Abstract
Endothelial cells (EC) were extracted through a lectin-based method from bone marrow of 57 patients with active multiple myeloma (MM) and compared with their healthy quiescent counterpart, human umbilical vein EC (HUVEC). MMECs exhibit specific antigens that indicate ongoing angiogenesis and embryo vasculogenesis; solid intercellular connections, hence stability of MM neovessels; and frequent interactions with plasma cells, hence tumor dissemination. They show heterogeneous antigen expression, hence existence of subsets. Their main genetic markers are indicative of a vascular phase. They show intrinsic angiogenic ability, because they rapidly form a capillary network in vitro, and extrinsic ability, because they generate numerous new vessels in vivo. They vividly secrete growth and invasive factors for plasma cells. They signal through kinases mandatory for development of neovascularization. Ultrastructurally, they are abnormal and show metabolic activation, like tumor ECs. Thalidomide heavily interferes with their functions. Vasculogenesis and angiogenesis might contribute to the MM vascular tree and progression, in the form of growth, invasion, and dissemination. In view of the heterogeneity of the antigenic phenotype of MMECs, a mixture (or a sequence) of antiangiogenic agents coupled with thalidomide would seem plausible for the biologic management of MM.
Introduction
Endothelial cells (ECs) of tumor vessels differ greatly from those of quiescent healthy vessels. They proliferate rapidly in keeping with the enhanced angiogenesis that accompanies tumor progression (growth, invasion, metastasis).1 The profile and level of their cell adhesion molecules are also different because their attachment to each other and the extracellular matrix during sprouting (that implies cell proliferation and migration) is greatly reduced.2 Their survival is markedly dependent on growth factors secreted by the tumor and its microenvironment (cells and matrix), and on their expression of specific receptors for these factors.3 They are abnormal in shape and highly permeable because of fenestrae, vesicles, transcellular holes, widened intercellular junctions, a discontinuous basement membrane, and scarce accessory stabilizing cells such as pericytes.4 They share the lining of new vessels with tumor cells able to mimic vessels.5 The fast growth of ECs and tumor cells coupled with the structural and functional abnormalities of ECs make tumor vessels tortuous and dilated, with uneven diameter, profuse branching, and shunts. Thus, tumor blood flow is chaotic and variable and leads to hypoxic and acidic regions that stimulate further angiogenesis.6
Studies of this type in hematologic tumors remain circumstantial.7-10 ECs of the bone marrow of multiple myeloma (MM) form thin, tortuous, and arborized vessels7 ; express some adhesion molecules8 ; and display colony-forming ability.9 Neovessels of non-Hodgkin lymphomas display overlapping morphology.10 ECs of the bone marrow of patients undergoing bone marrow transplantation for unspecified tumors show spindle-shaped or round morphology, variable growth rate, and expression of some vascular antigens and adhesion molecules.11 Similar observations have been made on the bone marrow of mice and rats,12 and healthy donors.13
Bone marrow angiogenesis is a constant hallmark of MM progression7,14 and partly sustained by vascular endothelial growth factor (VEGF),15 basic fibroblast growth factor (bFGF), and matrix metalloproteinases (MMPs)16 secreted by the plasma cells. No studies, however, have so far documented EC antigen profile (including that related to neovascularization), angiogenic ability in vitro and in vivo, genetic markers relevant for neovascularization, and ultrastructural morphology. The following comparison of the bone marrow ECs of active MM (MMECs) with healthy quiescent ECs, that is, human umbilical vein ECs (HUVECs), will make the picture clearer. In addition, some hypotheses will be presented on the mechanisms of MM neovascularization and progression.
Patients, materials, and methods
Patients
Fifty-seven patients who fulfilled the South West Oncology Group (SWOG) diagnostic criteria for MM17 were studied at diagnosis (n = 30), relapse (n = 16), and leukemic phase (n = 11): 32 men, 25 women, aged 41-87 years (median, 71.7 years); 9 staged17 IIA, 6 IIB, 31 IIIA, 11 IIIB; the M component was immunoglobulin G (IgG) in 31, IgA in 10, IgD in 2, κ in 9, λ in 5. The study was approved by the local ethics committee, and all patients gave their informed consent.
Cells, conditioned media (CM), and reverse transcriptase–polymerase chain reaction (RT-PCR)
Bone marrow aspirates were centrifuged on Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation, and the separated mononuclear cells were left to adhere to 25-cm2 polystyrene flasks in complete medium (RPMI-1640 medium supplemented with 10% fetal calf serum [FCS] and 1% glutamine) for 2 hours in culture conditions. Adherent cells were stromal cells. These include ECs.9 To isolate ECs, stromal cells were harvested in trypsin/ethylendiaminotetraacetate (EDTA) solution (0.05/0.02% in phosphate-buffered saline [PBS]), washed twice with PBS, suspended in FCS-free medium (SFM), and immunodepleted of macrophages and possible residual plasma cells by a 30-minute incubation in CD14 (a monocyte-macrophage marker) plus CD38 (a plasma cell and hematopoietic cell marker) monoclonal antibody (MoAb)–coated flasks (Immunotech, Coulter, Marseilles, France). Residual cells were suspended at 0.25 to 1 × 106/mL in SFM and incubated for 30 minutes at 37°C with magnetic microbeads (Dynal, Oslo, Norway) at 0.15 to 0.5 × 106/mL, respectively, coated with Ulex europaeus agglutinin-1 (UEA-1; Sigma Chemical, St Louis, MO) (a lectin binding a specific receptor highly expressed by and restricted to ECs18 ) in rotation. Microbeads with bound cells were recovered using a side-pool magnetic separation unit, transferred to 12-well plates in 3 mL complete medium/well, and left to migrate to the plate surface and grow. Fifteen to 20 days were needed to obtain 1 to 2 × 106 cells per patient. The MMEC population contained more than 95% factor VIII–related antigen (FVIII-RA)+ and CD31+ cells (both are endothelial markers, whereas CD31 is also expressed by leukocytes, including monocytes-macrophages and plasma cells), as assessed by fluorescence activated cell sorting (FACS; FACS Calibur; Becton Dickinson, San Jose, CA). Contamination by macrophages and plasma cells was either ruled out or very insignificant, as evaluated by FACS with the CD14 and CD38 MoAbs, respectively, and by RT-PCR and Western blot for CD38 (see “Antigen phenotype”). The trypan blue viability was more than 90%. To obtain their conditioned medium (CM), MMECs at 90% confluence were cultured in SFM (approximately 1 × 106/mL) for 24 hours. CM were collected, sequentially centrifuged at 1200 and 12 000 rpm for 10 minutes, respectively, filtered through sterilized 0.22-μm pore-size filters (Costar, Cambridge, MA), and stored at -80°C.
