Abstract
We performed a multivariable comparison of 125 consecutive patients with follicular lymphoma (FL) treated at our centers with either high-dose radioimmunotherapy (HD-RIT) using 131I-anti-CD20 (n = 27) or conventional high-dose therapy (C-HDT) (n = 98) and autologous hematopoietic stem cell transplantation. The groups were similar, although more patients treated with HD-RIT had an elevated pretransplantation level of lactate dehydrogenase (41% versus 20%, P = .03) and elevated international prognostic score (41% versus 19%, P = .02). Patients treated with HD-RIT received individualized therapeutic doses of 131I-tositumomab (median, 19.7 GBq [531 mCi]) to deliver 17 to 31 Gy (median, 27 Gy) to critical organs. Patients treated with C-HDT received total body irradiation plus chemotherapy (70%) or chemotherapy alone (30%). Patients treated with HD-RIT experienced improved overall survival (OS) (unadjusted hazard ratio [HR] for death = 0.4 [95% confidence interval (95% CI), 0.2-0.9], P = .02; adjusted HR, 0.3, P = .004) and progression-free survival (PFS) (unadjusted HR = .6 [95% C.I., 0.3-1.0], P = .06; adjusted HR, 0.5, P = .03) versus patients treated with C-HDT. The estimated 5-year OS and PFS were 67% and 48%, respectively, for HD-RIT and 53% and 29%, respectively, for C-HDT. One hundred-day treatment-related mortality was 3.7% in the HD-RIT group and 11% in the C-HDT group. The probability of secondary myelodysplastic syndrome/acute myeloid leukemia (MDS/AML) was estimated to be .076 at 8 years in the HD-RIT group and .086 at 7 years in the C-HDT group. HD-RIT may improve outcomes versus C-HDT in patients with relapsed FL. (Blood. 2003;102:2351-2357)
Introduction
Follicular lymphoma (FL) is the second most common type of non-Hodgkin lymphoma (NHL) and the most common indolent lymphoid malignancy.1 Advanced FL generally cannot be cured using standard therapies, and remission durations typically become progressively shorter over time, resulting in median survivals of 7 to 10 years from diagnosis.1-3 In an attempt to alter the natural history of this disease, many investigators have evaluated the use of high-dose therapy (HDT) and autologous hematopoietic stem cell transplantation (ASCT).4-6 Both single-arm and randomized trials using this HDT approach in relapsed FL have suggested improvements in remission durations without clear evidence of cure and no conclusive benefit in overall survival (OS).4,5,7 Many clinicians, therefore, remain reluctant to use HDT and ASCT for patients with relapsed FL.
Our group has explored and reported the use of radiolabeled antibodies with ASCT as a method to augment the doses of radiation that can be delivered to lymphomatous sites, while limiting radiation exposure and toxicity to healthy organs.8-10 FL is particularly radiosensitive, as indicated by long-term remissions in patients with localized disease when external beam radiotherapy alone is used for treatment.11,12 With these observations in mind, we now have the opportunity to specifically evaluate the relative safety and efficacy of high-dose radioimmunotherapy (HD-RIT) versus conventional high-dose therapy (C-HDT) conditioning regimens in a nonrandomized cohort of patients with relapsed or refractory FL. This study was carried out with the use of a multivariable analysis comparing these 2 groups of patients to primarily determine whether HD-RIT would result in improved overall and progression-free survival (PFS) and lower treatment-related mortality (TRM) compared with C-HDT.
Patients, materials, and methods
Selection of patients for radioimmunotherapy
Consecutive patients with the confirmed diagnosis of FL or transformed FL treated with myeloablative anti-CD20 radioimmunotherapy on 2 sequential trials for relapsed B-cell lymphomas (a phase 1 dose escalation study and a subsequent phase 2 trial at the maximally tolerated dose) were included in the study group.8-10 Patients with other histologies (2 patients with small lymphocytic lymphoma) were not included in this comparative analysis of FL. Patients were eligible for the radioimmunotherapy trials if they were between the ages of 18 and 60 years, had progressive or persistent disease following conventional chemotherapy, had evidence of disease at the time of treatment, had acceptable organ function, and were otherwise considered candidates for transplantation by the primary treating physician. Patients were ineligible if they had human antimouse antibodies (HAMAs), had received antilymphoma therapy within 30 days, or had received more than 20 Gy of external beam radiation to a critical organ (eg, lung, liver, kidney, spinal cord). Patients were also ineligible for therapeutic infusions if biodistribution studies predicted that critical organs would receive higher radiation doses than target tumor sites.
