Abstract
Multiple myeloma (MM) is an incurable form of cancer characterized by accumulation of malignant plasma cells in the bone marrow. During the course of this disease, tumor cells cross endothelial barriers and home to the bone marrow. In latter stages, myeloma cells extravasate through blood vessels and may seed a variety of organs. Insulin-like growth factor I (IGF-I) is one of several growth factors shown to promote the growth of MM cells. In the current study, we have assessed the ability of IGF-I to serve additionally as a chemotactic factor affecting the mobility and invasive properties of these cells. Results indicate that IGF-I promotes transmigration through vascular endothelial cells and bone marrow stromal cell lines. Analysis of endogenous signaling pathways revealed that protein kinase D/protein kinase Cμ (PKD/PKCμ) and RhoA were both activated in a phosphatidylinositol 3-kinase (PI-3K)–dependent manner. Inhibition of PI-3K, PKCs, or Rho-associated kinase by pharmacologic inhibitors abrogated migration, whereas mitogen-activated protein kinase (MAPK), Akt, and p70S6 kinase inhibitors had no effect. These results suggest that IGF-I promotes myeloma cell migration by activation of PI-3K/PKCμ and PI-3K/RhoA pathways independent of Akt. The identification of IGF-I as both a proliferative and migratory factor provides a rational basis for the development of targeted therapeutic strategies directed at IGF-I in the treatment of MM.
Introduction
Multiple myeloma (MM) is an incurable B-cell neoplasia characterized by bone marrow infiltration of malignant plasma cells and accounts for approximately 1% to 2% of all human cancers. The molecular lesions and biochemical abnormalities contributing to initiation or development of this disease are poorly understood although a number of studies have suggested that nonrandom chromosomal translocations and activating mutations in certain oncogenes1 may contribute to these processes. As a complementary approach to understanding the biology of MM, a number of recent studies have focused on elucidation of factors and their associated signaling pathways that promote growth or survival of these cells. For example, interlukin-6 (IL-6) has been identified as a growth and survival factor for MM both in vitro2 and in vivo.3,4 However, it has become apparent that myeloma cells respond to a variety of factors that may play important roles in disease development.5 A number of reports have suggested that IGF-I may play such a role both in vitro6-12 and in vivo.9,13 IGF-I stimulates proliferation of both IL-6–independent and –dependent cell lines and may act synergistically with IL-6. Furthermore, IGF-I rescues MM cells from dexamethasone (Dex)–induced apoptosis.10-12
In addition to its role as a growth and survival factor for MM, IGF-I plays an important role in migration of several normal and malignant cell types including human melanoma,14 pancreatic carcinoma,15 arterial smooth muscle cells,16,17 and preosteoclasts through bone endothelial cells.18 Recently, it has been reported that IGF-I also promotes migration of murine 5T2 plasma cells.19 As transendothelial migration is a principal requirement for homing of myeloma cells to the bone marrow microenviroment20 and subsequent spread of extramedullary disease during late stages, it is of obvious importance to understand the factors promoting these events.
Given the known role of IGF-I as a proliferative and antiapoptotic factor, we have sought to determine its potential role in the migration of myeloma cells through both endothelial barriers and associated bone marrow stroma. In the current study, we demonstrate that IGF-I acts as a chemotactic factor for MM cells and promotes both migration and tissue invasion. Furthermore, we identify 2 signaling pathways regulating these processes that are distinct from those involved in proliferation and antiapoptosis.
Materials and methods
Cell lines and conditioned medium
Human MM cell lines H929, OPM-2, and MM144 were cultured in RPMI 1640 (Biofluids, Rockville, MD) as previously described.10 These lines all proliferate in response to IGF-I and the biochemical pathways associated with proliferation have been described.9,10 Human bone marrow stromal cell lines HS-27A and HS-521 were cultured in RPMI 1640 containing heat-inactivated 5% fetal bovine serum (FBS). Human umbilical vein endothelial cells (HUVECs)22 were cultured in M199 containing 5% heat-inactivated human serum and 20% fetal calf serum (FCS), heparin (25 μg/mL), 2 mM l-glutamine, penicillin (100 U/mL), and streptomycin (100 μg/mL).
Antibodies and reagents
Human recombinant IGF-I and IL-6 were purchased from PeproTech (Rocky Hill, NJ). Antibodies used in the present study with their indicated specificities were purchased from the following sources: p-Ser473–Akt, p-Ser744/748–PKD/PKCμ, p-PKC (pan), p-Ser638/641–PKCα/β, p-Thr538–PKCθ, p-Thr410/403–PKCζ/λ, PKCμ, Cell Signaling Technology (Beverly, MA); RhoA, Santa Cruz Biotechnology (Santa Cruz, CA); p-Tyr861–FAK, Sigma (Saint Louis, MO); Rac, cdc42, and FAK, Transduction Laboratories (Lexington, KY); p-Thr32–FKHRL1 and antiphosphotyrosine (4G10), Upstate Biotechnology (Lake Placid, NY); horseradish peroxidase–conjugated antimouse or antirabbit antibodies, Transduction Laboratories. Inhibitors used were as follows: PD98059, LY294002, and rapamycin, Cell Signaling Technology; Go6976, Go6983, Y27632, and IL-6–hydroxymethyl-chiro-inositol2-[(R)-2-O-octadecylcarbonate] as an Akt inhibitor (AI), CalBiochem (San Diego, CA); wortmannin, Sigma. Inhibitors were dissolved in sterile simyl sulphoxide (DMSO; Sigma), aliquoted, and stored at –20°C. All compounds were diluted to final concentrations in RPMI 1640 medium immediately before use.
