Abstract
In Children's Cancer Group (CCG) study 2891, patients who were recently diagnosed with acute myelocytic leukemia (AML) were assigned randomly to standard- or intensive-timing induction chemotherapy. Patients in first complete remission (CR1) and who had a human leukocyte antigen (HLA)–identical, related donor or a donor disparate at a single class I or II locus were nonrandomly assigned to receive a bone marrow transplant (BMT) by using oral busulfan (16 mg/kg) and cyclophosphamide (200 mg/kg). Methotrexate only was given for graft-versus-host disease (GVHD) prophylaxis. One hundred fifty patients received transplants. Grade 3 or 4 acute GVHD occurred in 9% of patients. Patients younger than 10 years had a lower incidence of grade 3 or 4 GVHD (4.6%) compared with patients 10 years or older (17.4%) (P = .044). Disease-free survival (DFS) at 6 years was 67% and 42% for recipients of intensive- and standard-timing induction therapies, respectively. Multivariate analysis showed that receiving intensive-timing induction therapy (P = .027) and having no hepatomegaly at diagnosis (P = .009) was associated with favorable DFS, and grades 3 and 4 acute GVHD were associated with inferior DFS. Multivariate analysis showed that grades 1 or 2 GVHD (P = .008) and no hepatomegaly at diagnosis (P = .014) were associated with improved relapse-free survival (RFS). Our results show that children older than 10 years are at higher risk for developing severe GVHD; acute GVHD is associated with favorable RFS.
Introduction
Bone marrow (BM) transplantation using a human leukocyte antigen (HLA)–matched sibling donor has been effective for children who have acute myelocytic leukemia (AML) in first remission.1-8 Retrospective studies on adult recipients of allogeneic BM transplantation suggest that graft versus leukemia (GVL) may contribute to curing patients with AML.9,10 The role of GVL in curing pediatric AML has not been established. In Children's Cancer Group (CCG) 2891, patients who were recently diagnosed with AML were randomly assigned to intensive-timing induction therapy or standard-timing induction therapy. Patients in first complete remission and who had HLA-identical sibling donors or a family donor disparate for a single HLA-A, -B, or -DR locus were allocated to BM transplantation. Patients without donors were randomly assigned to receive intensification therapy or an autologous BM transplantation. We previously reported outcomes on the basis of the results of randomization (intent-to-treat) and showed that recipients of allogeneic BMT had improved disease-free survival (DFS) and overall survival (OS) compared with patients who received consolidation chemotherapy or autologous BMT, and that intensive-timing induction therapy resulted in improved DFS and OS regardless of consolidation therapy type received.1,2 To best examine the effects of clinical characteristics, type of induction therapy received, incidence and severity of transplant-related toxicities, and graft-versus-host disease (GVHD), as well as existence of GVL effect, we limited our analysis to patients who received induction therapy and underwent BM transplantation according to protocol.
Patients, materials, and methods
Induction therapy
CCG 2891 was open to patients who were recently diagnosed with AML and myelodysplastic syndrome. In this report we excluded AML as a second malignancy, patients with Down syndrome, granulocytic sarcoma without marrow involvement, and granulocytic sarcoma with de novo myelodysplastic syndrome. Thus, we included 887 children with de novo AML. Marrow was analyzed for cytogenetic abnormalities and immunophenotype at diagnosis. Cell markers analyzed included CD10, CD19, CD2, CD7, anti-2B/3A, terminal deoxynucleotidyl transferase (TDT), CD14, CD15, and CD33. Induction therapy consisted of dexamethasone, cytarabine, thioguanine, etoposide, and rubidomycin (called DCTER).1,2 Patients were randomly assigned to receive intensive-timing induction therapy or standard-timing DCTER. For patients who received standard-timing DCTER, if marrow was hypocellular on day 14 examination, the second course of DCTER was given when counts recovered. If day 14 marrow showed more than 40% blasts, the second course of DCTER was given regardless of counts. In the intensive-timing arm, a second course of DCTER was given after a 6-day rest, regardless of counts. All patients received 4 courses of induction therapy. Patients who entered a complete remission and who had an HLA-matched sibling or related donor disparate for one HLA-A, -B, or -DR locus were nonrandomly assigned to allogeneic BM transplantation. Patients without donors were randomly assigned to autologous BM transplantation or consolidation chemotherapy. The protocol allowed BM transplantation up to 12 weeks after completion of induction therapy.
