Abstract
Fanconi anemia (FA) is an autosomal recessive cancer susceptibility syndrome characterized by cellular sensitivity to genotoxic agents. In recent years, FA proteins have been associated with different molecules involved in signal transduction, which has raised the interest in FA-dependent signaling pathways. Here, we report that the c-Jun N-terminal kinase (JNK) fails to phosphorylate in response to UV radiation and treatment with mitomycin C in FA lymphoblast cells derived from type A patients (FA-A). Furthermore, defective kinase activity seems to be specific for JNK, because extracellular signal-regulated kinase (ERK) responded to the proper stimuli in FA-A cells. We also demonstrate that the early growth-response factor-1 (Egr-1), a JNK downstream target gene that is normally induced by genotoxic stress, is not upregulated in UV-treated FA-A cells. Moreover, FA-A cells are more sensitive to apoptosis than control lymphoblasts. Both JNK and Egr-1 may be part of a pathway triggered by FA proteins, because functional correction of FA-A cells by gene transfer restores, at least in part, JNK activation and Egr-1 expression after UV exposure. Together, our data suggest that activation of JNK and expression of Egr-1 gene in B lymphoblasts mediate a cellular response to genotoxic agents that may be induced by FA proteins.
Introduction
Fanconi anemia (FA) is an autosomal recessive disease characterized by developmental abnormalities, progressive bone marrow failure, and cancer susceptibility. At the cellular level, FA cells are hypersensitive to DNA cross-linking agents such as mitomycin C (MMC) or diepoxybutane.1,2 FA is a genetically heterogeneous disease, comprising at least 8 complementation groups: FA-A, -B, -C, -D1, -D2, -E, -F and -G, the FA-A group being the most frequently represented in these patients.3,4 Seven FA-associated genes have been cloned to date, and their products were found to interact with DNA damage–response proteins, including BRCA1, ATM, and NBS1.5-7 In line with this, it has been described that FA proteins may be involved in the process of repairing chromosome defects that occur during homologous recombination.5
A complex of 5 FA proteins (FANCA, FANCC, FANCE, FANCF, and FANCG) triggers the monoubiquitination of the FANCD2 protein and its interaction with BRCA1 in response to DNA damage.6 BRCA1 plays an important role in mediating cell cycle arrest, apoptosis, and cellular responses to DNA damage signals.8,9 Recently it has been shown that BRCA1 is cleaved by caspase-3 during UV-induced apoptosis, which produces a C-terminal fragment that triggers an apoptotic response through activation of c-Jun N-terminal kinase (JNK) and GADD45.10 Consistently, phosphorylation activity of JNK is abrogated in breast cancer cells after blockade of BRCA1 expression with specific ribozymes, indicating that JNK is a downstream target of BRCA1.11
Additionally, FANC proteins exhibit functions besides their role in the complex. To this end, a number of FANCC-dependent molecular mechanisms have been suggested to ensure the survival of hematopoietic cells. Notably, FANCC is involved in the activation of signal transducer and activator of transcription-1 (STAT1) through growth factor receptors,12 it cooperates with heat-shock protein 70 (Hsp70) to suppress the activity of proapoptotic interferon-inducible double-stranded RNA-dependent protein kinase (PKR),13 and binds to glutathione S-transferase P1-1 (GSTP1), inducing the detoxification of by-products of oxidative stress.14 Interestingly, GSTP1 regulates JNK by protein-protein interactions,15 although the significance of this link in FANC-dependent signaling is presently unknown.
DNA damage is associated with activation of JNK, which has been suggested to play an important role in cell homeostasis and cell growth by mediating enhanced DNA repair.16,17 JNK belongs to the mitogen-activated protein kinase (MAPK) family, which participates in the decision-making process that cells use to mount an appropriate response to specific stimuli. Consequently, the JNK signal transduction pathway is implicated in many pathologic conditions, including cancer, and represents a potential target for therapeutic intervention.18,19
Here we have analyzed the activity of JNK and its downstream effectors in FA-A lymphoblast cell lines exposed to genotoxic agents. In these cells we found a deficiency in the phosphorylation activity of JNK, as well as a diminished expression of the early growth-response factor-1 gene (Egr-1), compared with normal lymphoblasts. Furthermore, deficient activation/expression of these proteins was partially reverted by transducing the cells with the wild-type form of the FANCA gene.