MTT assay for cell proliferation
Proliferation was examined by colorimetric methyl thiazolyl tetrazolium (MTT) assay as previously described.23 Briefly, exponentially growing cells were washed with phosphate-buffered saline (PBS) and resuspended in serum-free RPMI 1640 for 2 hours. Following pretreatment with LY294002, PD98059, rapamycin, Go6976, Go6983, Y27632, or AI for 1 hour, cells were incubated at a density of 3 × 104/well in 96-well culture plates (Costar, Cambridge, MA) with or without 100 ng/mL IGF-I for 24 and 48 hours at 37°C. Ten microliters of 5 mg/mL MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazollium bromide (Sigma) was added to each well for 4 hours followed by incubation overnight in 100 μL of 10% sodium dodecyl sulfate (SDS) in 0.01 N HCl at 37°C. Optical density of plates was read on a Bio-Rad MR 5000 microplate reader (Hercules, CA) at 570 nm.
Transendothelial migration assay
IGF-I–induced MM transendothelial migration was determined using 24-well, 6.5-mm internal diameter transwell cluster plates with polycarbonate membranes (5-μM pore size) separating the 2 chambers (Corning Costar, Cambridge, MA). HUVECs (105) or bone marrow stromal cell lines HS-5 and HS-27A were grown on the insert for 24 hours to produce a confluent monolayer. IGF-I or IL-6 diluted to varying concentrations in RPMI 1640 was loaded in the lower chamber. MM cell suspensions starved for 3 hours in serum-free RPMI 1640 were loaded onto the insert (upper chamber). Plates were then incubated for 4 hours at 37°C. In experiments performed with specific inhibitors, cells not pretreated or pretreated with inhibitors for 1 hour were loaded onto inserts and incubated in the absence or presence of IGF-1 in the lower chamber for 4 hours. At the end of the incubation period, cells migrating through endothelial or bone marrow stromal cell layers into the lower chamber were harvested, stained with trypan blue, and counted under a microscope.
Western blotting
Cells (1 × 107) were grown in serum-free media for 12 hours and pretreated with indicated concentrations of inhibitors for 1 hour. Cultures were either stimulated with 100 ng/mL IGF-I for 5 minutes or indicated times, or not stimulated. Following treatment, cells were lysed as previous described10 in buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 2.5 mM EDTA (ethylenediaminetetraacetic acid), 10 mM leupeptin, 10 mM pepstatin, 10 mM aprotinin, 10 mM NaF, 10 mM sodium pyrophosphate, 1 mM 4-(2-aminoethyl)-benzenesulfony fluoride hydrochloride (AEBSF), and 250 mM Na3 VO4. After incubation for 30 minutes at 4°C, cell debris and nuclei were removed by centrifugation at 20 000g for 10 minutes at 4°C. Equal concentrations of total protein (50 μg per lane) combined with an equal volume of 2 × Laemmli buffer were boiled at 100°C for 3 minutes and then separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) followed by electrophoretic transfer to Immobilon polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membranes were blotted with specific antibodies. Detection was performed by a standard procedure using a panel of secondary horseradish peroxidase–conjugated antibodies and chemiluminescence (Pierce, Rockford, IL).
Rho family GTPase activation assay
RhoA-binding domain (RBD) and p21-binding domain (PBD) glutathione S-transferase (GST) fusion proteins were purified using GST beads from bacterial expression vector pGEX-2T transformed by hPAK-I-PBD plasmids (kindly provided by Dr Gary Bokoch, Scripps Research Institute, La Jolla, CA) and GST-RBD and GST-PBD binding assays were performed as previously described.24,25 Briefly, cells unstimulated or stimulated with IGF-I (10 ng/mL-200 ng/mL) for different times (1 min-120 min) were lysed in 50 mM Tris, pH 7.2, 1% Triton X-100, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2 for affinity precipitation of GTP-bound RhoA, and in 25 mM Tris, pH 7.5, 1% NP-40, 150 mM NaCl, 10 mM MgCl2 and a cocktail of protease inhibitors for precipitation of GTP-Rac and GTP-cdc42. Cell lysates were incubated with GST-RBD or -PBD for 50 minutes and washed 3 times. Precipitated GTP-RhoA, GTP-Rac, and GTP-cdc42 were detected by Western blotting using monoclonal anti-RhoA, -Rac, and -cdc42 antibodies.
Statistical analysis
Student t test was performed to analyze the statistical significance of differences between experimental groups using the Statview J 4.11 software statistical package (Abacus Concept, Berkeley, CA). P values less than .05 by the 2-tailed test were considered significant.
Results
IGF-I stimulates transendothelial migration by myeloma cells
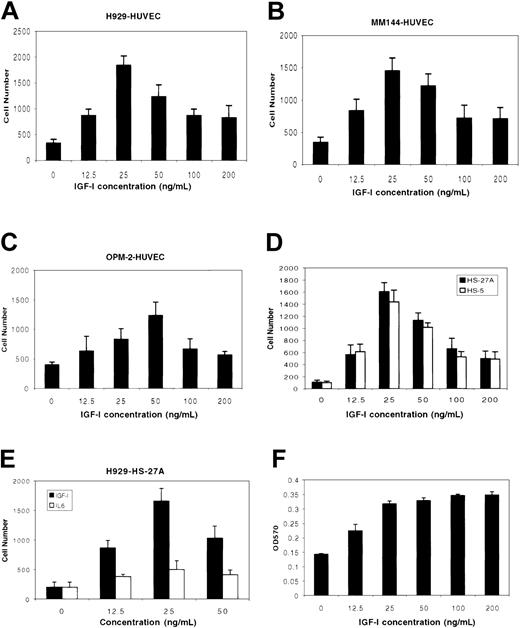
The effect of IGF-I on migration and tissue invasion by MM cell lines was examined in a transwell migration assay. IGF-I was added to the lower chamber of transwell plates containing micropore filters precoated with HUVECs, after which MM cells were added to the upper chamber. As shown in Figure 1, IGF-I stimulated migration of MM cell lines H929 (Figure 1A), MM144 (Figure 1B), and OPM-2 (Figure 1C) with a bell-shaped, dose-dependent curve that has been reported to be a classic chemotatic response.16,26 Maximal responses were seen at 25 ng/mL for H929 and MM144 cells (P < .01), and 50 ng/mL for OPM-2 (P < .05) compared with nonstimulated controls. At higher IGF-I concentrations (100 ng/mL to 200 ng/mL), responses were considerably diminished. In similar experiments, we examined IGF-I–mediated migration/invasion using 2 bone marrow–derived stromal cells, HS-27A (epithelial-like) and HS-5 (fibroblast-like). IGF-I induced transmigration of both cell lines comparable to that seen with HUVECs (Figure 1D). IL-6, another important growth factor for MM cells, did not significantly promote invasion of H929 (Figure 1E) or MM144 (not shown) through the HS-27A line. Interestingly, and in contrast to the decreased migratory responses seen at higher IGF-I concentrations, MM cells evidenced maximal proliferative responses in the 50 ng/mL-to-100 ng/mL range and this level of proliferation was sustained at the higher concentrations tested (Figure 1F). These results indicate that a threshold of IGF-I concentration affects the ability of these cells to migrate, but does not similarly impact on proliferation.