Biodistribution studies
The anti-CD20 antibody tositumomab (Corixa Pharmaceuticals, Seattle, WA) was radioiodinated with 131I as previously described.8,13 Patients enrolled in the phase 1 trial received 0.35, 1.7, and 7 mg/kg tositumomab trace labeled with 131I (185-370 MBq [5-10 mCi]) to evaluate biodistribution and to determine the optimal protein dose. Patients on the subsequent phase 2 trial received trace 131I-labeled infusions at a dose of 1.7 mg/kg, which was estimated to be the optimal dose in phase 1 studies. Thyroid uptake was blocked with oral potassium iodide starting 24 hours before antibody infusion and continuing for 30 days. Serial gamma camera images were obtained immediately after the trace-labeled antibody infusion and 48, 120, and 144 hours afterward to estimate the absorbed radiation dose to critical organs and whole body as previously described.10
Therapeutic antibody infusions and hematopoietic stem cell transplantation
Ten to 14 days following the trace-labeled infusion, patients received therapeutic tositumomab at a protein dose identical to the optimal trace-labeled dose for that patient (typically 1.7 mg/kg) radioiodinated with 131I calculated to deliver the desired radiation to the critical organ receiving the highest dose. In these trials, patients remained in radiation isolation until radiation exposure was less than or equal to 0.05 mSv/h (5 mR/h) at 1 meter. Patients included in this study did not receive chemotherapy as part of the conditioning regimen, and autologous bone marrow or peripheral blood hematopoietic stem cells were infused when total body radioactivity was less than 0.02 mSv/h (2 mR/h) at 1 meter. Patients undergoing bone marrow harvest were required to have less than 25% lymphomatous involvement of the bone marrow, and, as per standard of care, the marrow was treated with anti-CD9, -CD10, -CD19, and -CD20 antibodies and complement before cryopreservation. This purging method was capable of purging approximately 2 logs of B cells from the stem cell product. Patients receiving autologous bone marrow received either sargramostim (granulocyte-macrophage colony-stimulating factor [GM-CSF]) at 250 μg/m2 per day or filgrastim (granulocyte colony-stimulating factor [G-CSF]) at 5 μg/kg per day until neutrophil count was more than 1.0 × 109/L (1000/μL) for 2 consecutive days. Patients receiving mobilized peripheral blood hematopoietic stem cells were treated with GM-CSF or G-CSF at the discretion of the treating physician.
Selection of reference patients
Patients between the ages of 18 and 60 years with the diagnosis of FL or transformed FL who were treated with C-HDT and ASCT at our center between 1990 and 1998 were studied as a nonrandomized external control group. Patients were treated with C-HDT primarily on the basis of patient preference, physician preference, or insurance coverage for a specific procedure. Patients receiving C-HDT were required to have acceptable organ function, performance status, and otherwise be deemed candidates for transplantation by their primary treating physician. Bone marrow and peripheral blood hematopoietic stem cell harvesting procedures and supportive care paralleled that of study patients described in “Selection of patients for radioimmunotherapy.” Data collected from both the study and control patients included age, sex, bulk of disease at transplantation, stage at transplantation, serum lactate dehydrogenase (LDH) at transplantation, histology at transplantation (follicular grade, evidence of transformation to diffuse large B-cell lymphoma), presence of splenomegaly, number of prior regimens, prior radiation therapy, chemosensitivity at the time of transplantation (defined as a complete or partial remission from the regimen immediately prior to transplantation), hematopoietic stem cell source, development of secondary myeloid malignancies or myelodysplasia, date of last follow-up, date of progression of disease, date of death, and cause of death.
