Abstract
Deletions at the 3′ end of the human β-globin locus are associated with the hereditary persistence of fetal hemoglobin (HPFH) in adults, potentially through the juxtaposition of enhancer elements in the vicinity of the fetal γ-globin genes. We have tested how sequences at the HPFH-2, HPFH-3, and HPFH-6 breakpoints, which act as enhancers in vitro, affect the silencing of a locus control region Aγ (LCRAγ) transgene in the adult stage of mice. We found persistent Aγ expression in the adult blood of most of the multicopy HPFH-2, HPFH-3, or HPFH-6 lines, in contrast to the control LCRAγ lines which were silenced. Cre-mediated generation of single copy lines showed persistent γ gene expression maintained in some of the HPFH-2 and HPFH-6 lines, but not in any of the HPFH-3 or LCRAγ lines. In the HPFH-2 and HPFH-6 lines, persistent γ gene expression correlated with euchromatic transgene integrations. Thus, our observations provide support for a model whereby HPFH conditions arise from the juxtaposition of enhancers as well as permissive chromatin subdomains in the vicinity of the γ-globin genes.
Introduction
The human β-globin locus on the short arm of chromosome 11 comprises 5 developmentally regulated genes arranged in the following order: 5′-ϵGγAγδβ-3′ (Figure 1), coding for the synthesis of the β-like chains of hemoglobin. Lesions in the locus give rise to hemoglobinopathies, such as β-thalassemia. Activation of the locus depends on the locus control region, or LCR, located upstream of the gene cluster.1 The human globin genes undergo 2 developmental switches in their activation. Expression of the ϵ- and γ-globin genes, which are activated first during primitive erythropoiesis in the embryonic yolk sac, switches to expression of the γ genes at the start of definitive erythropoiesis in the fetal liver (with a small contribution by β-globin). The second switch occurs gradually around birth with the activation of the adult stage δ- and β-globin genes (with δ-globin making a minor contribution), whereas γ-globin expression is suppressed to very low levels (1%-2%) by the end of the first year of age.
Constructs used in the study and location of HPFH breakpoints in the human β-globin locus. The constructs comprising the LCR and the Aγ gene with or without the HPFH enhancers are shown at the top. The human β-globin locus is shown below the constructs, indicating the position of the LCR and the order of the genes. The HPFH-1, HPFH-2, HPFH-3, and HPFH-6 deletions are shown below the locus. Hatched boxes indicate the core enhancer elements associated with the HPFH breakpoints (not to scale). Solid vertical arrows indicate hematopoietic-specific DNase I hypersensitive sites (HS), and the gray arrow indicates a ubiquitous HS site.
Constructs used in the study and location of HPFH breakpoints in the human β-globin locus. The constructs comprising the LCR and the Aγ gene with or without the HPFH enhancers are shown at the top. The human β-globin locus is shown below the constructs, indicating the position of the LCR and the order of the genes. The HPFH-1, HPFH-2, HPFH-3, and HPFH-6 deletions are shown below the locus. Hatched boxes indicate the core enhancer elements associated with the HPFH breakpoints (not to scale). Solid vertical arrows indicate hematopoietic-specific DNase I hypersensitive sites (HS), and the gray arrow indicates a ubiquitous HS site.
Understanding the molecular basis of globin gene switching is of great interest because persistent expression of the fetal γ-globin genes in the adult ameliorates the effects of hemoglobinopathies. Most commonly, persistent γ-globin expression is caused by a number of naturally occurring deletions at the 3′ end of the β-globin locus (Figure 1). The persistence of fetal hemoglobin (HbF) associated with such deletions is classified in 2 related clinical syndromes: hereditary persistence of fetal hemoglobin (HPFH) and (δβ)0-thalassemia.2 HPFH results in substantial (14%-30%) pancellular γ-globin expression, whereas (δβ)0-thalassemias give rise to lower levels (2%-15%) of heterocellular γ-globin expression in the adult stage.3 Three nonexclusive hypotheses have been proposed for the persistent γ-globin expression resulting from these deletions. The first hypothesis proposes the deletion of silencer elements from the Aγ- to δ-globin intergenic region.4-6 The second hypothesis predicts that the deletions juxtapose distal enhancer elements in proximity to the γ genes.4,7,8 Finally, persistent HbF expression could also result from the loss of sequences delineating stage-specific functional chromatin subdomains in the locus.9-11
The analysis of 3′ HPFH breakpoints has provided evidence supporting the second hypothesis of enhancer element juxtaposition. Examples include the HPFH-1, HPFH-2, HPFH-3, and HPFH-6 deletions (Figure 1), with sequences located immediately downstream to their 3′ breakpoints shown to act as enhancers in transient transfection assays.4,8,12,13 HPFH breakpoint sequences have also been tested in transgenic mice for their effects on γ-globin regulation.4,14 Normally, LCR γ-globin constructs are expressed in the embryonic stage in transgenic mice, with γ expression extending into early fetal liver hematopoiesis and autonomously switching off later in the fetal liver and adult blood stages.15-17 Of importance, a single point mutation in the γ gene promoter (-117 HPFH) results in persistent expression of the γ genes in the adult in humans and in mice.18 The addition of the HPFH-2 or HPFH-3 breakpoints to γ-globin transgenes (the latter without the LCR) extended γ gene expression beyond the early fetal liver stage, this being more prominent on inclusion of the LCR,4,14 thus suggesting that HPFH breakpoints have the capacity to alter the developmental regulation of γ-globin in transgenic mice.
