 subunit has been demonstrated,63 implicating the EGF repeats of β1.63 Recently, it was demonstrated that a point mutation (Cys560Arg) in the cysteine-rich domain of the β3 subunit irreversibly activates αIIbβ3.7 It was also reported that while yet another point mutation in the cysteine-rich domain, Cys598Tyr, also locks αIIbβ3 in the active conformation, 2 mutations in the I-like domain of β3, Cys177Ala and Cys273Ala, had no such effect.6 Further, free sulfhydryls have been located within the β3 subunit,64 providing a possible site of disulfide exchange. The recent crystal structure of the extracellular domains of αvβ3 suggests that the cysteine-rich stem of the β3 subunit is the site of a conformational change that may be associated with the activation process.65 Together, these data are consistent with the concept that disulfide exchange is crucial for the sustained binding of integrins to their ligands, is common to different platelet integrins, and is independent of the presence of the I domain, which is found in the α2 but not in αIIb subunit. The 2 integrins are structurally similar in many other respects. By contrast, ligation of nonintegrins is independent of disulfide exchange. This was demonstrated here with respect to GPVI and previously with respect to GPIb-IX-V.12
How then, can PDI regulate the affinity of α2β1? We propose that α2β1 exists in 2 conformations, determined by the state of disulfide bridging (presumably within the β1 subunit)2,61,63 where the equilibrium between the 2 forms is enzymatically catalyzed by disulfide-exchange activity. It would therefore be necessary that the active conformation can be stabilized by ligand binding, and that a mechanism exists for this information to be relayed allosterically from the I domain, which is the ligand-binding domain in α2β1, to the rest of the integrin. Indeed, such a mechanism has recently been proposed, based on the conformational changes observed within the α2 I domain upon binding a GFOGER-containing peptide, determined at the level of their crystal structures.39
Prepublished online as Blood First Edition Paper, June 5, 2003; DOI 10.1182/blood-2002-06-1646.
Supported in part by grants from The Chief Scientist's office of the Ministry of Health, Israel; The Medical Research Council, United Kingdom; the British Heart Foundation; and The Netherlands Heart Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Pia Siljander for helpful advice and Tony Peachey and Mengru Li for technical assistance.