Follow-up and statistical analysis
Computerized tomography (CT) was performed at 1, 3, 6, and 12 months after transplantation and annually thereafter for study patients. Control patients were followed up according to the schedules of the treatment protocol (typically at 1 and 3 months after transplantation and annually thereafter) or as determined by the primary treating physician. All patients were assessed more frequently at the discretion of the primary physician. Bone marrow aspirates and biopsies with cytogenetic analyses were typically obtained 30 days after transplantation and annually thereafter for patients in the HD-RIT group.
Primary comparative endpoints of this study were OS and PFS. Secondary comparative endpoints included TRM and development of AML/MDS. Complete and overall response rates were considered descriptive data and were formally evaluated only for the patients in the HD-RIT group. Complete responses (CRs) were defined as complete disappearance of all tumor for at least 1 month and partial responses (PRs) as 50% to 99% regression. The Cotswold definition of complete response unconfirmed (CRu) was used.14 Categorical baseline variables were compared using the chi-square or Fisher exact test as appropriate. Continuous baseline characteristics were compared using the 2-sample t test or the Wilcoxon rank sum test, as appropriate. Age-adjusted International Prognostic Index (IPI) scores were based on maximal disease stage along with LDH and performance status immediately before transplantation. Progression was defined as any evidence (clinical, radiographic, bone marrow, flow cytometric, or molecular) of progressive disease, or administration of any antilymphoma therapy for suspected progression. OS and PFS were estimated by the method of Kaplan and Meier. Unadjusted comparisons of OS and PFS were made using the log-rank test. Adjusted comparisons were based on Cox regression models, in which an optimal “base” model was fit from the nontreatment variables listed earlier. An indicator for treatment (HD-RIT versus C-HDT) was then added to this base model to determine the effect of the conditioning therapy in relation to the effects of the nontreatment variables. All P values are 2-sided, and those values resulting from regression models were estimated using the Wald test. No adjustments were made for multiple comparisons.
TRM was defined as nonrelapse mortality through 100 days after transplantation. Nonrelapse mortality (NRM) was defined as death without prior relapse, regardless of cause. TRM and the probability of AML or MDS were summarized using cumulative incidence estimates. Relapse was regarded as a competing risk of TRM, and death without AML/MDS was a competing risk of AML/MDS. Definitions of secondary AML/MDS were based on World Health Organization (WHO) criteria or presence of clonal cytogenetic abnormalities of the bone marrow.15
Institutional approval and consent
Radioimmunotherapy studies were approved by the Institutional Review committees of the University of Washington and The Fred Hutchinson Cancer Research Center, and all patients provided written informed consent. Reference patients were either enrolled into institutionally approved studies or treated with standard transplantation protocols. Separate institutional approval was obtained to evaluate records of reference patients retrospectively.
Results
Baseline patient information
One hundred twenty-five consecutive patients aged younger than 60 years with FL were treated with high-dose therapy and autologous transplantation at our centers from January 1990 through May 1998. Of these 125 patients, 27 received treatment in sequential HD-RIT trials for relapsed B-cell NHL, and 98 nonrandomized control patients with FL were treated with conventional high-dose conditioning regimens and ASCT. A comparison of the clinical characteristics of these patients is detailed in Table 1. Seventeen (63%) of the patients in the HD-RIT group had histologies at diagnosis of grade I FL, 8 (30%) had grade II FL, and 2 (7%) had grade III FL. Forty-six (47%) patients in the C-HDT group had grade I FL, 40 (41%) had grade II FL, 9 (9%) had grade III FL, and 3 (3%) had insufficient tissue to determine the histologic grade. Nine (9%) of the 98 patients in the C-HDT group had more than one extranodal site of lymphomatous involvement (liver, 3; small bowel, 2; orbit, 2; stomach, 1; uterus, 1), compared with 1 (4%) of 27 patients receiving HD-RIT (liver).
