Abstract
Bcl-2 protein expression has been associated with poor prognosis in patients with noncutaneous diffuse large B-cell lymphomas. In primary cutaneous large B-cell lymphomas, the location on the leg, the round-cell morphology defined as the predominance of centroblasts and immunoblasts over large centrocytes, and multiple skin lesions were identified as adverse prognostic factors. The prognostic value of bcl-2 protein expression has not been studied in large series of patients. We evaluated 80 primary cutaneous large B-cell lymphomas collected by the French Study Group on Cutaneous Lymphomas. The prognostic value of age, sex, number of lesions, cutaneous extent, location, serum lactate dehydrogenase (LDH) level, B symptoms, morphology, and bcl-2 protein expression was studied. The overall 5-year specific survival rate was 65%. In univariate analysis, advanced age, multiple skin lesions (n = 48), location on the leg (n = 25), round-cell morphology (n = 32), and bcl-2 expression (n = 39) were significantly related to death from lymphoma. In multivariate analysis, bcl-2 expression (P = .0003), multiple skin lesions (P = .004), and age remained independent prognostic factors. The 5-year specific survival rates in bcl-2–positive and bcl-2–negative patients were 41% and 89%, respectively (P < .0001). A new prognostic classification of primary cutaneous B-cell lymphoma should be based primarily on bcl-2 protein expression rather than the location of skin lesions.
Introduction
Primary cutaneous B-cell lymphomas (PCBCLs) comprise approximately 20% of cutaneous lymphomas.1,2 Whereas most PCBCLs have an indolent clinical course, a variable proportion of patients with these lymphomas eventually dies of their disease.2-16
PCBCLs presenting with skin tumors of the leg were first recognized as having a poorer prognosis.4,8 These tumors of the leg were consistently composed of a majority of large B cells (centroblasts, large centrocytes, and immunoblasts in variable proportions). The term “primary cutaneous large B-cell lymphoma of the leg” (PCLBCL-leg) was used.2,8 In the European Organization for Research and Treatment of Cancer (EORTC) classification for primary cutaneous lymphomas,2 these PCLBCLs-leg were classified as a separate clinical entity of intermediate prognosis. Using the Revised European-American (REAL) classification, they were classified as diffuse large B-cell lymphomas.17
Patients with skin tumors at other sites (mostly the head or the trunk) that were composed of various proportions of small centrocytes, large centrocytes, centroblasts, and rarely immunoblasts appeared to have a more favorable prognosis.2,4,5,11,15 In the EORTC classification they were classified as primary cutaneous follicle center–cell lymphomas (PCFCCLs) and considered as indolent lymphomas irrespective of their cytologic features. These PCFCCLs included both small-cell and large-cell lymphomas. According to the REAL classification, they were classified as either follicle center–cell lymphomas or diffuse large B-cell lymphomas.
Although the EORTC classification highly contributed to a more uniform diagnosis and management of patients with primary cutaneous lymphomas, this subdivision of PCBCLs has been much disputed.5,18-24 The controversy particularly concerned PCBCLs with a predominance of large cells (primary cutaneous large B-cell lymphomas, PCLBCLs) since they were divided into 2 different categories (PCLBCL-leg and PCFCCL), primarily on the basis of the site of presentation (leg versus other sites).2,25 Indeed, it was found that PCLBCL-leg differed from PCFCCL not only by anatomic location and overall prognosis, but also by other characteristics including a higher age of onset and a more frequent bcl-2 protein expression.8,26 Therefore, it could be suspected that factors other than location might dictate the prognosis of PCLBCL.
In a previous study,13 we attempted to identify independent prognostic factors in a large series of PCLBCLs, including both PCLBCL-leg and PCFCCL as defined in the EORTC classification. Bcl-2 protein expression was not included in this prognostic analysis. We found that a round-cell morphology, the location on the leg, and multiple skin lesions were independent predictive parameters for survival. The round-cell morphology was defined as the predominance of centroblasts and/or immunoblasts and opposed to the cleaved-cell morphology in which large centrocytes predominated. This morphologic distinction appeared to be the strongest prognostic factor. However, its reproducibility among pathologists was insufficient.