Materials and methods
Cell culture
Peripheral blood mononuclear cells were obtained from healthy donors and FA patients after informed consent. To determine the complementation group of these patients, cells were transduced with retroviral vectors expressing FANCA, FANCC, FANCF, and FANCG proteins (kindly provided by H. Hanenberg, Heinrich-Heine University, Düsseldorf, Germany) and then exposed to increasing concentrations of MMC.20 All patients studied so far belong to FA complementation group A. Epstein-Barr virus (EBV)–transformed lymphoblasts from FA-A patients and healthy donors were maintained in RPMI media (Biochrom, Berlin, Germany) supplemented with 15% heat-inactivated fetal calf serum (FCS) and antibiotics.
Cells were seeded in 6-well plates with 3 mL culture medium and exposed to a UV dose of 700 J/m2, unless otherwise indicated, or treated with 10 μM MMC or 1 μM phorbol-12-myrisate-13-acetate (PMA) (both from Sigma, St Louis, MO) and then analyzed for MAPK activity.
Cell viability was determined by the trypan blue exclusion test by counting at least 200 cells from each individual culture.
Apoptosis was assessed by a quantitative analysis of histone-associated DNA fragments using an enzyme immunoassay kit (Roche, Mannheim, Germany) and further confirmed by Western blot, detecting the 85-kDa cleavage fragment of poly (adenosine diphosphate [ADP] ribose) polymerase (PARP).
RT-PCR analysis
Total RNA was prepared using TRIZOL reagent (Invitrogen, San Diego, CA). To assess mRNA expression, a semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) method was used as previously described.21 The generated cDNA was amplified by using primers for human Egr-1 (5′-ACCTCCTCTCTCTCTTCC and 5′-TGGATAGAGGTGAAGAACTTGG), p53 (5′-GCCATCTACAAGCAGTCACAGC and 5′-AGTGTGATGATGGTGAGGATGG), MDM2 (5′-AGTGAAGACTATTCTCAGCC and 5′-AGGAGAATGGTGCGAACC), p21 (5′-ATGAGTTGGGAGGAGGCA and 5′-AGAAGATGTAGAGCGGGC), Bax (5′-TGGAGCTGCAGAGGATGATTG and 5′-CCAGTTGAAGTTGCCGTCAGA), and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH).21 After 20 (GAPDH), 25 (p53), 31 (Egr-1), 25 (MDM2), 28 (p21), and 25 (Bax) amplification cycles, the expected PCR products were size-fractionated onto a 2% agarose gel and stained with ethidium bromide. When indicated, real-time PCR was used to quantify the Egr-1 mRNA levels as previously described.22
JNK immunoassay
JNK activity was detected by an immunocomplex assay as described elsewhere23 with slight modifications. In brief, cell lysates were cleared by centrifugation at 13 000 rpm for 10 minutes. Soluble proteins were incubated with anti-JNK1 (sc-474), anti-ERK2 (sc-154), or anti-p38 (sc-535) (all from Santa Cruz Biotechnology, Santa Cruz, CA) for 2 hours at 4°C and then incubated with Gammabind Sepharose beads (Amersham Biosciences, Piscataway, NJ). The immune complexes were washed and resuspended in kinase buffer containing [γ-32P]adenosine triphosphate ([γ-32P]ATP) (3000 Ci/mmol [110 TBq/mmol]; Amersham Biosciences) and 1 μg gluathione S-transferase–activating transcription factor-2 (GST-ATF2) or 5 μg myelin basic protein (MBP) (Sigma) for JNK/p38 and extracellular signal-regulated kinase (ERK), respectively. After incubation, samples were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. To ensure an even immunoprecipitation of MAPKs, part of the immune complexes were run on a 10% SDS-PAGE, transferred to nitrocellulose filters, and developed with the corresponding anti-MAPK antibody.
Western blot analysis
Cell lysates were prepared as previously described.24 Proteins (100 μg) were resolved in SDS-PAGE and transferred to nitrocellulose filters. Blots were incubated with rabbit anti–Egr-1 or anti-PARP (Santa Cruz) or mouse anti–β-tubulin antibodies (Sigma) and then incubated with goat antirabbit or antimouse antibodies conjugated with peroxidase (Applied Biosystems, Foster City, CA). Bound antibody was detected by a chemiluminescence system (Applied Biosystems).