IGF-1 induces migration and invasion by MM cells. MM cells H929 (A, D), MM144 (B), and OPM-2 (C) starved in serum-free medium for 3 hours were plated on polycarbonate pore membranes (5-μM pore size) on which HUVECs (A-C) or bone marrow stromal cell lines (D-E) were previously grown for 24 hours to form a continuous monolayer. MM cells were exposed to IGF-I (12.5 ng/mL to 200 ng/mL) or IL-6 (12.5 ng/mL to 50 ng/mL; E) added to the lower chamber. After 4 hours of incubation, cells in the lower chamber were harvested and counted microscopically following trypan blue staining. Results are representative of 3 independent experiments. H929 cells (F) starved in serum-free medium for 3 hours were incubated in the presence or absence of IGF-I as indicated. After 48 hours, cells were subjected to MTT assay. Results are shown as means ± SE (n = 4) and are representative of 3 separate experiments.
IGF-1 induces migration and invasion by MM cells. MM cells H929 (A, D), MM144 (B), and OPM-2 (C) starved in serum-free medium for 3 hours were plated on polycarbonate pore membranes (5-μM pore size) on which HUVECs (A-C) or bone marrow stromal cell lines (D-E) were previously grown for 24 hours to form a continuous monolayer. MM cells were exposed to IGF-I (12.5 ng/mL to 200 ng/mL) or IL-6 (12.5 ng/mL to 50 ng/mL; E) added to the lower chamber. After 4 hours of incubation, cells in the lower chamber were harvested and counted microscopically following trypan blue staining. Results are representative of 3 independent experiments. H929 cells (F) starved in serum-free medium for 3 hours were incubated in the presence or absence of IGF-I as indicated. After 48 hours, cells were subjected to MTT assay. Results are shown as means ± SE (n = 4) and are representative of 3 separate experiments.
IGF-I induces tyrosine phosphorylation of FAK
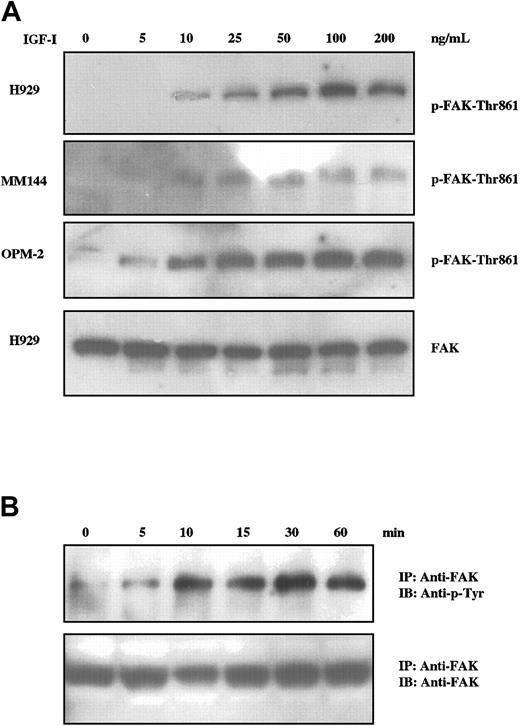
The focal adhesion kinase p125FAK (FAK) is a widely expressed cytosolic tyrosine kinase activated by a number of integrins and some growth factors and is a candidate for an important element in cell adhesion and migration.27,28 Additionally, FAK activation has been associated with IGF-I–induced motility of neuronal cells.29,30 We therefore assessed the status of FAK activation in IGF-I–treated MM cells by Western blot analysis using an antibody specific for phosphorylation of FAK at tyrosine-861 (Figure 2A). IGF-I stimulation of H929, MM144, and OPM-2 cells resulted in phosphorylation of FAK at tyrosine-861 in a dose-dependent manner for all cell lines tested. Maximal phosphorylation was observed between 50 ng/mL and 100 ng/mL, varying with individual lines. Time-course studies (Figure 2B) revealed detectable FAK phosphorylation at 5 minutes with peak levels being maintained from 10 minutes to at least 60 minutes. These experiments were performed by immunoprecipitation and blotting with antiphosphotyrosine, as FAK can be phosphorylated at multiple sites. Whereas IGF-I induces tyrosine phosphorylation of FAK in a concentration- and time-dependent manner, it is unlikely that FAK activation alone is sufficient for IGF-I–mediated transmigration (see “Dissection of proliferative and migratory pathways” and “Discussion”).
