Abstract
In previous studies amphotropic MFGS-gp91phox (murine onco-retrovirus vector) was used in a clinical trial of X-linked chronic granulomatous disease (X-CGD) gene therapy to achieve transient correction of oxidase activity in 0.1% of neutrophils. We later showed that transduced CD34+ peripheral blood stem cells (CD34+ PBSCs) from this trial transplanted into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice resulted in correction of only 2.5% of human neutrophils. However, higher rates of transduction into stem cells are required. In the current study we demonstrate that the same vector (MFGS-gp91phox) pseudo-typed with RD114 envelope in a 4-day culture/transduction regimen results in a 7-fold increase in correction of NOD/SCID mouse repopulating X-CGD CD34+ PBSCs (14%-22% corrected human neutrophils; human cell engraftment 13%-67%). This increase may result from high expression of receptor for RD114 that we demonstrate on CD34+CD38– stem cells. Using RD114-MFGS encoding cyan fluorescent protein to allow similar studies of normal CD34+ PBSCs, we show that progressively higher levels of gene marking of human neutrophils (67%-77%) can be achieved by prolongation of culture/transduction to 6 days, but with lower rates of human cell engraftment. Our data demonstrate the highest reported level of functional correction of any inherited metabolic disorder in human cells in vivo with the NOD/SCID mouse system using onco-retrovirus vector.
Introduction
Chronic granulomatous disease results from inherited defects of phagocyte oxidase, leading to recurrent infections.1 Allogeneic transplantation can cure chronic granulomatous disease (CGD), but treatment-related complications remain a problem.2,3 Gene therapy is a promising therapeutic alternative, but clinical trials of p47phox-deficient autosomal recessive CGD or gp91phox-deficient X-linked CGD (X-CGD) using amphotropic pseudotyped MFGS oncoretrovirus vector resulted in 0.1% or less of circulating neutrophils, demonstrating functional correction with effect lasting less than a year.3,4 The nonobese diabetic/severe combined immunodeficient (NOD/SCID) mouse human-xenograft model is used as a surrogate in vivo system for assessment of transduction and engraftment of primitive human hematopoietic stem cells,5 although there remains controversy whether it is predictive of outcome in humans. More recently with the amphotropic MFGS-gp91phox onco-retrovirus vector used in our clinical study of X-CGD gene therapy we showed that transduced X-CGD CD34+ peripheral blood stem cells (CD34+ PBSCs) from this trial transplanted into NOD/SCID mice resulted in correction of only 2.5% of human neutrophils in vivo, despite ex vivo transduction of up to 70% of CD34+ PBSCs.6
Low numbers of corrected neutrophils in the clinical trial indicate a need to achieve higher levels of transduction of primitive stem cells. Although the newly developed third-generation self-inactivating lentivector systems appear to improve stem cell targeting,6 issues of clinical scale-up of the transient production systems of lentivector production, as well as problems achieving adequate levels of transgene expression from internal promoters, may impede clinical application of lentivector. Not only have MFGS vectors already been used safely in clinical trials, but also in the case of gp91phox subunit correction for X-CGD the MFGS-gp91phox achieves high levels of gp91phox transgene protein production that is equal to native protein present in normal neutrophils; a level that could not be achieved even with one of the strongest internal promotors (elongation factor 1 α) that has been tried with lentivector.6 Thus, there are compelling reasons to attempt to improve performance of MFGS vector in the correction of X-CGD, because versions of this vector will have fewer barriers to clinical application.
Studies report high-level transduction efficiency in hematopoietic stem cells using feline endogenous virus (RD114) envelope, which binds to neutral amino acid transporter (RDR).7-11 The current study examines the potential of feline endogenous virus envelope RD114-pseudotyping to significantly improve gene transfer of MFGS-gp91phox into human X-CGD CD34+ PBSCs to achieve high levels of functional correction of oxidase defect in human X-CGD neutrophils in vivo arising from transduced XCGD CD34+ PBSCs transplanted into NOD/SCID mice. In our study we focus on the use of CD34+ PBSCs as the target because this stem cell source has been used previously for CGD gene therapy trials. However, it is of note that in previously published gene marking studies, the level of engraftment and transduction efficiency of CD34+ PBSCs in the NOD/SCID model is lower compared with bone marrow (BM) or cord blood CD34+ cells.12-15 In our current study we also examine one potential basis for the performance of RD114-pseudotyped vectors by demonstrating for the first time high levels of RDR expression on hematopoietic stem cells with primitive CD34+CD38– phenotype. Finally, we used RD114-MFGS vector encoding cyan fluorescent protein (CFP) to transduce normal CD34+ PBSCs to explore the effect on the outcome of transplantation into NOD/SCID mice of prolongation of ex vivo culture to allow time for additional transductions. We will show that prolongation of culture and transduction from 4 to 6 days can greatly increase the observed percentage of marked human cells in vivo, although at the cost of reduction in human cell engraftment.
Materials and methods
Collection of normal and X-CGD CD34+ PBSCs
After obtaining informed consent (protocols 94-I-0073 and 95-I-0134, approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases) 1 healthy adult volunteer and 2 patients with X-CGD received 5 daily subcutaneous injections with 10 μg/kg granulocyte colony stimulating factor (G-CSF; Filgrastim; Amgen, Thousand Oaks, CA). PBSCs were collected by apheresis on day 5 (CS3000 Plus; Baxter Healthcare, Fenwal Division, Deerfield, IL), and CD34+ cells were selected from the apheresis product (ISOLEX 300i; Nexell Therapeutics, Irvine, CA).
Generation of MFGS vector encoding gp91phox and CFP
To generate MFGS-gp91phox and MFGS-CFP transfer vectors, open reading frames (ORFs) of human gp91phox cDNA or CFP (Clontech, Palo Alto, CA), respectively, were inserted into the NcoI-BamHI cloning site of MFGS.16 To obtain RD114-pseudotyped MFGS-gp91phox and MFGS-CFP vectors we transduced FLYRD18-packaging cells17 with amphotropic–MFGS-gp91phox and transfected with MFGS-CFP plasmid, respectively. To obtain gibbon ape leukemia virus (GALV)–pseudotyped MFGS-CFP vector we transduced PG13-packaging cells with RD114-packaged MFGS-CFP in medium containing tunicamycin.18 For each vector a high-titer producer clone was selected.
Virus supernatant production and ultracentrifugation concentration
FLYRD18 MFGS vector producers were plated at 2 × 106 per 185-cm2 flask for 48 to 60 hours, and virus supernatant was collected in serum-free X-VIVO 10 (BioWhittaker, Walkersville, MD) with 1% human serum albumin (X-VIVO10/1% HSA). We performed transductions with RD114-pseudotyped vector particles separated from conditioned medium by ultracentrifugation (25 000 RPM [83 000g], 90 minutes, 4°C).9,19,20 Preliminary experiments showed that biologic activity was preserved after ultracentrifugation (data not shown). After ultracentrifugation, virus pellet was suspended in 1% of original volume of fresh X-VIVO10/1% HSA.19 Virus titer of original neat supernatant determined on CD34+ PBSCs by terminal dilution studies was 2 × 106 to 4 × 106 infectious units/mL for both MFGS-CFP and MFGS-gp91phox RD114-pseudotyped vectors, respectively. For GALV-pseudotyped MFGS-CFP vector, PG13 MFGS-CFP producer cells were plated at 2 × 106 per 185-cm2 flask for 60 hours, and supernatant was collected after 12 hours in X-VIVO10/1% HSA. Virus titer determined by terminal dilution studies on CD34+ PBSCs was 6 × 106 infectious units/mL.