Treatment received
High-dose radioimmunotherapy. Patients receiving high-dose radioimmunotherapy were treated with variable doses of 131I-tositumomab according to the design of phase 1 and phase 2 trials. Targeted radiation doses to the critical organ receiving the highest radiation dose were approximately 17 Gy in 2 patients, 20 Gy in 4 patients, 24 Gy in 3 patients, 26 Gy in 3 patients, 27 Gy in 14 patients, and 31 Gy in 1 patient. The critical organ receiving the highest dose was lung in 26 patients and kidney in 1 patient. Patients received a median of 19.7 GBq (531 mCi) of I-131 (range, 10.4-29.1 GBq [280-785 mCi]). Twenty-four patients from the HD-RIT group received purged bone marrow (as described earlier) as the source of hematopoietic stem cells, 2 patients received unmanipulated mobilized peripheral blood hematopoietic stem cells, and 1 patient at the lowest dose level had autologous reconstitution of hematopoiesis without transplantation. Hematopoietic stem cells were infused a median of 16 days following therapeutic 131I-tositumomab infusion (range, 12-27 days). The median numbers (range) of nucleated cells infused in the hematopoietic graft were 144 × 108 (67-302 × 108) and 678 × 108 (66-2510 × 108) for bone marrow and peripheral blood sources, respectively.
Conventional high-dose therapy. The 98 reference patients were treated as dictated by an alternative clinical trial (88 patients) or as standard of care (10 patients) using a variety of conditioning regimens, including total body irradiation (TBI)/cyclophosphamide (CY)/etoposide (VP-16) in 58 patients (59%), TBI/CY in 11 patients (11%), busulfan (Bu)/melphalan (Mel)/thiotepa (TT) in 21 patients (21%), CY/carmustine/VP-16 in 6 patients (6%), Bu/CY in 1 patient, and Bu/TT in 1 patient. Fifty patients from the C-HDT group received marrow as the source of hematopoietic stem cells. Forty-eight of these 50 patients had their bone marrow grafts purged with anti-CD9, anti-CD10, anti-CD19, and anti-CD20 antibody and complement treatment. The remaining 48 patients in the C-HDT group received mobilized peripheral blood hematopoietic stem cells, and 13 of these grafts underwent CD34 selection on immunoabsorption columns prior to cryopreservation. The CD34 selection method used was capable of depleting approximately 2 logs of tumor cells from the stem cell product. The median numbers (range) of nucleated hematopoietic cells infused were 251 × 108 (83-660 × 108) and 877 × 108 (3.65-4108 × 108) for bone marrow and peripheral blood sources, respectively, and did not differ significantly from those treated with HD-RIT (P = .34 for bone marrow, P = .59 for peripheral blood).
Response to therapy, progression, and survival
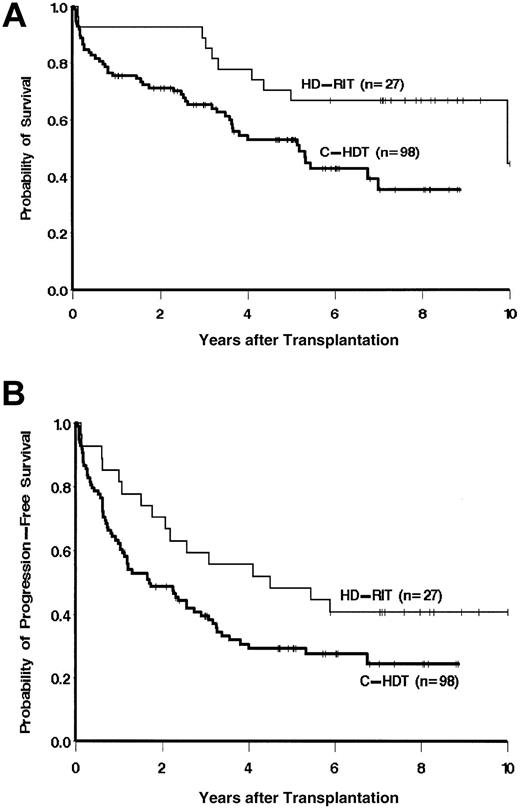
Twenty-three (85%) of 27 patients with FL treated with HD-RIT attained a CR or CRu following therapy. Two patients had a PR, 1 had a minor response, and 1 had progressive disease yielding an overall response rate (CR/CRu + PR) of 93%. Patients in the HD-RIT group had an improved overall survival as compared with recipients of C-HDT (unadjusted hazard ratio [HR] for death = 0.4; 95% confidence interval [95% CI], 0.2-0.9; P = .02) as shown in Figure 1. Likewise, patients receiving HD-RIT were also more likely to be alive and progression free compared with those who received C-HDT (unadjusted HR for death or progression = 0.6; 95% CI, 0.3-1.0; P = .06, Figure 1). Ten (37%) of 27 patients in the HD-RIT group remain alive and progression free more than 7 years following therapy. The 5-year point estimates of OS and PFS for the patients in the HD-RIT group were 67% and 48%, respectively, as compared with 53% and 29% for the C-HDT group. There was no statistically significant difference between the OS and PFS of control patients receiving TBI versus non-TBI-containing regimens, or those patients who received peripheral blood stem cell (PBSC) versus bone marrow grafts.