With the exception of this study, prognostic analyses of patients with PCLBCL were performed only in small groups of patients.12,16,27,28
Bcl-2 protein is an antiapoptotic protein whose overexpression has been found to be associated with a poor prognosis in noncutaneous diffuse large B-cell lymphomas.29-32 The prognostic value of bcl-2 protein expression has not been studied previously in a large series of patients with PCLBCL.
In the present multicenter study, we analyzed the prognostic value of bcl-2 protein expression together with other potential prognostic factors in patients with PCLBCLs.
Patients, materials, and methods
Inclusion criteria
Clinical and histologic data on all patients included in the registry of the French Study Group on Cutaneous Lymphomas (FSGCL) for a diagnosis of B-cell lymphoma were reviewed. Cases were selected for analysis if they met the following criteria: (1) diagnosis of cutaneous B-cell lymphoma between January 1, 1984, and September 30, 2001; (2) absence of extracutaneous disease detected by a comprehensive staging procedure at diagnosis; (3) histologic and immunophenotypic features showing a majority (ie, > 50%) of large cells among neoplastic B cells.
Staging procedure at diagnosis included in all cases physical examination; routine laboratory tests; chest radiograph or thoracic computed tomographic (CT) scan; abdominal ultrasound tomography or abdominal CT scan; and bone marrow aspirate (7.5% of cases), bone marrow biopsy (25%), or both (67.5%). Due to incomplete staging procedure at diagnosis, 6 cases—most of whom were elderly patients who had no bone marrow examination—were excluded from the study. There were 14 cases excluded because of a positive initial staging. One patient was excluded because he had a previous history of nodal B-cell lymphoma, and 7 patients were excluded since no material was available for the histologic review.
Included in the study were 80 patients with a PCLBCL. Of the patients, 33 had been included in a previous study.13
Histologic review and bcl-2 study
Skin biopsies were reviewed by a panel of 3 expert pathologists (T.P., P.C., J.W.) from different centers, without previous knowledge of the clinical data.
For each case, hematoxylin-eosin slides and CD3 and CD20 stainings were studied. Histologic subclassification was essentially based on the relative proportions of immunoblasts, centroblasts (large noncleaved cells), and large centrocytes (large cleaved cells), including multilobated cells, as previously described.13 Cases including more than 50% large B cells with round nuclei (ie, centroblasts and immunoblasts) were classified as round-cell lymphomas. Cases that showed a predominance of large cleaved cells were classified as cleaved-cell lymphomas. When a disagreement was observed within the pathology panel, final classification was obtained by consensus using a multiheaded microscope.
Bcl-2 protein expression was studied in all cases using formalin-fixed and/or Bouin liquid–fixed, paraffin-embedded sections, deparaffinized and stained with an appropriate monoclonal antibody (clone 124; DAKO, Copenhagen, Denmark). In order to avoid technical-dependent variability in bcl2 staining, bcl2 immunostaining was performed in the same laboratory (T.P.) and at the same time. Positive and negative controls were done using reactive lymph node and bcl-2–positive nodal follicular lymphoma. Keeping in mind that the small reactive lymphocytes generally express bcl-2 protein, the small cells were not counted, with the help of CD20 and CD3 markers when necessary. An exact quantification of the proportion of neoplastic large cells that showed an unequivocal bcl-2 positivity was performed by consensus among the 3 pathologists. Bcl-2 staining was finally considered positive if this proportion exceeded 50%.
Since bcl-2 protein expression was the variable of interest in prognostic analysis, the reproducibility of this measure was studied. Four pathologists who had not taken part in the expert panel were asked to separately review bcl-2 slides in all cases. Results were compared with those obtained by the expert panel.
Data collection
Variables analyzed for prognostic value included demographic characteristics, bcl-2 protein expression, and all factors that were significantly associated with survival in previous multivariate studies on PCBCLs.13,14 These variables were as follows: age at diagnosis; sex; anatomic site (head and neck, arm, anterior aspect of the trunk, posterior aspect of the trunk including the buttock, or leg); number of skin lesions; cutaneous extent (referred to as “localized” when either one or multiple skin lesions were restricted to one anatomic site, and “disseminated” when several anatomic sites or several limbs were involved); duration of skin lesions before diagnosis; serum lactate dehydrogenase (LDH) level; histologic group (round-cell versus cleaved-cell morphology); and bcl-2 protein expression. In addition, patients were classified according to the number of risk factors of the International Prognostic Index (IPI) using age (≤ 60 vs > 60), LDH level (normal vs elevated), and cutaneous extent (localized vs disseminated). The number of extranodal sites was not usable since patients with extracutaneous disease were not included in the study. Performance status was not used because it had not been registered in a large number of patients at the beginning of the study period.