Conditioning
BM transplantations were performed at CCG-approved centers. Patients received oral busulfan (4 mg/kg/d × 4 days) in 4 divided doses from day –9 through day –6, followed by cyclophosphamide (50 mg/kg/d × 4 days) on days –5 through –2. After a day of rest, bone marrow cells were infused. We recommended that patients receive 3 × 108 nucleated donor marrow cells/kg. Donor bone marrow was processed only in cases of major ABO incompatibility.
Supportive care
Posttransplantation supportive care was given according to institutional guidelines. All patients required placement of indwelling central venous catheter and received total parenteral nutrition as needed. The protocol recommended trimethoprim/sulfamethoxazole for Pneumocystis carinii prophylaxis and nystatin or clotrimazole be given until the absolute neutrophil count (ANC) exceeded 1 × 109/L. Cytokines were not used routinely after transplantation. Acyclovir was recommended for prophylaxis against recurrent herpes simplex stomatitis in seropositive patients.
Definitions
Myeloid engraftment was defined as the third consecutive day the ANC exceeded 0.5 × 109/L. Platelet engraftment was defined as the third day the platelet count exceeded 20 × 109/L without platelet transfusion. Toxicity was graded by using the CCG toxicity scale.
GVHD prophylaxis
All patients, including those who received HLA-disparate grafts, received methotrexate until day 100 according to the Seattle regimen.11 The staging and grading of GVHD was performed according to the Seattle criteria.11 The treatment of acute and chronic GVHD was left to the discretion of individual transplantation centers.
Statistical analysis
Analysis was limited to 150 patients who had AML and underwent allogeneic BM transplantation while in first remission and includes data collected through August 11, 2000. The Kaplan-Meier method12 was used to calculate estimates of OS, DFS, and relapse-free survival (RFS) for patients in remission at the end of induction. DFS was defined as the time from the end of induction to relapse or death by any cause. RFS was defined as the time from the end of induction to relapse or death as a result of progressive disease, censoring deaths as a result of other causes. Patients lost to follow-up were censored at their last known point of study, with a cutoff of February 11, 2000. Confidence intervals were calculated by using Greenwood estimate of the standard error.13 Differences in OS, DFS, and RFS were tested for significance by using the log-rank statistic.14
Univariate analysis of prognostic factors was examined by using the approach of Kaplan and Meier and of Cox regression for fixed and time-dependent factors, respectively.15 All factors were considered by using defined discrete categories. Factors significant in univariate analysis at a P value less than .05 were considered for inclusion in multivariate Cox regression models. The likelihood ratio test was used to determine whether variables should be added or dropped from the multivariate model. Multivariate analyses included patients with complete covariate data. Acute GVHD status was analyzed with 3 levels: none, grades 1 to 2, and grades 3 to 4 acute GVHD. Chronic GVHD status was analyzed as a dichotomous factor (no chronic GVHD or chronic GVHD). Because acute and chronic GVHD status can vary with time, fixed and time-dependent analyses of these factors were considered. Fixed and time-dependent analyses of acute GVHD yielded almost identical results, so only results from the fixed analysis are reported. Conversely, fixed and time-dependent analysis of chronic GVHD yielded qualitatively different results, so only results from the time-dependent analysis are reported.
Results
Patients
One hundred eighty-one patients had matched family donors and thus were assigned to undergo allogeneic BM transplantation. One hundred forty-nine (82%) received induction therapy and underwent allogeneic BM transplantation according to protocol. One patient randomly assigned autologous BM transplantation received an allogeneic BMT. Thirty-two patients who were assigned allogeneic BM transplantation did not receive it for the following reasons: one patient underwent autologous BM transplantation, 16 did not receive transplants for unspecified reasons, 2 patients relapsed before BM transplantation, 7 received only 1 course of induction therapy, and 6 patients received preparative therapy other than that specified in the protocol. Thus, 150 patients were analyzed. Three (2%) patients required an additional course of induction therapy to achieve a remission; the remainder went into remission after one course of induction therapy. The median number of days from the beginning of the second course of induction therapy to BM transplantation was 79 days (range, 51-197 days). All patients who underwent BM transplantation were in remission as defined as an unsupported platelet count of more than 100 × 109/L, absolute neutrophil count of more than 1 × 109/L, and less than 5% bone marrow blasts. One hundred forty-four patients received grafts from HLA-identical donors and 6 received marrow disparate at a single HLA locus. Patient characteristics are shown in Table 1.