Overall survival (A) and progression-free survival (B) of patients treated either with HD-RIT using 131I-tositumomab and ASCT or C-HDT and ASCT.
Overall survival (A) and progression-free survival (B) of patients treated either with HD-RIT using 131I-tositumomab and ASCT or C-HDT and ASCT.
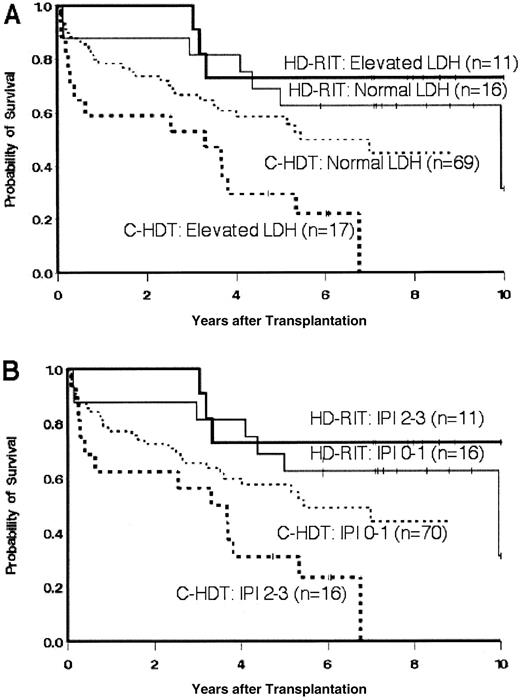
Because these patients were not randomly assigned to receive a given therapy, we performed a multivariable analysis, adjusting for variables that were either associated with outcome or that influenced the association of treatment with outcome. Nontreatment variables included the number of prior therapies, prior radiation therapy, bulk of disease at transplantation, chemosensitivity at transplantation, presence of transformed disease, hematopoietic stem cell source, purging/selection of hematopoietic stem cells, and elevated LDH. The nontreatment variables included in the base regression model were LDH, presence of transformed disease, and IPI score. After adjustment for these factors, the hazard of mortality in the HD-RIT group was still statistically significantly less than in the C-HDT group (HR = 0.3, P = .004). The suggestive decrease in the hazard of disease progression or death in the HD-RIT group also held up after adjustment for these factors (HR = 0.5, P = .03) as summarized in Table 2. The group of patients receiving C-HDT with missing LDH data performed similarly to those with normal levels of LDH. Moreover, inclusion of an indicator for missing LDH data in the regression model had virtually no effect on the association of treatment with outcome. The effect of an elevated LDH and the resultant age-adjusted IPI score on OS is further demonstrated as a stratification variable in Figure 2.
Overall survival of patients treated either with HD-RIT or C-HDT and ASCT stratified by (A) elevated or normal levels of LDH at the time of transplantation or (B) age-adjusted IPI score (0-1 versus 2-3) at the time of transplantation.
Overall survival of patients treated either with HD-RIT or C-HDT and ASCT stratified by (A) elevated or normal levels of LDH at the time of transplantation or (B) age-adjusted IPI score (0-1 versus 2-3) at the time of transplantation.