Gene reporter assay
A genomic PCR fragment of 688 base pair (bp) (–666 to +22, referred to the initiation of transcription) from the promoter region of Egr-1 (Egr-1pt) was cloned into the pGL2–basic luciferase reporter vector (Promega, Madison, WI). Lymphoblast cells were cotransfected with 10 μg pGL2–Egr-1pt and 1 μg pRSV–β-gal in triplicate by electroporation (1700 μF, 110 V). After 20 hours of transfection, cell extracts were prepared and analyzed for the relative luciferase activity by using a reporter gene assay system (Applied Biosystems). Results were normalized for transfection efficiency with values obtained with pRSV–β-gal as described.22
Results
UV activation of JNK is impaired in FA-A B lymphoblasts
JNK is activated by exposure of cells to many forms of environmental stress and genotoxic agents and connects the cellular damage sensors to effector proteins that stop the cell cycle, activate the DNA-repairing machinery, or trigger the apoptotic pathway.25 Because FA cells lack an efficient response to genotoxic agents, we studied the UV-dependent activation of JNK in EBV-transformed B lymphoblasts from FA-A patients and healthy donors. Five control B lymphoblast cell lines responded to UV exposure by increasing the phosphorylation of ATF2, a substrate of activated JNK. However, UV-dependent activation of JNK in 4 FA-A cell lines was significantly impaired (Figure 1A). It is well documented that FA cells are sensitive to DNA cross-linking agents such as MMC.26 Thus, we studied whether the JNK response to MMC in FA-A cells was also defective. When lymphoblasts were treated with MMC for different time intervals, JNK activity clearly increased in control cells by 6 hours of treatment, whereas no activation of JNK was observed in FA-A cells at any incubation time (Figure 1B). An increase in JNK activity was observed at concentrations of MMC above 50 nM, although the highest levels of phosphorylation were achieved at 10 μM (data not shown); thus we set this concentration for JNK studies.
Activation of JNK and ERK in control and FA-A lymphoblasts. (A) Lymphoblast cell lines were exposed to UV radiation, cultured for 30 minutes, and subjected to immunoprecipitation with anti-JNK. The immunoprecipitates were then incubated with GST-ATF2 and [γ-32P]ATP. GST-ATF2 phosphorylation was assessed by SDS-PAGE and autoradiography (top panel). The lysates were analyzed by Western blot with anti-JNK (bottom panel). (B) Control and FA-A lymphoblasts were incubated in the presence of MMC for the indicated times and analyzed for the activity of JNK as assessed by phosphorylation of the GST-ATF2 substrate (top panel). The lysates were analyzed by Western blot with anti-JNK (bottom panel). (C) Control and FA-A cell lines were treated with PMA for 1 hour. Anti-ERK immunoprecipitates were subjected to immune complex kinase assays with MBP as substrate (top panel). The lysates were also analyzed by Western blot with anti-ERK (bottom panel).
Activation of JNK and ERK in control and FA-A lymphoblasts. (A) Lymphoblast cell lines were exposed to UV radiation, cultured for 30 minutes, and subjected to immunoprecipitation with anti-JNK. The immunoprecipitates were then incubated with GST-ATF2 and [γ-32P]ATP. GST-ATF2 phosphorylation was assessed by SDS-PAGE and autoradiography (top panel). The lysates were analyzed by Western blot with anti-JNK (bottom panel). (B) Control and FA-A lymphoblasts were incubated in the presence of MMC for the indicated times and analyzed for the activity of JNK as assessed by phosphorylation of the GST-ATF2 substrate (top panel). The lysates were analyzed by Western blot with anti-JNK (bottom panel). (C) Control and FA-A cell lines were treated with PMA for 1 hour. Anti-ERK immunoprecipitates were subjected to immune complex kinase assays with MBP as substrate (top panel). The lysates were also analyzed by Western blot with anti-ERK (bottom panel).
To get insight into the specificity of this effect, we studied the functional responsiveness of the MAPKs, ERK, and p38. ERK is activated by growth factors and mitogenic stimuli (eg, PMA) and plays an important role in regulating the G1/S transition. Analysis of the levels of MBP phosphorylation normalized to those of ERK expression demonstrated that the kinase activity of ERK was similar in both control and FA-A cell lines following treatment with PMA (Figure 1C; densitometry analysis not shown). We also investigated the activation of p38 in FA-A cells. However, although we exposed the lymphoblast cell lines to a number of stress stimuli, the level of p38-dependent phosphorylation of ATF2 was too low to obtain conclusive results (data not shown).