Patient characteristics (n = 150)
. | No. . | % . |
|---|---|---|
| Sex, M/F | 75/75 | 50/50 |
| FAB | ||
| M0 | 6 | 4 |
| 1 | 19 | 13 |
| 2 | 46 | 31 |
| 3 | 8 | 5 |
| 4 | 41 | 27 |
| 5 | 20 | 13 |
| 6 | 2 | 1 |
| 7 | 7 | 5 |
| No data | 1 | 1 |
| Cytogenetics | 85 | 57 |
| Normal | 17 | 20 |
| t(15;17) | 6 | 7 |
| t(8;21) | 17 | 20 |
| Abnormal 16* | 13 | 15 |
| Abnormal 11† | 12 | 14 |
| -7/7q- | 3 | 4 |
| +8 | 3 | 4 |
| Other | 14 | 16 |
| Induction chemotherapy | ||
| Intensive timing | 94 | 63 |
| Standard timing | 52 | 35 |
| Intensive/standard timing | 4 | 3 |
| Splenomegaly at diagnosis | ||
| None | 95 | 63 |
| Enlarged | 55 | 37 |
| Hepatomegaly at diagnosis | ||
| None | 95 | 63 |
| Enlarged | 55 | 37 |
| Donor | ||
| Parent | 11 | 7 |
| Sibling | 138 | 92 |
| Unknown | 1 | 1 |
. | No. . | % . |
|---|---|---|
| Sex, M/F | 75/75 | 50/50 |
| FAB | ||
| M0 | 6 | 4 |
| 1 | 19 | 13 |
| 2 | 46 | 31 |
| 3 | 8 | 5 |
| 4 | 41 | 27 |
| 5 | 20 | 13 |
| 6 | 2 | 1 |
| 7 | 7 | 5 |
| No data | 1 | 1 |
| Cytogenetics | 85 | 57 |
| Normal | 17 | 20 |
| t(15;17) | 6 | 7 |
| t(8;21) | 17 | 20 |
| Abnormal 16* | 13 | 15 |
| Abnormal 11† | 12 | 14 |
| -7/7q- | 3 | 4 |
| +8 | 3 | 4 |
| Other | 14 | 16 |
| Induction chemotherapy | ||
| Intensive timing | 94 | 63 |
| Standard timing | 52 | 35 |
| Intensive/standard timing | 4 | 3 |
| Splenomegaly at diagnosis | ||
| None | 95 | 63 |
| Enlarged | 55 | 37 |
| Hepatomegaly at diagnosis | ||
| None | 95 | 63 |
| Enlarged | 55 | 37 |
| Donor | ||
| Parent | 11 | 7 |
| Sibling | 138 | 92 |
| Unknown | 1 | 1 |
FAB indicates French-American-British classification.
Thirteen cases had inversion 16, 1 patient had a balanced translocation, and 1 had a partial deletion of chromosome 16.
All had abnormalities of 11q23.
Engraftment and toxicity
The median number of days to reach an absolute neutrophil count of 0.5 × 109/L was 23 (range, 0-46 days). The median time to reach an unsupported platelet count of 20 × 109/L was 24 days (range, 1-113 days). Grades 3 and 4 nonhematologic toxicities occurred in 63.3% of patients (95 of 150) (Table 2). Toxicities that occurred in more than 10% of cases included stomatitis (26%), hyperglycemia (10%), hematuria (10%), renal insufficiency (21%), hypertension (7%), increased alanine aminotransferase (ALT) (27%), increased aspartate aminotransferase (AST) (19%), hyperbilirubinemia (17%), and hepatic veno-occlusive disease (12%). Grades 3 and 4 nonhematologic toxicities were more frequent in patients with acute GVHD. Grade 3 or 4 toxicities occurred in 53% of patients without GVHD, whereas 75% of patients with acute GVHD experienced at least one grade 3 or 4 toxicity (P = .007). Patients with acute GVHD had significantly higher incidences of hyperbilirubinemia (25% versus 9%, P = .008), hyperglycemia (18% versus 3%, P = .002), renal insufficiency (35% versus 8%, P < .001), hematuria (18% versus 3%, P = .002), diarrhea (11% versus 1%, P = .014), and skin rashes (13% versus 0%, P = .001) compared with patients without GVHD.