Transduction of CD34+ PBSCs
Cultures were initiated in Retronectin (Takara Shuzo, Otsu, Japan) precoated 6-well plates with 1 × 106 CD34+ PBSCs in 3 mL growth medium (X-VIVO10/1% HSA; 50 ng/mL FLT3-ligand, 50 ng/mL stem cell factor, 20 ng/mL interleukin 6 (IL6), 10 ng/mL thrombopoietin, 10 ng/mL IL3) per well. Beginning 16 hours after culture initiation, CD34+ PBSCs were transduced 6 hours/d times 4 days using dilutions of concentrated virus vector equivalent to 8 × 106 to 1 × 107 infectious units (2- to 5-fold concentrated from the original neat supernatant) and 5 μg/mL protamine. Some CD34+ PBSCs were transduced 6 hours/d times 5 days (Table 1, mice III-C and III-D) or 6 days (Table 1, mice III-E and III-F). Naive nontransduced X-CGD CD34+ PBSCs served as negative control for gp91phox expression and as one of the negative controls for CFP expression, whereas CFP-transduced normal CD34+ PBSCs served as positive control for native gp91phox expression. Cell number relative to initiation of culture increased 4.4-fold (range, 2.6- to 6.8-fold) over 4 days. For ex vivo comparison of the transduction efficiency of RD114- and GALV-pseudotyped MFGS-CFP vectors, CD34+ PBSCs were transduced under optimum conditions for GALV-pseudotyped MFGS-CFP vector using 60% of neat supernatant and RD114-pseudotyped MFGS-CFP vector diluted from concentrated stock to a similar titer (3.6 × 106 infectious units/mL).
Transplantation of CD34+ PBSCs into NOD/SCID/β2m–/– or NOD/SCID mice
NOD/SCID/β2-microglobulin–/– (β2m–/–) and NOD/SCID mice21,22 (Jackson Laboratory, Bar Harbor, ME) were housed in microisolator cages provided with autoclaved food and acidified water. Cultured human transduced CD34+ PBSCs cells (3 to 20 × 106 day 4 [and for some mice day 5 and 6, Table 1]) were injected via tail vein into sublethally irradiated (300 cGy) 7-week-old NOD/SCID/β2m–/– or NOD/SCID mice. An aliquot of cells was retained in ex vivo culture.
Harvest of BM, peripheral blood (PB), splenocytes, and human CD34+ cell selection from mice
Mice were killed 6 to 9 weeks after transplantation, and BM from tibias and femurs was flushed into X-VIVO10/1% HSA. PB and spleens were collected from some mice. Spleens were minced and filtered (100-μm mesh). Red cells in BM, PB, and spleen preparations were lysed with ACK lysing buffer (Quality Biological, Gaithersburg, MD).
Engrafted human CD34+ cells were positively selected from chimeric mouse BM using the Dynal CD34+ Progenitor Cell Selection System (Dynal AS, Oslo, Norway) following the manufacturer's protocol. A portion of selected cells was cultured in fresh growth medium (liquid culture), and a portion was plated for colony assays in semisolid collagen medium (CollagenCult; StemCell Technologies, Vancouver, BC, Canada). Human growth factors for liquid culture were the same as that used for transductions, except for the addition of G-CSF 10 ng/mL. For colony assays IL6 and thrombopoietin (TPO) were omitted, but G-CSF 40 ng/mL, granulocyte-macrophage colony-stimulating factor (GM-CSF) 10 ng/mL, and erythropoietin 3 U/mL were added.
Analysis of human cell engraftment and transgene expression by flow cytometry
Antihuman fluorochrome-conjugated monoclonal antibodies were used to identify human hematopoietic cells (CD45-PerCP [peridinin chlorophyll protein] or CD45-CyChrome), human myeloid cells (CD13-FITC [fluorescein isothiocyanate] or CD13-PE [phycoerythrin]), and human B lymphocytes (CD19-PE). Human gp91phox expression was determined by indirect staining with murine monoclonal antibody 7D5 followed by Cy5- or FITC-conjugated goat antimouse immunoglobulin G (IgG) antibody.23 For analyses not requiring detection of CFP, a FACSsort (Argon laser; Becton Dickinson, San Jose, CA) was used, whereas CFP detection required a Vantage cell sorter (Becton Dickinson) equipped with a Krypton-UV laser (excitation at 413 nm).
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in ex vivo differentiating cultures of human CD34+ cells
With the use of the chemiluminescence assay, we measured phorbol 12-myristate 13-acetate (PMA)–stimulated superoxide production on day 16 in human myeloid cells differentiating in ex vivo culture from nontransduced and transduced-corrected X-CGD CD34+ PBSCs or normal CD34+ PBSCs.4
Analysis of human CD34+ cells purified from chimeric mouse BM
We measured PMA-stimulated superoxide and H2O2 production by granulocytes arising in culture from human CD34+ cells selected from chimeric mouse BM. Liquid cultures at 20 days were analyzed using the flow cytometry dihydrorhodamine 123 (DHR) assay.4,24 Superoxide generation by granulocytes and monocytes in myeloid colonies at 14 days of culture was indicated by formazan precipitate formed from nitroblue tetrazolium (NBT) dye reduction.4 For the NBT test 0.5 mL 0.1% NBT plus 0.5 μg/mL PMA in phosphate-buffered saline was layered over colony cultures. After 1 hour, 2 mL 1.5% paraformaldehyde in buffered saline was added to halt the reaction and to fix cells. After washes, collagen gels were transferred from the wells to slides for drying, counterstained with safranin to delineate NBT-negative (oxidase negative) colonies. NBT colony assays of human CD34+ cells selected from chimeric BM of mice receiving transplants of nontransduced X-CGD CD34+ and normal MFGS-CFP CD34+ cells served as negative and positive controls for superoxide generation, respectively. CFP expression in colony assays of human CD34+ cells selected from chimeric BM of mice receiving transplants of MFGS-CFP–transduced CD34+ cells were assessed with living cultures prior to any staining, fixation, or drying using a fluorescence microscope (CFP-excitation peak, 433 nm; emission peak, 475 nm).