Follow-up data
The end point was March 30, 2002. Follow-up information recorded until the end point included therapy, achievement of a complete response, relapse, nodal or visceral progression of the disease, final status, and date and cause of death. The follow-up time ranged from 3 to 167 months (median, 35 months; mean, 43 months). Status at the end point was known for 77 patients (96%). Only 3 patients (4%) were lost to follow-up before March 30, 2002. For these patients, the follow-up time was 4, 5, and 7 years.
Statistical analysis
Comparison between subgroups of patients was performed using the usual χ2 test or Fisher exact test for categoric variables and Student t test or Mann-Whitney test for continuous variables. Specific survival duration was calculated from diagnosis to date of disease-related death or censoring. Patients whose deaths were unrelated to lymphoma were considered censored. Survival rates were estimated in the entire study group and in subgroups of patients according to bcl-2 protein expression. Survival curves were computed using the method of Kaplan and Meier.34
Prognostic factors were evaluated by specific survival univariate and multivariate analyses using a Cox proportional-hazards model.35 Factors significant at the .2 level in univariate analysis were included in stepwise regression multivariate analyses.
Results
Clinical and histologic characteristics of the patients at diagnosis and follow-up data
Included in the study were 80 patients. Their clinical characteristics and follow-up data are summarized in Table 1. Of the patients, 43 (54%) were men and 37 (46%) were women. Age ranged from 28 to 98 years (mean, 62 years; median, 64 years). Clinically, patients presented with cutaneous nodules or tumors (90%) or deeply infiltrated plaques (10%). Of the patients, 32 (40%) had only 1 lesion, 36 (45%) had 2 lesions, and 12 (15%) had more than 2 lesions; 31 patients (39%) had lesions on the trunk, 28 (35%) on the head, 25 (31%) on the leg, and 6 (7.5%) on the arm.
A high LDH level was present in 8 cases (10%). There were 4 patients (5%) who had B symptoms. The number of IPI risk factors was 0 in 28 cases (35%), 1 in 43 cases (54%), 2 in 7 cases (9%), and 3 in 2 cases (2%).
After histologic review, 48 cases (60%) were classified as cleaved-cell PCLBCL and 32 cases (40%) were classified as round-cell PCLBCL. The results of bcl-2 protein expression assessment are shown in Table 2. There were 39 cases (49%) who exhibited a high expression of bcl-2 protein (≥ 50%) and were considered positive.
The initial treatment consisted of local radiotherapy in 41 cases (51%), a systemic polychemotherapy in 20 cases (25%), and the association of both therapies in 10 cases (12.5%). The last 9 patients (11.5%) underwent excision alone, interferon, or simple observation.
Among 66 patients (82.5%) who achieved a complete response, 38 (58%) had no relapse, whereas 28 (42%) experienced one or several relapses. Of the 80 patients, 21 (26%) developed an extracutaneous disease. Time until extracutaneous dissemination ranged from 2 to 63 months (mean, 20 months; median, 16 months). In 4 of 21 patients, extracutaneous progression occurred within 6 months after the histologic diagnosis of cutaneous lymphoma. Progression was restricted to the lymph nodes in all 4 cases. These patients had experienced multiple cutaneous tumors for 2 to 12 months (mean, 6 months) prior to the histologic diagnosis of cutaneous lymphoma and 5 to 15 months (mean, 10 months) prior to the diagnosis of nodal progression.