Human umbilical vein ECs (HUVECs) were obtained from 22 samples as described19 and grown in gelatin-coated plates in complete medium (M199 medium supplemented with 10% FCS, 0.02% extract of bovine brain, and 0.015 porcine heparin, Sigma). Cell viability and collection of the CM were performed as described earlier. HUVECs were chosen because they are typical mature, quiescent ECs, and available in a large number.19 In contrast, ECs from the bone marrow of nonmalignant hematologic diseases and healthy donors are available only in a limited number1,13 (A.V., unpublished observations, January 11, 2003) because of the absence of the vascular phase as in MM.7 However, these ECs can be considered as mature, quiescent cells as well, because their morphology, culture behavior, and phenotype closely overlap those of HUVECs.11,13 HUVECs were obtained in proportion of 1-2 × 106 per vein. Cells were tested at the second passage, ie, after 15 to 20 days of culture. Thus, both MMECs and HUVECs were tested after a similar sufficiently long culture time to abolish any previous activating step as a result of the harvesting.
FACS analysis for FVIII-RA, CD14, and CD38 was followed by RT-PCR and Western blot for FVIII-RAand CD38. RT-PCR was performed as described20 on 2 μg total RNA extracted with Trizol reagent (Invitrogen, Life Technologies, Carlsbad, CA) and reverse transcribed by Moloney murine leukemia virusreverse transcriptase (MMLV-RT; Invitrogen). Then, 1 μg cDNA was amplified by 22 to 35 cycles using human FVIII-RA primers (5′-GTTCGTCCTGGAAGGATCGG-3′ and 5′-CACTGACACCTGAGTGAGAC-3′), human CD38 primers (5′-ACCCCGCCTGGAGCCCTATG-3′ and 5′-GCTAAAACAACCACAGCGACTGG-3′), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control primers (5′-CCCTCCAAAATCAAGTGGGG-3′ and 5′-CGCCACAGTTTCCCGGAGGG-3′) (Invitrogen).
Cell preparations with more than 95% FVIII-RA+ cells and no (or very insignificant) CD14+ and CD38+ cells were admitted to the sequence of tests.
Antigen phenotype
Antigen phenotype was studied by Western blot and FACS analysis according to the best antigen resolution. Western blot was performed as described,20 using antibodies to FVIII-RA, tyrosin kinase with immunoglobulin and EGF homology-2 (Tie2/Tek), the receptor of angiopoietin 1 (Ang-1) and Ang-2,21 VEGF receptor 2 (VEGFR-2) (or kinase insert domaincontaining gene [KDR]) and VEGFR-3 (or fms-like tyrosine kinase-4 [flt-4]), bFGF receptors (bFGFRs)-1, -2, -3, and erythropoietin receptor (EpoR) (all from Santa Cruz Biotechnology, Santa Cruz, CA). Also used were VEGFR-1 (or flt-1; Sigma); endoglin (or CD105; Serotec, Kidlington, Oxford, United Kingdom), a regulatory component of the transforming growth factor-β (TGF-β) receptor complex22 ; thrombospondin (Santa Cruz), an angiogenesis inhibitor23 ; aquaporin 1 (a gift from Dr A. Frigeri, Department of Physiology, University of Bari, Italy), a water channel protein-enhancing cell permeability24 ; and CD38 (Dako, Glostrup, Denmark). Proteins of total EC extracts (50 μg) were subjected to 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, transferred electrophoretically to a polyvinylidene difluoride (PVDF) membrane (NEN, Life Science, Boston, MA), incubated with the primary and secondary antibody for 1 hour, then with enhanced chemiluminescence (NEN), and revealed for signal by Kodak Biomax film (Eastman Kodak, Rochester, NY). The band intensity was expressed as arbitrary optical density (OD) units by reading with Fluorad (Bio-Rad Labs, Richmond, CA).
FACS analysis was performed with the fluorescein isothiocyanate (FITC)–conjugated UEA-1 lectin and FITC- or phycoerythrin (PE)–conjugated murine MoAbs to FVIII-RA, CD14, CD34 (a glycoprotein expressed by ECs and early lymphohematopoietic stem or progenitor cells), CD133 (or AC133, a glycoprotein expressed by hematopoietic stem or progenitor cells, including hemangioblasts25 ), CD138 (or syndecan-1, a heparan sulfate proteoglycan expressed by ECs, epithelial cells, and plasma cells26 ), CD61 (or β3 integrin/GPIIIA, the receptor of fibronectin, vitronectin, thrombospondin, and fibrinogen expressed by ECs, B cells, monocytesmacrophages, and tumor cells27 ), CD144 (or vascular endothelial [VE]–cadherin, a molecule restricted to ECs and contained in their adherent junctions2 ) (all from Immunotech, Coulter), CD31, and CD62E (or E-selectin, an EC adhesion molecule28 ) (Chemicon, Temecula, CA). FITC- or PE-conjugated antisera to mouse IgG (Sigma) were used as secondary antibodies in indirect assays. ECs (1 × 105) were incubated for 30 minutes at 4°C with 20 μL fluorochrome-conjugated or unconjugated antibody. In indirect assays, cells were washed with PBS and incubated with the fluorochrome-conjugated secondary antibody. A fluorochrome-conjugated indifferent murine IgG1 was used as the negative control.
Functional studies
A matrigel capillarogenesis assay was performed on 21 MMEC and 15 HUVEC samples to assess the ability of their cells to form a 3-dimensionally organized capillary network.16 Cells were plated in duplicate in 24-well plates (2 × 105 cells/well) precoated with matrigel (300 μL/well; Becton Dickinson) in 1 mL specific complete medium. After an 8-hour incubation in culture conditions, their 3-dimensional organization was examined with a reverted, phase-contrast photomicroscope, and their planimetric parameters were measured by computed image analysis (QWin image analysis software; Leica Imaging Systems, Cambridge, United Kingdom): (1) after contour enhancement and thresholding, a geometric filter was applied to remove profiles of very small size or circular and allow identification of the network formed by the cells and assessment of the percentage of area it covered (area percentage); (2) skeletonization of the binary image was then performed and the total length per field (“length”) of the cell network evaluated. The binary image was further processed to extract topical parameters, ie, the regions of the field of view delimited by cells (ie, the mesh of the network) and the points where branching occurred; (3) the number of meshes per field (“mesh”); and (4) the number of branching points per field (“branching”) were then estimated and used to characterize the topologic structure of the EC organization.
A chick embryo chorioallantoic membrane (CAM) assay was performed in 32 MMEC and 20 HUVEC CM to assess their angiogenic ability in vivo.16 On incubation day 8, the CAMs of White Leghorn eggs were implanted in duplicate with 1-mm3 inert gelatin sponges (Gelfoam; Upjohn, Kalamazoo, MI) loaded with 3 μL CM, whereas sponges loaded with the RPMI-1640 medium alone or supplemented with bFGF (200 μg/mL) were used as negative and positive control, respectively. On incubation day 12, when the angiogenic response peaked, blood vessels entering the sponge within the focal plane of the CAM were recognized macroscopically, counted at × 50 by 2 observers (D.R. and B.N.) in a double-blind fashion with a stereomicroscope, and photographed. CAMs and sponges were then routinely processed for histology and counted for microvessels at × 250 in 0.125-mm2 fields with a planimetric method, as described.16
Gelatin zymography was performed in 16 MMEC and 12 HUVEC CM to visualize the gelatinolytic activity of secreted MMP-2 and MMP-9.16 CM proteins (5 μg) were applied in duplicate to 7.5% SDS-polyacrylamide gels copolymerized with type A gelatin (Sigma). After electrophoresis and staining in 0.1% Coomassie brilliant blue, the intensity of gelatinolytic bands was expressed as arbitrary OD units by reading with the Fluorad.