Analysis of vector copy number by real-time PCR (TaqMan)
Vector copy number in genomic DNA of transduced human cells was determined by real-time quantitative TaqMan polymerase chain reaction (PCR; PE Applied Biosystems, Foster City, CA). We used a common forward primer for MFGS vectors that was located just upstream of the respective transgene; a 6-carboxyfluorescein (6FAM)–labeled probe that overlapped the start of the transgene sequence; and a reverse primer located within the coding region of the specific transgene close to the 5′ end: MFGS-CFP, forward primer (GTGAAGGCTGCCGACCC), 6FAM-labeled probe (TGGACCATCCTCTAGACTGCCATGGC), and reverse primer (CTCGCCCTTGCTCACCAT); MFGS-gp91phox, forward primer (GTGAAGGCTGCCGACCC), 6FAM-labeled probe (TGGACCATCCTCTAGACTGCCATG), and reverse primer (CCAAACCAGAATGACAAAAATGG).
For TaqMan PCR analysis the following incubation periods were applied for all primer sets: 2 minutes at 50°C, 10 minutes at 95°C, 40 cycles of 15 seconds at 95°C, and 60 seconds at 60°C. Standard curves for the TaqMan PCR analysis were obtained by using CD34+ cells or K562 cells transduced with the indicated vector at known copy number.
Reverse transcriptase (RT) real-time TaqMan PCR analysis of mRNA levels of receptors for RD114 and GALV in human hematopoietic stem cells
Expression of mRNAencoding the receptor for RD114 envelope was determined by reverse transcribing mRNA isolated from unfractionated human mononuclear cells from BM, cord blood, and mobilized apheresis product and CD34+ subsets from the mononuclear cells (obtained by flow cytometric cell sorting) containing human CD34+CD38+ or CD34+CD38– cells and analyzing the cDNA by real-time PCR under conditions described earlier. K562 cultured cell mRNA was used as a control with results from all sources normalized to mRNA encoding β2-microglobulin. Primers and probes are shown as follows: β2-microglobulin, forward primer (GGAGGGCATCCAGCGTACTCC), 6FAM-labeled probe (TCAGGTTTACTCACGTCATCCAGCAGAGAAT GGAA), and reverse primer (CGGATTGATGAAACCCAGACAC); RD114 receptor, forward primer (CCTGGATCATGTGGTACGCC), 6FAM-labeled probe (ATGTTCCTGGTGGCTGGCAAGATCGT), and reverse primer (GCGGGCAAAGAGTAAACCC); GALV receptor, forward primer (GCATAGATAGCACCGTGAATGG), 6FAM-labeled probe (CAGTGCAGTTGCCTAATGGGAACCTTGT), and reverse primer (GCTGACGGCTTGACTGAACTG).
Results
RDR mRNA is highly expressed in human CD34+CD38– cells
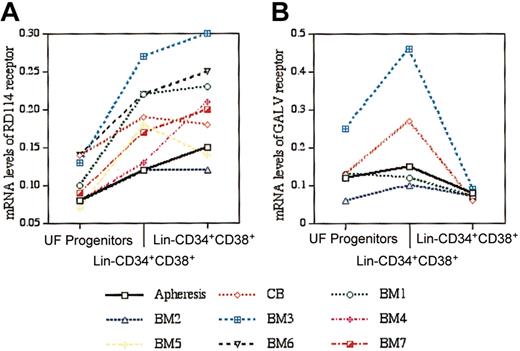
Previous studies show that mRNA expression for GALV and amphotropic receptors (Pit1 and Pit2, respectively) are lower in primitive than in more differentiated or lineage-positive human cells.11,25,26 With the use of RT TaqMan PCR analyses we found 7 of 9 samples to have the highest levels of RDR mRNA in the primitive (lin–CD34+CD38–) subset of hematopoietic progenitors (Figure 1A). In contrast, Pit1 mRNA levels in all human lin–CD34+CD38– samples analyzed were lower than in lin–CD34+CD38+ cells (Figure 1B).
RDR and GALV mRNA levels relative to β2-microglobulin mRNA in human apheresis product, cord blood, and bone marrow. (A) RDR mRNA levels were determined by RT TaqMan PCR in unfractionated (UF) mononuclear progenitor cells (mean, 0.1 ± 0.05), lin–CD34+CD38+ (mean, 0.18 ± 0.05), and lin–CD34+CD38– (mean, 0.20 ± 0.06) cells. (B) GALV mRNA levels were determined by RT TaqMan PCR in UF mononuclear progenitor cells (mean, 0.13 ± 0.07), lin–CD34+CD38+ (mean, 0.21 ± 0.14), and lin–CD34+CD38– (mean, 0.08 ± 0.01) cells. In contrast to the RDR mRNA data, with each stem cell source analyzed, the level of GALV mRNA in lin–CD34+CD38– was lower than in the lin–CD34+CD38+ cells.
RDR and GALV mRNA levels relative to β2-microglobulin mRNA in human apheresis product, cord blood, and bone marrow. (A) RDR mRNA levels were determined by RT TaqMan PCR in unfractionated (UF) mononuclear progenitor cells (mean, 0.1 ± 0.05), lin–CD34+CD38+ (mean, 0.18 ± 0.05), and lin–CD34+CD38– (mean, 0.20 ± 0.06) cells. (B) GALV mRNA levels were determined by RT TaqMan PCR in UF mononuclear progenitor cells (mean, 0.13 ± 0.07), lin–CD34+CD38+ (mean, 0.21 ± 0.14), and lin–CD34+CD38– (mean, 0.08 ± 0.01) cells. In contrast to the RDR mRNA data, with each stem cell source analyzed, the level of GALV mRNA in lin–CD34+CD38– was lower than in the lin–CD34+CD38+ cells.
Ultracentrifugation-concentrated RD114-pseudotyped MFGS-gp91phox achieves high transduction of CD34+ PBSCs ex vivo with functional correction of X-CGD
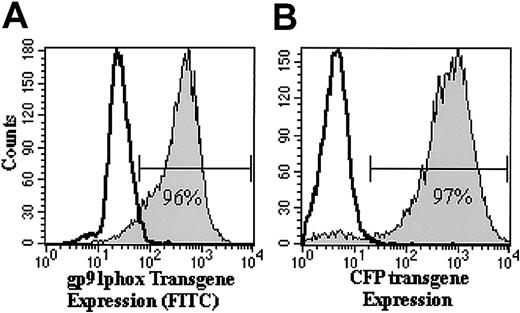
Ex vivo transduction efficiency of X-CGD or normal CD34+ PBSCs transduced with RD114-pseudotyped MFGS-gp91phox or MFGS-CFP vector, respectively, ranged from 82% to 99% on culture day 9 (Figure 2; Table 1, column 2). By day 30, more than 70% of cultured cells expressed transgene (Figure 3).
Flow cytometric analyses of transgene expression in human CD34+ PBSCs on day 9 of ex vivo culture. Shown are representative histogram analyses of RD114-MFGS-gp91phox (A) and RD114-MFGS-CFP (B) transgene expression of transduced (filled histograms) human X-CGD CD34+ PBSCs and normal human CD34+ PBSCs, in which the gp91phox and CFP transgene expression is 96% and 97%, respectively. Nontransduced X-CGD CD34+ PBSCs served as negative control as represented by the open histogram in each plot.
