Abstract
Sepsis is associated with systemic inflammation, coagulopathy, and disrupted protein C (PC) pathway function. The effect of prothrombotic polymorphism, factor V Leiden (Arg506Gln; FV Leiden), was examined in a large clinical trial (PROWESS) of severe sepsis and a mouse endotoxemia model. In PROWESS, 4.1% (n = 65) of patients were heterozygous FV Leiden (VL+/–) carriers. The 28-day mortality was lower in VL+/– (13.9%) than in non-FV Leiden (VL–/–; 27.9%) patients (P = .013). The mortality benefit of recombinant human activated PC (rhAPC) treatment was similar in VL+/– (placebo, 15.6%; rhAPC,12.1%) and VL–/– patients (placebo, 31.0%; rhAPC, 24.7%; interaction P = .981). VL+/– status did not appear to influence baseline biomarkers of coagulopathy and inflammation or disease severity, with the exception that vasopressor usage was less in VL+/– patients (46.2% versus 63.0%; P = .009). In a median lethal dose (40 mg/kg) endotoxin mouse model, VL+/– mice had lower mortality than wild-type mice (19% versus 57%; P = .008), whereas the mortality of homozygous (VL+/+) mice was almost identical to that of wild-type mice (65% versus 57%; P = .76). The findings suggest that FV Leiden constitutes a rare example of a balanced gene polymorphism that maintains the FV Leiden mutation in the general gene pool due to a survival advantage of VL+/– in severe sepsis.
Introduction
Coagulation activation and inflammation are both parts of the innate host response to infection that may contribute to organ dysfunction and death when control of these systems is compromised.1,2 The protein C (PC) pathway suppresses excessive activation of blood coagulation and protects against inadvertent blood clots by inactivation of coagulation factors Va and VIIIa. Three independent point mutations in factor V (Arg506Gln, FV Leiden; Arg306Thr, FV Cambridge; and Arg306Gly, FV Hong Kong) disrupt the activity of this natural anticoagulant pathway by rendering factor Va partially resistant to inactivation by activated protein C (APC). Despite increasing the risk of thrombosis in heterozygous and homozygous carriers,3 the FV Leiden allele is highly prevalent in the Caucasian population with an incidence between 4% and 6%.4,5 Whereas FV Cambridge is very rare, the FV Hong Kong allele, which is associated with uncertain thrombotic risk,6,7 is present in about 3% to 5% of Southeastern Asians.8,9 The relatively high prevalence of FV Hong Kong and FV Leiden has prompted speculation about an associated survival advantage exerting a positive selection pressure,10,11 for example, by reducing blood loss during childbirth or by enhancing, via some unknown mechanism, embryo implantation.12-14
Here we examine the hypothesis that the FV Leiden mutation confers a survival advantage in severe sepsis. This hypothesis is based on several observations. First, animal studies have shown that natural anticoagulants, in particular the components of the PC pathway (eg, thrombomodulin [TM] and PC/APC), can reduce mortality associated with septic shock.15,16 Second, a recent large clinical trial (recombinant human activated Protein C Worldwide Evaluation in Severe Sepsis [PROWESS]) demonstrated that treatment with recombinant human APC (rhAPC) reduced relative all-cause 28-day mortality by 19.4% in patients with severe sepsis as compared to patients treated with placebo.17 Third, although a study of 184 in-hospital patients with meningococcemia found increased morbidity in heterozygous FV Leiden carriers, there was a trend toward reduced mortality in FV Leiden carriers compared with noncarriers.18 Fourth, infusion of low concentrations of thrombin in dogs significantly protected the animals from endotoxin-induced mortality.19 The infused thrombin likely resulted in augmented activation of endogenous PC by the TM-thrombin complex, thus potentially increasing endogenous APC levels. Fifth, both antithrombin III and tissue-factor pathway inhibitor failed to demonstrate a significant reduction in 28-day mortality in phase 3 clinical trials.20,21 Potentially these compounds may have prevented the generation of APC from its zymogen, either by inhibiting the active site of thrombin or by preventing the generation of thrombin. These data taken together suggest that mutations that enhance thrombin formation, such as FV Leiden, might have a similar beneficial effect on survival in sepsis, due to augmentation of endogenous PC activation.
An alternative hypothesis is that FV Leiden mutation is detrimental to patients with severe sepsis. It is believed that the consequence of an exaggerated and uncontrolled systemic host inflammation and coagulation (increased thrombin generation) response to the infecting pathogen(s) may lead to microvascular thrombosis, multiple organ dysfunction syndrome, and death in severe sepsis.2,22 Thrombin is a multifunctional protein that causes endothelial cell perturbation as well as thrombosis. The presence of the prothrombotic FV Leiden mutation, together with a loss of TM function due to endothelial damage in severe sepsis,23 may result in increased thrombin generation, microvascular thrombosis, and tissue injury. These changes could result in higher morbidity, as seen in the study of patients with meningococcemia,18 or higher mortality.