IGF-I induces tyrosine phosphorylation of FAK in MM cells. MM cells starved for 12 hours were not stimulated or stimulated with varying concentrations of IGF-I (5 ng/mL to 200 ng/mL; A) for 10 minutes or with 100 ng/mL for times indicated (B). Cell lysates were subjected to SDS-PAGE (A) or immunoprecipitated with anti-FAK and subjected to SDS-PAGE (B), transferred to membranes, and blotted with indicated antibodies. Membranes in panel A were stripped and reprobed with anti-FAK as controls for loading. Control blot is shown only for H929, but comparable results were observed with all lines.
IGF-I induces tyrosine phosphorylation of FAK in MM cells. MM cells starved for 12 hours were not stimulated or stimulated with varying concentrations of IGF-I (5 ng/mL to 200 ng/mL; A) for 10 minutes or with 100 ng/mL for times indicated (B). Cell lysates were subjected to SDS-PAGE (A) or immunoprecipitated with anti-FAK and subjected to SDS-PAGE (B), transferred to membranes, and blotted with indicated antibodies. Membranes in panel A were stripped and reprobed with anti-FAK as controls for loading. Control blot is shown only for H929, but comparable results were observed with all lines.
IGF-I induces phosphorylation of PKD/PKCμ
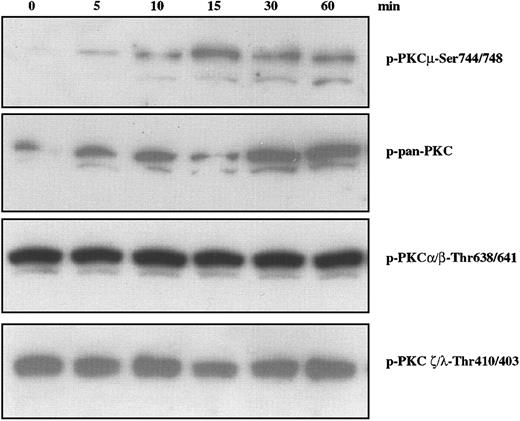
Several pathways have been implicated in migratory properties associated with a variety of cell types. Among these is the phosphatidylinositol 3-kinase (PI-3K) downstream cascade involving 3-phosphoinositide–dependent protein kinase (PDK)–1 and protein kinases C (PKCs).31 PKCs have been implicated in a wide range of biologic responses including cytoskeletal changes, cell adhesion, and motility.32,33 Based on their structures and cofactor requirements, 13 isoenzymes of intracellular serine/threonine kinase PKCs have been classified into 3 major groups: Ca2+-dependent, classic PKCs (cPKCs); Ca2+-independent, novel PKCs (nPKCs); and Ca2+- and lipid-independent atypical PKCs (aPKCs). A fourth subgroup contains PKCμ (also known as PKD), a serine/threonine kinase34,35 that has distinct structural and enzymatic properties, is ubiquitously expressed, and is activated by lipids and platelet-derived growth factor (PDGF).36 We examined the status of PKC family members using antibodies specific for phosphorylation of residues associated with activation. As shown in Figure 3, IGF-I stimulation of H929 cells leads to phosphorylation of PKD/PKCμ as detected by antibody specific for p-Ser744/748 (Figure 3, upper band). The lower band in Figure 3 is assumed to result from a cross reaction with nonphosphorylated PKCμ and is consistent with the lower band seen with a p-pan–PKC reagent (second panel). Phosphorylation is observed by 5 minutes, peaks at 15 minutes, and remains for at least 60 minutes. IGF-I treatment increases phosphorylation detected by a polyclonal pan-PKC which recognizes the conserved carboxy terminal Ser-660 present on most PKCs with the exception of aPKCs. In contrast, IGF-I treatment did not alter the phosphorylation of PKCα/βII at Thr638/641 or PKC ζ/λ at residues Thr410/403, which are constitutively phosphorylated in H929 cells. Phosphorylation of PKCθ was similarly unaffected (data not shown). Loading of all gels was uniform as assessed by stripping the blots and reprobing with pan-PKC (not shown). Thus, IGF-1 stimulated phosphorylation of at least one PKC family member, PKD/PKCμ, in a time-dependent manner.
Effect of IGF-I on phosphorylation of PKC family members in H929 cells. H929 cells starved for 12 hours in serum-free medium were not stimulated or stimulated with 100 ng/mL IGF-I for varying times. Cell lysates were subjected to SDS-PAGE, transferred to membranes, and blotted with indicated antibodies.
Effect of IGF-I on phosphorylation of PKC family members in H929 cells. H929 cells starved for 12 hours in serum-free medium were not stimulated or stimulated with 100 ng/mL IGF-I for varying times. Cell lysates were subjected to SDS-PAGE, transferred to membranes, and blotted with indicated antibodies.
IGF-I induces formation of GTP-RhoA
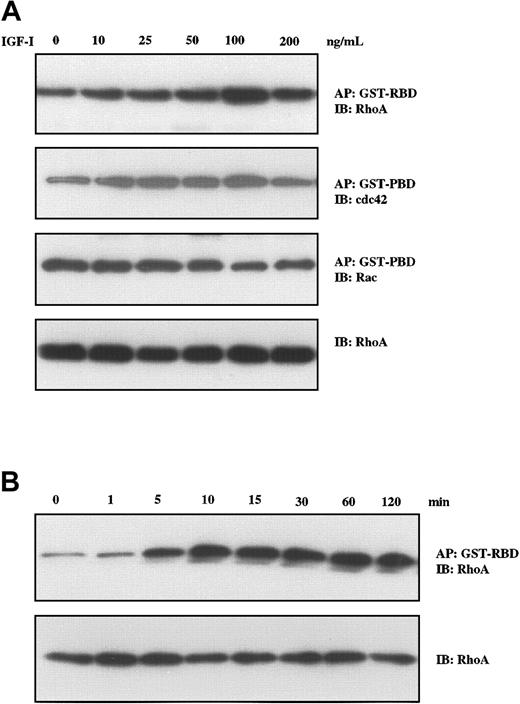
We have recently described the critical role of RhoA in Wnt–induced morphologic changes in MM cells.37 As members of this family of proteins with guanosine triphosphatase (GTPase) activity including RhoA, Rac, and cdc42 have been implicated in changes in cell morphology, formation of stress fibers, focal adhesion,38 and cell motility,39 we next evaluated their status following IGF-I stimulation in a series of pull-down experiments. A GST-RBD construct was used for binding GTP-RhoA, and GST-PBD was used for binding GTP-Rac and GTP-cdc42. As seen in Figure 4A, IGF-I treatment led to increased levels of GTP-RhoA and GTP-cdc42, whereas GTP-Rac levels were unchanged. Increases were seen in the concentration range from 10 ng/mL to 100 ng/mL. Time-course studies (Figure 4B) revealed that increases in GTP-RhoA were observed at 5 minutes, reached maximum levels at 10 minutes, and remained elevated for at least 120 minutes.