Flow cytometric analyses of transgene expression in human CD34+ PBSCs on day 9 of ex vivo culture. Shown are representative histogram analyses of RD114-MFGS-gp91phox (A) and RD114-MFGS-CFP (B) transgene expression of transduced (filled histograms) human X-CGD CD34+ PBSCs and normal human CD34+ PBSCs, in which the gp91phox and CFP transgene expression is 96% and 97%, respectively. Nontransduced X-CGD CD34+ PBSCs served as negative control as represented by the open histogram in each plot.
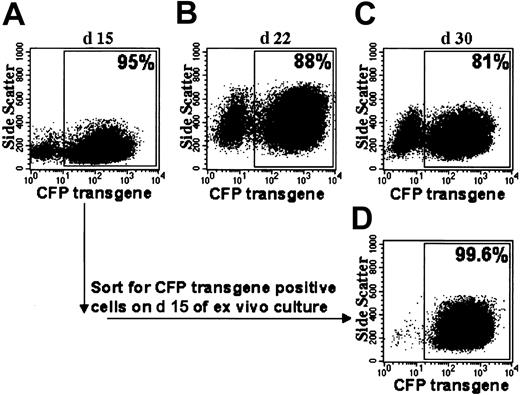
Flow cytometric analyses of CFP transgene expression after prolonged ex vivo culture. (A-C) Shown are dot plot analyses of MFGS-CFP–transduced normal CD34+ PBSCs on different days. (Day 9 of ex vivo culture for the same sample is shown in Figure 2B). (D) Cells sorted for CFP transgene expression on day 15 maintain CFP expression. The percentage of CFP-positive cells is indicated for each plot.
Flow cytometric analyses of CFP transgene expression after prolonged ex vivo culture. (A-C) Shown are dot plot analyses of MFGS-CFP–transduced normal CD34+ PBSCs on different days. (Day 9 of ex vivo culture for the same sample is shown in Figure 2B). (D) Cells sorted for CFP transgene expression on day 15 maintain CFP expression. The percentage of CFP-positive cells is indicated for each plot.
We assessed PMA-stimulated superoxide production (chemiluminescence assay) by myeloid cells differentiating from ex vivo cultured naive (nontransduced) and transduced-corrected X-CGD CD34+ PBSCs or normal CD34+ PBSCs. Cultures of naive X-CGD CD34+ PBSCs had oxidase activity less than 0.5% of cultures of normal CD34+ PBSCs. MFGS-gp91phox–transduced X-CGD CD34+ PBSC cultures demonstrated supranormal levels of superoxide production equivalent to 5-fold that of normal controls. It is important to note that in cultures of normal CD34+ PBSCs at day 16 only 14% to 27% of cells in culture expressed native gp91phox (not shown), whereas more than 85% of MFGS-gp91phox– transduced X-CGD CD34+ PBSCs expressed gp91phox transgene (results for day 9 in Table 1, column 2). Furthermore, the mean fluorescence intensity (MFI) of anti-gp91phox labeling in cultured transduced X-CGD CD34+ PBSCs was consistently 3-fold higher than the MFI of labeling of native gp91phox in the positive cells arising from normal CD34+ PBSCs in similar culture conditions.
CFP transgene expression persists after prolonged ex vivo culture
Ex vivo–cultured MFGS-CFP–transduced normal CD34+ PBSCs that expressed CFP in 95% of cells were sorted at day 15 into CFP-positive and CFP-negative populations. Two weeks later CFP-positive sorted cells continued to express CFP in more than 99% of cells (Figure 3), whereas cells sorted negative for CFP demonstrated CFP expression in less than 1% of cells (not shown). Vector copy number on day 23 in the CFP-positive versus CFP-negative sorted populations was 5.9 versus 0.1, respectively, indicating that transgene integration was highly associated with expression of transgene product.
Relationship between length of ex vivo culture, transduction efficiency, and engraftment in NOD/SCID mice
A culture initiated with 18 × 106 CD34+ PBSCs was transduced daily from culture day 1 to culture day 6 with RD114-MFGS-CFP. One third of the culture, respectively, was transplanted into 2 NOD/SCID mice on day 4 (culture expanded to 15 × 106 cells per mouse; Table 1, mice III-A and B), day 5 (30 × 106 cultured cells per mouse; Table 1, mice III-C and D), and day 6 (55 × 106 cultured cells per mouse; Table 1, mice III-E and F). The ex vivo transduction rates at days 4, 5, and 6 were 97%, 98%, and 99%, respectively, analyzed on day 9 of ex vivo culture (Table 1, column 2). When chimeric BM was analyzed 6 weeks later, the 4-, 5-, and 6-day cultured cells demonstrated a highly significant drop in total human cell engraftment on day 5 and 6 (Figure 4, dashed line), whereas the percentage of transgene expression in the engrafted human granulocytes progressively increased (Figure 4, solid line; Table 1, column 5). Therefore, for all subsequent experiments with NOD/SCID mice the 4-day culture and transduction regimen was used.
Increased transgene expression in NOD/SCID repopulating CD34+ PBSCs during prolonged ex vivo culture at the cost of human cell engraftment. CD34+ PBSCs were cultured ex vivo and transduced with RD114-MFGS-CFP for 4 (mice III-A, III-B, Table 1) 5 (mice III-C, III-D), or 6 (mice III-E, III-F) days before transplantation into NOD/SCID mice. Although transgene expression (solid line) increases with each extra day of transduction on days 5 and 6, human cell engraftment (dashed line) is lost substantially with ex vivo culture longer than 4 days.
Increased transgene expression in NOD/SCID repopulating CD34+ PBSCs during prolonged ex vivo culture at the cost of human cell engraftment. CD34+ PBSCs were cultured ex vivo and transduced with RD114-MFGS-CFP for 4 (mice III-A, III-B, Table 1) 5 (mice III-C, III-D), or 6 (mice III-E, III-F) days before transplantation into NOD/SCID mice. Although transgene expression (solid line) increases with each extra day of transduction on days 5 and 6, human cell engraftment (dashed line) is lost substantially with ex vivo culture longer than 4 days.
Human CD34+ PBSC engraftment in NOD/SCID/β2m–/– and NOD/SCID mice
NOD/SCID/β2m–/– mice received transplants of either naive-cultured X-CGD CD34+ PBSCs, RD114-MFGS-gp91phox–transduced X-CGD CD34+ PBSCs, or RD114-MFGS-CFP–transduced normal CD34+ PBSCs. The last group of cells also was transplanted into NOD/SCID mice for comparison with experiments using NOD/SCID/β2m–/– mice. Human hematopoietic cell engraftment of the 4-day ex vivo–cultured cells at 6 to 9 weeks after transplantation averaged 35% (range, 13%-67%) in BM (Table 1, column 3 for BM), 15% (range, 7%-33%) in spleens, and 9% (range, 4%-16%) in peripheral blood.