This study addresses the question of whether thrombin generation in sepsis is beneficial (eg, via increased APC generation) or detrimental (eg, via increased microvascular thrombosis and worsened multiorgan dysfunction). By (1) analyzing the effect of FV Leiden carrier status on mortality in a large clinical study on the efficacy of rhAPC in the treatment of severe sepsis and (2) using a mouse FV Leiden endotoxemia model, we attempt to resolve the question whether the prothrombotic FV Leiden mutation protects against or aggravates the lethal consequences of infection.
Patients, materials, and methods
Patients
Clinical study in patients with severe sepsis. PROWESS was a randomized, double-blind, placebo-controlled phase 3 trial evaluating the efficacy of rhAPC (drotrecogin alfa [activated]) in severe sepsis. The primary efficacy end point was 28-day all-cause mortality. Details of the trial design including inclusion and exclusion criteria were previously reported.17 The study was conducted in 164 hospitals in 11 countries (Australia, Belgium, Brazil, Canada, Germany, Spain, France, Netherlands, New Zealand, United States, and South Africa) after approval from the respective ethics review boards. Patients entered into the study were randomized 1:1 to a 96-hour infusion of drotrecogin alfa (activated) at 24 μg/kg/h (n = 850) or placebo (n = 840). Approximately two thirds of the 1690 patients enrolled in PROWESS were from North America and the 5 participating European countries. Informed consent obtained from all patients included permission for a blood sample for the determination of the 3 factor V single nucleotide polymorphisms, the only genetic marker determinations in this study.
Statistical analysis. Baseline comparisons used Fisher exact tests for categorical and Wilcoxon rank-sum tests for continuous variables. As recommended by the Consolidated Standards of Reporting Trials (CONSORT) guidelines24 for subgroup analyses, we reported the relative risk point estimates and the 95% confidence intervals (CIs) for the complementary subgroups. We assessed potential treatment-by-subgroup interactions using the Breslow-Day interaction test for homogeneity of odds ratios across strata. Stepwise logistic regression was used to model odds of 28-day all-cause mortality as a function of FV Leiden status, therapy, age, sex, the Acute Physiology, Age, and Chronic Health Evaluation II (APACHE II) score, vasopressor use, preexisting conditions (hypertension, chronic obstructive pulmonary disease, diabetes, malignancy, and cardiomyopathy). Forward and backward selection were used with P < .1 to retain terms in the model. D-dimer and activated partial thromboplastin time (APTT) data were transformed using natural logarithms to normalize residuals and analyzed with repeated measures and an unstructured covariance matrix. Results of pair-wise comparisons between treated and untreated patients are noted at each time point in Figure 1. Two-sided P < .05 was regarded as significant and 95% CIs are reported. SAS for Windows versions 8.2 and 6.09 (SAS Institute, Cary, NC) were used for these analyses.
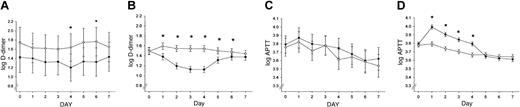
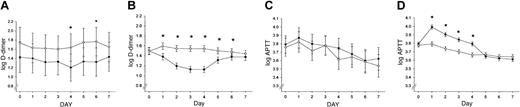
Effect of recombinant human APC treatment and FV Leiden mutation status on coagulation markers. Log APTT values and log D-dimer levels (fibrin degradation product) are shown for the first 7 study days in PROWESS (4 days of drug infusion, 3 days after infusion) for VL+/– patients (A,C) and VL–/– patients (B,D) receiving either placebo (○) or rhAPC (•). The 95% CIs are indicated. *P < .05.
Effect of recombinant human APC treatment and FV Leiden mutation status on coagulation markers. Log APTT values and log D-dimer levels (fibrin degradation product) are shown for the first 7 study days in PROWESS (4 days of drug infusion, 3 days after infusion) for VL+/– patients (A,C) and VL–/– patients (B,D) receiving either placebo (○) or rhAPC (•). The 95% CIs are indicated. *P < .05.