IGF-I induces Rho-GTP formation in H929 cells. H929 cells were starved in serum-free medium for 12 hours and were not stimulated or stimulated with varying concentrations of IGF-I (10 ng/mL to 200 ng/ml; A) or with 100 ng/mL IGF-1 for different times (5 min to 120 min; B). Fresh lysates were then affinity precipitated (AP) using GST-RBD glutathione beads or GST-PBD glutathione beads. After washing 3 times, the bound proteins were eluted and analyzed by Western blotting using RhoA-, Rac-, and cdc42-specific antibodies. The same lysates were directly subjected to SDS-PAGE, transferred to membranes, and blotted with anti-Rho antibodies as a control for loading.
IGF-I induces Rho-GTP formation in H929 cells. H929 cells were starved in serum-free medium for 12 hours and were not stimulated or stimulated with varying concentrations of IGF-I (10 ng/mL to 200 ng/ml; A) or with 100 ng/mL IGF-1 for different times (5 min to 120 min; B). Fresh lysates were then affinity precipitated (AP) using GST-RBD glutathione beads or GST-PBD glutathione beads. After washing 3 times, the bound proteins were eluted and analyzed by Western blotting using RhoA-, Rac-, and cdc42-specific antibodies. The same lysates were directly subjected to SDS-PAGE, transferred to membranes, and blotted with anti-Rho antibodies as a control for loading.
PI-3K inhibitors block IGF-I–stimulated phosphorylation of PDK/PKCμ and GTP-RhoA formation
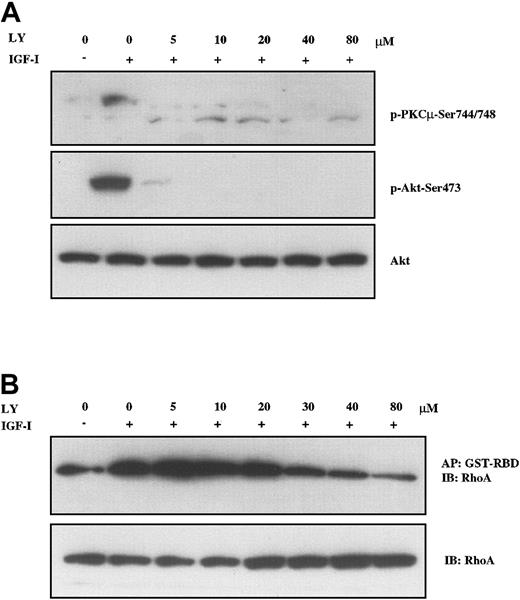
Having identified FAK, PKD/PKCμ, and RhoA as downstream targets in IGF-I signaling, we next sought to determine which pathways were involved in their activation. To examine the PI-3K pathway, we used 2 structurally distinct inhibitors, LY294002 and wortmannin, to block PI-3K activation. The effect of these compounds in H929 cells was examined by analyzing the phosphorylation of PKD/PKCμ and formation of GTP-RhoA. As shown in Figure 5A, pretreatment with LY294002 at concentrations as low as 5 μM led to nearly complete inhibition of PKD/PKCμ phosphorylation at positions Ser744/748 (upper band). IGF-I–induced phosphorylation of Akt at Ser473 was included as a positive control for LY294002 and was similarly inhibited. IGF-I–induced formation of active GTP-RhoA was also inhibited by LY294002 in a dose-dependent manner but higher concentrations were required (Figure 5B). Inhibition was observed starting at 20 μM with a maximal effect seen at 80 μM. Wortmannin produced a similar inhibition on IGF-I–stimulated phosphorylation of PKD/PKCμ and GTP-RhoA formation (data not shown). Neither of these compounds inhibited FAK phosphorylation. These results indicate that IGF-I mediates activation of PKD/PKCμ and Rho-kinase via PI-3K.
PI-3K inhibition blocks IGF-I–mediated activation of PKC and RhoA. H929 cells starved in serum-free medium and then pretreated with indicated concentrations (5 μM to 80 μM) of PI-3K inhibitor LY294002 for 1 hour were not stimulated or stimulated with 100 ng/mL IGF-I for 10 minutes. (A) Cell lysates were directly subjected to SDS-PAGE or (B) precipitated (AP) with GST-RBD glutathione beads and then subjected to SDS-PAGE, transferred to membranes, and blotted with indicated antibodies. Blot in panel A was stripped and reprobed with anti-Akt.
PI-3K inhibition blocks IGF-I–mediated activation of PKC and RhoA. H929 cells starved in serum-free medium and then pretreated with indicated concentrations (5 μM to 80 μM) of PI-3K inhibitor LY294002 for 1 hour were not stimulated or stimulated with 100 ng/mL IGF-I for 10 minutes. (A) Cell lysates were directly subjected to SDS-PAGE or (B) precipitated (AP) with GST-RBD glutathione beads and then subjected to SDS-PAGE, transferred to membranes, and blotted with indicated antibodies. Blot in panel A was stripped and reprobed with anti-Akt.