Four patients (15%) in the HD-RIT group and 8 patients (8%) in the C-HDT group had FL that had transformed into diffuse large B-cell lymphoma prior to transplantation. Because the natural history of transformed FL is very different from nontransformed FL, we analyzed the outcomes of these 2 subsets separately. Patients with nontransformed FL were more likely to be alive if they received HD-RIT as compared with C-HDT (hazard ratio for death, 0.3; 95% CI, 0.1-0.7; P = .005) (Figure 3). Likewise, progression-free survival was superior in the HD-RIT cohort (hazard ratio for death or progression, 0.5; 95% CI, 0.3-1.0; P = .04). The numbers of patients with transformed FL were too small to make significant statistical comparisons.
OS (A) and PFS (B) of patients with nontransformed follicular lymphoma treated either with HD-RIT using 131I-tositumomab and ASCT or C-HDT and ASCT.
OS (A) and PFS (B) of patients with nontransformed follicular lymphoma treated either with HD-RIT using 131I-tositumomab and ASCT or C-HDT and ASCT.
Engraftment and toxicities
Patients in each arm experienced comparable periods of neutropenia and thrombocytopenia after infusion of hematopoietic stem cells (Table 3). The 100-day TRM for the HD-RIT group was 3.7% as compared with 11% (P = .1) for the C-HDT group (Table 4). The 100-day TRM was not significantly different (P = .7) between patients in the C-HDT group who received a TBI-containing (10%) or chemotherapy-only regimen (14%). Overall, NRM also trended lower in the HD-RIT group after adjusting for LDH (HR for NRM = 0.2; 95% CI, 0.1-1.0; P = .06) (Figure 4). To determine if the higher 100-day TRM in the C-HDT group was the major contributor to inferior survival in this treatment arm, we performed a survival analysis that excluded patients who died without relapse by day 100 after transplantation. Even after excluding these patients, HD-RIT resulted in improved survival (P = .03, log rank) as compared with C-HDT.
Nonrelapse mortality of patients treated either with HD-RIT using 131I-tositumomab and ASCT or C-HDT and ASCT.
Nonrelapse mortality of patients treated either with HD-RIT using 131I-tositumomab and ASCT or C-HDT and ASCT.
We additionally evaluated the long-term risk of AML or MDS for each of these groups. Two (7.4%) patients in HD-RIT group developed AML or MDS at 4.7 and 6.8 years after transplantation, and 6 (6.1%) patients in the C-HDT group developed AML or MDS at 0.7, 1.2, 2.2, 3.0, 5.1, and 5.3 years after transplantation. The estimated probability of AML or MDS by 7 years after transplantation is .086 in the C-HDT group and .076 at 8 years after transplantation in the HD-RIT group. Follow-up is such that estimates beyond these times are not reliable.
Discussion
Anti-CD20-radiolabeled antibodies represent a promising new therapy for patients with relapsed follicular lymphoma.8,16-20 Randomized studies evaluating these agents at nonmyeloablative doses have demonstrated superior response rates compared with unconjugated antibodies, although the median time to progression has been 6 to 11 months.16,21 An alternative approach has been to deliver potentially curative doses of radiation to tumor sites by escalating the quantity of radioactivity infused and using autologous hematopoietic stem cell support.10,22 In this study we rigorously evaluated a cohort of patients with relapsed FL who were treated either with high-dose radioimmunotherapy or with traditional high-dose conditioning regimens and ASCT. Unlike the setting of relapsed aggressive NHL, however, the decision to refer patients with FL for high-dose therapy remains controversial because prospective randomized data do not exist that clearly establish its role in prolonging survival. Nevertheless, selected patients are commonly treated with this approach in an attempt to thwart the inexorable decline in remission durations over time. Reluctance to use HDT in this setting stems primarily from concerns about the predictable morbidity from expected toxicities and the possibility of fatal complications.