PCR determinations of FV gene mutation. Whole blood anticoagulated with either citrate or EDTA (ethylenediaminetetraacetic acid) was spotted onto an FTA card (Fitzco, Spring Park, MN), air dried at room temperature, and shipped to Lilly Research Laboratories in a desiccant pack for the determination of FV Leiden and FV Hong Kong/Cambridge. Blood samples were available from 1605 of the 1690 patients in the PROWESS study. Samples from the remaining patients were not collected, improperly collected, or not received by the central testing laboratory. Samples from 4 and 22 patients failed to amplify for the FV Leiden and FV Hong Kong/Cambridge polymerase chain reaction (PCR) analyses, respectively. Factor V mutation genotypings by PCR was performed on a block thermal cycler for amplification, followed by digestion with restriction enzyme Mnl1 (New England Biologicals, Beverly, MA) or restriction enzyme BstNI (New England Biologicals) for FV Leiden or FV Cambridge/Hong Kong, respectively, and separation/identification by ethidium-bromide stained agarose gel electrophoresis. Primers were obtained from Genosys: 5′-GGAACAACACCATGATCAGAGCA and 3′-TAGCCAGGAGACCTAACATGTTC for FV Leiden; 5′-TGTCTTCTGTCCTAAC and 3′-TCTTGAACC for FV Cambridge/Hong Kong. An alternate method was also used for FV Leiden genotyping. The FV Leiden mutation was detected by fluorescent resonance energy transfer probes with the LightCycler (Roche Molecular Biochemicals, Indianapolis, IN) and the FV Leiden mutation kit (Roche Diagnostics, Indianapolis, IN). DNA from a subject with FV Hong Kong genotype was a generous gift from Professor Raymond H. Liang, Department of Medicine, University of Hong Kong. A segment of DNA containing the mutation was cloned and the plasmid used as a positive control. FV Cambridge and Hong Kong genotypes in patients were confirmed by sequencing.
Measurement of endogenous levels of APC in placebo-treated patients. Three to 7 blood samples were collected from placebo-treated patients enrolled in North America in the PROWESS study for the measurement of endogenous APC levels over the first 4 study days. Details of the sampling time, sampling method, and assay methodology have been published.25 The lower limit of quantification of the immunocapture assay for APC was 10 ng/mL.
Measurement of coagulation and inflammation biomarkers. Serial blood samples were drawn before infusion and on days 1 to 7 after the start of study drug infusion. All biomarkers except interleukin 6 (IL-6) levels were determined at each time point from citrated plasma. IL-6 levels were determined from serum. Citrated plasma and serum samples were stored at –70°C until analyzed centrally. The last 403 patients enrolled were analyzed for at least one of the following additional biomarkers: prothrombin fragment F1.2 (F1.2); thrombin-antithrombin complex (TAT); plasminogen activator inhibitor 1 (PAI-1); thrombin activatable fibrinolysis inhibitor (TAFI); α2-antiplasmin (α2-AP); plasminogen; and soluble TM (sTM). The following assays were performed on either STA or STA compact coagulation analyzers (Diagnostica Stago, Asnieres, France) using Diagnostica Stago test kits. Activated partial thromboplastin time (APTT, STA-APTT), prothrombin time (PT, STA-Neoplastine CI plus), PC (Staclot Protein C), and protein S (Staclot Protein S) were all measured with coagulation-based activity assays. D-dimer levels were measured immunoturbidimetrically with the STA Liatest D-DI immunoassay. Antithrombin (AT, Stachrom ATIII), α2-AP (Stachrom Antiplasmin), plasminogen (Stachrom Plasminogen), and PAI-1 (Stachrom PAI) levels were quantitated with chromogenic activity assays. TAFI (enzyme-linked immunosorbent assay [ELISA], Enzyme Research Laboratories, South Bend, IN), sTM (Asserachrom Thrombomodulin, Diagnostica Stago), F1.2 (Behring Diagnostics, Westwood, MA), TAT (Behring Diagnostics), and IL-6 (Quantikine Human IL-6 HS kit; R&D Systems, Minneapolis, MN) antigen levels were measured by enzyme immunoassay. Platelet counts were performed using flow cytometric methodology.
FV Leiden mouse endotoxemia model
Animals. All animals were housed and the experiments were performed in the Oscar F. Peterson Animal Resource Center at the Medical College of Wisconsin, following standards and procedures approved by the Animal Care and Use Committee (protocol no. 318-97-1). TMPro and FV Leiden mice (a kind gift of David Ginsburg, University of Michigan, Ann Arbor) were maintained on a C57BL/6 genetic background and are as previously described.26-28 In this study we used TMPro mice that were homozygous for the Glu404Pro mutation. Age- and sex-matched littermate mice derived from heterozygotic (VL+/–) matings were used for all experiments. Genotyping was performed as previously described using a multiplex PCR technique.26
Enzyme capture assay for APC. Because no assay for analysis of mouse APC is currently available, the potential for activation of endogenous PC was estimated by infusing human PC and measuring its conversion to APC. In vivo levels of APC were determined using a previously described enzyme capture assay.29 Briefly, 20 μg human PC (Enzyme Research Laboratories) was injected into the femoral vein. Blood samples were collected from the inferior vena cava after 10 minutes into a mixture of 0.38% sodium citrate and 50 mM benzamidine HCl (final concentrations). APC was captured using a monoclonal antibody to human APC (HAPC 1555; kind gift of C. Esmon, Oklahoma Medical Research Foundation, Oklahoma City). Captured APC activity was determined with an APC-specific chromogenic substrate (PCa; American Diagnostica, Greenwich, CT)30 measured against a human APC (APC 1380; Enzyme Research Laboratories) standard curve.