Dissection of proliferative and migratory pathways
The above data and previous studies have now identified a number of downstream targets in the PI-3K pathway, including Akt, Forkhead family members, p70S6 kinase, PKD/PKCμ, and RhoA. How these various elements are involved in regulation of proliferation and migration/invasion of MM cells is not clear. We, therefore, sought to dissect the function of these downstream targets using a series of kinase inhibitors and employing an MTT assay as an indicator of proliferation and transendothelial migration as an indicator of migration/invasion. The following inhibitors were evaluated: AI as an inhibitor of Akt; LY294002 as an inhibitor of PI-3K; Y27632 as an inhibitor of the Rho-associated kinase (ROCK); PD98059 as a MAPK inhibitor; rapamycin as an inhibitor of mTor in the p70S6 pathway branch; Go6976 as a broad spectrum PKC inhibitor; and Go6983 as an inhibitor which blocks all PKCs with the exception of PKCμ.
We first assessed the ability of AI40 to block signaling at the level of Akt as opposed to other points in the PI-3K pathway. As seen in Figure 6A, AI does not inhibit phosphorylation of Akt, but does block its ability to subsequently phosphorylate the Akt downstream target FKHRL1. Importantly, even at the highest concentrations tested, AI failed to inhibit RhoA activation, indicating RhoA is not downstream of Akt. Thus, AI inhibits Akt activity without interfering with the ability of PI-3K to phosphorylate Akt or, presumably, other PI-3K targets. We then proceeded to evaluate the effects of the various inhibitors described in the previous paragraph on IGF-I–mediated proliferation (Figure 6B). Treatment of myeloma cells with IGF-I for 24 and 48 hours resulted in significant proliferation (P < .001). This proliferation was blocked by pretreatment with either PI-3K inhibitor LY294002 (10 μM) or Akt inhibitor AI (20 μM). Pretreatment of cells with rapamycin (10 μM), an inhibitor of p70S6 kinase, partly abrogated IGF-I–promoted proliferation as previously reported.10 In contrast, PKC inhibitors Go6976 (0.5 μM) and Go6983 (0.5 μM), MAPK inhibitor PD98059 (20 μM), and Rho-associated kinase inhibitor Y27632 (10 μM) did not significantly inhibit IGF-I–induced proliferation. Taken together, these data indicate that IGF-I–mediated proliferation is dependent on PI3K/Akt and downstream Akt targets which affect both cell division and antiapoptosis, but not PKC or RhoA pathways.
IGF-I–stimulated proliferation of H929 cells occurs through the PI-3K/Akt pathway and does not involve PKCs or RhoA. (A) H929 cells starved for 12 hours in serum-free medium were pretreated with indicated concentrations (2.5 μM to 40 μM) of the Akt inhibitor AI and either stimulated or not stimulated with 100 ng/mL IGF-I for 10 minutes. Cell lysates were subjected to SDS-PAGE or precipitated (AP) with GST-RBD glutathione beads and then subjected to SDS-PAGE, transferred to membranes, and blotted with indicated antibodies. (B) H929 cells were grown in medium with or without IGF-I and the following inhibitors: PI-3K inhibitor LY294002 (LY, 10 μM); Akt inhibitor AI (20 μM); Rho-associated kinase inhibitor Y27632 (10 μM); MAPK inhibitor PD98059 (PD; 20 μM) p70S6 kinase inhibitor rapamycin (Rap; 10 μM); PKC inhibitor Go6983 (500 nM) and PKC inhibitor Go6976 (500 nM) for 24 and 48 hours. Cell proliferation was measured by MTT assay. Results are shown as means ± SE (n = 4) and are representative of 3 independent experiments. * indicates P < .01; **, P < .001.
IGF-I–stimulated proliferation of H929 cells occurs through the PI-3K/Akt pathway and does not involve PKCs or RhoA. (A) H929 cells starved for 12 hours in serum-free medium were pretreated with indicated concentrations (2.5 μM to 40 μM) of the Akt inhibitor AI and either stimulated or not stimulated with 100 ng/mL IGF-I for 10 minutes. Cell lysates were subjected to SDS-PAGE or precipitated (AP) with GST-RBD glutathione beads and then subjected to SDS-PAGE, transferred to membranes, and blotted with indicated antibodies. (B) H929 cells were grown in medium with or without IGF-I and the following inhibitors: PI-3K inhibitor LY294002 (LY, 10 μM); Akt inhibitor AI (20 μM); Rho-associated kinase inhibitor Y27632 (10 μM); MAPK inhibitor PD98059 (PD; 20 μM) p70S6 kinase inhibitor rapamycin (Rap; 10 μM); PKC inhibitor Go6983 (500 nM) and PKC inhibitor Go6976 (500 nM) for 24 and 48 hours. Cell proliferation was measured by MTT assay. Results are shown as means ± SE (n = 4) and are representative of 3 independent experiments. * indicates P < .01; **, P < .001.