Of the 21 patients who developed extracutaneous disease, the progression was restricted to the lymph nodes in 7 cases. The remaining 14 cases had a visceral disease either associated with lymph node involvement (7 cases) or not (7 cases). The central nervous system (CNS) was the most frequent site of visceral dissemination (4 cases). CNS involvement was observed in 2 male and 2 female patients older than 65 years, with a bcl-2–positive (3 cases) or –negative (1 case) PCLBCL that was primarily located either on the lower leg (2 cases) or at other sites (2 cases). Other locations of visceral dissemination included the liver (2 cases), the testis (2 cases), the bones (1 case), the lung (1 case), the pancreas (1 case), the kidney (1 case), the breast (1 case), the pelvis (1 case), and the brachial plexus (1 case).
Of the 80 patients, 25 (31%) died of lymphoma, whereas 5 patients (6%) died of unrelated disease. Death from lymphoma occurred in 18 of 25 patients within 3 years after diagnosis. The 3-year and 5-year disease-specific survival rates were 75% and 65%, respectively.
Univariate and multivariate analyses
Univariate analysis showed that the following variables were related to death from lymphoma: advanced age (P < .0001); the number of IPI risk factors (P < .0001); the location on the leg (P < .0001); the presence of more than one skin lesion at diagnosis (P = .007); round-cell morphology (P < .0001); and positive bcl-2 protein expression (P < .0001). The most predictive cutoff point for age was 70 years. Sex, duration of lesions before diagnosis, LDH level, extent of skin lesions, and initial therapy (either including a polychemotherapy or not) had no significant effect on death from lymphoma. Multivariate analysis of disease-specific survival using all candidate variables identified bcl-2 protein expression (P = .0003), multiple skin lesions at diagnosis (P = .001), and age older than 70 years (P = .004) as independent factors associated with a poor prognosis (Table 3). When the number of IPI risk factors was forced in the model as a unique parameter (0 vs ≥ 1), bcl-2 expression remained the strongest predictive factor of survival (P = .0001; RR = 8.4) independently of the number of IPI risk factors (P = .02; RR = 6.7) and the number of skin lesions (P = .0006; RR = 4.9). Similar results of univariate and multivariate analyses were obtained when the 4 patients who underwent an extracutaneous dissemination within 6 months after diagnosis were excluded from the study. Multivariate analysis also showed similar results when therapy was forced in the model. Bcl-2 protein expression was the strongest prognostic factor of survival both in the group of patients whose therapy included a polychemotherapy and in those who were given radiotherapy or other treatments.
Characteristics of the patients and survival rates according to bcl-2 protein expression
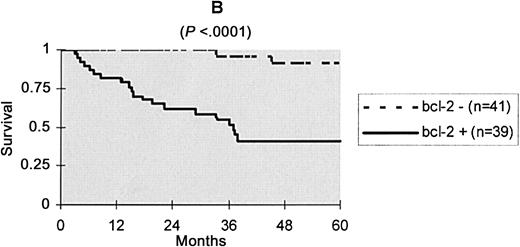
Since bcl-2 protein expression was the strongest prognostic factor, the main features at diagnosis and in follow-up data according to this variable were analyzed (Table 1). Patients with a bcl-2–positive PCLBCL differed from patients with a bcl-2–negative lymphoma by a higher age of onset, a female predominance, a shorter duration of skin lesions before diagnosis consistent with more rapidly growing tumors, a higher number of IPI risk factors, and more frequent location on the leg and round-cell morphology. Bcl-2 expression and morphology were in high concordance, with a kappa coefficient of 0.72. This coefficient was only 0.50 when concordance between bcl-2 expression and location on the leg was studied. Initial therapy was not significantly different between patients with bcl-2–negative and bcl-2–positive PCLBCL. However, there was a tendency for multiagent chemotherapies to be used more frequently in bcl-2–positive cases, possibly due to a more aggressive presentation and/or a more frequent location on the leg. Despite this tendency, patients with a bcl-2–positive lymphoma had a poorer prognosis, with a 5-year specific survival rate of 41%, versus 89% in patients with a bcl-2–negative PCLBCL. Kaplan Meier disease-specific survival curves in both groups are shown in Figure 1.
Kaplan-Meier specific survival curves according to bcl-2 protein expression.
The reproducibility study of bcl-2 protein expression assessment showed the following results: 67 (84%) of the 80 cases were classified consistently as either bcl-2 positive or negative by all of the 4 pathologists, in accordance with the consensus of the panel review. In 9 cases (11%), all but 1 of the 4 pathologists agreed with the classification. In only 4 cases (5%), 2 or more pathologists disagreed with the others and with the consensus of the panel review.