Either 50 (VEGF assay) or 100 (bFGF) μL of the same CM was tested in duplicate for concentrations of these factors with the sandwich enzymelinked immunosorbent assay (ELISA, Quantikine; R & D Systems, Minneapolis, MN) according to the manufacturer's instructions. The interassay and intra-assay coefficients of variations were 5.4% (VEGF) and 6.2% (bFGF), and 7.3% (VEGF) and 8.7% (bFGF), respectively.
Fifteen MMEC and 9 HUVEC samples exposed to 10 ng/mL bFGF (Sigma) in complete medium for 30 minutes were examined by the above described Western blot with MoAbs to the mitogen-activated protein (MAP) extracellular signal-regulated kinase-1 (ERK-1) and ERK-2 in their activated (phosphorylated) form (Cell Signaling Technology, Beverly, MA).
Gene expression assay
Total RNA was isolated from 10 MMEC and 3 HUVEC samples using Trizol reagent as described earlier, and its quality was checked by agarose-formaldehyde gel electrophoresis. Total RNA (5 μg/assay) was used in duplicate as template for α32 P]cDNA probe synthesis (α32 P]dCTP; Amersham; gene-specific primer mixture; SuperArray, Bethesda, MD) by the MMLV-RT, according to the manufacturer's instructions. cDNA probes were denatured and then hybridized with nylon-based arrays spotted with cDNA fragments from 96 genes subdivided into 4 functionally characterized groups: (1) growth factors and receptors; (2) cytokines, chemokines, and adhesion molecules; (3) matrix, proteases, and inhibitors; and (4) other angiogenesis-related genes (GEArray Q Series, Human Angiogenesis Gene Array, SuperArray). We started with this highly selected microchip for gene array instead of an all-encompassing gene array because the selected genes entail a well-characterized profile governing neovascularization (both angiogenesis and embryo vasculogenesis), hence facilitating interpretation of data, simplifying data acquisition and analysis, and avoiding rationalization of genes not functionally characterized. Data acquired with a phosphor screen scanner (Cyclone and OptiQuant software; Packard Instruments, Meriden, CT) were exported to Microsoft Excel spreadsheets and analyzed (pUC 18 plasmid DNA background subtraction, and normalization to GAPDH and β-actin–positive control genes) with GEArray Analyzer software (SuperArray). The relative expression level of each gene was compared with the signal derived from GAPDH and β-actin, whereas negative values were transformed to zeroes. Results were analyzed by the Mann-Whitney nonparametric test with P < .05 as the significance cutoff. Data are expressed as the mean ± 1 SD.
Electron microscopy
T25 flasks of 10 MMEC and 10 HUVEC samples at 90% confluence were washed by PBS, fixed in 3% glutaraldehyde-0.1% mol/L PBS for 3 hours, washed in the same buffer for 12 hours, and postfixed in 1% osmium tetroxide. They were then scraped with a rubber bar, dehydrated in graded ethanol, and embedded in Epon 812. Ultrathin sections were cut on LKB ultratome, stained with uranyl acetate-lead citrate sequence, and examined in a Zeiss EM109 transmission electron microscope.
Thalidomide assays
Thalidomide (Biomol Research Laboratories, Plymouth Meeting, PA) was solubilized in dimethylsulfoxide (DMSO) at 10 mM and stepwise diluted in the medium at the time of assay. The final concentration of DMSO was 0.08% or less. ECs from 18 patients were plated in duplicate (2 × 103/well) in 96-well plates in complete medium. After 24 hours, the medium was removed and replaced on days 0, 2, 4, and 6 by the same medium (positive control), by this medium supplemented with thalidomide 6, 10, 20, and 30 μmol/L, or by SFM (negative control); 6, 10, and 20 μmol/L are the interstitial fluid concentrations of a 70-kg adult who receives 180, 300, and 600 mg/d.29 The cell number was estimated on day 8 by a crystal-violet colorimetric method,16 using a known number of cells, and calculated as mean ± 1 SD.
In the matrigel assay, the cell samples plated as described earlier (see “Functional studies”) were exposed to the control and complete media containing the thalidomide doses. Planimetric parameters of exposed cultures were compared with those of control cultures.
Results
Cell preparations and phenotype
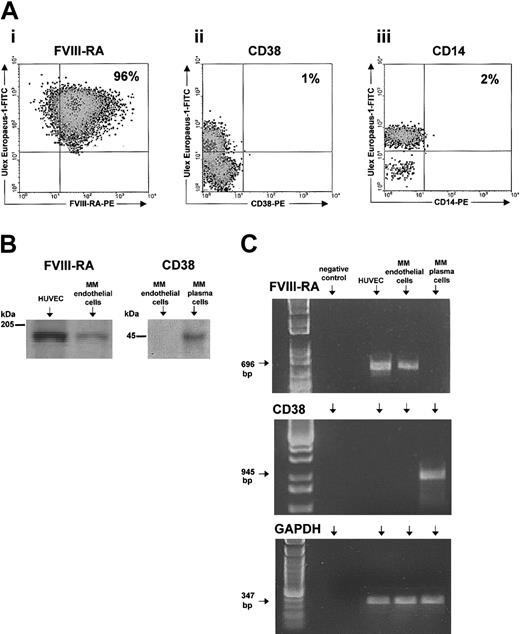
FACS analysis of all patients showed that bone marrow cells extracted with UEA-1 microbeads were endothelial, because more than 95% expressed the FVIII-RA and were devoid (or substantially devoid in some instances) of plasma cells (CD38 cells) and macrophages (CD14). A representative analysis is shown in Figure 1. The expression of FVIII-RA and absence (or insignificance) of CD38 were always confirmed by Western blot and RT-PCR (Figure 1). Both techniques showed that MMECs always expressed the FVIII-RA less than HUVECs (Western blot OD = 0.87 ± 0.42 × 103 versus 1.54 ± 0.78; P < .01 by Fisher and Kruskal Wallis test followed by paired Duncan [t], Bonferroni [t], and Wilcoxon tests; similar significance by RT-PCR). An example is given in Figure 1.
Purity of MMEC preparations assessed by FACS, Western blot, and RT-PCR analyses. (A) One representative patient showing by FACS that 96% of ECs express FVIII-RA, and very little CD38 and CD14. At least 10 000 cells were read for each labeling. (B) Western blot shows that MMECs express less FVIII-RA than HUVECs and do not express CD38 by contrast with paired plasma cells. (C) Confirmatory results are obtained by RT-PCR; bp indicates base pair.