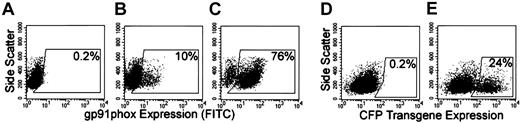
The gp91phox transgene expression and oxidase correction in X-CGD CD34+ PBSCs isolated from chimeric NOD/SCID/β2m–/– mice
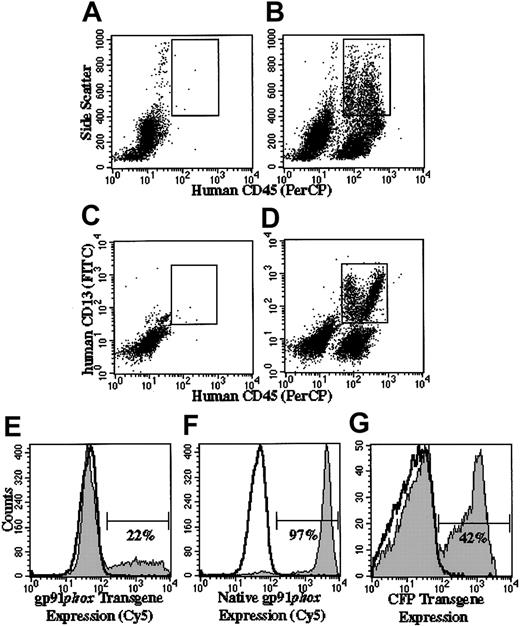
Naive-cultured human X-CGD CD34+ PBSCs transplanted into NOD/SCID/β2m–/– mice gave rise to human CD45+CD13+ high side scatter cells (human granulocyte gate) that did not express human gp91phox (nonfilled overlay in Figure 5E-F). In mice receiving transplants of MFGS-gp91phox–transduced X-CGD CD34+ PBSCs, 14% to 22% of cells from chimeric BM in the human granulocyte gate expressed gp91phox transgene (Table 1, column 4; Figure 5E). Mice that received transplants of MFGS-CFP–transduced normal CD34+ PBSCs served as a control for native gp91phox expression in which 94% to 98% of chimeric BM cells in the human granulocyte gate expressed native gp91phox (representative analysis shown in Figure 5F). CFP transgene expression in normal CD34+ PBSCs did not influence the native expression of gp91phox relative to that seen in the same cells not transduced (data not shown). The gp91phox-positive populations in Figure 5E-F were distinct from the gp91phox-negative cells. However, in contrast to observations ex vivo, the mean fluorescence intensity was higher for native gp91phox expression in normal granulocytes (Figure 5F) than for gp91phox transgene expression in corrected X-CGD cells (Figure 5E).
Flow cytometric analyses of engraftment and transgene expression in human cells in chimeric NOD/SCID and NOD/SCID/β2m–/– mice. Panels A-D indicate how flow cytometry gates were set to analyze human granulocytes arising in mice that received transplants. The gate shown in panels A (control mouse not receiving transplant) and B (mouse receiving transplant of human CD34+ PBSCs) confines human cells (human CD45+) with high side scatter characteristics, whereas the gate in panels C and D confines human CD45+/CD13+ cells. The analyses shown in panels E-G are delimited to cells that satisfy the characteristics confined to both gates and define a human granulocyte gate. In panels E-G, the gp91phox-positive (E-F) and CFP-positive (G) regions are indicated by the horizontal bars. The percentages indicate the percentage of cells in that region. Panels B and D demonstrate representative analyses of bone marrow from a mouse that received transplants of human RD114-MFGS-gp91phox–transduced X-CGD CD34+ PBSCs, in which total human cell engraftment (all human CD45+ irrespective of side scatter) was 36% (B) and the percentage of CD45+/CD13+ cells was 17% (D). Panels A and C show representative analyses of bone marrow from a mouse not receiving a transplant, indicating that the combined gating eliminates almost all murine cell background signal. Panels E-F show representative analyses of gp91phox expression in the human granulocyte gate in the bone marrow of mice that received transplants of RD114-MFGS-gp91phox–transduced X-CGD CD34+ PBSCs or RD114-MFGS-CFP–transduced normal CD34+ PBSCs (the latter used to demonstrate expression of native gp91phox in normal human cells). Panel G shows a representative analysis of CFP transgene expression in the human granulocyte gate in the bone marrow of a NOD/SCID mouse that received transplants of RD114-MFGS-CFP–transduced normal CD34+ PBSCs. Naive human X-CGD CD34+ PBSCs transplanted into NOD/SCID/β2m–/– mice gave rise to human granulocytes that did not express gp91phox or CFP (nonfilled overlays in panels E-G).
Flow cytometric analyses of engraftment and transgene expression in human cells in chimeric NOD/SCID and NOD/SCID/β2m–/– mice. Panels A-D indicate how flow cytometry gates were set to analyze human granulocytes arising in mice that received transplants. The gate shown in panels A (control mouse not receiving transplant) and B (mouse receiving transplant of human CD34+ PBSCs) confines human cells (human CD45+) with high side scatter characteristics, whereas the gate in panels C and D confines human CD45+/CD13+ cells. The analyses shown in panels E-G are delimited to cells that satisfy the characteristics confined to both gates and define a human granulocyte gate. In panels E-G, the gp91phox-positive (E-F) and CFP-positive (G) regions are indicated by the horizontal bars. The percentages indicate the percentage of cells in that region. Panels B and D demonstrate representative analyses of bone marrow from a mouse that received transplants of human RD114-MFGS-gp91phox–transduced X-CGD CD34+ PBSCs, in which total human cell engraftment (all human CD45+ irrespective of side scatter) was 36% (B) and the percentage of CD45+/CD13+ cells was 17% (D). Panels A and C show representative analyses of bone marrow from a mouse not receiving a transplant, indicating that the combined gating eliminates almost all murine cell background signal. Panels E-F show representative analyses of gp91phox expression in the human granulocyte gate in the bone marrow of mice that received transplants of RD114-MFGS-gp91phox–transduced X-CGD CD34+ PBSCs or RD114-MFGS-CFP–transduced normal CD34+ PBSCs (the latter used to demonstrate expression of native gp91phox in normal human cells). Panel G shows a representative analysis of CFP transgene expression in the human granulocyte gate in the bone marrow of a NOD/SCID mouse that received transplants of RD114-MFGS-CFP–transduced normal CD34+ PBSCs. Naive human X-CGD CD34+ PBSCs transplanted into NOD/SCID/β2m–/– mice gave rise to human granulocytes that did not express gp91phox or CFP (nonfilled overlays in panels E-G).
The flow cytometry gating for human granulocytes in chimeric mouse PB probably included cells other than human granulocytes, because native gp91phox expression was observed in only 89% of PB cells in the human granulocyte gate from a chimeric mouse that received transplants with normal human CD34+ PBSCs. Mice that received transplants of MFGS-gp91phox–transduced human X-CGD CD34+ PBSCs expressed human gp91phox transgene in 10% to 25% of PB cells in the human granulocyte gate (Table 1, column 7).