Egr-1 expression is down-regulated in FA-A cell lines
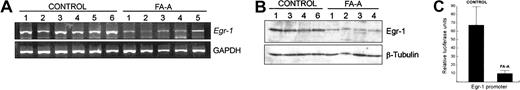
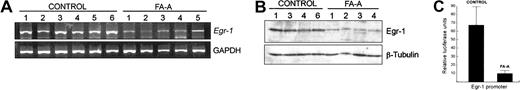
Recently, it has been demonstrated that JNK but not ERK induces the expression of Egr-1 in NIH3T3 cells after exposure to UV radiation.27 Because Egr-1 plays a role in the survival of B lymphoblasts,28 we analyzed whether the expression of this transcription factor is deregulated in FA-A lymphoblast cell lines and showed that the mRNA and protein levels of Egr-1 in untreated FA-A cells were significantly lower than those in control cells (Figure 2A-B). To further confirm these data, a 688-bp fragment of the Egr-1 promoter was cloned into a promoterless luciferase vector, and this construct was transiently transfected into control and FA-A lymphoblasts. Figure 2C shows that the transcriptional activity of the Egr-1 promoter in FA-A cells was about 7-fold lower than the activity detected in control lymphoblasts.
Constitutive expression of Egr-1 is reduced in FA-A cells. Total RNA and cell lysates were purified from control and FA-A lymphoblast cell lines and analyzed for Egr-1 mRNA and protein levels by semiquantitative RT-PCR (A) and Western blot (B), respectively. GAPDH mRNA was used as an amplification control. The levels of β-tubulin were analyzed to assure equal loading. (C) Control and FA-A cells were transfected with a 688-bp fragment of the Egr-1 promoter in the presence of a β-galactosidase reporter vector, and the Egr-1 promoter-dependent transcription was determined. Units of luciferase activity were normalized based on values of β-gal activity to control for transfection efficiency. Histograms represent the means ± SD of triplicate analyses.
Constitutive expression of Egr-1 is reduced in FA-A cells. Total RNA and cell lysates were purified from control and FA-A lymphoblast cell lines and analyzed for Egr-1 mRNA and protein levels by semiquantitative RT-PCR (A) and Western blot (B), respectively. GAPDH mRNA was used as an amplification control. The levels of β-tubulin were analyzed to assure equal loading. (C) Control and FA-A cells were transfected with a 688-bp fragment of the Egr-1 promoter in the presence of a β-galactosidase reporter vector, and the Egr-1 promoter-dependent transcription was determined. Units of luciferase activity were normalized based on values of β-gal activity to control for transfection efficiency. Histograms represent the means ± SD of triplicate analyses.
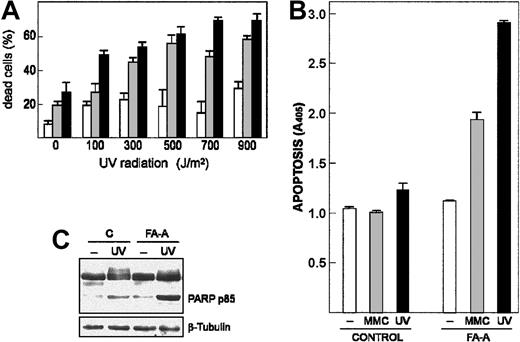
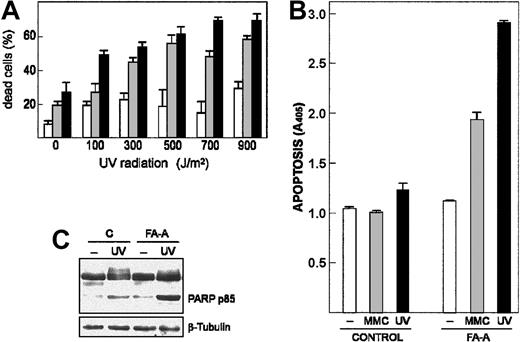
We asked whether FA-A cells were more sensitive than control lymphoblasts to apoptosis induced by DNA damage, which would be consistent with the cell survival activity associated with Egr-1. Thus, 2 FA-A cell lines and control cells were exposed to increasing doses of UV radiation (from 100 to 900 J/m2), and then cell viability was determined by trypan blue exclusion. As shown in Figure 3A, the percentage of dead cells in the FA-A populations was significantly higher than that observed in the control cell line at any UV dose. Moreover, loss of cell viability induced by either UV exposure or treatment with MMC was due to activation of an apoptotic process, as assessed by quantification of histone-associated DNA fragments (Figure 3B), and cleavage of PARP protein (Figure 3C).