Survival rates according to bcl-2 expression and number of skin lesions
The presence of multiple skin lesions and bcl-2 protein overexpression were statistically unrelated (Table 1). Since these 2 variables were the strongest independent prognostic factors (Table 3), we studied survival in the 3 at-risk groups defined by the presence of none (group 1 = 18 cases), one (group 2 = 37 cases), or both (group 3 = 25 cases) of these factors. The 5-year specific survival rates in groups 1, 2, and 3 were 93%, 82%, and 18%, respectively.
Discussion
In the present report, we have studied bcl-2 protein expression and prognostic factors in a series of 80 patients with PCLBCL. We found that bcl-2 was expressed by 50% or more of tumor cells in about 50% of these lymphomas. Univariate and multivariate analyses showed that bcl-2 protein expression was the strongest predictive factor of death from lymphoma. This result has not been demonstrated previously in PCLBCL. It extends previous observations of the negative prognostic value of bcl-2 overexpression in systemic and nodal lymphomas.
Although primary cutaneous lymphomas were theoretically defined in the EORTC classification by the absence of extracutaneous progression within 6 months after diagnosis, we adopted in the present study the pragmatic, widely used definition based on a complete negative staging at diagnosis.5,7,10,11,13,28,36 This definition was recently recommended for 2 major reasons: (1) aggressive lymphomas that arise in the skin may show dissemination before a period of 6 months; (2) patients need to be treated at presentation, thus a clear-cut diagnosis must be established immediately, not 6 months afterward.5 In our series, 4 of 80 patients had extracutaneous progression (restricted to lymph nodes) within 6 months after diagnosis. Since these patients had experienced multiple cutaneous tumors for an average of 10 months prior to nodal progression, it seems likely that they had a primary cutaneous disease rather than secondary cutaneous involvement by a nodal lymphoma. When these patients were excluded from the prognostic analysis, similar results were observed. Therefore, it can be assumed that adverse prognostic factors identified in the present study, including bcl-2 protein expression, do not reflect undiagnosed associated systemic diseases and would apply to primary cutaneous lymphomas, either defined restrictively or not.
Among 14 patients who underwent a visceral progression of their disease, 4 (29%) had a CNS localization. It is noteworthy that no patient in our series had an intravascular large-cell lymphoma, an entity in which CNS involvement is a common feature. CNS involvement was recently reported in 4 of 160 patients with a primary cutaneous B-cell lymphoma36 and briefly mentioned in 2 of 4 cases in another study.28 No clinical or pathologic feature predictive of a secondary CNS involvement can be identified from these few cases. Clinicians may therefore be aware of this rare site of progression when following patients with PCLBCL.
We identified bcl-2 protein expression as the main negative prognostic factor in PCLBCL. The 5-year specific survival rate was 41% in patients with a bcl-2–positive PCLBCL versus 89% in patients with a bcl-2–negative PCLBCL. After adjustment for other prognostic factors, the relative risk for death associated with bcl-2 overexpression was 7.6 (95% confidence interval, 2 to 28; Table 2).
Previous studies of the prognostic value of bcl-2 protein expression have been performed only in patients with noncutaneous diffuse large B-cell lymphomas. Whereas some of these studies, including fewer than 65 patients, led to negative results,37-40 more powerful analyses of more than 150 patients found that bcl-2 protein overexpression was independently related to relapse,29 disease-free survival,30,31 or overall survival.32 In view of recent in vitro studies in human leukemia cell lines,41,42 it has been suspected that a high bcl-2 expression may confer resistance to several antineoplastic agents.30,39,40 In our study, the high prognostic value of bcl-2 expression was independent of therapy (ie, polychemotherapy versus other treatments), suggesting that mechanisms other than drug resistance may explain the poor prognosis related to bcl-2 overexpression in patients with PCLBCL.