Transduction efficiency of MFGS-CFP–transduced normal CD34+ PBSCs engrafted in chimeric mice
In NOD/SCID/β2m–/– or NOD/SCID mice that received transplants of 4-day MFGS-CFP–transduced normal CD34+ PBSCs, 20% to 42% of cells from chimeric BM in the human granulocyte gate expressed CFP transgene (Table 1, column 5; Figure 5G). In the PB of 4 of these mice, CFP expression was detected in 35% to 77% of cells in the human granulocyte gate (Table 1, column 8) and in 19% to 50% of human CD45+/CD19+ cells (human B-lymphocyte gate; Table 1, column 9), demonstrating high CFP transgene expression in both the B-lymphoid and myeloid lineages in PB. In spleens from these same 4 mice, 9% to 13% of human B lymphocytes expressed CFP transgene (Table 1, column 6). Inspection of the results shown in Table 1, column 5, suggests that when sufficient cells are transplanted to achieve more than 10% human cell engraftment in NOD/SCID mice, there is no difference in either the human engraftment or in the high-transduction efficiencies seen in NOD/SCID/β2m–/– (6 mice, I-4a, I-4b, and II-4a through II-4d) versus the NOD/SCID mice (6 mice, IIIA-IIIF, Table 1, column 1). In NOD/SCID mice that received transplants of normal CD34+ PBSCs transduced with MFGS-CFP for 5 and 6 days, respectively, 42% to 48% and 67% to 77% of cells from chimeric BM in the human granulocyte gate expressed CFP transgene (Table 1, column 5; Figure 4).
Analysis of cultured human CD34+ cells selected from chimeric mouse BM
Human CD34+ cells that were isolated from the chimeric mouse BM were cultured in liquid medium or plated in semisolid collagen as outlined in “Materials and methods.” At 14 days of ex vivo liquid culture after human CD34+ isolation from chimeric BM of mice that received transplants of MFGS-gp91phox–transduced XCGD CD34+ PBSCs, 7% to 10% of differentiating cultured cells expressed gp91phox transgene (Table 1, column 10; Figure 6B). For the nontransduced X-CGD CD34+ group of chimeric mice, no expression of gp91phox was detected (Figure 6A). For comparison, in a culture of human CD34+ cells isolated from chimeric BM from one mouse that received transplants of MFGS-CFP–transduced normal CD34+ PBSCs, 76% of the cells in culture expressed native gp91phox (Figure 6C). Similarly, CFP expression by flow cytometry of this group of cultures of human CD34+ cells isolated from chimeric BM of mice that received transplants of MFGS-CFP–transduced normal CD34+ PBSCs ranged from 6% to 24% (Table 1, column 11; Figure 6E).
Transgene expression by human CD34+ cells that were cultured in vitro for 14 days after immunobead selection from the chimeric mouse bone marrow. Immunobead-selected and cultured nontransduced X-CGD CD34+ cells served as negative control for gp91phox (A) and CFP (D) expression, respectively. (B-C) Shown are representative analyses of gp91phox expression of transduced MFGS-gp91phox X-CGD CD34+ PBSCs and MFGS-CFP–transduced normal CD34+ PBSCs, respectively, in which gp91phox transgene expression was detected in 10% of cells, and native gp91phox expression was detected in 76% of cells. (E) Shown is a representative analysis of CFP transgene expression by MFGS-CFP–transduced normal CD34+ PBSCs in culture, in which CFP expression was detected in 24% of cells.
Transgene expression by human CD34+ cells that were cultured in vitro for 14 days after immunobead selection from the chimeric mouse bone marrow. Immunobead-selected and cultured nontransduced X-CGD CD34+ cells served as negative control for gp91phox (A) and CFP (D) expression, respectively. (B-C) Shown are representative analyses of gp91phox expression of transduced MFGS-gp91phox X-CGD CD34+ PBSCs and MFGS-CFP–transduced normal CD34+ PBSCs, respectively, in which gp91phox transgene expression was detected in 10% of cells, and native gp91phox expression was detected in 76% of cells. (E) Shown is a representative analysis of CFP transgene expression by MFGS-CFP–transduced normal CD34+ PBSCs in culture, in which CFP expression was detected in 24% of cells.
We performed a DHR analysis of oxidase activity of granulocytes maturing at day 20 in liquid cultures of human CD34+ cells isolated from BMs of chimeric mice. With normal CD34+ cell cultures, 17.5% of cells were DHR positive, consistent with our previously published data that, at 2 to 3 weeks, cultures of normal CD34+ PBSCs contain 10% to 20% oxidase-positive cells.27 Only mature myeloid cells in the culture have all the oxidase subunit components required for demonstrating a positive DHR response. At day 20 in liquid cultures of RD114-MFGS-gp91phox–transduced human X-CGD CD34+ PBSCs isolated from chimeric BM, an average of 1.8% of cells were DHR positive. When compared with the 17.5% DHR-positive cells in the normal control, it suggested that the level of oxidase correction was about 10% of the similarly cultured normal human CD34+ cells isolated from the chimeric mouse BM as noted earlier. No DHR-positive cells were seen in cultures from human CD34+ cells selected from chimeric mice that received transplants of nontransduced X-CGD CD34+ PBSCs. Aliquots of human CD34+ cells selected from BM of chimeric mice that received transplants of MFGS-gp91phox–transduced XCGD CD34+ PBSCs, naive X-CGD CD34+ PBSCs, or MFGSCFP–transduced normal CD34+ PBSCs were plated in semisolid collagen cultures. Colonies were assessed for stimulated superoxide production at 14 days of culture using the NBT assay or examined for CFP expression as indicated in “Materials and methods.” From 11% to 16% of all colonies from the MFGS-gp91phox X-CGD group were NBT positive (Table 1, column 12; Figure 7A). No NBT-positive colonies were seen in the naive X-CGD group (negative control for NBT), whereas 75% to 97% of all colonies from the MFGS-CFP group (normal positive control for NBT) were NBT positive (Table 1, column 12). With the MFGS-CFP transductions, CFP expression was detected in 3% to 22% of colonies from human normal CD34+ cells isolated from chimeric mice (Table 1, column 13; Figure 7B).
Photomicroscopic images demonstrating oxidase correction (NBT-positive staining in gp91phox–transduced X-CGD cells) and CFP transgene expression (fluorescence of CFP–transduced normal cells), panels A and B, respectively, in colonies derived from transduced CD34+ cells purified from chimeric mice. (A) NBT-positive colony from RD114-MFGSgp91phox–transduced X-CGD CD34+ PBSCs, 14 days after immunobead selection of human CD34+ cells from mouse bone marrow. Original magnification, × 60. NBT-negative colonies do not form formazan precipitate but can be delineated by safranin counter staining (not shown). (B) CFP-positive colony from RD114-MFGS-CFP–transduced normal CD34+ PBSCs, 14 days after immunobead selection of human CD34+ cells from mouse bone marrow. Original magnification, × 100.