Grades 3 and 4 toxicities that occurred in at least 5% of patients
. | All patients, n = 150 . | . | No acute GVHD, n = 79 . | . | Acute GVHD, n = 71 . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | N . | % . | N . | % . | N . | % . | P* . | |||
| Patients who had more than one grade 3 or 4 toxicity | 95 | 63.3 | 42 | 53.2 | 53 | 74.7 | .007 | |||
| Liver | 59 | 39.3 | 26 | 32.9 | 33 | 46.5 | .097 | |||
| Elevated AST | 29 | 19.3 | 12 | 15.2 | 17 | 23.9 | .216 | |||
| Elevated ALT | 40 | 26.7 | 18 | 22.8 | 22 | 31.0 | .273 | |||
| Hyperbilirubinemia | 25 | 16.7 | 7 | 8.9 | 18 | 25.4 | .008 | |||
| Veno-occlusive disease | 18 | 12.0 | 10 | 12.7 | 8 | 11.3 | 1.000 | |||
| Pancreas | 16 | 10.7 | 3 | 3.8 | 13 | 18.3 | .007 | |||
| Hyperglycemia | 15 | 10.0 | 2 | 2.5 | 13 | 18.3 | .002 | |||
| Kidney | 31 | 20.7 | 6 | 7.6 | 25 | 35.2 | < .001 | |||
| Elevated BUN | 7 | 4.7 | 1 | 1.3 | 6 | 8.5 | .053 | |||
| Hematuria | 15 | 10.0 | 2 | 2.5 | 13 | 18.3 | .002 | |||
| Diastolic hypertension | 9 | 7.0 | 4 | 5.1 | 5 | 7.0 | .736 | |||
| Gastrointestinal tract | 47 | 31.3 | 18 | 22.8 | 29 | 40.9 | .022 | |||
| Stomatitis | 39 | 26.0 | 18 | 22.8 | 21 | 29.6 | .358 | |||
| Abdominal pain | 7 | 4.7 | 2 | 2.5 | 5 | 7.0 | .256 | |||
| Diarrhea | 9 | 6.0 | 1 | 1.3 | 8 | 11.3 | .014 | |||
| Nausea and vomiting | 11 | 7.3 | 5 | 6.3 | 6 | 8.5 | .757 | |||
| Skin | 9 | 6.0 | 0 | 0.0 | 9 | 12.7 | .001 | |||
. | All patients, n = 150 . | . | No acute GVHD, n = 79 . | . | Acute GVHD, n = 71 . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | N . | % . | N . | % . | N . | % . | P* . | |||
| Patients who had more than one grade 3 or 4 toxicity | 95 | 63.3 | 42 | 53.2 | 53 | 74.7 | .007 | |||
| Liver | 59 | 39.3 | 26 | 32.9 | 33 | 46.5 | .097 | |||
| Elevated AST | 29 | 19.3 | 12 | 15.2 | 17 | 23.9 | .216 | |||
| Elevated ALT | 40 | 26.7 | 18 | 22.8 | 22 | 31.0 | .273 | |||
| Hyperbilirubinemia | 25 | 16.7 | 7 | 8.9 | 18 | 25.4 | .008 | |||
| Veno-occlusive disease | 18 | 12.0 | 10 | 12.7 | 8 | 11.3 | 1.000 | |||
| Pancreas | 16 | 10.7 | 3 | 3.8 | 13 | 18.3 | .007 | |||
| Hyperglycemia | 15 | 10.0 | 2 | 2.5 | 13 | 18.3 | .002 | |||
| Kidney | 31 | 20.7 | 6 | 7.6 | 25 | 35.2 | < .001 | |||
| Elevated BUN | 7 | 4.7 | 1 | 1.3 | 6 | 8.5 | .053 | |||
| Hematuria | 15 | 10.0 | 2 | 2.5 | 13 | 18.3 | .002 | |||
| Diastolic hypertension | 9 | 7.0 | 4 | 5.1 | 5 | 7.0 | .736 | |||
| Gastrointestinal tract | 47 | 31.3 | 18 | 22.8 | 29 | 40.9 | .022 | |||
| Stomatitis | 39 | 26.0 | 18 | 22.8 | 21 | 29.6 | .358 | |||
| Abdominal pain | 7 | 4.7 | 2 | 2.5 | 5 | 7.0 | .256 | |||
| Diarrhea | 9 | 6.0 | 1 | 1.3 | 8 | 11.3 | .014 | |||
| Nausea and vomiting | 11 | 7.3 | 5 | 6.3 | 6 | 8.5 | .757 | |||
| Skin | 9 | 6.0 | 0 | 0.0 | 9 | 12.7 | .001 | |||
AST indicates aspartate aminotransferase; ALT, alanine aminotransferase; and BUN, blood urea nitrogen.
P values for comparison of toxicity incidence for patients with and without acute GVHD.