DNA damage–induced apoptotic response is increased in FA-A cells. (A) Control (□) and 2 FA-A lymphoblast cell lines (▦, ▪) were exposed to different doses of UV radiation and left in culture for 12 hours. The percentage of dead cells was then measured by trypan blue dye exclusion. (B-C) Control and FA-A lymphoblasts were exposed to UV radiation (700 J/m2) and left in culture for 5 hours or treated with 50 nM MMC for 48 hours, and then apoptotic cell death was determined by an enzyme immunoassay method that quantifies the histone-associated DNA fragments present in the cytosol (B) or by Western blot with an antibody that recognizes both the full-length (112 kDa) and the cleavage fragment (85 kDa) of PARP protein (C). The levels of β-tubulin were determined to assure equal loading. A405, units of absorbance at 405 nm. Histograms represent the means ± SD of triplicate analyses.
DNA damage–induced apoptotic response is increased in FA-A cells. (A) Control (□) and 2 FA-A lymphoblast cell lines (▦, ▪) were exposed to different doses of UV radiation and left in culture for 12 hours. The percentage of dead cells was then measured by trypan blue dye exclusion. (B-C) Control and FA-A lymphoblasts were exposed to UV radiation (700 J/m2) and left in culture for 5 hours or treated with 50 nM MMC for 48 hours, and then apoptotic cell death was determined by an enzyme immunoassay method that quantifies the histone-associated DNA fragments present in the cytosol (B) or by Western blot with an antibody that recognizes both the full-length (112 kDa) and the cleavage fragment (85 kDa) of PARP protein (C). The levels of β-tubulin were determined to assure equal loading. A405, units of absorbance at 405 nm. Histograms represent the means ± SD of triplicate analyses.
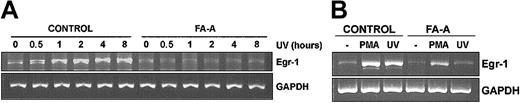
Then, we exposed cells to UV light and analyzed the expression of Egr-1 mRNA at different times. As shown in Figure 4A, Egr-1 was readily increased after 30 minutes and reached the highest level of expression by 2 hours of culture following UV exposure. Conversely, the same treatment did not result in an increased expression of Egr-1 mRNA in FA-A cells at any incubation time (Figure 4A).
Expression of Egr-1 mRNA in FA-A lymphoblasts treated with UV and PMA. Control and FA-A cell lines were exposed to UV and left in culture for the indicated time intervals (A) or treated with PMA for 1 hour (B), and then total RNA was extracted and analyzed for the expression of Egr-1 by semiquantitative RT-PCR. GAPDH mRNA was used as an amplification control.
Expression of Egr-1 mRNA in FA-A lymphoblasts treated with UV and PMA. Control and FA-A cell lines were exposed to UV and left in culture for the indicated time intervals (A) or treated with PMA for 1 hour (B), and then total RNA was extracted and analyzed for the expression of Egr-1 by semiquantitative RT-PCR. GAPDH mRNA was used as an amplification control.
Because ERK is also able to induce Egr-1 expression,29 we investigated whether treatment of these cells with ERK activators might up-regulate Egr-1 mRNA. As expected, both PMA and UV induced the expression of Egr-1 in control lymphoblasts. Furthermore, consistent with our results that JNK but not ERK phosphorylation activity was defective in FA-A cell lines, treatment of FA-A cells with PMA induced the expression of Egr-1 at levels similar to those observed in control cells, as determined by RT-PCR and densitometry analysis (Figure 4B and data not shown).