Photomicroscopic images demonstrating oxidase correction (NBT-positive staining in gp91phox–transduced X-CGD cells) and CFP transgene expression (fluorescence of CFP–transduced normal cells), panels A and B, respectively, in colonies derived from transduced CD34+ cells purified from chimeric mice. (A) NBT-positive colony from RD114-MFGSgp91phox–transduced X-CGD CD34+ PBSCs, 14 days after immunobead selection of human CD34+ cells from mouse bone marrow. Original magnification, × 60. NBT-negative colonies do not form formazan precipitate but can be delineated by safranin counter staining (not shown). (B) CFP-positive colony from RD114-MFGS-CFP–transduced normal CD34+ PBSCs, 14 days after immunobead selection of human CD34+ cells from mouse bone marrow. Original magnification, × 100.
TaqMan PCR analysis of genomic vector insert copy number in transduced CD34+ PBSCs ex vivo and in human CD34+ cells isolated from chimeric mouse BM
In 2 experiments in which X-CGD CD34+ PBSCs were transduced with RD114-MFGS-gp91phox (transduction rates were 92% and 96%, Table 1, column 2), the vector insert copy number in the ex vivo–transduced cells was 7.2 and 8.1, respectively, calculated per gp91phox transgene-positive cell by flow cytometry. Similarly, in 4 experiments in which normal CD34+ PBSCs were transduced for 4 days with RD114-MFGS-CFP (transduction rates were 82%-97%, Table 1, column 2), the vector insert copy number in the ex vivo–transduced cells was 7.1 to 8.4, respectively, calculated per CFP transgene-positive cell by flow cytometry.
We have noted earlier how we purified the human CD34+ cells that had engrafted in vivo in the chimeric mouse BM. When these cells were differentiated in liquid cultures for up to 3 weeks, the resultant populations consisted of only human CD45+ cells. In addition to assessing expression of transgene and oxidase activity, we also analyzed genomic insert copy number. The average copy number of MFGS-gp91phox in human CD34+ cells isolated from the chimeric mice engrafted with transduced X-CGD CD34+ PBSCs was 2.53 ± 0.45 copies per transgene-expressing cell. Similarly, the average copy number of MFGS-CFP in human CD34+ cells isolated from the chimeric mice engrafted with transduced normal CD34+ PBSCs was 1.65 ± 0.24 copies per transgene-expressing cell. Thus, we note that the average insert copy number per transgene-expressing positive cell in ex vivo–transduced human CD34+ PBSCs before transplantation into the mice was 3- to 4-fold higher than the copy number in the transduced human CD34+ cells engrafted in vivo in the mice.
With 2 of the cultures of human CD34+ cells isolated from chimeric BM of mice that received transplants of MFGS-CFP–transduced normal CD34+ PBSCs (I-4a and I-4b, Table 1) the cells were sorted into CFP-positive and CFP-negative populations at day 17. Subsequent analyses after sorting revealed a purity of 93% and 95% CFP-positive cells in the positive groups and no CFP-positive cells in the negative groups. Vector copy number in genomic DNA isolated from the sorted cell populations was determined by real time TaqMan PCR. Analyses of the sorted, cultured human cells derived from chimeric marrow of the 2 mice showed that CFP-positive sorted cells had 2.0 and 2.1 vector copies per cell, whereas the CFP-negative sorted cells had 0.025 and 0.031 copies per cell. These data are consistent with the vector copy numbers determined without sorting as described in the previous paragraph. The results demonstrate that most CFP-negative cells have no integrated copies of the vector, suggesting minimal vector silencing of integrated MFGS-CFP vector in the human cells that had engrafted in the mice.
Discussion
In this study, we show that ultracentrifugation-concentrated RD114-pseudotyped MFGS-gp91phox and MFGS-CFP routinely achieves unprecedented levels of ex vivo transduction of human CD34+ PBSCs of more than 95%, resulting in 14% to 77% transgene expression (gp91phox or CFP) in vivo in human cells engrafted in NOD/SCID mice.
For this study we concentrated the RD114 vector by ultracentrifugation without loss of activity. Although highly concentrated vector was not toxic to the cells, use of vector concentrations more than 10-fold over neat culture supernatant did not result in higher levels of transduction of NOD/SCID repopulating cells. Another reason for vector concentration was that culture of human CD34+ cells in conditioned medium from the FLYRD18 line appears to induce loss of NOD/SCID repopulating cells.20 This effect appears to be eliminated when ultracentrifugation-concentrated RD114-pseudotyped vector that is resuspended in fresh medium is used for transduction.9
The monoclonal antibody we use to detect expression of human gp91phox does not bind to mouse gp91phox. This allowed straightforward assessment of expression of gp91phox transgene in the human neutrophils arising in NOD/SCID mice. However, in our study we have for the first time also measured the level of functional correction of oxidase activity of the corrected, engrafted human cells. However, this assessment required the isolation of the human cells from the chimeric mouse marrow, because murine neutrophils in NOD/SCID animals have normal levels of oxidase that would interfere with the functional assay. Because we also wanted to assess the number of transduced progenitors present 6 weeks after transplantation, we isolated human CD34+ cells from the chimeric BM. Isolated human CD34+ cells from mice that received transplants of RD114-MFGS-gp91phox–transduced X-CGD CD34+ PBSCs gave rise to oxidase normal neutrophils in NBT colony assays (12% NBT positive for transduced X-CGD cells versus 86% NBT positive for control normal cells). The DHR assay was performed on the same cells grown in liquid culture, demonstrating a similar level of correction relative to cultures of normal CD34+ cells isolated from mice that received transplants. Furthermore, up to 25% of human granulocytes circulating in the PB of mice that received transplants of MFGS-gp91phox–transduced CD34+ PBSCs expressed gp91phox transgene. Similar observations are made with the experiments in which normal CD34+ PBSCs were transduced with RD114-pseudotyped MFGS-CFP and transplanted into both NOD/SCID/beta2m–/– and NOD/SCID mice, in which 20% to 42% and 35% to 77% of human granulocytes in the chimeric BM and PB, respectively, were CFP positive. When CD34+ cells are isolated from the chimeric BM of these mice, we observe that 13.3% ± 2.3% of colonies arising from these cells express CFP.
In normal human PB, flow cytometry detection of native gp91phox is limited to granulocytes and monocytes, in which the mean fluorescence intensity for granulocytes is 3- to 4-fold higher than that for monocytes. The cytochrome b558 component of the phagocyte NADPH oxidase is a heterodimer of p22phox and gp91phox, in which protein synthesis and stability of both subunits are tightly linked. Thus, transgene gp91phox protein expression may be limited to myeloid cells as well. In fact, it was only in the granulocytes that we were able to detect expression of both human native gp91phox and human transgene gp91phox in cells from chimeric mice. We were not able to detect high-level expression of gp91phox in the human B-lymphocyte gate by flow cytometry. With CFP expression there is no lineage restriction, making it possible to assess and compare expression of this transgene not only in human granulocytes but also in the human B lymphocytes arising in chimeric mice. We found that 19% to 50% of human CD45+CD19+ cells from the PB and 9% to 13% of human CD45+CD19+ cells from spleens of these chimeric mice were CFP positive.