Endotoxin challenge. Endotoxin from Escherichia coli serotype O55:B5 (Sigma Chemical, St Louis, MO; lot no. 96H4015) was reconstituted in sterile, tissue-culture–grade phosphate-buffered saline (Gibco BRL, Grand Island, NY) and delivered by intraperitoneal injection in a volume of 100 to 160 μL. Blood was collected 2 or 18 hours after endotoxin administration from the inferior vena cava into 0.38% sodium citrate (final concentration).
ELISAs for TAT, D-dimer, and cytokines. Plasma samples were analyzed for TAT using the commercially available Enzygnost TAT micro kit (Dade Behring, Marburg, Germany). D-dimers were quantified with the Asserachrom D-Di (Diagnostica Stago) ELISA. These assays have been validated with respect to cross-reactivity with mouse antigens as described.27 Cytokine assays were performed utilizing Quantikine M kits specific for the indicated murine cytokines (R&D Systems). In all cases the assays were performed according to the manufacturer's directions.
Results
FV Leiden carrier status in the PROWESS patient population
Blood samples were obtained from all patients with severe sepsis (defined as patients with a confirmed or clinically suspected infection associated with one or more acute organ dysfunctions) enrolled in the PROWESS study to identify the FV mutation carrier status (Arg506Gln as factor V Leiden, Arg306Thr as factor V Cambridge, and Arg306Gly as factor V Hong Kong). No homozygous FV Leiden patients were found in the PROWESS patient population, which is not inconsistent with the estimated frequency of 0.06% to 0.25% in the general Caucasian population.31 The incidence of VL+/– carriers (4.1%; Table 1) is consistent with the known incidence in the general population of the countries from which the patients were enrolled. The 65 FV Leiden carriers in the PROWESS study were enrolled from 10 of the 11 participating countries and 44 of the 164 participating study hospitals. Most (61 of 65) of the patients with the FV Leiden mutation were Caucasian. All 4 non-Caucasian patients who were carriers of the FV Leiden mutation were enrolled from North America: one had ancestry from the Indian subcontinent, 2 were of Hispanic descent, and one had African ancestry.
There were only 29 Asian patients (11 Western Asians and 18 East and Southeast Asians) enrolled in the PROWESS study; FV mutation data were available from 27 of these 29 patients. The prevalence of FV Cambridge and Hong Kong6,9,32 among PROWESS patients was similar to the frequency of these mutations in the general population and by ethnicity (Table 1).6,7,32,33 Because only 3 cases of these 2 mutations (2 for heterozygous FV Cambridge and one for heterozygous FV Hong Kong) were found among all the PROWESS patients, no further analysis was performed.
Baseline characteristics by FV Leiden status in PROWESS
Except for a less frequent need of vasopressor treatment at baseline for maintaining blood pressure (also reflected in baseline cardiovascular Sepsis-Related Organ Failure Assessment [SOFA] score34 ), VL+/– carriers were not significantly different from non-Leiden patients with respect to baseline clinical disease severity indices, preexisting conditions, or baseline status of biomarkers of coagulation activation, fibrinolysis, inflammation, and endothelial function (Tables 2-3). The CIs for these biomarkers were wide and there was no significant imbalance between the 2 groups of patients. Endogenous APC levels over the first 4 study days in the placebo-treated VL+/– carriers were all below the lower limit of quantification (10 ng/mL) of the assay.