Purity of MMEC preparations assessed by FACS, Western blot, and RT-PCR analyses. (A) One representative patient showing by FACS that 96% of ECs express FVIII-RA, and very little CD38 and CD14. At least 10 000 cells were read for each labeling. (B) Western blot shows that MMECs express less FVIII-RA than HUVECs and do not express CD38 by contrast with paired plasma cells. (C) Confirmatory results are obtained by RT-PCR; bp indicates base pair.
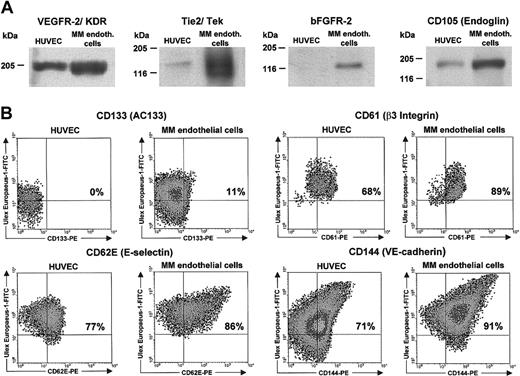
MMECs were then used to determine their antigen phenotype and functions. The phenotype coincided with several vascularassociated cellular markers modulated in nascent compared with quiescent vessels.30 It was studied by Western blot or FACS analysis, according to the most precise demonstration of antigen (Table 1). The expression of Tie2/Tek, VEGFR-2, bFGFR-2, CD105 (endoglin), CD133 (AC133), aquaporin 1, and CD61 (β3 integrin) was greater on MMECs than on HUVECs, whereas that of CD62E (E-selectin), CD144 (VE-cadherin), CD138 (syndecan-1), and EpoR equalled HUVECs, and thrombospondin was lower (Table 1). Some examples are given in Figure 2. FACS analysis shows that expression of antigens is not shared by the entire cell population, which thus consists of subsets.
Western blot and FACS analyses of several vascular cell markers in a representative patient and a HUVEC sample. (A) Western blot analysis. (B) FACS analysis. At least 10 000 cells were read for each FACS labeling. In 23 patients VEGFR-2 gave a dimeric band (not shown).
Western blot and FACS analyses of several vascular cell markers in a representative patient and a HUVEC sample. (A) Western blot analysis. (B) FACS analysis. At least 10 000 cells were read for each FACS labeling. In 23 patients VEGFR-2 gave a dimeric band (not shown).
Functions of MMECs compared with HUVECs
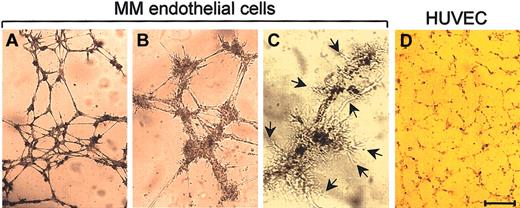
The in vitro capillarogenic activity of 21 MMEC and 15 HUVEC samples was studied by the matrigel assay. After an 8-hour incubation, the MMECs had spread throughout the matrigel surface and aligned to form branching, anastomosing and thick tubes with multicentric junctions that gave rise to a closely knit network of capillary-like structures (Figure 3A-B). Extroversions of cell membranes suggesting sprouting were common (Figure 3C, arrows). Their area percentage was 32.6 ± 1.4, length was 8480 ± 1108, branching was 52 ± 8, and mesh was 45 ± 11. HUVECs produced a loose and thin network with few junctions (Figure 3D). Their area percentage was 12.8 ± 0.8, length was 5270 ± 650, branching was 28 ± 3, mesh was 19 ± 4 (P < .01 or better; same paired tests).
Capillarogenic activity of MMECs compared with HUVECs. Cells were seeded on matrigel in the specific complete medium and after an 8-hour incubation their 3-dimensional organization was examined planimetrically. A patient's cells formed (A) branching, anastomosing tubes with (B) multicentric junctions, resulting in a network of capillary-like structures and (C) extroversions of cell membranes (arrows), suggesting sproutive growth. (D) HUVECs formed a loose and poorly organized network. The network in (A) has area percentage of 29.2, length of 7670, branching of 43, mesh of 38; that in (D) has area percentage of 16.3, length of 4110, branching of 21, mesh of 12. Bar = 70 μm (A,D), 30 μm (B), and 18 μm (C).
Capillarogenic activity of MMECs compared with HUVECs. Cells were seeded on matrigel in the specific complete medium and after an 8-hour incubation their 3-dimensional organization was examined planimetrically. A patient's cells formed (A) branching, anastomosing tubes with (B) multicentric junctions, resulting in a network of capillary-like structures and (C) extroversions of cell membranes (arrows), suggesting sproutive growth. (D) HUVECs formed a loose and poorly organized network. The network in (A) has area percentage of 29.2, length of 7670, branching of 43, mesh of 38; that in (D) has area percentage of 16.3, length of 4110, branching of 21, mesh of 12. Bar = 70 μm (A,D), 30 μm (B), and 18 μm (C).
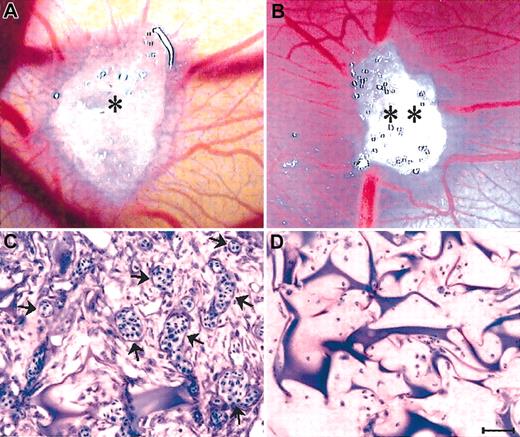
The ability of CM from 32 MMEC and 20 HUVEC samples to induce an angiogenic response in vivo was assessed with CAMgelatin sponge assay. Vessels entering the sponge were recognized macroscopically (× 50) and counted (Table 2). When the sponges were loaded with the medium alone (negative control) or HUVEC CM, physiologic angiogenesis was observed in the form of a few allantoic vessels partly around and partly converging toward the sponge. By contrast, CAM implanted with the medium containing bFGF (positive control) or the MMEC CM gave significantly higher vessel counts and numerous allantoic vessels converging like spokes toward the sponge (Table 2; Figure 4A-B). Histologic examination (× 250) and planimetric vessel counting in 0.125mm2 fields showed that both bFGF- and MMEC CM-loaded sponges displayed a dense collagenous matrix intermingled with numerous blood vessels among the sponge trabeculae and at the boundary between the sponge and the CAM mesenchyme (Table 2; Figure 4C). Vessels pierced the sponge at some points. By contrast, no vessels could be detected inside the medium alone– or HUVEC CM–loaded sponges, and significantly fewer vessels were found at the boundary (Table 2; Figure 4D).