Most studies of human CD34+ stem cell engraftment in NOD/SCID mice are performed using the ontologically younger cord blood CD34+ cells that have a relative advantage for engraftment in this in vivo model. In studies in which cord blood was compared with BM or mobilized peripheral blood as the source of human transduced CD34+ cells transplanted into NOD/SCID mice, cord blood cells demonstrated signifi-cantly higher levels of transgene expression in human cells in vivo.28,29 Thus, the high level of transgene expression in NOD/SCID repopulating cells reported in this study is notable for the fact that we used CD34+ PBSCs, a cell source that would have much broader clinical relevance for autologous stem cell gene therapy than the use of cord blood CD34+.
Prior to our current studies it had been reported that infusion of up to 20 × 106 freshly isolated CD34+ PBSCs appeared to be required to achieve human blood cell engraftment levels of more than 20% in sublethally irradiated NOD/SCID mice.30 For the NOD/SCID/β2m–/– model, higher human cell engraftment potential was reported because of elimination of natural killer (NK) cell activity.21,22 In preliminary studies, we found that engraftment of 4-day cultured human CD34+ PBSCs in NOD/SCID/β2m–/– mice was superior to NOD/SCID mice, when the cell inoculum was less than 1 × 106 (data not shown). However, when more than 3 to 5 × 106 of 4-day cultured cells per mouse were injected, the engraftment observed with both immunodeficient strains of mice became similar. Because we are only able to obtain limited numbers of CD34+ PBSCs from our patients with X-CGD for preclinical studies, we chose the NOD/SCID/β2m–/– mouse model for studies with X-CGD CD34+ PBSCs, but we found that as we continued our studies we did not see a substantial difference between the 2 models as observed in the data shown in Table 1.
As previously noted, amphotropic-pseudotyped onco-retrovirus vectors yield low transduction of NOD/SCID repopulating CD34+ PBSCs.6 In a marking study comparing amphotropic and RD114-pseudotyped onco-retrovirus vectors in NOD/SCID repopulating human cord blood CD34+ cells, the RD114-pseudotyped vector greatly outperformed the amphotropic vector.20 Compared with amphotropic-pseudotyped vectors, GALV-pseudotyped vectors have achieved higher transduction in some human, nonhuman primate, and dog transplantation models,11,31,32 although in one report of a dog model of gene transfer, GALV- and RD114-pseudotyped vectors appeared to be equivalent.10 In preliminary studies, transduction of CD34+ PBSCs under optimal conditions with our GALV-pseudotyped MFGS-CFP vector resulted in 38% to 60% ex vivo marking. This finding was in contrast to our ultracentrifugation-concentrated RD114-pseudotyped MFGSCFP vector that, even when diluted to a titer similar to the GALV-pseudotyped vector (as determined on a K562 human erythroleukemia cell line), achieved transduction rates of 80% to 97% in human CD34+ cells under the same conditions. In a direct comparison of GALV and RD114-pseudotyped vectors by van der Loo et al,33 the RD114-pseudotyped vector achieved a 20.9-fold higher level of gene transfer into NOD/SCID repopulating CD34+ PBSCs than the GALV-pseudotyped vector. The higher ex vivo transduction rates with RD114 versus amphotropic and GALV-pseudotyped vectors seen by us and in the other studies20,33 and the relative lower marking of GALV and amphotropic vector-transduced human cells engrafted in the NOD/SCID mice suggest a correlation between the level of ex vivo transduction of the bulk population of CD34+ PBSCs and that of the more primitive NOD/SCID repopulating cells. However, the 1 to 2 order of magnitude increase in targeting of the NOD/SCID repopulating cells with the RD114 vector is far out of proportion to the percentage increase in overall bulk transduction and likely relates to better targeting of the more primitive cell population. This high transduction level of RD114-pseudotyped vectors in NOD/SCID repopulating cells might relate in part to high expression levels of mRNA for the neutral amino acid transporter (RD114 receptor) on primitive lin–CD34+CD38– cells, as demonstrated in Figure 1. However, other physiologic or biochemical factors such as receptor-envelope affinity might greatly influence transduction efficiency and overshadow the influence of receptor expression.
Although the 14% to 77% transgene expression of human cells engrafted in chimeric BM of NOD/SCID/beta2m–/– or NOD/SCID mice in our studies targeting human CD34+ PBSCs with RD114-pseudotyped vectors is encouraging, this represents a marked decrease from the initial ex vivo bulk transduction of 82% to 97% transgene expression (7.1-8.4 transgene copies per transgene expressing cell ex vivo). To evaluate if gene silencing was a reason for this decrease, we analyzed the vector copy number in MFGSCFP–transduced human CD34+ cells selected from chimeric mice and sorted into CFP-positive and -negative populations. We found that vector copy number in the CFP-negative population was 0.03, whereas that in the CFP-positive population was 2.05 copies per cell. This suggests that silencing is not the major cause of the difference between ex vivo and in vivo transgene expression rates, but rather the higher engraftment potential of the nontransduced cells. The lower insert copy number in the engrafted CFP-expressing human CD34+ cells versus the ex vivo insert copy number before transplantation most likely represents the lower efficiency of transduction of primitive NOD/SCID repopulating cells that divide late and thus less frequently during the 4-day ex vivo transduction period34,35 than more mature cells that, while going through several cell divisions, acquire several vector inserts but do not engraft this mouse model.
When we examined the effect of additional days of transduction, we found that extending the transductions from day 4 to day 6 almost doubled the observed percentage of human cells expressing transgene in NOD/SCID mice in vivo as seen in Figure 4. It is important to note that even on day 4 the observed ex vivo transduction for this experiment was 97%, whereas on day 6 it had increased to 99%. Thus, the extraordinarily high level of ex vivo transduction on both days was barely distinguishable, yet the outcome in vivo in the mice was quite different, indicating that substantial changes were occurring with the very small number of stem cells that mediate engraftment in the NOD/SCID mouse. Again as noted earlier, it is likely that substantial numbers of stem cells do not go into the cell division required for onco-retrovirus integration until after day 4 of culture. However, although the extension of the time for culture and transduction enhances integration of vector, it also reduces the engraftment potential of the stem cell compartment. Thus, we conclude that for our transduction conditions a 4-day culture with 3 daily transductions is the right compromise to achieve both goals. Alternative culture conditions might be found that allow preservation of engraftment potential during the time required for additional transductions. These observations emphasize the importance of considering preservation of total engraftment potential when evaluating methods that maximize transduction efficiency of primitive human hematopoietic stem cells.
Prepublished online as Blood First Edition Paper, June 26, 2003; DOI 10.1182/blood-2002-05-1482.
Supported by a grant from Deutsche Forschungsgemeinschaft (BR 2057/1) (S.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.