The use of prognostic parameters defined by immunohistochemistry may be limited in clinical practice by the lack of a clear definition and poor reproducibility. Variable percentages of bcl-2–positive tumor cells have been used to define bcl-2 overexpression.37,38,40 However, major studies in noncutaneous diffuse large B-cell lymphomas consistently classified cases as bcl-2–expressing lymphomas if the protein was detected in more than 50%31,32 or 60%30 of tumor cells. In our patients with a PCLBCL, the 50% cutoff had a high clinical pertinence and a strong independent prognostic value. In 80% of cases the percentage of cells expressing the bcl-2 protein was either less than 20% or more than 80% (Table 1). Therefore, the assessment of bcl-2 positivity could seem easy in most cases. However, its reproducibility had not previously been evaluated in cutaneous lymphomas. When it was tested in our series, we found that 95% of cases were classified by all (84%) or all but 1 (11%) of 4 pathologists in accordance with the expert consensus. These data indicate that interpretation of bcl-2 immunostaining in PCLBCL is reproducible and therefore reliable.
In the present study, tumors were not investigated for the presence of the t(14;18) translocation. Increased expression of bcl-2 protein has been detected in lymphomas with t(14;18). After this translocation, the BCL2 oncogene, located on chromosome 18q, is subject to regulatory elements of the immunoglobulin heavy-chain gene, which leads to constitutive activation of the gene.43 However, it has been demonstrated that the increased bcl-2 expression in a series of PCLBCLs was not associated with the t(14;18).26 Pathologic mechanisms other than the t(14;18) may increase the expression of bcl-2, as has been hypothesized in noncutaneous lymphomas.29,30
Beside bcl-2 protein expression, other prognostic factors in PCLBCL must be taken into account. In a previous study,13 we found that the round-cell morphology, the location on the leg, and multiple skin lesions were independent adverse prognostic factors. When bcl-2 protein expression was included in the present study, neither morphology nor location of skin tumors gave any additional prognostic information. There was a high concordance between bcl-2 protein expression and round-cell morphology (κ = 0.72) (Figure 2). However, bcl-2 protein expression had the strongest prognostic value. Furthermore, we found previously that the reproducibility of the morphologic distinction between round- and cleaved-cell PCLBCL was insufficient.13 It seems therefore preferable, in clinical practice, to use the primary distinction between bcl-2–negative and bcl-2–positive PCLBCL.
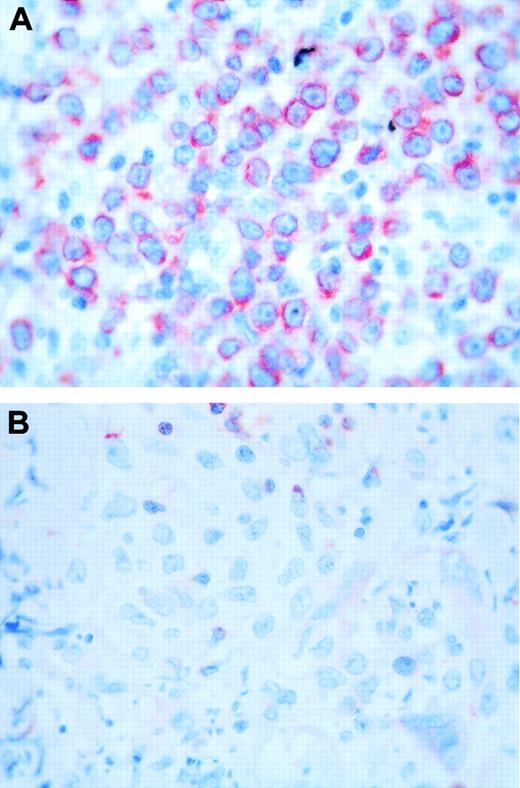
Bcl-2 staining. (A) Case with a round-cell morphology and high bcl-2 expression. (B) Case with a cleaved-cell morphology and negative bcl-2 staining. Original magnification, × 400.
Bcl-2 staining. (A) Case with a round-cell morphology and high bcl-2 expression. (B) Case with a cleaved-cell morphology and negative bcl-2 staining. Original magnification, × 400.
It has been previously suggested that the subdivision of PCLBCLs according to location (leg versus other sites) was quite simple and reproducible.2 However, it may be difficult, using this topographic distinction, to classify cases with multiple skin tumors located both on the leg and at other sites, or those with lesions at borderline sites like the buttock. In view of this and of the absence of independent prognostic value of location after taking into account other factors including bcl-2, we think that the primary subdivision of PCLBCL according to anatomic location, which has been previously proposed, should be reconsidered.