In this study, we wanted to further test in transgenic mice whether the juxtaposition of the HPFH enhancers and/or chromatin position effects play a role in the persistent adult γ-globin expression. To this end, we have undertaken a rigorous analysis of the effects of the HPFH-2, HPFH-3, and HPFH-6 breakpoint enhancers on γ-globin regulation in mice, by comparing their effects in multicopy versus single copy animals and relating them to chromosomal sites of integration. We find that all 3 HPFH elements can drive γ-globin expression in the adult blood of multicopy animals; however, only the HPFH-2 and HPFH-6 enhancers were capable of maintaining detectable γ gene expression in the adult blood of single copy mice. These properties of the HPFH enhancers appear to be directly correlated to euchromatic (ie, noncentromeric or nontelomeric) sites of transgene integration. These findings support the hypothesis for the juxtaposition of enhancers in generating HPFH phenotypes4,7 and provide evidence for the involvement of positive chromatin position effects.10
Materials and methods
DNA constructs
A loxP site was cloned into the ClaI site 5′ to the Aγ gene in the LCR-Aγ-3′HS cosmid,17 generating cosmid LCR-loxP-Aγ-3′HS. The Aγ gene was subcloned as a ClaI-KpnI fragment into pBluescript KS. The HPFH-2 (0.7-kb ScaI-NruI) core element was blunt-end ligated into an NsiI site 3′ of the Aγ gene. The HPFH-3 (0.7-kb HphI) core element was amplified using Deep Vent DNA polymerase (New England Biolabs, Beverly, MA) and NsiI-fitted primers and cloned into the NsiI site 3′ of the Aγ gene. The HPFH-6 enhancer (1.45-kb EcoRI-BglII fragment) was blunt-end ligated into the KpnI site of the pBluescript-Aγ plasmid. The final cosmid constructs were generated by ligation and packaging into phage of the following fragments: (1) a 27-kb PvuI-ClaI fragment from LCR-loxP-Aγ-3′HS containing the cos site of the pTCF cosmid vector,19 the loxP site, and the LCR; (2) a ClaI-KpnI fragment from the pBluescript-Aγ plasmid containing the Aγ gene (5.6 kb) alone or with each of the HPFH enhancers; (3) a 14-kb KpnI-NotI fragment from the LCRϵ cosmid20 containing a cos site and sequences downstream of the ϵ-globin gene. Packaging was performed using Gigapack Gold extracts (Stratagene, La Jolla, CA) according to the manufacturer's instructions.
Transgenic mice
Fragments containing the LCR-loxP-Aγ inserts with or without the HPFH enhancers were released by SalI-KpnI digestion, purified by a 5%-25% NaCl salt gradient centrifugation, and prepared for microinjection, essentially as previously described.21 Purified DNA was injected into the pronucleus of fertilized eggs of FVB/N mice, as previously described.15 Transgenic founders were identified by Southern blotting using the LCR HS5 3.3-kb EcoRI fragment and the 2.3-kb EcoRI fragment 3′ to the Aγ gene as probes. Integrity of cosmid transgenes was checked by cosmid hybridization according to Strouboulis et al.20 Transgene copy numbers were determined by using a 5′ Aγ probe (1.7-kb EcoRI-BamHI fragment) and a 0.9-kb PvuI fragment from the endogenous mouse CA-II gene, and ratios of intensities of the 2 probes were compared with those obtained for the single copy human β-globin locus transgenic lines 2 and 72.20 PhosphorImager analysis was performed using ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Selected multicopy transgenic mouse lines were bred with the Zp3Cre22 or the CAGCre23 transgenic lines to generate single copy animals. The integrity and the copy numbers of the single copy mice were determined as described earlier.