Functional complementation with FANCA restores JNK activity and Egr-1 expression in FA-A cells
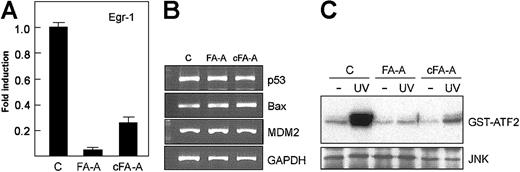
Transduction of EBV-transformed FA lymphoblasts with the corresponding FANC cDNA, including FANCA, corrects the sensitivity of FA cells to DNA cross-linking agents and restores the FA protein complex.30 FA-A lymphoblasts were transduced with a retroviral vector containing both FANCA and EGFP cDNAs.20 Analysis of transduced cells (about 35% were green fluorescent protein [EGFP]–positive) revealed a partial correction of the sensitivity to MMC. By 5 days of treatment with 100 nM MMC, more than 60% of control vector–transduced cells were dead as assessed by the uptake of propidium iodide, whereas 81% of FANCA-transduced cells were viable (data not shown). Consistently, in functionally corrected FA-A cells the mRNA levels of Egr-1 were 5-fold higher than those observed in FA-A lymphoblasts, as determined by real-time PCR (Figure 5A), whereas other genes involved in cell cycle control and apoptosis (p53, MDM2, Bax) maintained the same expression level in both wild-type and corrected FA-A cells (Figure 5B).
Restoration of the JNK–Egr-1 pathway in FA-A cell lines corrected with FANCA cDNA. (A) FANCA-corrected (cFA-A) and uncorrected FA-A cells (transduced with an empty control vector) were exposed to UV radiation, and then total RNA was extracted and analyzed for the expression of Egr-1 mRNA by real-time PCR. Histograms represent the means ± SD of triplicate analyses. (B) The levels of p53, Bax, and MDM2 were also analyzed by semiquantitative RT-PCR. GAPDH mRNA was used as an amplification control. (C) After exposure of cells to UV, lysates were immunoprecipitated with anti-JNK and subjected to immune complex kinase assay with GST-ATF2 as substrate (top panel). The lysates were also analyzed by Western blot with anti-JNK (bottom panel). c indicates control lymphoblast cells.
Restoration of the JNK–Egr-1 pathway in FA-A cell lines corrected with FANCA cDNA. (A) FANCA-corrected (cFA-A) and uncorrected FA-A cells (transduced with an empty control vector) were exposed to UV radiation, and then total RNA was extracted and analyzed for the expression of Egr-1 mRNA by real-time PCR. Histograms represent the means ± SD of triplicate analyses. (B) The levels of p53, Bax, and MDM2 were also analyzed by semiquantitative RT-PCR. GAPDH mRNA was used as an amplification control. (C) After exposure of cells to UV, lysates were immunoprecipitated with anti-JNK and subjected to immune complex kinase assay with GST-ATF2 as substrate (top panel). The lysates were also analyzed by Western blot with anti-JNK (bottom panel). c indicates control lymphoblast cells.
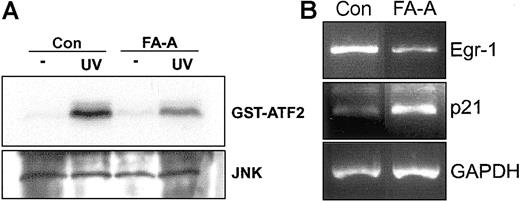
We also studied the kinase activity of JNK in FANCA-transduced FA-A cells after UV exposure and found a modest but significant increase of ATF2 phosphorylation when compared with the signal detected in UV-treated control cells (Figure 5C).
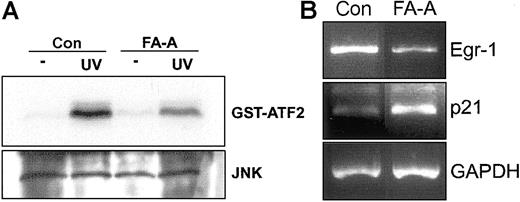
Finally, to extend our previous findings to a more clinically relevant cellular model, we exposed peripheral blood mononuclear cells from healthy donors and FA-A patients to UV radiation. Induction of JNK phosphorylation activity was lower in FA-A than in normal cells (Figure 6A). Furthermore, the expression of Egr-1 decreased in FA-A cells as previously observed in lymphoblast cell lines. To assess the specificity of this expression pattern, the mRNA levels of p21, which is highly expressed in FA-C lymphoblast cell lines,31 were analyzed and found to be increased in FA-A cells compared with control lymphoblasts (Figure 6B).