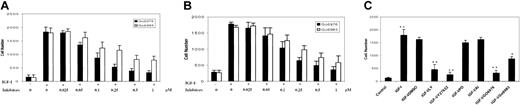
Having demonstrated that PKCμ and RhoA were activated by IGF-I but not involved in proliferation, we next sought to assess their potential roles in transmigration. For these experiments, we took advantage of the 2 PKC inhibitors Go6976 and Go6983, which selectively inhibit PKC isoenzyme activity.41 The indolocarbazole Go6976 inhibits cPKCs (α, β, γ), nPKCs (δ, ϵ, η, θ), and PKCμ, whereas the bisindolylmaleimide Go6983 suppresses kinase activity of PKC isoenzymes from 3 of the subgroups, but does not effectively inhibit PKCμ kinase activity. Thus, Go6983 can be used to differentiate PKCμ from other PKC isoenzymes. Pretreatment of H929 cells with Go6976 or Go6983 for 1 hour inhibited migration through the HS-27 cell line in a dose-dependent manner with maximal effect at 0.5 μM to 1 μM (Figure 7A). The effect with Go6983 was only partial with approximately 50% inhibition observed even at maximal concentration. Similar results were obtained in IGF-I–induced trans–HS-5 stromal cell migration (Figure 7B). Taken together, these results suggest that IGF-I–mediated migration is at least partly PKC dependent and that PKCμ is one member of this family facilitating the migratory process.
IGF-I–induced migration is blocked by inhibition of the PKC and RhoA pathways. H929 cells pretreated with increasing concentrations (0.025 μM to 1 μM) of PKC inhibitors were plated on polycarbonate pore membranes (5-μM pore size) on which 2 bone marrow stromal cell lines, HS-27A (A) or HS-5 (B), were pregrown for 24 hours. (C) H929 cells pretreated with the indicated inhibitors (Figure 6B) were plated on HS-27A layered membranes as in panel A. Medium containing IGF-I and the indicated inhibitors was then added to the bottom chambers and plates were incubated for 4 hours. Cells in the lower chamber were harvested and counted following trypan blue staining. Results are shown as means ± SE (n = 3) and are representative of 3 independent experiments. * indicates P < .01; **, P < .001.
IGF-I–induced migration is blocked by inhibition of the PKC and RhoA pathways. H929 cells pretreated with increasing concentrations (0.025 μM to 1 μM) of PKC inhibitors were plated on polycarbonate pore membranes (5-μM pore size) on which 2 bone marrow stromal cell lines, HS-27A (A) or HS-5 (B), were pregrown for 24 hours. (C) H929 cells pretreated with the indicated inhibitors (Figure 6B) were plated on HS-27A layered membranes as in panel A. Medium containing IGF-I and the indicated inhibitors was then added to the bottom chambers and plates were incubated for 4 hours. Cells in the lower chamber were harvested and counted following trypan blue staining. Results are shown as means ± SE (n = 3) and are representative of 3 independent experiments. * indicates P < .01; **, P < .001.
As IGF-I stimulation resulted in formation of GTP-RhoA (Figure 4), we next investigated the possible involvement of the Rho-kinase and other cascades in migration/invasion. For these experiments, the same inhibitors as used to assess proliferation (Figure 6B) were evaluated at the same concentrations. As seen in Figure 7C, in the presence of the PI-3K inhibitor LY294002, transendothelial migration was reduced by approximately 70%. Similarly, the Rho-associated kinase inhibitor Y27632 reduced migration by 80% and the PKC inhibitors Go6976 and Go6983 by 75% and 47%, respectively. In contrast, the Akt inhibitor (AI) and MAPK inhibitor (PD98059) did not show any significant effect on IGF-I–induced migration. These results strongly suggest that IGF-I–mediated migration is largely regulated by the PI-3K/PKC and PI-3K/RhoA pathways, but does not involve Akt or the MAPK cascade. Furthermore, Western blot analysis (not shown) revealed that none of these inhibitors blocked FAK phosphorylation, indicating that FAK is not a downstream element in any of the pathways analyzed and that FAK activation is not sufficient for IGF-I–mediated migration/invasion.
Discussion
For the past several years, considerable effort has been directed toward identifying the biochemical lesions and signaling pathways that contribute to either survival or growth of myeloma cells. Among growth factors that fulfill this role is IGF-I, which several laboratories have shown acts as both a proliferative and antiapoptotic factor in vitro6-8,10-12 and in vivo.9,13 One aspect of myeloma biology that has received relatively little attention is that of the signals and biochemical pathways that contribute to the important properties relating to myeloma cell motility and ability to invade other tissues. In the initial stage of this disease, circulating myeloma cells specifically home to the bone marrow by processes wherein they must bind to the surface of blood vessel endothelium and penetrate the endothelial membrane to reach the bone marrow compartment. Once in the bone marrow, these cells must interact with, and likely move through, bone marrow stromal cells from primary to secondary sites. Finally, in late-stage disease myeloma cells again extravasate through blood vessels and invade multiple tissues to produce disseminated disease. As IGF-I has been shown to act as a chemotactic factor for neuronal cells,29,30 vascular smooth muscle,16 and murine plasma cells,19 we have, herein, investigated its ability to act as an inducer of migration and invasion in MM cells.
Initial studies (Figure 1) revealed that IGF-I readily induced migration of MM cells and facilitated invasion of vascular endothelial monolayers. Similar results were obtained using 2 bone marrow stromal cell lines: HS-5, characterized as fibroblastoid; and HS-27A, characterized as epithelioid.21 IL-6, a second prominent MM growth factor, had no significant effect in this assay. Interestingly, the response curve for IGF-I–mediated migration is bell shaped wherein migration is promoted at lower concentrations (< 50 ng/mL) and inhibited at higher concentrations. In contrast, proliferation is greatest at higher concentrations and is not inhibited as concentrations increase. These results raise the currently unanswered question of how a cell decides whether to migrate or proliferate upon exposure to IGF-I. Although it should be cautioned that migration and proliferation assays are very different especially in terms of time (4 hours versus 24-48 hours), it is nonetheless interesting to speculate that there might exist a threshold concentration which, to some extent, differentiates migratory from proliferative responses. Since migration may be associated with lower IGF-I concentrations, the possibility is raised that IGF-I gradients may have significant implications for myeloma biology. As IGF-I is produced by a number of cell types in the bone marrow microenvironment including stromal cells, osteoblasts, and endothelial cells,42-44 the potential exists to establish gradients that could initially facilitate the migration of myeloma cells to areas where local concentrations are higher, thus shifting the IGF-I effect to that of survival and proliferation.