GVHD
Fifty-three percent of patients (n = 79) never developed acute GVHD. Twenty-three percent of patients (n = 34) developed grade 1 acute GVHD, 16% (n = 24) grade 2, 5% (n = 7) grade 3, and 4% (n = 6) grade 4 acute GVHD. Grade 3 or 4 acute GVHD occurred in 6.4% of patients who received intensive-timing induction therapy and in 13.5% of patients who received standard-timing induction therapy (P = .223). Among the 87 patients younger than 10 years, 4.6% (4 of 87) developed grade 3 or 4 acute GVHD, whereas among the 63 patients 10 years or older, 17.5% (11 of 63) developed severe GVHD (P = .044). Grade 3 or 4 acute GVHD was not seen in any of the 6 patients who received grafts from one-antigen–mismatched donors. Limited chronic GVHD occurred in 7.3% of patients (n = 11) and extensive chronic GVHD occurred in 14% of patients (n = 21). Chronic GVHD was seen in 21.2% of patients who received standard-timing induction therapy and in 21.3% of patients who received intensive-timing induction therapy.
Survival
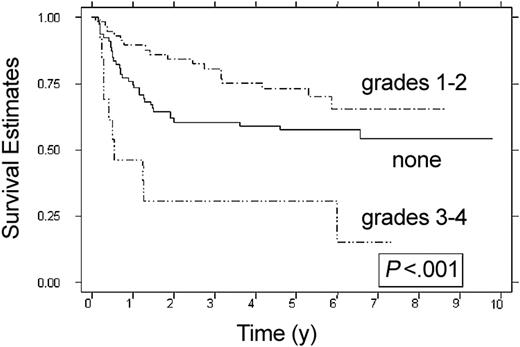
Overall survival and DFS at 6 years were 67% (95% confidence interval [CI], 58%-74%) and 57% (95% CI, 48%-65%), respectively. Patients who received intensive-timing induction therapy had a DFS of 67% (95% CI, 56%-75%) compared with 42% (95% CI, 27%-56%) in recipients of standard-timing therapy (P = .005). Overall survival for recipients of intensive-timing induction therapy was 75% (95% CI, 64%-83%) at 6 years compared with 54% (95% CI, 38%-67%) in recipients of standard-timing induction therapy (P = .008). DFS, OS, and RFS for patients who underwent BM transplantation in less than 79 days from the beginning of the second course of induction therapy was no different than that seen in patients who underwent BM transplantation more than 79 days from the start of the second course of induction therapy (data not shown). Patients who received grafts from one-antigen–mismatched donors (n = 6) had a DFS of 60% (95% CI, 13%-88%), which was not statistically different than DFS for patients who received HLA-identical grafts. Univariate analysis of factors associated with favorable DFS (Table 3) included receiving intensive-timing induction therapy (P = .005), no hepatomegaly at diagnosis (P = .013), no splenomegaly at diagnosis (P = .03), lack of TDT expression (P = .019), and having acute GVHD (P = .001). The effect of specific cytogenetic abnormalities (inversion 16, t(8;21), monosomy 7) on OS, DFS, and RFS were not significant; however, informative cytogenetic data were available in only 57% (85 of 150) of cases. Patients with grade 1 or 2 acute GVHD had a DFS of 65% (95% CI, 49%-78%) compared with 58% (95% CI, 46%-68%) seen in patients who never developed acute GVHD (P = .076). Patients with grade 3 or 4 acute GVHD had inferior DFS (15% at 6 years; 95% CI, 1%-45%) compared with patients without acute GVHD or grade 1 or 2 acute GVHD (P < .001) (Figure 1). Factors associated with a favorable OS included receiving intensive-timing induction therapy (P = .008) and lack of grade 3 or 4 acute GVHD (P < .001).