UV-mediated activation of JNK and expression of Egr-1 in FA-A primary leukocytes. (A) Peripheral blood mononuclear cells from a healthy donor (Con) and a patient with FA-A were exposed to UV radiation, and then cell lysates were immunoprecipitated with anti-JNK and subjected to immune complex kinase assay with GST-ATF2 as substrate (top panel). The lysates were analyzed by Western blot with anti-JNK (bottom panel). (B) Total RNA from UV-treated cells was extracted and analyzed for the expression of Egr-1 mRNA by semiquantitative RT-PCR. The levels of p21 were also analyzed. GAPDH mRNA was used as an amplification control.
UV-mediated activation of JNK and expression of Egr-1 in FA-A primary leukocytes. (A) Peripheral blood mononuclear cells from a healthy donor (Con) and a patient with FA-A were exposed to UV radiation, and then cell lysates were immunoprecipitated with anti-JNK and subjected to immune complex kinase assay with GST-ATF2 as substrate (top panel). The lysates were analyzed by Western blot with anti-JNK (bottom panel). (B) Total RNA from UV-treated cells was extracted and analyzed for the expression of Egr-1 mRNA by semiquantitative RT-PCR. The levels of p21 were also analyzed. GAPDH mRNA was used as an amplification control.
Discussion
DNA damage–inducible activation of FANCD2 requires an intact FANC-BRCA pathway. In FA cells, this pathway is disrupted because of inactivating mutations in a defined FANC gene. We found that JNK phosphorylation is not induced in B lymphoblast cell lines derived from patients with FA complementation group A after exposure to UV or treatment with MMC, which was further confirmed in FA-A peripheral blood mononuclear cells. Although the increased sensitivity of FA cells to MMC is well documented, the relevance of FANC proteins in transducing UV-induced signals has not been established. Therefore, our data suggest a broader spectrum of sensitivities to DNA damage agents in FA-A lymphoblast cells.
Activation of JNK has been often observed in apoptosis induced by different stimuli. However, JNK is implicated in various, often opposing cellular responses resulting, for example, in inhibition or induction of apoptosis.32,33 Despite these apparent contradictions, there is a growing consensus that the effects of JNK on cellular responses depend strongly on the cell type and the context of other regulatory influences that the cell is receiving.25,34 Recently, it has been described that JNK signals cell survival in transformed B lymphoblasts, suggesting that it may contribute to the pathogenesis of some proliferative disorders.35 In line with this, the lack of JNK activation in our FA-A lymphoblast cell lines correlated with an increased susceptibility to cell death induced by DNA damage.
Because BRCA1 is involved in the activation of the JNK stress pathway,36 a potential explanation for our results is that JNK acts as a downstream effector molecule of the FANC-BRCA1 complex. Alternatively, FANCA protein could be connected with JNK through a FANC complex–independent pathway as already described for FANCC, which inhibits apoptosis in ways that do not require an intact FANC protein complex.13,14
Although FANCA has been described to associate with signaling molecules such as IκB kinase (IKK),37 most of the information about FANC-dependent signaling refers to FANCC. Thus, it would be interesting to study FANCA-binding proteins in B lymphoblast cells and other cell systems as a way to reveal novel molecular mechanisms regulated by FANCA.
We found also that UV-treated FA-A cells failed to increase the mRNA levels of Egr-1. Previous data have shown that activated JNK induces the expression of Egr-1, a transcription factor that may play a key regulatory role by linking injurious stimuli to the induction of effector molecules (ie, interleukin-2, superoxide dismutase, intercellular adhesion molecule-1, and others).27,38 Furthermore, blockade of Egr-1 expression by ligation of the B-cell receptor or treatment with antisense oligonucleotides causes growth inhibition and apoptosis in an immature B-cell lymphoma.28 Thus, Egr-1 gene and its upstream activating kinase JNK may be part of a signaling pathway triggered by FANCA in EBV-transformed B lymphoblasts. With this in mind, we investigated whether transduction of FA-A cells with the FANCA cDNA restored the responsiveness of the JNK–Egr-1 pathway to UV radiation. Interestingly, we observed a significant increase in both JNK phosphorylation activity and Egr-1 expression, which correlates with previous data showing the formation of a functional FANC protein complex in EBV-transformed B lymphoblast cells corrected with FANC cDNAs.30
In conclusion, we propose that JNK may be in a pathway triggered by a FANCA-dependent mechanism and that activation of JNK and expression of its downstream target gene Egr-1 mediate a cellular response to genotoxic agents in normal B lymphoblasts that is defective in FA cells.