Having demonstrated that IGF-I promotes migration/invasion by MM cells, a series of experiments was performed to identify downstream signaling pathways participating in this response. Western blotting of elements previously found to be associated with cell movement revealed activation of members of 2 such families, PKCs and small GTPases. Among the PKCs, only the PKD/PKCμ isoenzyme evidenced a change in phosphorylation state (Figure 3). Other PKC isoenzymes including members of the classic, novel, and atypical subgroups were expressed and constitutively phosphorylated, but appeared unchanged following IGF-I treatment. Analysis of the Rho family of GTPases demonstrated activation of both RhoA and cdc42 (Figure 4). As previous studies had demonstrated that the RhoA pathway was responsible for Wnt–induced morphologic changes in myeloma cells,37 we focused on the roles of PKCμ and RhoA in migration. Inhibition studies revealed that both PKCμ phosphorylation and RhoA activation were blocked by the PI-3K inhibitor LY294002 (Figure 5), suggesting that both are downstream elements in this pathway. Inhibition of PKCs did not affect RhoA activation and RhoA inhibition did not affect PKCμ phosphorylation (not shown), indicating that these are separate branches downstream of PI-3K.
Previous studies10,11,45 have demonstrated that the PI-3K pathway is the major regulator of IGF-I–mediated proliferative and antiapoptotic effects. Akt appears to be a critical element in this cascade, as it is responsible for subsequent activation of the Forkhead family of transcription factors and p70S6 kinase, both of which have been shown to play significant roles in myeloma cell growth. Having demonstrated that PKCμ and RhoA were also activated through PI-3K, a series of kinase inhibitors were used to determine whether these effectors were downstream of Akt and their respective roles in proliferation or migration. As previously reported10 and confirmed in Figure 6B, inhibition of PI-3K completely blocks proliferation, and inhibition of p70S6 kinase produces a partial effect. The Akt inhibitor AI was found to prevent proliferation to the same extent as the PI-3K inhibitor LY. This result provides biologic confirmation of the importance of Akt in proliferation in agreement with a previous report using constitutively activated Akt in MM cells to show resistance to apoptosis induced by Apo2L/TRAIL and doxorubicin.11 However, neither the Rho-associated kinase inhibitor Y27632 nor 2 PKC inhibitors significantly reduced proliferation, indicating a lack of involvement in this process.
The same series of inhibitors were also employed in transmigration assays (Figure 7) to elucidate pathway contributions to motility and invasion. These experiments revealed that treatment with the PI-3K inhibitor LY or the PKC inhibitor Go6976 leads to nearly complete abrogation of migration, whereas Go6983 produces only a partial effect. Go6976 inhibits cPKCs (α, β, γ), nPKCs (δ, ϵ, η, θ), and PKCμ, whereas Go6983 suppresses kinase activity of PKC isoenzymes from the 3 major subgroups but does not effectively inhibit PKCμ kinase activity.41,46 These observations suggest that multiple PKCs are requisite for IGF-I–mediated migration/invasion and that PKCμ is a critical participant in this process. PKCμ activation and its role in myeloma cell migration has not been previously described, although PKC isoenzymes have been associated with cytoskeleton reorganization and increase in motility of several cell types. For example, PKCα mediates the vascular endothelial growth factor and beta-1 integrin–promoted migration of MM cells47 and blockade of activation of PKCα inhibits melanoma cell metastasis.48 Of relevance to the present observation is the finding that PKCμ has been shown to mediate Wnt-5A–induced melanoma cell motility and invasion.49
Similar to PKC inhibition, complete blockage of migration was obtained with an inhibitor to the downstream target of GTP-RhoA, Rho-associated kinase (ROCK). The GTP forms of RhoA, Rac, and cdc42 are the active states of these small GTPases that regulate specific morphologic change in the actin microfilament-based cytoskeleton. Reorganization of this cytoskeleton facilitates the dynamic changes necessary for cellular adhesion, motility, and tumor metastasis. RhoA has been shown to regulate stress fiber formation and focal adhesions,50 whereas cdc42 is associated with induced peripheral actin microspikes (filopodia)51 and Rac1 with membrane ruffling (lamellipodia).52 Activation of these GTPases is required for PDGF-induced cell migration53 and RhoA induces metastasis in vivo.54 Although the present study clearly defines an important role for RhoA in myeloma cell migration, the potential involvement of cdc42 (which is also activated) in this process remains to be determined. In contrast, neither the MAPK inhibitor PD98059 nor the Akt inhibitor AI had any effect on migration.
Several important conclusions can be drawn from the above observations. IGF-I is clearly a potent inducer of myeloma cell migration/invasion, suggesting a possibly critical role in both bone marrow homing in early-stage disease and metastasis in latter stages. Whereas IGF-I–mediated proliferation is largely regulated by the PI-3K/Akt pathway, migration does not involve Akt, but requires activation of PI-3K/PKC and PI-3K/RhoA. Inhibition of either the PKC or Rho pathways largely abolishes migration, suggesting that neither alone is sufficient for migration and that activation of both is likely to be required for manifestation of this phenotype. Activation of FAK is not sufficient to induce migration, although it may be necessary, and FAK activation is PI-3K independent. It should be cautioned that the above studies have been limited to cell lines that are likely to be representative of late-stage disease. It will clearly be necessary to perform similar studies on patient samples and to compare the responses in earlyversus late-stage disease. The elucidation of IGF-I in a new role (in addition to its proliferative effects) in the pathophysiology of multiple myeloma further indicates that attempts to therapeutically target this pathway may prove to be of significant benefit in the treatment of this disease.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-06-2066.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.