Univariate analysis of prognostic factors
Factor . | No. . | Overall survival, % . | P . | Disease-free survival, % . | P . | Relapse-free survival, % . | P . |
|---|---|---|---|---|---|---|---|
| Timing | .008 | .005 | .378 | ||||
| Intensive | 94 | 75 | 67 | 74 | |||
| Standard | 52 | 54 | 42 | 66 | |||
| Acute GVHD | |||||||
| None | 79 | 72 | NA | 58 | NA | 62 | NA |
| Grades 1-2 | 58 | 72 | .768* | 65 | .076* | 80 | .006* |
| Grades 3-4 | 13 | 15 | < .001* | 15 | .004* | 100 | .84* |
| Age, y | .32 | .758 | .05 | ||||
| Birth-2 | 19 | 79 | 68 | 68 | |||
| 3-10 | 68 | 69 | 52 | 62 | |||
| 11+ | 63 | 61 | 60 | 85 | |||
| Chloroma | .699 | .687 | .827 | ||||
| Yes | 12 | 67 | 58 | 70 | |||
| No | 137 | 67 | 57 | 72 | |||
| Liver | .161 | .013 | .01 | ||||
| Normal | 95 | 70 | 63 | 78 | |||
| Enlarged | 55 | 62 | 48 | 59 | |||
| Spleen | .073 | .03 | .011 | ||||
| Normal | 95 | 71 | 61 | 78 | |||
| Enlarged | 55 | 59 | 49 | 59 | |||
| CNS disease | .149 | .941 | .443 | ||||
| Yes | 5 | 100 | 60 | 60 | |||
| No | 144 | 65 | 57 | 71 | |||
| WBC | .311 | .266 | .17 | ||||
| Less than 20 × 103/μL | 81 | 72 | 62 | 75 | |||
| 20-100 × 103/μL | 56 | 61 | 52 | 71 | |||
| Greater than 100 × 103/μL | 13 | 66 | 51 | 51 | |||
| TDT | .16 | .019 | .342 | ||||
| 0%-25% | 61 | 67 | 51 | 66 | |||
| Greater than 25% | 7 | 50 | 17 | 33 | |||
| FAB M5 | .724 | .210 | .027 | ||||
| Yes | 20 | 66 | 66 | 95 | |||
| No | 129 | 67 | 56 | 67 | |||
| Cytogenetics | |||||||
| t(15;17) | 6 | 67 | .893 | 67 | .751 | 80 | .773 |
| t(8;21) | 17 | 53 | .265 | 47 | .385 | 79 | .831 |
| Abnormal 16 | 13 | 77 | .584 | 54 | .505 | 64 | .316 |
| Abnormal 11 | 12 | 53 | .375 | 53 | .830 | 83 | .778 |
Factor . | No. . | Overall survival, % . | P . | Disease-free survival, % . | P . | Relapse-free survival, % . | P . |
|---|---|---|---|---|---|---|---|
| Timing | .008 | .005 | .378 | ||||
| Intensive | 94 | 75 | 67 | 74 | |||
| Standard | 52 | 54 | 42 | 66 | |||
| Acute GVHD | |||||||
| None | 79 | 72 | NA | 58 | NA | 62 | NA |
| Grades 1-2 | 58 | 72 | .768* | 65 | .076* | 80 | .006* |
| Grades 3-4 | 13 | 15 | < .001* | 15 | .004* | 100 | .84* |
| Age, y | .32 | .758 | .05 | ||||
| Birth-2 | 19 | 79 | 68 | 68 | |||
| 3-10 | 68 | 69 | 52 | 62 | |||
| 11+ | 63 | 61 | 60 | 85 | |||
| Chloroma | .699 | .687 | .827 | ||||
| Yes | 12 | 67 | 58 | 70 | |||
| No | 137 | 67 | 57 | 72 | |||
| Liver | .161 | .013 | .01 | ||||
| Normal | 95 | 70 | 63 | 78 | |||
| Enlarged | 55 | 62 | 48 | 59 | |||
| Spleen | .073 | .03 | .011 | ||||
| Normal | 95 | 71 | 61 | 78 | |||
| Enlarged | 55 | 59 | 49 | 59 | |||
| CNS disease | .149 | .941 | .443 | ||||
| Yes | 5 | 100 | 60 | 60 | |||
| No | 144 | 65 | 57 | 71 | |||
| WBC | .311 | .266 | .17 | ||||
| Less than 20 × 103/μL | 81 | 72 | 62 | 75 | |||
| 20-100 × 103/μL | 56 | 61 | 52 | 71 | |||
| Greater than 100 × 103/μL | 13 | 66 | 51 | 51 | |||
| TDT | .16 | .019 | .342 | ||||
| 0%-25% | 61 | 67 | 51 | 66 | |||
| Greater than 25% | 7 | 50 | 17 | 33 | |||
| FAB M5 | .724 | .210 | .027 | ||||
| Yes | 20 | 66 | 66 | 95 | |||
| No | 129 | 67 | 56 | 67 | |||
| Cytogenetics | |||||||
| t(15;17) | 6 | 67 | .893 | 67 | .751 | 80 | .773 |
| t(8;21) | 17 | 53 | .265 | 47 | .385 | 79 | .831 |
| Abnormal 16 | 13 | 77 | .584 | 54 | .505 | 64 | .316 |
| Abnormal 11 | 12 | 53 | .375 | 53 | .830 | 83 | .778 |
CNS indicates central nervous system; WBC, white blood count; and NA, not applicable.
For comparison to patients who did not develop GVHD.
DFS according to acute GVHD. None (n = 79), grades 1-2 (n = 58), and grades 3-4 (n = 13). Estimates at 6 years are 58%, 65%, and 15%.
DFS according to acute GVHD. None (n = 79), grades 1-2 (n = 58), and grades 3-4 (n = 13). Estimates at 6 years are 58%, 65%, and 15%.