Prepublished online as Blood First Edition Paper, September 4, 2003; DOI 10.1182/blood-2003-06-2091.
Supported by a grant from “Fundacion Marcelino Botin” (proyecto Terapia Genica) and by grant G03/073 from “Fondo de Investigacion Sanitaria” (programa Redes de Grupos) (J.L.F.-L. and J.A.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to H. Hanenberg for providing plasmids and J. Surralles for helpful discussion.

![Figure 1. Activation of JNK and ERK in control and FA-A lymphoblasts. (A) Lymphoblast cell lines were exposed to UV radiation, cultured for 30 minutes, and subjected to immunoprecipitation with anti-JNK. The immunoprecipitates were then incubated with GST-ATF2 and [γ-32P]ATP. GST-ATF2 phosphorylation was assessed by SDS-PAGE and autoradiography (top panel). The lysates were analyzed by Western blot with anti-JNK (bottom panel). (B) Control and FA-A lymphoblasts were incubated in the presence of MMC for the indicated times and analyzed for the activity of JNK as assessed by phosphorylation of the GST-ATF2 substrate (top panel). The lysates were analyzed by Western blot with anti-JNK (bottom panel). (C) Control and FA-A cell lines were treated with PMA for 1 hour. Anti-ERK immunoprecipitates were subjected to immune complex kinase assays with MBP as substrate (top panel). The lysates were also analyzed by Western blot with anti-ERK (bottom panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-06-2091/6/m_h80145411001.jpeg?Expires=1769388575&Signature=cYe-OmaKeSq7yd8ivLSBYRlmG8vXzmEzoPJ7IiumCfgl3pyFniUQsAGi5QYdlMqCQ2JnAqmdtwdE3VeQ~VnYye3RqMQq5sy2sg-AWZizrKuVH6GSe-H1riZkqZ5eS1dPqy25vqoQc6DniyeeeGpR28Qyg-oajABbNVbGBMRJnwCV4DKLQZ8W~hmq05~GKoaQ9JdXJ9CcS767tEer9sRvMrHZ1GWi8gY96P3u6oMHC8sGdr8U~1lj7rxHzbGLsflw0ha~fBIkWSgU75CSEULQUpAjoUrVsRnxs9AF65sfNaPMQV9Sh7mUJd0K16k33C~zX4mzGl3mMTdxHu9v8bJffg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 1. Activation of JNK and ERK in control and FA-A lymphoblasts. (A) Lymphoblast cell lines were exposed to UV radiation, cultured for 30 minutes, and subjected to immunoprecipitation with anti-JNK. The immunoprecipitates were then incubated with GST-ATF2 and [γ-32P]ATP. GST-ATF2 phosphorylation was assessed by SDS-PAGE and autoradiography (top panel). The lysates were analyzed by Western blot with anti-JNK (bottom panel). (B) Control and FA-A lymphoblasts were incubated in the presence of MMC for the indicated times and analyzed for the activity of JNK as assessed by phosphorylation of the GST-ATF2 substrate (top panel). The lysates were analyzed by Western blot with anti-JNK (bottom panel). (C) Control and FA-A cell lines were treated with PMA for 1 hour. Anti-ERK immunoprecipitates were subjected to immune complex kinase assays with MBP as substrate (top panel). The lysates were also analyzed by Western blot with anti-ERK (bottom panel).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/1/10.1182_blood-2003-06-2091/6/m_h80145411001.jpeg?Expires=1769388576&Signature=mIci5eOet5UeT5DCNdtMfuD06CE07rL~h2KuG7zFwXytHBCCwR0ng7KlSmWlYs9xwxiHB0FaqTx1WgSmHi5T9rNNqrOBMeyuDgkyA796wKh~lBo6YJW9iAIfBfyTzZ~ZQeWO3clPaZ4XMUvN8JqA9yt001o9UzTXBO05mPQKT9FDeDjAZF2EF2ncRsVigJg9TcM22ZnC0W20kbK3sJF~E9FIimBxaA63RobqpueEJMBSGLYeynGG~kI9V3az7gBvHvfQSDfLylQvv9ww6CqPuMDcEG-N3Vg5S2GP6n74jiW5c377SvbsZBNjb1pRE6cPp2CpISi529sAufzVwlLq4A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)