Angiogenic activity of MMEC CM in the CAM sponge assay. (A) The positive control (RPMI-1640 + bFGF) and (B) the CM were loaded onto gelatin sponges (* and **) implanted on top of the CAM on day 8. Macroscopic view of the CAM on day 12, showing numerous new capillaries with a “spoked wheel” pattern around both sponges. (C) Histologic sections of the sponge shown in panel B, showing a collagenous matrix pierced by winding blood vessels (arrows) cut on different planes and containing blood cells. (D) Histologic section of a sponge loaded with the HUVEC CM, showing no vessels among the trabeculae. Similar results (not shown) were produced by the negative control (RPMI-1640 medium). Bar = 4mm (A-B) and 90 μm (C-D).
Angiogenic activity of MMEC CM in the CAM sponge assay. (A) The positive control (RPMI-1640 + bFGF) and (B) the CM were loaded onto gelatin sponges (* and **) implanted on top of the CAM on day 8. Macroscopic view of the CAM on day 12, showing numerous new capillaries with a “spoked wheel” pattern around both sponges. (C) Histologic sections of the sponge shown in panel B, showing a collagenous matrix pierced by winding blood vessels (arrows) cut on different planes and containing blood cells. (D) Histologic section of a sponge loaded with the HUVEC CM, showing no vessels among the trabeculae. Similar results (not shown) were produced by the negative control (RPMI-1640 medium). Bar = 4mm (A-B) and 90 μm (C-D).
MMP-2 and MMP-9 secretion was assessed by SDS-PAGE zymography of 16 MMEC and 12 HUVEC CM samples. Both MMECs and HUVECs secreted activated (62-kDa form) MMP-2 and lower levels of activated (88-kDa form) MMP-9. The MMEC values were, on average, 3 and 4 times higher for MMP-2 and MMP-9, respectively (Figure 5). Evaluation of bFGF and VEGF levels in the CM by immunoassay showed that those of bFGF were 4 times higher and those of VEGF were 40 times higher in the MMEC CM (Figure 5).
MMP-2 and MMP-9 secretion by an MMEC and a HUVEC sample. (A) SDS-PAGE zymography of the cell CM. Note the white bands against a blue background with an apparent molecular weight of 62 kDa and 88 kDa, corresponding to the gelatinolytic regions of activated (cleaved) MMP-2 and MMP-9, respectively. (B) The intensity of the bands is evaluated as optical density (OD) units by the Fluorad in 16 MMEC and 12 HUVEC samples. Significance by analysis of variance by Fisher and Kruskal-Wallis test followed by Duncan [t], Bonferroni [t], and Wilcoxon paired tests. (C) The same conditioned media were evaluated for VEGF and bFGF levels by an ELISA system. Significance by the same paired tests. Data shown in B and C are mean ± one SD. (D) Western blot for phosphorylated (p) forms of 42-kDa ERK-1 and p44 ERK-2 in a representative HUVEC and patient's cells.
MMP-2 and MMP-9 secretion by an MMEC and a HUVEC sample. (A) SDS-PAGE zymography of the cell CM. Note the white bands against a blue background with an apparent molecular weight of 62 kDa and 88 kDa, corresponding to the gelatinolytic regions of activated (cleaved) MMP-2 and MMP-9, respectively. (B) The intensity of the bands is evaluated as optical density (OD) units by the Fluorad in 16 MMEC and 12 HUVEC samples. Significance by analysis of variance by Fisher and Kruskal-Wallis test followed by Duncan [t], Bonferroni [t], and Wilcoxon paired tests. (C) The same conditioned media were evaluated for VEGF and bFGF levels by an ELISA system. Significance by the same paired tests. Data shown in B and C are mean ± one SD. (D) Western blot for phosphorylated (p) forms of 42-kDa ERK-1 and p44 ERK-2 in a representative HUVEC and patient's cells.
Fifteen MMEC and 9 HUVEC samples were stimulated by exogenous bFGF for 30 minutes and analyzed by Western blot for the expression of the activated (phosphorylated) 42-kDa (p42) ERK-1 and p44 ERK-2. Both cell types displayed these activated kinases (Figure 5).
cDNA macroarrays
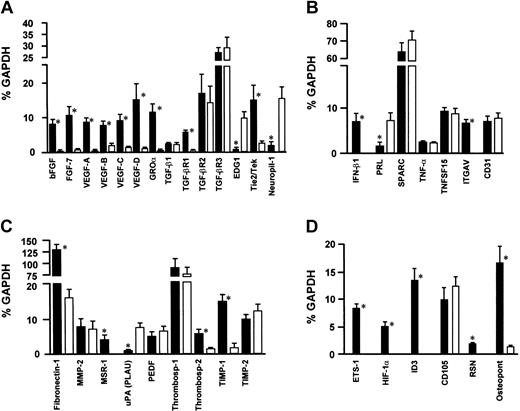
The angiogenesis-related gene expression profile of 10 MMEC samples was investigated and compared with that of 3 HUVEC samples by using cDNA macroarrays containing 96 genes known to be involved in neovascularization. Results relative to genes, whose expression in MMECs and/or HUVECs was detectable, are shown in Figure 6, as percentage of expression of GAPDH-positive control gene.
cDNA macroarray. (A) Growth factors and receptors. (B) Cytokines, chemokines, and adhesion molecules. (C) Matrix, proteases, and inhibitors. (D) Other angiogenesis-related genes. The figure summarizes the expression profile of 96 angiogenesis-related genes of 10 MMEC and 3 HUVEC samples. Genes were clustered in 4 functional groups. Only results for genes whose expression in MMECs and/or HUVECs was detectable are shown; no bar is shown for instances when gene expression was undetectable. The gene symbol is reported on the x-axis, and its expression is shown as a percentage of GAPDH housekeeping gene. Data shown are mean ± 1 SD for both MMECs (▪) and HUVECs (□). * Indicates the difference of gene expression between the MMEC and HUVEC is statistically significant (P < .05 or better by Mann-Whitney nonparametric test).
cDNA macroarray. (A) Growth factors and receptors. (B) Cytokines, chemokines, and adhesion molecules. (C) Matrix, proteases, and inhibitors. (D) Other angiogenesis-related genes. The figure summarizes the expression profile of 96 angiogenesis-related genes of 10 MMEC and 3 HUVEC samples. Genes were clustered in 4 functional groups. Only results for genes whose expression in MMECs and/or HUVECs was detectable are shown; no bar is shown for instances when gene expression was undetectable. The gene symbol is reported on the x-axis, and its expression is shown as a percentage of GAPDH housekeeping gene. Data shown are mean ± 1 SD for both MMECs (▪) and HUVECs (□). * Indicates the difference of gene expression between the MMEC and HUVEC is statistically significant (P < .05 or better by Mann-Whitney nonparametric test).