The HD-RIT-based conditioning regimen tested in this study had well-tolerated nonhematopoietic toxicity. We have previously demonstrated that such patients have comparatively low rates of vomiting (28%), transaminase elevations (29%), stomatitis requiring treatment with parenteral opiates (3%), alopecia (3%), and venoocclusive disease of the liver (0%).22 The risk of TRM (3.7%) observed after HD-RIT compared favorably with the risk of TRM after C-HDT (11%) and likely was a contributor to the improved outcomes in the HD-RIT group. Rates of TRM in the C-HDT group were less than or equal to those reported in other trials evaluating high-dose therapy for lymphoma at our center.23-25 Similar durations of cytopenias and rates of early fatal infection in both groups (3.7% versus 4.1%) suggest that the apparent reduction in TRM with HD-RIT is due to diminished nonhematopoietic organ toxicity as compared with other regimens. This low rate of organ injury may be related to the lack of chemotherapy use in the RIT group, the targeted delivery of the high-dose radioimmunotherapy, or the individualized treatment that precisely limits the effective total radiation dose to critical organs.
In this study, HD-RIT provided more improvement in OS than in PFS as compared with the C-HDT arm. This observation in part may have resulted from the lower TRM in patients receiving HD-RIT, who then continue to be at risk of relapse after treatment. Nevertheless, the higher TRM alone in the C-HDT group does not account for the entire difference in OS, because exclusion of patients who had early transplant-related mortality did not eliminate survival differences between the groups. The apparent late plateau in the overall survival curves may be also related to the improved ability of patients who relapse after HD-RIT to better tolerate further therapy. In addition, the close follow-up of patients on the HD-RIT arm may have identified evidence of progression sooner than those in the control group, further narrowing the apparent difference in PFS between the cohorts.
One major concern regarding both high-dose therapy and the therapeutic use of radionuclides is the potential for leukemogenesis. Secondary cancers in patients heavily treated before transplantation are often difficult to attribute to any single specific agent. Despite the rigorous follow-up, including annual bone marrow evaluations for patients who received radioimmunotherapy, our data do not suggest an increase in AML/MDS in the patients in the HD-RIT group relative to the control group. Furthermore, the follow-up-adjusted rates observed in both cohorts fall within the range reported in other studies evaluating high-dose therapy for FL.4,6,26 More recent large studies specifically evaluating AML/MDS following autologous transplantation for lymphoma have suggested that the incidence of this complication peaks at a median of 2.5 years after transplantation,27 paralleling our observations in the C-HDT group (median, 2.6 years). In contrast, patients receiving HD-RIT developed AML/MDS a median of 5.8 years after transplantation, suggesting that a longer follow-up may be required to more accurately portray the true risk of developing a secondary myeloid malignancy in this group.
As with any study, the final interpretation of the results must be tempered by the limitations of the design. The nonrandomized nature of this comparison may have led to unrecognized imbalances between the groups. For example, we were not able to adjust for the HD-RIT eligibility requirements of absent HAMA and favorable biodistribution, although these factors are not known to correlate with outcome. Furthermore, the relatively small sample size may have limited our ability to detect differences that truly exist in known baseline prognostic factors between the 2 groups. Moreover, these results do not address the fundamental and controversial question of whether high-dose therapy should be used as a treatment for relapsed FL, but rather focus on which conditioning regimen might be optimal if ASCT is selected as a treatment approach. Nevertheless, the apparent efficacy and limited toxicity of HD-RIT offer an attractive alternative to conventional transplantation or nontransplant regimens.
Despite the improved survival observed in the patients with FL who received ablative HD-RIT, disappointingly, most patients eventually developed recurrent lymphoma. Our ongoing strategy is attempting to improve these results by adding high doses of chemotherapy to RIT in patients aged younger than 60 years.28 Given the excellent safety and tolerability of this approach, we are also currently evaluating HD-RIT without chemotherapy in adults aged 60 to 80 years, who might not have the opportunity for potentially curative high-dose therapy because of concerns about regimen-related mortality.29 Other plans also include the use of radioimmunotherapy as a less-toxic preparative regimen for allogeneic transplantation.
FL, with its long natural history, considerable patient-to-patient variability, and unclear potential for cure remains an ongoing challenge for clinicians and investigators alike. As new therapeutic agents such as radioimmunoconjugates become available for the treatment of FL, prospective randomized clinical trials will be needed to determine whether the course of this disease truly can be altered.
Prepublished online as Blood First Edition Paper, May 15, 2003; DOI 10.1182/blood-2003-02-0622.
Supported by National Cancer Institute grants P01CA44991 and K23CA85479 and American Society of Clinical Oncology Young Investigator Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.