DNA FISH analysis
Peripheral blood cells were cultured for 72 hours in RPMI 1640 medium. Chromosome preparations were made according to standard procedures. Fluorescence in situ hybridization (FISH) was carried out as described by Mulder et al.24 The probe used to detect the transgene was the biotin-labeled human β-globin LCR, which was immunochemically detected with fluorescein. Chromosomal DNA was counter-stained with DAPI (4,6 diamidino-2-phenylindole), which stains centromeric domains more intensely.
S1 nuclease protection assays
S1 nuclease protection assays were carried out on total RNA isolated from 12.5- and 16.5-day postcoitum (dpc) fetal livers and from adult blood. RNA was isolated with Trizol, according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Conditions for S1 nuclease protection assays and polyacrylamide gel electrophoresis have been previously described.15,25 The probes used were as follows: γ globin 5′ probe, 320-bp AvaII fragment, protected fragment size 165 bp; mouse βmaj 5′ probe, 700-bp HindIII/NcoI fragment, protected fragment size 100 bp. Specific activities of probes were determined as previously described26 and are indicated in the figure legends. PhosphorImager quantitation was done by ImageQuant software (Molecular Dynamics).
RNA in situ hybridization
Fetal liver cells (16.5 dpc) were disrupted by pipetting in phosphate-buffered saline (PBS) and fixed onto poly-lysine–coated slides (Sigma, St Louis, MO) according to van de Corput and Grosveld.27 For detection of each globin gene transcript, a mixture of 3 or 4 different 50-mer oligonucleotide probes was used, as previously described.27-29 Fluorescence was detected by epifluorescence microscopy, and photographs were recorded with a CCD camera. At least 400 cells per slide were scored for each line.
Immunofluorescence
Preparation of blood smears and immunofluorescence was performed according to Papayannopoulou and Nakamoto.30 Mouse antihuman hemoglobin (γ-chain) diluted 1:100 (Cortex Biochem, San Leandro, CA) and rabbit antimouse hemoglobin diluted 1:250 (ICN Pharmaceuticals, Costa Mesa, CA) antibodies were applied on the smears for 1 hour at 37°C. Subsequently, goat antimouse fluorescein isothiocyanate (FITC) 1:500 (Sigma) and goat antirabbit A594 diluted 1:500 (Molecular Probes, Leiden, the Netherlands) antibodies were applied for 1 hour at 37°C. Fluorescence was detected by epifluorescence microscopy, and photographs were recorded with a CCD camera. At least 400 cells were scored for γ staining in each line.
Results
LCRAγ-HPFH transgenic mice
To investigate in vivo the effects of the HPFH-2, HPFH-3, and HPFH-6 enhancers on γ-globin expression, we introduced each element separately downstream of a 5.6-kb Aγ gene in an LCRAγ cosmid previously shown to be autonomously silenced in the adult stage.17 The constructs used were fitted with a loxP site upstream of the Aγ gene to allow generation of single copy animals from the multicopy lines by Cre-mediated recombination. This procedure allows a direct comparison of the effects of copy numbers on the influence of the HPFH elements on γ gene expression by keeping the same transgene flanking regions.
The LCRAγ HPFH-2, HPFH-3, and HPFH-6 cosmids, as well as the control LCRAγ cosmid, were microinjected into mouse fertilized eggs. We obtained 10 founders transgenic for the control LCRAγ cosmid, which were bred to establish lines (Table 1). For the LCRAγ HPFH-2 construct 9 transgenic lines were obtained from 7 transgenic founders as a result of independently segregating integration events. For the LCRAγ HPFH-3 construct, 4 lines were generated from 3 founders, and for the LCRAγ HPFH-6 construct 4 founders were obtained, of which 3 transmitted the transgene (Table 1).
The γ-globin gene is expressed in the adult stage of multicopy LCRAγ-HPFH transgenic mice
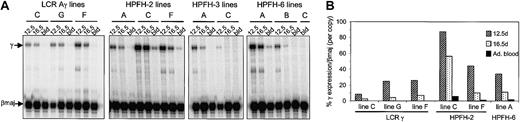
We first analyzed γ expression in RNA isolated from the adult blood of transgenic animals from the control LCRAγ and the HPFH lines, many of which were multicopy (Table 1). Expression of γ-globin is normally switched off by this stage in mice.17,20,31-33 For the multicopy LCRAγ founders, analysis of adult stage γ globin expression was carried out in adult blood isolated from the founder (mosaic) animals, because they were directly cross-bred with the Cre-expressing transgenic lines to generate single copy animals, or from F1 animals (Table 1). An estimate of the transgene copy number in the LCRAγ multicopy mosaic founders was determined from the ratio of end versus joining fragments obtained in Southern blots hybridized with the LCR's HS5 probe or the 2.3-kb EcoRI 3′ Aγ probe.