Expression of genes belonging to the specific promoters and inhibitors group (ie, angiomotin, angiogenin, angiopoietin 1 and 2, and vasostatin) was undetectable. In the functional group of growth factors and receptors, a number of genes involved in neovascularization were detected in MMECs. These genes include bFGF, FGF-7, VEGF-A, VEGF-B, VEGF-C, VEGF-D, and GROα growth factors, and their expression was about 10% of housekeeping GADPH expression. In contrast, HUVECs showed a very low expression of growth factor genes, in most cases less than 1% of GAPDH. Genes encoding for receptor molecules were also expressed by MMECs, in the order transforming growth factor-β receptor-3 (TGF-βR3) > TGF-βR2 > TGF-βR1 for TGF receptors [27%, 17%, and 6% of GAPDH, respectively]). HUVECs gave a similar pattern for TGF-βR3 and TGF-βR2, whereas TGF-βR1 was undetectable. Finally, Tie2/Tek receptor gene was more expressed in MMECs than in HUVECs (15% and 2% of GAPDH, respectively). Interestingly, the expression of EDG1 gene (whose product is involved in the final maturation events of tumor neovessels) was undetectable in MMECs, whereas it rose up to 10% of GAPDH in HUVECs. A similar pattern was observed for neuropilin 1 (a VEGF coreceptor) gene (15% and 1% of GAPDH in HUVECs and MMECs, respectively). In the group of cytokines, chemokines, and adhesion molecules, MMECs and HUVECs expressed similar levels of SPARC, TNF-α, TNFSF15, and CD31 genes. On the contrary, only MMECs expressed IFN-β1 and ITGAV genes, whereas HUVECs showed a greater expression of PRL gene (7.5% and 1.2% of GAPDH in HUVECs and MMECs, respectively). In the functional group of matrix, proteases, and inhibitors genes, MMECs and HUVECs expressed MMP-2, PEDF, thrombospondin-1, and TIMP2 (the MMP-2 inhibitor) genes at similar levels. In contrast, fibronectin-1, MSR-1, thrombospondin-2, and TIMP1 (the MMP-9 inhibitor) were more expressed in MMECs (140%, 4.1%, 6.3%, and 15% of GAPDH, respectively) than in HUVECs (16%, 0%, 2.2, and 1.8% of GAPDH, respectively). Expression of urokinase-type plasminogen activator (uPA or PLAU) gene alone was higher in HUVECs (7.7% and 0.45% of GAPDH, respectively). In Figure 6D, the expression of other angiogenesis-related genes is shown. Endoglin gene expression of MMECs overlapped HUVECs, whereas genes coding for transcription factors such as ETS-1, hypoxia-inducible factor-1α (HIF1A), and ID3 were expressed only by MMECs, which also showed a higher expression of the osteopontin gene (18% and 1.3% of GAPDH, respectively).
Ultramicroscopic morphology
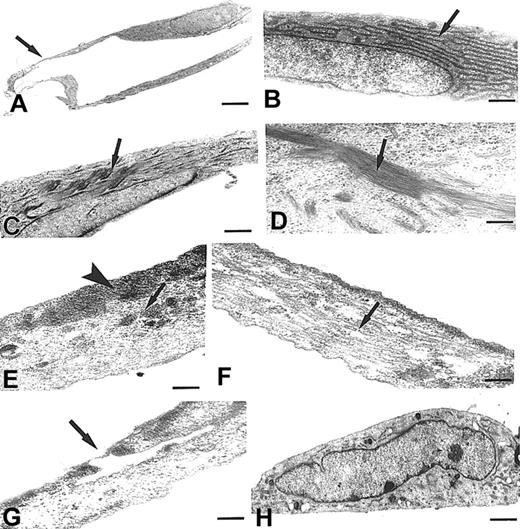
MMECs frequently gave thin extroversions of cytoplasm that were arranged in tubelike structures (Figure 7A,G). Cells were very elongated and thin, with a smooth surface, a central euchromatic nucleus, and cytoplasm containing a well-developed Golgi complex and mitochondria, numerous lysosome-like structures, and cisternae of parallel-arranged rough endoplasmic reticulum (Figure 7B). Moreover, alterations of cytoskeleton microfilaments in the form of patchy addensation and depolymerization were found (Figure 7C-F). Bundles of thickened, variously electron-dense microfilaments, irregularly scattered in the cytoplasm or located at the subcortical plasma membrane level were also common (Figure 7E). In the sharp contact regions between adjacent cells the plasma membranes were tightly closed. In contrast, HUVECs were variously shaped and sized with short protrusions and dark cytoplasms containing lipid droplets and only some units of rough endoplasmic reticulum (Figure 7H). Vesicles, vacuoles, and a network of microfilaments and microtubules extending across the cytoplasm and thickened near the subcortical plasma membrane were recognizable.
Utrastructural features of MMECs and a HUVEC. (A-G) MMECs. (H) HUVEC. (A,G) Two MMECs showing very thin cytoplasmic regions arranged in the form of a tubelike structure (arrows). (B) Another cell showing numerous and parallel arranged cisternae of rough endoplasmic reticulum (arrow). (C-F) Cells showing bundles of thickened microfilaments, located to nucleus (C-D, arrows) or to subcortical plasma membranes (E, arrowhead), or scattered in the cytoplasm (E, arrow), or showing disarranged microtubules (F, arrow). (H) A HUVEC showing lipid droplets and a normal distribution of cytoskeleton organelles. Scale bar, 2.7 μm (A); 0.4 μm (B); 0.8 μm (C); 0.2 μm (D); 0.4 μm (E-F); 0.3 μm (G); and 0.7 μm (H).
Utrastructural features of MMECs and a HUVEC. (A-G) MMECs. (H) HUVEC. (A,G) Two MMECs showing very thin cytoplasmic regions arranged in the form of a tubelike structure (arrows). (B) Another cell showing numerous and parallel arranged cisternae of rough endoplasmic reticulum (arrow). (C-F) Cells showing bundles of thickened microfilaments, located to nucleus (C-D, arrows) or to subcortical plasma membranes (E, arrowhead), or scattered in the cytoplasm (E, arrow), or showing disarranged microtubules (F, arrow). (H) A HUVEC showing lipid droplets and a normal distribution of cytoskeleton organelles. Scale bar, 2.7 μm (A); 0.4 μm (B); 0.8 μm (C); 0.2 μm (D); 0.4 μm (E-F); 0.3 μm (G); and 0.7 μm (H).
Effects of thalidomide on MM endothelial cells
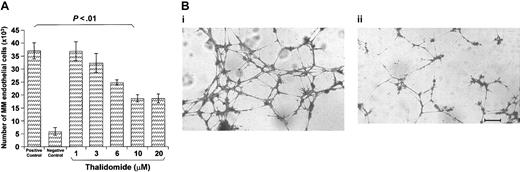
MMECs from 18 patients were exposed on days 0, 2, 4, and 6 to complete medium alone (positive control) or supplemented with each thalidomide dose, or to starvation SFM (negative control), and their proliferation rate was measured on day 8 by a colorimetric method. Figure 8A shows that thalidomide significantly inhibited proliferation at 6 and 10 μM in a dose-dependent fashion (-34% and -42% of the positive control, P < .05 and P < .02; Wilcoxon rank test), whereas 20 and 30 μM gave a plateau. Twelve HUVEC samples gave overlapping results (data not shown).