Mortality outcome by FV Leiden status in PROWESS
The 28-day mortality rate among VL+/– patients (9 of 65 patients; 13.9%) was about half that observed for non-Leiden patients (428 of 1536 patients; 27.9%; P = .013). Modeling with stepwise logistic regression confirmed that, after adjusting for other important predictors of mortality including age, disease severity (ie, APACHE II score), and cardiomyopathy as a preexisting condition, VL+/– patients had a 2.8 times better odds of survival than non-Leiden patients (1.3-5.8; P = .006). Explicitly adjusting for the differences in baseline vasopressor use or ethnicity did not significantly influence these results (odds of survival for VL+/– carriers increased 2.7 or 2.8 times; P = .008 or .007). The relative risk of death with recombinant human APC treatment in VL+/– carriers was not significantly different than in non-Leiden patients (Table 4) and, using the above model, the odds of survival with rhAPC treatment were about 1.3 times that of placebo-treated patients (1.1-1.7; P = .013). The Breslow-Day interaction P (.981) was nonsignificant, supporting the conclusion that the effect of rhAPC therapy was similar in VL+/– and non-Leiden patients. Further, the protective effect of heterozygous FV Leiden polymorphism and the beneficial effect of rhAPC therapy appeared to be independent of one another (interaction P = .974). These observations suggest, but do not prove, that VL+/– carriers derive a similar benefit from treatment with rhAPC as non-Leiden patients.
Reduced anticoagulant response of rhAPC in FV Leiden carriers
No significant differences in clinically obvious thrombosis were found between VL+/– and non-Leiden patients (placebo and treatment group) during the 28-day study period. No serious bleeding events were reported in any of the VL+/– carriers. Serious bleeding was significantly more frequent in the non-Leiden patients (P = .048) and occurred in 1.0% of patients treated with placebo and in 2.3% of patients receiving rhAPC. Infusion of rhAPC into VL+/– carriers with severe sepsis failed to prolong the APTT and resulted in a less pronounced decrease in plasma D-dimer levels than in non-Leiden patients (Figure 1A-D), confirming a reduced anticoagulant effect of rhAPC in VL+/– carriers.
Reduced mortality of heterozygous FV Leiden mice in endotoxin model of sepsis
We have previously used genetically engineered mice with reduced TM function (TMPro mice) to determine the consequence of PC pathway disruption in an experimental model of endotoxemia.27,28 TMPro mice carry a single amino acid substitution in TM (Glu404Pro) that eliminates the cofactor activity of TM in the thrombin-dependent activation of PC. A dose of endotoxin producing about 50% lethality in wild-type controls resulted in 100% mortality of TMPro mice, confirming that genetic disruption of the PC pathway at the level of TM function increased mortality after a severe inflammatory stimulus.27 The detrimental effect of the TMPro mutation is likely caused by a combination of reduced PC activation, the loss of TM cofactor function for activation of TAFI, and reduced TM expression, which might concomitantly reduce the anti-inflammatory effects of the TM lectin-like domain.35
To further investigate the contribution of augmented coagulation per se to the reduced survival of TMPro mice, we used FV Leiden mice. We predicted that the FV Leiden mutation would disrupt the anticoagulant function of the PC system to a similar extent as the TMPro mutation, leading to substantially augmented thrombin formation in either strain of mice. However, whereas the TMPro mutation impairs PC activation,28 the FV Leiden mutation was predicted to result in enhanced PC activation. Hence, these 2 models permit comparison of the effect of increased thrombin generation with either enhanced APC generation (FV Leiden) or diminished APC generation (TMPro) on the magnitude of the inflammatory response and survival.
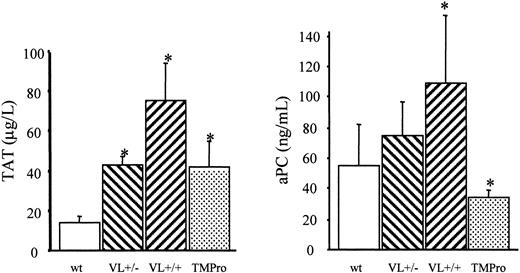
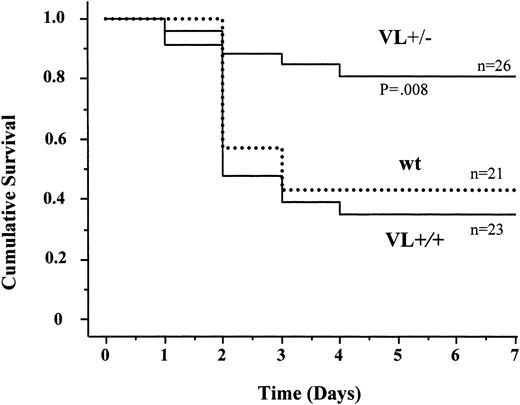
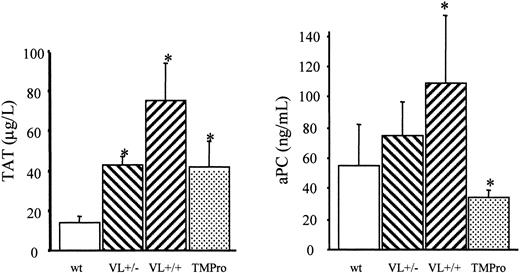