As expected, γ-globin expression was not detected in the adult blood of most of the LCRAγ control animals (Figure 2; Table 1). Low, but detectable, γ gene expression was observed in the blood of the multicopy control lines E and H (Figure 2; Table 1), as previously observed.17 In contrast, most of the HPFH lines showed persistent γ-globin expression in the adult blood (Figure 2). Seven of the 9 HPFH-2 lines showed γ expression levels ranging from 0.5% to 19.2% per copy, compared with endogenous mouse βmaj per copy expression levels (Table 1). Of those lines, the single copy line (line C) showed appreciable levels of γ expression in blood (Table 1). Lines G1, G2, and G3 were derived from one founder animal that had a single detectable integration site as determined by FISH. Only line G1 (5-6 copies) gave detectable γ expression, whereas the 2 low copy lines, line G2 (2 copies) and line G3 (single copy), did not show any observable γ gene expression (Table 1).
S1 nuclease protection analysis of expression of human Aγ-globin versus mouse βmaj globin in adult blood RNA of transgenic founders and lines generated. The protected fragments are indicated to the left of the panel. In this experiment, the specific activity of the βmaj probe was 2.35 times that of the γ probe (f indicates founder).
S1 nuclease protection analysis of expression of human Aγ-globin versus mouse βmaj globin in adult blood RNA of transgenic founders and lines generated. The protected fragments are indicated to the left of the panel. In this experiment, the specific activity of the βmaj probe was 2.35 times that of the γ probe (f indicates founder).
Of the 4 HPFH-3 lines, line A (13 copies) and line B2 (∼25 copies) were expressing γ-globin in the adult blood at levels ranging from 4.4% to less than 1% per copy, compared with βmaj (Figure 2; Table 1). The highest γ-expressing of the HPFH-6 mice was a founder animal, which, although mosaic, expressed γ-globin at approximately 28% of total mouse βmaj levels (Figure 2; Table 1). This founder did not transmit the transgene. Of the 3 established HPFH-6 transgenic lines (1-4 copies), γ gene expression per copy ranged from approximately 0.45% (line 6B) to 1.8% (line 6A) of mouse βmaj (Table 1). These findings demonstrate that the HPFH enhancers can alter the developmental expression of the Aγ gene in the adult blood in the majority of lines.
Persistent adult stage γ-globin expression in single copy HPFH-2 and HPFH-6 lines
To distinguish whether the persistence of γ gene expression in the HPFH lines was specifically due to the presence of the HPFH enhancers or due to a nonspecific effect of multicopy integrants of LCR-containing globin transgenes,17,34 we generated single copy mice (Figure 3) from selected multicopy animals by cross-breeding with lines expressing the Cre recombinase either exclusively in the growing oocyte (ie, Zp3-Cre mice22 ) or before the 2-cell stage of embryonic development (ie, CAG-Cre mice23 ).
Cre-mediated generation of single copy mice from multicopy lines. Genomic DNA was digested with EcoRI and probed with a human 5′Aγ probe and an endogenous mouse CAII probe, as loading control. The sizes of fragments detected are indicated by arrows. The blot includes DNA from all lines used in the single copy expression analysis shown in Figure 4. Original multicopy lines are shown next to the single copy lines obtained after Cre-mediated recombination, except line HPFH-3C where no multicopy DNA sample was available. Single copy β-globin locus lines L72 and L220 were included as copy number controls. LCRAγ single copy lines were generated directly by breeding founder mice with Cre-expressing mice.
Cre-mediated generation of single copy mice from multicopy lines. Genomic DNA was digested with EcoRI and probed with a human 5′Aγ probe and an endogenous mouse CAII probe, as loading control. The sizes of fragments detected are indicated by arrows. The blot includes DNA from all lines used in the single copy expression analysis shown in Figure 4. Original multicopy lines are shown next to the single copy lines obtained after Cre-mediated recombination, except line HPFH-3C where no multicopy DNA sample was available. Single copy β-globin locus lines L72 and L220 were included as copy number controls. LCRAγ single copy lines were generated directly by breeding founder mice with Cre-expressing mice.