Effect of thalidomide on proliferation of ECs and capillarogenesis assay. (A) Effect of thalidomide on the proliferation of ECs from 18 patients. Cells (2 × 103) were incubated on days 0, 2, 4, and 6 in the complete medium (positive control), the starvation (FCS-free) medium (negative control), and in the positive control medium containing each thalidomide dose. Cells were counted on day 8. Significance determined by Wilcoxon rank test. Data shown are mean ± one SD. (B) Capillarogenesis assay on matrigel of one patient's ECs. Cells (2 × 105) were plated on matrigel and grown for 8 hours in the complete medium alone (i) or containing 10 μM thalidomide (ii). The medium gave cells arranged in branching, reciprocally anastomosing tubes forming a closely knit capillary-like plexus. The culture has area percentage of 26.2, length of 6814, branching of 41, and mesh of 35. Disaggregation of the plexus paralleled the exposure to thalidomide: area percentage of 15.5, length of 3250, branching of 25, and mesh of 8. Bar = 70 μm.
Effect of thalidomide on proliferation of ECs and capillarogenesis assay. (A) Effect of thalidomide on the proliferation of ECs from 18 patients. Cells (2 × 103) were incubated on days 0, 2, 4, and 6 in the complete medium (positive control), the starvation (FCS-free) medium (negative control), and in the positive control medium containing each thalidomide dose. Cells were counted on day 8. Significance determined by Wilcoxon rank test. Data shown are mean ± one SD. (B) Capillarogenesis assay on matrigel of one patient's ECs. Cells (2 × 105) were plated on matrigel and grown for 8 hours in the complete medium alone (i) or containing 10 μM thalidomide (ii). The medium gave cells arranged in branching, reciprocally anastomosing tubes forming a closely knit capillary-like plexus. The culture has area percentage of 26.2, length of 6814, branching of 41, and mesh of 35. Disaggregation of the plexus paralleled the exposure to thalidomide: area percentage of 15.5, length of 3250, branching of 25, and mesh of 8. Bar = 70 μm.
The effects of thalidomide on capillarogenesis were also investigated. After an 8-hour incubation, unexposed MMECs gave a closely knit network whose area percentage was 27.5 ± 1.1, length was 6314 ± 708, branching was 48 ± 6, and mesh was 33 ± 8. MMECs exposed to 10 μM were less organized, because the area percentage was 17.1 ± 0.7 (-38%), and length was 4146 ± 560 (-34%). The network was also less developed structurally, because branching was 28 ± 4 (-42%) and mesh was 10 ± 3 (-70%) (P < .01 or better; Student t test for paired data). Lowering of all planimetric parameters was slightly more evident at 20 and 30 μM. Unexposed and exposed (10 μM) cells of a patient are shown in Figure 8B. Similar results were obtained with HUVECs (data not shown).
Discussion
The vascular tree in the bone marrow of patients with myeloma seems to closely overlap that of both human and murine melanoma and colon and ovary carcinomas.1-6,30 Its ECs intensely express markers of vivid angiogenesis such as VEGFR-2 and Tie2/Tek. Overexpression implies synergistic activity of the cognate angiogenic factors VEGF and Ang-2 in the induction of sprouts from existing vessels.31 Ang-1 also binds Tie2/Tek and promotes sprouting and especially circumferential growth of vessels, leading to an increase in their number and size, reduces vessel leakage, and stabilizes the newly formed vessels.31 All these factors are produced by plasma cells.16,32,33 MMECs sizably express CD133 (AC133), a marker of the progenitor ECs that take part in embryo vasculogenesis, ie, in the formation of an archaic vascular network.34 Perhaps, (UEA-1)-AC133 cells normally harbored in the bone marrow in a resting state35 are recruited and conveyed into vasculogenesis by plasma cell growth factors (VEGF, bFGF, hepatocyte growth factor).36 In turn, vasculogenesis may contribute to the full vascular tree in MM. Low expression of FVIII-RA suggests dedifferentiation of existing ECs while entering neovascularization, compared with healthy stabilized cells. Low expression of thrombospondin, an angiogenesis inhibitor,23 further confirms ongoing neovascularization. High expression of β3 integrin, which prevents apoptosis of ECs and favors their adhesion to the matrix (via fibronectin, vitronectin, and thrombospondin), proliferation, migration, and capillarogenesis,27 also implies vivid neovascularization. High expression of endoglin is another hallmark of tumor vessels that distinguishes them from healthy quiescent vessels.22 Because endoglin enhances the expression of the adhesion molecule CD31, which is the ligand of the plasma cell CD38, its overexpression by MMECs suggests enhanced opportunities for plasma cells to interact with their neovessels, enter circulation, and disseminate. The high expression of E selectin (another adhesion molecule) by MMECs also suggests frequent interactions between plasma cells (via sialyl Lewis x-a complex)28 and neovessels and a further way to disseminate. MMECs intensely express aquaporin 1. This enhances permeability and thus facilitates plasma extravasation, increases interstitial pressure, induces hypoxia, and generates further HIF1α and VEGF-mediated neovascularization.24 Expression of VE-cadherin suggests solid intercellular junctions2 within MM neovessels. The MMEC signal through ERK-1 and ERK-2 in response to bFGF, which is a product of plasma cells16 and inflammatory cells37 and takes part in the myeloma-associated angiogenesis.16 Of note, ERK-1 and ERK-2 kinases are mandatory in ECs for transmission to the nucleus of signals for proliferation and chemotaxis,30 which are essential functions for development of neovascularization.
Accordingly, DNA array (studied, however, in 10 patients only) shows enhanced expression of inducers of the tumor vascular phase,23 such as bFGF, FGF-7, VEGF-A, -B, -C, and -D, GROα chemokine, Tie2/Tek, TGF-β receptor 1, fibronectin-1, the transcription factors ETS-1, HIF-1α, ID3, RSN, and osteopontin, which has recently been shown to be a potent angiogenic factor secreted by ECs.38 Some inhibitors (thrombospondin 1 and 2, MMP inhibitors [or TIMPs]) are also expressed, but they are probably suppressed at the cytoplasm level or overcome by inducers.23,30
MMECs are thus indicative of both a vasculogenic and an angiogenic state. They form more rapidly than HUVECs a new and organized vascular network on matrigel. Thus, they show a growth advantage over HUVECs and high intrinsic ability to form vessels, perhaps because of the autocrine loops of growth (probably, VEGF/VEGFR, bFGF/bFGFR). They are highly angiogenic themselves because they generate numerous new vessels in the in vivo CAM assay. They also show high extrinsic angiogenic ability, perhaps via secretion of their VEGF and bFGF found in CM by ELISA, and of MMP-2 and MMP-9 found by zymography. VEGF, bFGF, and MMPs also act as growth and invasive factors for plasma cells,39-41 indicating another way by which new vessels support MM progression.