Multivariate analysis of factors associated with favorable DFS demonstrated that patients who had grade 3 or 4 acute GVHD were 2.73 times more likely to die compared with patients with no GVHD or grades 1 and 2 GVHD (P = .007). Patients who received standard-timing induction therapy were 1.8 times less likely to survive than those who received intensive-timing induction therapy (P = .027), and patients with an enlarged spleen at diagnosis were 2 times as likely not to survive as those who had a normal-sized spleen at diagnosis (P = .009). Factors associated with a favorable OS included lack of grade 3 or 4 GVHD (P < .001) and having received intensive-timing induction therapy (P = .041). Causes of death other than relapse included infection (n = 11), hemorrhage (n = 3), toxicity (n = 6), and GVHD (n = 6). Eight patients died of unknown causes.
Thirty-three patients had a marrow relapse and 6 had an isolated CNS relapse. The median time to relapse was 9.1 months after induction remission (range, 1.2-70.5 months). Thirteen patients died as a result of recurrent disease. Univariate analysis of factors associated with recurrent leukemia demonstrated that hepatomegaly (P = .01), splenomegaly (P = .011), French-American-British (FAB) M5 (P = .027), and not having any acute GVHD (P = .006) were associated with a greater risk for relapse (Table 3).
Graft-versus-leukemia
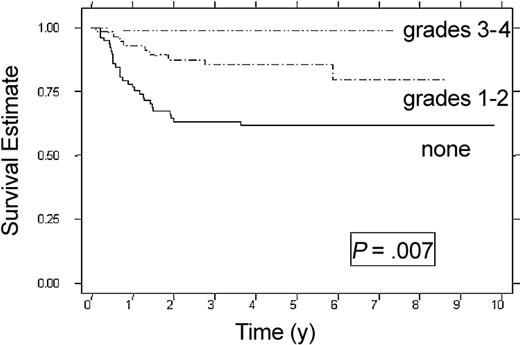
RFS correlated with the severity of acute GVHD (Figure 2; Table 3). Patients with no acute GVHD had a 62% RFS at 6 years (95% CI, 50%-72%). Patients with grade 1 acute GVHD had a 76% RFS (95% CI, 50%-90%) (P = .046) and patients with grade 2 acute GVHD had a 87% RFS (95% CI, 65%-96%) (P = .03). Of the 13 patients with grade 3 or 4 acute GVHD who survived, no relapses were observed. Although chronic GVHD was associated with improved RFS (RR = 0.24, P = .05), the reduced rate of relapse did not result in improved OS or DFS. Multivariate analysis showed that patients with grades 1 to 2 acute GVHD had one third the relapse risk as those with no GVHD (P = .008), and patients with grades 3 to 4 acute GVHD (n = 13) had the lowest risk for relapse. Patients with hepatomegaly at diagnosis were twice as likely to relapse as those with normal liver size (P = .014) (Table 4).
RFS according to acute GVHD. None (n = 79), grade 1-2 (n = 58), and grades 3-4 (n = 13). Estimates at 6 years are 62%, 80%, and 100%.
RFS according to acute GVHD. None (n = 79), grade 1-2 (n = 58), and grades 3-4 (n = 13). Estimates at 6 years are 62%, 80%, and 100%.
Multivariate analysis of factors affecting OS, DFS, and RFS
. | No. . | Relative risk . | 95% CI . | P . |
|---|---|---|---|---|
| DFS | ||||
| Acute GVHD | ||||
| None | 79 | 1.0 | NA | NA |
| Grades 1-2 | 58 | 0.66 | 0.37-1.2 | .165 |
| Grades 3-4 | 13 | 2.73 | 1.3-5.6 | .007 |
| Timing | ||||
| Intensive | 94 | 1.0 | NA | NA |
| Standard | 52 | 1.8 | 1.1-3.0 | .027 |
| Hepatomegaly | ||||
| No | 95 | 1.0 | NA | NA |
| Yes | 55 | 2.0 | 1.2-3.3 | .009 |
| RFS | ||||
| Acute GVHD | ||||
| None | 79 | 1.0 | NA | NA |
| Grades 1-2 | 58 | 0.36 | 0.17-0.76 | .008 |
| Grades 3-4 | 13 | 0 | NA | NA |
| Hepatomegaly | ||||
| No | 95 | 1.0 | NA | NA |
| Yes | 55 | 2.2 | 1.2-4.2 | .014 |
| OS | ||||
| Acute GVHD | ||||
| None | 79 | 1.0 | NA | NA |
| Grades 1-2 | 58 | 1.0 | 0.53-2.0 | .918 |
| Grades 3-4 | 13 | 4.5 | 2.1-9.7 | < .001 |
| Timing | ||||