We analyzed γ gene expression in the adult blood as well as in the early (12.5 dpc) and late (16.5 dpc) fetal liver stages of the single copy HPFH lines to determine whether the HPFH elements affected the γ-globin suppression that normally occurs during the fetal liver stage.17,20 As a control, we also included developmental time points from 3 single copy LCRAγ lines (Figure 4). As expected, γ gene expression declined rapidly throughout the fetal liver stages of the 3 LCRAγ single copy lines analyzed, becoming undetectable in the adult (Figure 4A-B; Table 2). The apparently higher levels of γ gene expression in the later fetal liver stages compared with transgenic mice carrying the complete human β-globin locus20 is due to the greater proximity of the γ gene to the LCR in the cosmid constructs. Differences in the levels of γ gene expression between the 3 lines could be accounted for by differences in the precise developmental timing of the embryos when they were dissected. The remaining single copy LCRAγ lines generated were analyzed only at the adult blood stage and also showed very low (< 0.5% per copy) to undetectable γ gene expression in adult blood (not shown).
Developmental pattern of γ-globin expression in the single copy mouse lines. (A) S1 nuclease protection analysis of human Aγ gene expression versus mouse βmaj in fetal liver RNA from day-12.5 and day-16.5 embryos and in adult blood RNA (bld) of the single copy transgenic lines. The protected fragments are indicated to the left of the panel. The specific activity of the βmaj probe was 2.35 times that of the γ probe. (B) Quantitation of γ-globin expression levels of the single copy lines shown in panel A, as a percentage of βmaj expression per copy.
Developmental pattern of γ-globin expression in the single copy mouse lines. (A) S1 nuclease protection analysis of human Aγ gene expression versus mouse βmaj in fetal liver RNA from day-12.5 and day-16.5 embryos and in adult blood RNA (bld) of the single copy transgenic lines. The protected fragments are indicated to the left of the panel. The specific activity of the βmaj probe was 2.35 times that of the γ probe. (B) Quantitation of γ-globin expression levels of the single copy lines shown in panel A, as a percentage of βmaj expression per copy.
Three single copy lines were generated from the HPFH-2 multicopy mice (Figure 4A). Of those, lines C and F expressed clearly detectable γ-globin levels in the adult blood, with line C expressing up to 6% of γ-globin per copy of mouse βmaj (Figure 4A; Table 2). Expression of γ-globin in the fetal liver of these 2 lines is also higher compared with the control mice (Figure 4B). By contrast, in line A the temporal pattern of γ-globin repression is indistinguishable to that of the control lines (Figure 4A; Table 2). Two single copy lines were generated from the multicopy HPFH-3 lines A and C. Line A originally carried 13 copies of the transgene and expressed robust levels of γ-globin in the adult blood (Figure 2). By contrast, when made single copy, there was no γ gene expression detectable in adult blood and expression in the fetal liver was indistinguishable to that of the LCRAγ control lines (Figure 4A; Table 2). This finding suggests that the expression observed in the original animal was due to the multicopy nature of the transgene. For reasons that are not clear, but could be related to negative position effects, line HPFH-3C gave lower levels of γ gene expression both in the fetal liver and adult stages (Figure 4A).
From the HPFH-6 mice, 3 single copy lines were generated. Line HPFH-6A expressed clearly detectable levels of γ-globin in adult blood with γ expression, also being higher throughout the fetal liver stage compared with the control lines (Figure 4A-B; Table 2). By contrast, lines B and C did not show persistent γ gene expression in the adult blood when made single copy (Figure 4A; Table 2). These results suggest that in single copy transgenics the HPFH-2 enhancer leads to clearly detectable levels of expression in the adult, whereas the HPFH-6 enhancer has some activity in the adult, and the HPFH-3 element displays no activity in single copy mice.