In the present study, the number of skin lesions remained a strong independent prognostic factor. This result is in accordance with our previous study on PCLBCLs13 and with more general studies that underlined the prognostic value of variables related to tumor burden in cutaneous or noncutaneous lymphomas.11,44,45 Beside bcl-2 protein expression, we found the number of skin lesions to be of major importance. Only patients with a bcl-2–positive PCLBCL and multiple skin tumors had a very poor prognosis, with a 5-year specific survival rate of 18%. In contrast, 5-year survival rates of patients with none or only 1 of these 2 adverse prognostic factors were 93% and 82%, respectively.
The IPI including age, LDH level, performance status, clinical stage, and number of extranodal sites was defined as the most effective tool for predicting outcome in patients with noncutaneous aggressive lymphomas.46 Although the IPI is not directly transposable to primary cutaneous lymphomas, an adaptation of this index using extent of skin lesions for clinical staging, as in the present study, and excluding the (inappropriate) number of extranodal sites, could be validated and used in further studies on cutaneous lymphomas. However, the IPI reflects only factors linked to the patient's characteristics and to the disease's extension and does not encompass molecular abnormalities of tumor cells that may play a critical role in determining outcome. In our patients, the prognostic value of bcl-2 protein expression was found to be independent of age, LDH level, extent of skin lesions, and a clinical index combining these factors. Although this analysis did not include the performance status, it extends previous observations of the independent prognostic value of bcl-2 expression and IPI risk factors in noncutaneous diffuse large B-cell lymphomas.31
In conclusion, we have identified bcl-2 protein expression as the strongest prognostic factor in PCLBCL and found bcl-2 immunostaining to be quite reproducible in these lymphomas, allowing its use in routine examination. Therefore, further classifications of primary cutaneous B-cell lymphomas should be based primarily on this biologic parameter. Additional prognostic factors (ie, age and number of skin lesions) should be taken into account for the choice of treatment in patients with PCLBCL. On the basis of these prognostic parameters, further therapeutic trials should be performed. Combinations of anthracyclin-containing polychemotherapies and rituximab were found to be more effective than chemotherapies alone in elderly patients with noncutaneous diffuse large B-cell lymphomas,47 particularly in cases with bcl-2 overexpression.48 Such combined therapies should be evaluated for patients with PCLBCL and adverse prognostic factors, as identified in the present study.
Appendix
The members of the French Study Group on Cutaneous Lymphomas are as follows: L. Andrac, M.F. Avril, M. Bagot, S. Barette, P. Berbis, P. Bernard, M. Beylot-Barry, C. Bodemer, J. Bosq, A. Carlotti, E. Cassagnau, T. Clerici, A. Colson, P. Cornillet, P. Courville, M. D'Incan, S. Dalac, J. P. Dales, A. de Muret, P. Dechelotte, M. Delaunay, M. H. Delfau-Larue, V. Descamps, B. Dreno, P. Dubus, A. Durlach, E. Esteve, S. Fraitag, C. Frances, F. Franck, N. Franck, F. Grange, P. Joly, F. Kerdraon, L. Laroche, F. Le Pelletier, B. Lenormand, L. Machet, F. Maitre, E. Marinho, J. P. Merlio, T. Petrella, H. Poirel, G. Quereux, P. Saiag, P. Souteyrand, M. C. Tortel, L. Vaillant, B. Vergier, and J. Wechsler.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-08-2726.
A complete member list of the French Study Group on Cutaneous Lymphomas appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the following clinicians, biologists, pathologists, and epidemiologists who actively participated in the study: M. H. Delfau-Larue, J. P. Merlio, B. Audhuy, A. Carlotti, B. Dreno, J. Bosq, A. Colson, A. Durlach, J. C. Guillaume, G. Hedelin, F. Maître, C. Michel, A. de Muret, B. Schubert, P. Souteyrand, L. Vaillant, T. Clerici, M. C. Tortel. We also thank Sylvie Espin and Marie-Antoinette Lignier (Centre de Pathologie de Dijon) for their technical assistance.