We therefore examined the survival of FV Leiden mice after challenge with a median lethal dose (LD50) of endotoxin. Intraperitoneal injection of 40 mg endotoxin/kg body weight caused death in approximately 60% of wild-type mice within 5 days (Figure 2). Mortality of VL+/+ mice was almost identical to that of wild-type mice (65% versus 57%; P = .76). VL+/– mice were significantly more likely to survive than wild-type mice (81% versus 43% survival; P = .008), or VL+/+ mice. VL+/+ and VL+/– mice challenged with 40 mg E coli endotoxin/kg body weight consistently produced more α-thrombin than wild-type mice, as deduced from plasma TAT levels (Figure 3). The conversion of infused human PC to APC (“Patients, materials, and methods”) was augmented in lipopolysaccharide (LPS)–challenged VL+/– and VL+/+ mice, demonstrating that the excess thrombin formed in FV Leiden mice was available for interaction with TM and PC. Of note, procoagulant activity in TMPro mice was almost identical to that in VL+/– mice, but APC formation was reduced, consistent with previous data.28 Eighteen hours after endotoxin injection, plasma TAT levels were similar in wild-type and VL+/– mice (21.7 ± 6 versus 16.3 ± 6 μg/L; P > .05) but were still much higher in VL+/+ mice (47.6 ± 13 μg/L; P = .003) indicating a persistent augmentation of thrombin formation only in VL+/+ mice. Plasma inflammatory cytokine levels of IL-6, IL-1β, tumor necrosis factor α (TNF-α), and IL-10, measured at baseline, and 2 and 18 hours after endotoxin injection, were similar in wild-type and FV Leiden mice.
Endotoxin-induced mortality in mice. Wild-type (wt), heterozygous FV Leiden (VL+/–), and homozygous FV Leiden (VL+/+) mice were injected intraperitoneally with 40 mg E coli endotoxin/kg body weight. Survival under standard husbandry conditions was monitored over a 7-day period.
Endotoxin-induced mortality in mice. Wild-type (wt), heterozygous FV Leiden (VL+/–), and homozygous FV Leiden (VL+/+) mice were injected intraperitoneally with 40 mg E coli endotoxin/kg body weight. Survival under standard husbandry conditions was monitored over a 7-day period.
Endotoxin-induced formation of thrombin and APC. A 40-mg dose of E coli endotoxin/kg body weight was injected intraperitoneally into wild-type (wt), heterozygous FV Leiden (VL+/–), homozygous FV Leiden (VL+/+), and homozygous TMPro mice (n = 8 per group). After 2 hours, 20 μg human PC was infused through the femoral vein, and 10 minutes after, blood was harvested for determination of TAT and APC levels in plasma. *P < .05. Error bars indicate standard deviation.
Endotoxin-induced formation of thrombin and APC. A 40-mg dose of E coli endotoxin/kg body weight was injected intraperitoneally into wild-type (wt), heterozygous FV Leiden (VL+/–), homozygous FV Leiden (VL+/+), and homozygous TMPro mice (n = 8 per group). After 2 hours, 20 μg human PC was infused through the femoral vein, and 10 minutes after, blood was harvested for determination of TAT and APC levels in plasma. *P < .05. Error bars indicate standard deviation.
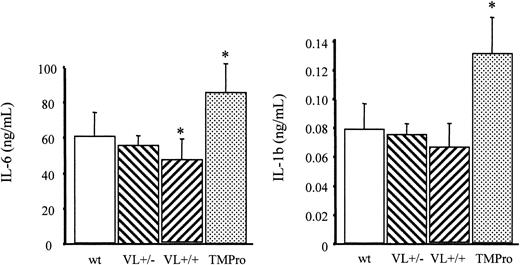
As reported earlier, TMPro mice challenged with a lower dose of endotoxin (5 mg endotoxin/kg body weight) exhibited higher plasma levels of IL-6 and IL-1β compared with wild-type mice.27 In contrast, VL+/+ mice subjected to the same treatment exhibit on average lower plasma concentrations of IL-6 and IL-1β than wild-type mice. This trend was statistically significant only for IL-6 levels and was still noticeable, albeit to a lesser extent, in VL+/– mice (Figure 4). These findings confirm that the FV Leiden and the TMPro mutations both augment the procoagulant response associated with endotoxemia, but exert opposite effects on APC generation and, depending on the dose of endotoxin, on IL-1β and IL-6 elaboration. The unaltered survival of VL+/+ mice on challenge with LPS, despite a more pronounced coagulation response than that seen in TMPro mice, provides direct experimental evidence (1) that the compromised survival of mice with reduced TM function is not the result of augmented coagulation per se, and (2) hence cannot be attributed to the loss of the anticoagulant function of APC. More importantly, these experiments yielded the surprising result that heterozygous carrier status for the FV Leiden mutation affords a significant survival advantage in an animal model of endotoxemia.