Correlation of persistent γ-globin expression with euchromatic sites of integration
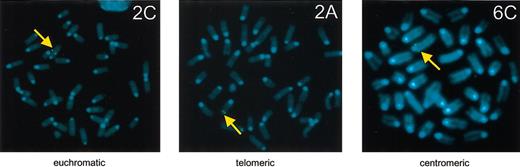
Although the data demonstrate that the HPFH-2 and HPFH-6 enhancers can lead to persistent γ expression in single copy mice, this is not always the case (eg, lines HPFH-2A or HPFH-6B). One explanation for these differences could be position effects dependent on the chromatin structure at the sites of transgene integration.35,36 We, therefore, mapped transgene integration sites in all transgenic lines using FISH in metaphase spreads. We classified chromosomal sites as (near) telomeric, (near) centromeric, and euchromatic, the latter including all integrations that did not map close to a telomere or a centromere. Examples are shown in Figure 5, and the results are summarized in Tables 1 and 2. Correlating γ gene expression patterns to integration sites revealed that in the control single copy LCRAγ lines, γ-globin switches off regardless of integration site (eg, euchromatic line LCRAγ-C, Table 2). The same appears to be true for the HPFH-3 single copy lines both of which were euchromatic integrants (Table 2). However, in the HPFH-2 and HPFH-6 lines there is a correlation between euchromatic sites of integration and persistence of γ gene expression in the adult stage (Table 2). The euchromatic HPFH-2 lines C and F showed clearly persistent levels of γ gene expression in the fetal liver and adult stages (Figure 4B), whereas telomeric line A (which expresses γ-globin when multicopy) did not display persistent γ gene expression when single copy. A similar effect is observed when comparing the euchromatic adult γ-expressing line HPFH-6A to the nonexpressing lines HPFH-6B or -6C, which are telomeric and centromeric, respectively (Table 2). From these observations we postulate that the HPFH-2 and HPFH-6 enhancers, but not HPFH-3, can lead to persistent γ expression in the adult stage only in “permissive” chromatin environments.
Chromosomal sites of transgene integration by FISH. Examples are shown of euchromatic (line HPFH-2C, left), telomeric (line HPFH-2A, middle), and centromeric (line HPFH-6C, right) sites of transgene integration (arrow), mapped by FISH on metaphase spreads using the human β-globin LCR as a probe.
Chromosomal sites of transgene integration by FISH. Examples are shown of euchromatic (line HPFH-2C, left), telomeric (line HPFH-2A, middle), and centromeric (line HPFH-6C, right) sites of transgene integration (arrow), mapped by FISH on metaphase spreads using the human β-globin LCR as a probe.
Cellular distribution of persistent γ-globin expression in HPFH-2 and HPFH-6 single copy mice
A distinguishing feature of HPFH syndromes is the pancellular distribution of γ-globin expression in heterozygotes.3 We investigated the cellular distribution of γ expression in 16.5-dpc fetal liver cells in the single copy HPFH-2C and HPFH-6A lines by RNA FISH using exon probes detecting γ-globin and mouse α-globin mRNA (Figure 6A). In HPFH-2C, 69% of the erythroid fetal liver cells expressed γ globin (Figure 6A). This finding compares with 56% per copy γ expression in this line, as determined by S1 nuclease protection (Table 2). In line HPFH-6A, 56% of the erythroid cells expressed γ globin (Figure 6A), whereas per copy γ expression levels by nuclease protection were 11.6% (Table 2). In both lines, therefore, the γ expression levels determined by nuclease protection analysis are distributed in a higher percentage of cells. There is also a greater difference between the HPFH-6A line (ie, 11.6% RNA versus 56% of cells) and the HPFH-2C line (56% RNA versus 69% of cells). The differences between the 2 lines may be due to the presence of different HPFH elements and/or due to position effects.
RNA in situ hybridization of 16.5-dpc fetal liver cells and immunofluorescence of adult blood cells from single copy lines HPFH-2C and HPFH-6A. (A) The signals obtained with exonic probes against γ-globin (red) and α-globin (green) are shown. The overlay of the 2 images shows double-positive cells as staining yellow. The percentages of γ-positive cells for the 2 transgenic lines are also indicated. A nontransgenic embryo was included as a control. (B) Representative fields of cells labeled with antihuman γ hemoglobin (green signal; FITC) and antimouse hemoglobin (red signal) antibodies. Percentages of cells with γ-globin signals are indicated. At least 400 cells were counted in each case.
RNA in situ hybridization of 16.5-dpc fetal liver cells and immunofluorescence of adult blood cells from single copy lines HPFH-2C and HPFH-6A. (A) The signals obtained with exonic probes against γ-globin (red) and α-globin (green) are shown. The overlay of the 2 images shows double-positive cells as staining yellow. The percentages of γ-positive cells for the 2 transgenic lines are also indicated. A nontransgenic embryo was included as a control. (B) Representative fields of cells labeled with antihuman γ hemoglobin (green signal; FITC) and antimouse hemoglobin (red signal) antibodies. Percentages of cells with γ-globin signals are indicated. At least 400 cells were counted in each case.