Although not all angiogenesis inducers and inhibitors23,30 nor their balance have been studied here, the overall phenotypic, genetic, and functional features of MMECs suggest that the balance leans toward inducers and hence to the vascular phase in the bone marrow. Cohabitation of plasma cells with ECs in the marrow microenvironment may reprogram the latter to become MMECs, ie, to establish angiogenic phenotype and functions, like HUVECs when cocultured with tumor (glioma) cells,42 and bone marrow ECs cocultured with MM cell lines.33 Cross talk between soluble factors may occur in the reprogramming. Besides antigenic phenotype, the “face” of MMECs also differs in depth from healthy cells because of a wide Golgi complex, numerous mitochondria, hyperplasia of rough endoplasmic reticulum, lysosome-like structures, and thickened microfilaments: these are absent in HUVECs and suggest structurally abnormal and metabolically activated cells. Abnormalities of this type are common in Kaposi sarcoma and other angiosarcomas, solid tumors,30 and B-cell lymphomas.43 Several features of MMECs compared with HUVECs, such as vivid secretion of bFGF, VEGF, MMP-2, and MMP-9, growth advantage, genetic profile, and ultrastructural morphology seem to point to a tumoral EC inside the bone marrow of MM. Further studies on a possible hybridization between plasma cells and existing (healthy) ECs (probably, MMEC are hybrids) or a derivation from a common precursor (probably, plasma cells and MMEC are sisters) are warranted.
Proliferation and capillarogenesis of MMECs (and HUVECs) are inhibited by thalidomide at 6 and 10 μM, ie, the levels observed in the interstitial fluids of a 70-kg adult who receives 180 or 300 mg daily. Data further support the hypothesis that thalidomide is, at least partly, antiangiogenic,44 a finding confirmed in an in vivo model of MM,45 and agree with its therapeutic power.46 Thalidomide needs liver hydroxylation (probably related to cytochrome P450) to become active.47 However, both thalidomide as intact drug and hydroxylated metabolites exert an overlapping activity in inhibiting HUVEC proliferation in vitro, whereas metabolites are 3 to 4 times more antiangiogenic in the CAM in vivo model.48 Thus, we would expect that hepatic metabolites be more active on MMECs being in the patient.
Our study suggests that MMECs displaying antigenic, functional, genetic, and morphologic features indicative of ongoing neovascularization (in the form of both angiogenesis and vasculogenesis) are a component of the MM bone marrow, where disease progresses, ie, grows, invades, and disseminates. Because we show that MMECs secrete growth (bFGF, VEGF) and invasive (MMP-2, MMP-9) factors for plasma cells and express adhesion molecules facilitating dissemination of these cells, halting MMECs may be a tool to block progression. However, we also show that MMECs are a heterogeneous population with a dissimilar expression of antigens, as evaluated by FACS. Antiangiogenic therapy in MM should thus include different drugs23 (together or in sequence) to target each subpopulation: MoAbs against VEGF, β3 integrin, and endoglin, inhibitors of VEGFR-2 and ERK kinases, and Tie2/Tek might all be candidates coupled with thalidomide. Carefully planned trials with mixtures of antiangiogenic and other biologic drugs49 are warranted to assess whether the vascular phase of MM, and eventually progression, can be halted.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-04-1338.
Supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan) and the Ministry for Education, the Universities and Research (MIUR, “Molecular Engineering–C03” funds, and Interuniversity Funds for Basic Research [FIRB], Rome), Italy. R.R. is the recipient of a fellowship from the European Union (94/342/CE).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





![Figure 5. MMP-2 and MMP-9 secretion by an MMEC and a HUVEC sample. (A) SDS-PAGE zymography of the cell CM. Note the white bands against a blue background with an apparent molecular weight of 62 kDa and 88 kDa, corresponding to the gelatinolytic regions of activated (cleaved) MMP-2 and MMP-9, respectively. (B) The intensity of the bands is evaluated as optical density (OD) units by the Fluorad in 16 MMEC and 12 HUVEC samples. Significance by analysis of variance by Fisher and Kruskal-Wallis test followed by Duncan [t], Bonferroni [t], and Wilcoxon paired tests. (C) The same conditioned media were evaluated for VEGF and bFGF levels by an ELISA system. Significance by the same paired tests. Data shown in B and C are mean ± one SD. (D) Western blot for phosphorylated (p) forms of 42-kDa ERK-1 and p44 ERK-2 in a representative HUVEC and patient's cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/9/10.1182_blood-2003-04-1338/6/m_h82135159005.jpeg?Expires=1766223232&Signature=170VD5PG92ARJ2vm9lc7K~32L3uM3v6jHDJcJ4ZZxJcjeJdxB8MgJ6N7LokqIsj6APxXfL1hTYvYYEsucNUvjbeFi5Vz~oX7~uAEfZdNj92qyFqQ6S40SiH1LyjtkS0YmkKI0-gA9zFCu~FsVA7zR~MrYSflb2Bb64mkyxKhQ1CXYnNjkoiAOZU0fSmFAyinQ-G5bJniS4SuSCQoCMHsgzbrx~crqZVAzm5s0e535NiC6QuY~D5b0i2KyGKsQ7tQ5S7MMU6X1tkezvwiFDaYu7H2sVbdALqnae6sFFD0WjiOexZ2pLAdC1epV4gi6~WM-e2Xi0D81kDe9liLhfGVwg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Figure 5. MMP-2 and MMP-9 secretion by an MMEC and a HUVEC sample. (A) SDS-PAGE zymography of the cell CM. Note the white bands against a blue background with an apparent molecular weight of 62 kDa and 88 kDa, corresponding to the gelatinolytic regions of activated (cleaved) MMP-2 and MMP-9, respectively. (B) The intensity of the bands is evaluated as optical density (OD) units by the Fluorad in 16 MMEC and 12 HUVEC samples. Significance by analysis of variance by Fisher and Kruskal-Wallis test followed by Duncan [t], Bonferroni [t], and Wilcoxon paired tests. (C) The same conditioned media were evaluated for VEGF and bFGF levels by an ELISA system. Significance by the same paired tests. Data shown in B and C are mean ± one SD. (D) Western blot for phosphorylated (p) forms of 42-kDa ERK-1 and p44 ERK-2 in a representative HUVEC and patient's cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/9/10.1182_blood-2003-04-1338/6/m_h82135159005.jpeg?Expires=1767293744&Signature=Cn6-fZBT-ZXtwk3dmkgyMnhKEMqFUyKDAxO032qCpx0VTLWPo0JCbOm~s4NGJmQVn3ypfJvRkk0-fA8GxD4qF26neuZIKj~SURv0BNSfipwy7mqjSCGaQKdng16V9z5E23Tt2VAF-1cYicZozBb227kJgj~0ETj5hGnnlAzgTcOiPM3O-E2qG~pvF4WdT-PxuSD7M2-9t3cv10UaRNCThnDnjX9tZ8iTMZMxd1CsCBgBMM7a0ZXHfboj4iiLNcWWgYiXP~0ooVKitw42BEAOhcKfVZJJVi-IsLhvYx~FwgRP~KLURIh6mE1uEbr5tIdb95~Gjfz87iw1U9wr3~lJiQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)