Endotoxin-induced inflammatory cytokine elaboration. Wild-type (wt), heterozygous FV Leiden (VL+/–), homozygous FV Leiden (VL+/+), and homozygous TMPro mice (≥ 5/group) were injected intraperitoneally with 5 mg E coli endotoxin/kg body weight. After 2 hours, blood was collected for determination of IL-6 and IL-1β levels in plasma. *P < .05. Error bars indicate standard deviation.
Endotoxin-induced inflammatory cytokine elaboration. Wild-type (wt), heterozygous FV Leiden (VL+/–), homozygous FV Leiden (VL+/+), and homozygous TMPro mice (≥ 5/group) were injected intraperitoneally with 5 mg E coli endotoxin/kg body weight. After 2 hours, blood was collected for determination of IL-6 and IL-1β levels in plasma. *P < .05. Error bars indicate standard deviation.
Discussion
The combined human and murine data in this report demonstrate that VL+/– carrier status is associated with a survival benefit in severe sepsis in humans and confers a survival advantage onto mice in LPS-induced endotoxemia. Due to the low frequency of homozygosity (0.06%-0.25%)36 of FV Leiden in the general human population, it was not possible to establish the effect of homozygous carrier status on the outcome of severe sepsis in humans. In the mouse endotoxemia model, homozygous carrier status did not improve survival, suggesting that the FV Leiden polymorphism may be an example of a balancing selection polymorphism in inflammatory disease, where heterozygous carrier status for a polymorphic allele lends a survival advantage over homozygosity for either allele,37 akin to the classic sickle cell–malaria paradigm.
The endotoxemia model recapitulates only certain aspects of sepsis, and the FV Leiden mutation may affect the progression of septicemia by different or additional mechanisms than those underlying the benefit seen in mice exposed to endotoxin, for example, by reducing bacterial dissemination secondary to augmented fibrin deposition around inflammatory foci.38 Yet, the prevalence of the FV Leiden polymorphism among patients enrolled in the PROWESS study was very similar to that of the general population, indicating that human heterozygous carriers of FV Leiden mutations are neither protected from infection and sepsis per se, nor from disease progression into clinically defined severe sepsis.
The clinical trial (PROWESS) and the animal experiments described here were initiated and conducted independently, and neither approach was specifically designed to reveal the mechanism by which the FV Leiden mutation alters the outcome of severe inflammatory disease. The animal experiments were based on the working hypothesis that the protective effect of the FV Leiden mutation might be mediated by enhanced activation of endogenous PC, thus replicating the benefit derived from treatment with exogenous rhAPC seen in human patients. We showed that VL+/– mice, and to a greater extent VL+/+ mice, respond to an LPS-induced inflammatory stimulus with augmented thrombin formation, and concomitantly increased activation of protein C. However, thrombin generation and concomitant PC activation were augmented only mildly and transiently in VL+/– mice, as compared with wild-type controls. Later on in the course of endotoxemia, that is, shortly before the onset of mortality in this model, the coagulation response in VL+/– mice was indistinguishable from that in wild-type mice. Likewise, human VL+/– carriers with severe sepsis in PROWESS did not exhibit a more intense coagulopathy as compared to non-Leiden patients, and endogenous APC levels remained below 10 ng/mL in the VL+/– carriers receiving placebo over the first 4 days of the study. In independent studies of human patients (with unknown FV Leiden carrier status) prior to and during episodes of severe sepsis and septic shock, endogenous APC levels were likewise only moderately and transiently elevated and did not parallel the rise in thrombin generation.23,25,39-42
If the protective effect of the FV Leiden mutation were indeed attributable to enhanced PC activation, this probably occurs in a transitory fashion during the early stage of disease and would not be observed by the time patients progressed to the stage of severe sepsis, when the endogenous capacity for PC activation is substantially impaired secondary to the suppression of TM activity and the consumption of PC. Indeed, the survival advantage seen in study patients might reflect in part a carryover of a benefit established in an earlier stage of disease, because VL+/– carriers were distinguished from non-Leiden patients already at study entry by a less frequent need for vasopressor use and a corresponding reduction in SOFA cardiovascular scores. The notion of an advantage established early during the disease course is consistent with the result of the logistic regression model analysis of PROWESS patient data, which suggested the survival benefit from treatment with rhAPC and VL+/– carrier status were independent of each other. The administration of exogenous APC in a stage of disease characterized by reduced capacity for endogenous PC activation could indeed augment the protective effect of the FV Leiden mutation without overriding it, due to the dissociation of both effects. We wish to point out, however, that the analysis of treatment effects (both benefit and risk) of rhAPC in the FV Leiden subgroup must be interpreted with caution due to the relatively small number of FV Leiden carriers in the study. However, in adherence to the CONSORT guidance24 for analyzing and interpreting complementary subgroups within a large trial, there was no evidence to suggest that VL+/– carriers with severe sepsis would not derive a treatment benefit from rhAPC similar to non-Leiden patients.