We also investigated the cellular distribution of persistent γ gene expression in the adult blood of the single copy HPFH-2C and HPFH-6A lines by immunofluorescence using antibodies against human γ-globin chains and mouse hemoglobin (Figure 6B). In line HPFH-2C we find 11% of the cells expressing γ-globin chains, whereas in line HPFH-6A we find 1% of the cells expressing γ-globin chains (Figure 6B). These values are close to RNA expression levels as determined by S1 nuclease protection (6% and 2% per copy expression for HPFH-2C and HPFH-6A, respectively; Table 2).
Discussion
In this paper we have tested in transgenic mice whether the juxtaposition of positive regulatory elements, such as enhancers, or chromatin position effects or both play a role in the persistent adult stage γ-globin expression in HPFH syndromes associated with large deletions in the 3′ end of the human β-globin locus. We linked the breakpoint sequences of the HPFH-2, HPFH-3, and HPFH-6 deletions, previously shown to act as enhancers in transient transfection assays, to an LCRAγ construct and tested their ability to influence γ-globin developmental regulation in transgenic mice. Because adult stage γ-globin expression has been observed in mice with LCRγ transgene arrays17 or as a result of chromosomal position effects,14,17,37-39 we adopted a number of criteria so as to ensure that the effects we observed in our analysis of HPFH breakpoints in mice are specific to the presence of the HPFH elements. First, we included a large number of transgenic lines carrying the control LCRAγ construct. Second, the analysis was carried out in both multicopy and single copy animals generated from the original multicopy lines by Cre-mediated recombination, thus maintaining the same chromosomal integration sites. Third, we mapped the chromosomal sites of integration in all our mice in an effort to correlate γ gene expression patterns to chromosomal position effects, especially in the single copy mice.
We found that all HPFH enhancers can lead to persistent γ gene expression in the adult stage of multicopy animals, whereas the analysis of several single copy LCRAγ control transgenic lines showed virtually no γ-globin expression in the adult blood. When made single copy, persistent adult stage γ gene expression was observed only in a subset of the HPFH-2 and HPFH-6 transgenic lines, a property that directly correlated with euchromatic sites of transgene integration. These findings are consistent with a model whereby persistent γ gene expression in deletion HPFH is a result of the juxtaposition of regulatory elements and/or accessible chromatin structures in the vicinity of the γ-globin genes.4,7-11
Our in vivo findings are in accordance with our previous in vitro data from transient transfection assays in erythroid K562 cells, which showed HPFH-2 to be the strongest enhancer,12 HPFH-6 showing intermediate activity, and HPFH-3 being the weakest element (Michael McArthur and N.P.A., unpublished data, 2003). In addition, previous work using mouse erythroid MEL cell hybrids carrying human chromosomes with the HPFH-1 or HPFH-2 deletions, showed the HPFH-2 × MEL cell hybrid (representing an adult stage of erythropoiesis) resulted in higher γ-globin expression levels compared with HPFH-1.40
Our data are also in agreement with 2 previous studies on the effects of HPFH breakpoints on γ gene regulation in transgenic mice. In the first study, Anagnou et al4 showed that HPFH-3 breakpoint sequences could extend expression of an Aγ-globin transgene (not linked to an LCR) in the fetal liver stage in a small number of multicopy transgenic fetuses (in the absence of the LCR, expression of γ-globin transgenes are restricted to the embryonic stage15,16 ). In the second study by Arcasoy et al,14 the HPFH-2 breakpoint (which includes the HPFH-1 enhancer) was linked to a GγAγ transgene, with or without an LCR. Linking the LCR to the GγAγHPFH-2 transgene resulted in persistent γ gene expression in the adult stage of 6 multicopy lines.14 Although these results are in agreement with our findings with the HPFH-2 mice, Arcasoy et al14 argued that position effects contributed little to the persistent adult stage γ gene expression. Because their analysis included only multicopy lines, it is impossible to confirm whether this is indeed the case. Furthermore, the LCRGγAγHPFH-2 construct carried both HPFH-2 and HPFH-1 enhancers, which masks the individual effects of each enhancer. On the basis of our analysis, we would argue that positive position effects are a requirement for the HPFH-2 (and HPFH-6) sequences to mediate persistent γ gene expression in adult mice.
We also tested the cellular distribution of γ-globin RNA and protein chains in 16.5-dpc fetal liver cells and adult blood, respectively, in the HPFH-2C and HPFH-6A lines. We found that γ expression (RNA or protein) was distributed in a higher percentage of cells compared with the γ per copy RNA levels determined by S1 nuclease protection. This finding was particularly pronounced in the late fetal liver stage. A similar effect was also observed by Arcasoy et al14 in that a high percentage (> 50%) of adult blood cells expressed γ globin chains, compared with γ RNA per copy expression levels ranging from 3.4% to 8%. The authors determined this pattern to be heterocellular, in contrast to the pancellular γ chain distribution observed in individuals with HPFH deletion. It is difficult from our data to conclude whether the pattern of γ expression is heterocellular or pancellular. Differences between the cellular patterns observed in transgenic mouse models and in human HPFH individuals could be due to position effects, the absence from the transgenic constructs of additional elements required to reproduce a pancellular distribution, differences in the threshold sensitivities of the anti–γ-globin antibodies, or differences between mouse and human erythropoiesis.
Our observations on HPFH-mediated persistent γ gene expression and positive position effects are also related to those of Elder et al41 on the chromatin structure of the domain in which the 3′ breakpoints of the HPFH-1 and HPFH-2 deletions map. These 2 breakpoints fall within 5 to 6 kb of each other and reside in a domain of DNase I hypersensitivity in erythroid cells, extending at least 8 kb upstream of the HPFH-1 breakpoint (Figure 1). Three DNase I hypersensitive sites, 2 erythroid specific and 1 ubiquitous, were mapped within an approximately 9-kb region spanning the HPFH-1 and HPFH-2 breakpoints.41 The sequence associated with the HPFH-1 breakpoint was also shown to be largely unmethylated in erythroid cells.8 An olfactory receptor gene also maps within the same hypersensitive domain and is expressed, albeit at low levels, in human erythroid cells.42 Therefore, the HPFH-2 deletion, although removing the 5′-most erythroid hypersensitive site, results in the juxtaposition of the downstream (ubiquitous and erythroid) hypersensitive sites to within 12 kb of the Aγ gene.41 The HPFH-3 and HPFH-6 breakpoints are also in close proximity to each other and, although there is presently no information regarding the chromatin structure of this region, it is possible that they lie in the same chromatin subdomain, which is distinct to that of the more 3′ HPFH-1 and HPFH-2 breakpoints.
It is conceivable that the levels of persistent γ gene expression observed in patients with HPFH deletions are due to the juxtaposition of combinations of the elements described here. For example, the HPFH-3 deletion brings all 4 HPFH elements (HPFH-3, HPFH-6, HPFH-2, and HPFH-1) close to the γ genes, the HPFH-6 deletion juxtaposes elements HPFH-6 and HPFH-2 (and HPFH-1), whereas the HPFH-2 deletion brings in HPFH-2 (and HPFH-1). Because the latter appears to be the strongest activator of the γ genes in our experiments, it is not unreasonable that all 3 HPFH deletions have similar levels of persistent γ globin expression in patients, as the HPFH-2 enhancer (and chromatin subdomain) is retained in all 3 deletions. In addition, all of these deletions remove the 3′HS1, which we have recently shown to participate in the active interactions between the LCR and the globin genes in the locus, thus forming an active chromatin hub (ACH).43 Hence, removal of 3′HS1 in these deletions may allow increased interactions of the HPFH elements with the ACH. In contrast, in (δβ)0 thalassemias the 3′ breakpoints occur either before 3′HS1 (most cases), or well past the HPFH-2 (and HPFH-1) breakpoints (reviewed in Stamatoyannopoulos and Grosveld3 ), which means either that the HPFH-6, HPFH-3, and HPFH-2 elements may not be able to interact with the ACH because of the presence of 3′HS1 or that the elements are absent altogether. This situation may account for the generally lower persistent γ gene expression levels associated with (δβ)0 thalassemias compared with HPFH syndromes.
In summary, our results relating the persistent γ-globin expression in transgenic mouse models to the juxtaposition of HPFH enhancer sequences and, potentially, to specific chromatin domains, provide a conceptual framework for the further elucidation of the molecular basis for the phenotypic differences between deletion syndromes leading to persistent HbF, as well as for the further investigation of the domain-wide transcriptional regulation of the human β-globin locus.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-05-1681.
Supported by the Netherlands Organisation for Scientific Research (NWD) and the European Union (EU) (F.G. and J.S.) and by the EU and the National Secretariat of Research and Technology of Greece (grant EKBAN No. 94) (N.P.A.). E.Z.K. was supported by a short-term European Molecular Biology Organization (EMBO) fellowship and by a studentship of the Joint Graduate Program in Molecular Biology and Biomedicine at the University of Crete.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank John Kong a San, Ton de Wit, and Sylvia Dekker for help with microinjections and Erasmus Dierexperimenteel Centrum for animal care. We also thank Drs R. Pavlou, D. Kardassis (University of Crete), and W. de Laat (Erasmus MC) for helpful discussions.