A recent study43 of rhAPC effects in a human endotoxemia model provides circumstantial evidence for a common mechanism underlying the benefit of FV Leiden carrier status and of rhAPC therapy. rhAPC, given at 24 μg/kg/h, significantly attenuated the drop in mean arterial blood pressure in healthy subjects challenged with a low dose of endotoxin (2 ng/kg). Likewise, one of the most significant improvements in organ function associated with rhAPC treatment in PROWESS was reflected in the cardiovascular SOFA score, due to reduced vasopressor requirement.44 The diminished need for vasopressor use among FV Leiden carriers at study entry appears congruent with these rhAPC treatment effects.
Taken together, the proposed APC-dependent mechanism of action of the survival benefit of the FV Leiden mutation, although compatible with the presented data, must await stringent experimental verification. Similarly, the reason why the FV Leiden mutation affords protection only in VL+/–, but not in VL+/+ mice subjected to endotoxemia remains unclear. Because only VL+/+ mice but not VL+/– or wild-type mice exhibit a lasting augmentation of coagulation in the later stage of endotoxemia, it is conceivable that the persistent prothrombotic state may produce more pronounced thrombotic organ damage in VL+/+ mice, which may negate the benefit of any early protective effect. Alternatively, the lack of a survival benefit in VL+/+ mice might be due to the loss of some unknown, potentially coagulation-independent function of the normal FV molecule.
Our findings raise a host of important questions about the general role of coagulation in the pathogenesis of sepsis, about the specific role of FV in this context, and about the therapeutic approach to septic patients, in particular FV Leiden carriers. Do drugs that inhibit thrombin generation without compensating for reduced APC rob heterozygous carriers of the FV Leiden mutation of their inherent survival advantage? Does rhAPC therapy provide additional benefit in FV Leiden carriers, and, if so, what is the contribution of APC's anticoagulant function, as compared to its vasoactive and cytoprotective effects on vascular endothelial cells?45,46 Do other mutations that affect the interaction of APC and FV, such as the FV Hong Kong or FV Cambridge mutations, confer a similar survival advantage? Do other prothrombotic gene polymorphisms, such as the prothrombin Gly0210Ala variant, replicate the FV Leiden effect? Similar results would argue strongly for a beneficial role for thrombin generation leading to endogenous APC generation in severe sepsis. Does the FV Leiden mutation exert a protective effect in inflammatory diseases other than sepsis? The answer to these questions will likely emerge in equal parts from future clinical studies encouraged by our findings and from further basic studies addressing the precise molecular mechanism by which the FV Leiden mutation prevents death in severe sepsis.
Prepublished online as Blood First Edition Paper, July 17, 2003; DOI 10.1182/blood-2003-06-1789.
Supported by grant HL60655 from the National Heart Lung and Blood Institute (H.W.), Grant-in-Aid 0150403N from the American Heart Association (H.W.), and Eli Lilly & Co.
S.B.Y., J.T.B., B.R.B., and D.E.J. are employees and stockholders of Eli Lilly & Co. J.-F.D. is a consultant for Eli Lilly & Co.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Bruce Gerlitz and Nancy Bauer for setting up and validating the PCR assays for the 3 factor V mutations; Kim Cocke, Crystal Dotson, Jeff Fill, and Linda Gfell for performing all the PCR assays; Cynthia Lindemann and Brian Cooley for their technical support; Samiha Sarwat and Jeff Helterbrand for their statistical support; and Gerald Johnson III and Delores Graham for their editorial assistance. We are grateful to Dr Ginsburg (University of Michigan) for providing FV Leiden mice.
The mouse model study was conducted by B.A.K., B.H.I., R.S., and H.W. independent of the human clinical study in this article and was not funded or supported in any way by Eli Lilly & Co. B.A.K., B.H.I., R.S., and H.W. are responsible for the mouse model data; S.B.Y., J.T.B., B.R.B., D.E.J., and J.-F.D. are responsible for the human patient data.