| Intensive | 94 | 1.0 | NA | NA |
| Standard | 52 | 1.9 | 1.0-3.4 | .041 |
. | No. . | Relative risk . | 95% CI . | P . |
|---|---|---|---|---|
| DFS | ||||
| Acute GVHD | ||||
| None | 79 | 1.0 | NA | NA |
| Grades 1-2 | 58 | 0.66 | 0.37-1.2 | .165 |
| Grades 3-4 | 13 | 2.73 | 1.3-5.6 | .007 |
| Timing | ||||
| Intensive | 94 | 1.0 | NA | NA |
| Standard | 52 | 1.8 | 1.1-3.0 | .027 |
| Hepatomegaly | ||||
| No | 95 | 1.0 | NA | NA |
| Yes | 55 | 2.0 | 1.2-3.3 | .009 |
| RFS | ||||
| Acute GVHD | ||||
| None | 79 | 1.0 | NA | NA |
| Grades 1-2 | 58 | 0.36 | 0.17-0.76 | .008 |
| Grades 3-4 | 13 | 0 | NA | NA |
| Hepatomegaly | ||||
| No | 95 | 1.0 | NA | NA |
| Yes | 55 | 2.2 | 1.2-4.2 | .014 |
| OS | ||||
| Acute GVHD | ||||
| None | 79 | 1.0 | NA | NA |
| Grades 1-2 | 58 | 1.0 | 0.53-2.0 | .918 |
| Grades 3-4 | 13 | 4.5 | 2.1-9.7 | < .001 |
| Timing | ||||
| Intensive | 94 | 1.0 | NA | NA |
| Standard | 52 | 1.9 | 1.0-3.4 | .041 |
NA indicates not applicable.
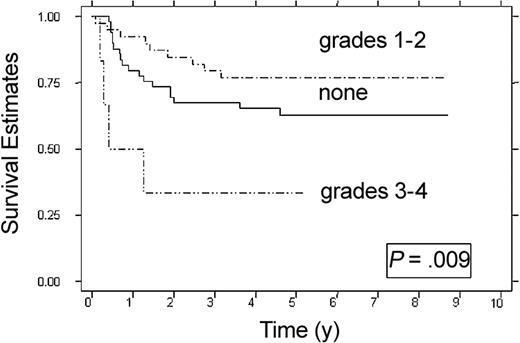
Because the choice of induction therapy is a controllable variable and patients who received intensive-timing induction had superior DFS, the effect of grade 1 or 2 acute GVHD on DFS in patients who received intensive-timing induction therapy was studied (Figure 3). Recipients of intensive-timing induction therapy who did not develop acute GVHD (n = 49) had a 63% DFS (95% CI, 47%-75%), whereas recipients of intensive-timing induction therapy with grade 1 or 2 acute GVHD (n = 39) had a 79% DFS (95% CI, 60%-87%) (P = .009).
Effect of grades 1 or 2 acute GVHD on DFS in recipients of intensive-timing induction therapy. None (n = 49), grades 1-2 (n = 39), and grades 3-4 (n = 6). Estimates at 6 years are 63%, and 79%; at 3 years, 33%.
Effect of grades 1 or 2 acute GVHD on DFS in recipients of intensive-timing induction therapy. None (n = 49), grades 1-2 (n = 39), and grades 3-4 (n = 6). Estimates at 6 years are 63%, and 79%; at 3 years, 33%.
Discussion
CCG 2891 represents the largest prospective series of pediatric patients with AML who underwent allogeneic BM transplantation in first remission. Outcomes presented here were based on patients who received induction therapy and preparative therapy according to protocol, instead of based on the results of randomization (intent to treat). This basis allowed a more accurate estimation of toxicities and severity of acute and chronic GVHD because the data reflect outcomes in patients who received induction therapy and underwent BM transplantation according to protocol.
This study demonstrates a GVL effect. Univariate analysis showed that patients with grades 1 or 2 acute GVHD had better DFS compared with patients who never developed GVHD, and acute and chronic GVHD were associated with improved RFS. In the multivariate analysis, acute GVHD was the most significant factor associated with improved RFS. The demonstration of a GVL effect is supported by studies that showed a higher relapse rate in patients who received methotrexate and cyclosporine A for GVHD prophylaxis16,17 and improved leukemia-free survival in adults with AML who had acute or chronic GVHD.9,10 Jernberg et al18 reported that chronic, but not acute, GVHD was associated with improved leukemia-free survival in children who had acute lymphoblastic leukemia (ALL) or AML. In that report, GVHD prophylaxis regimens were not standardized, and almost 40% of patients received grafts from unrelated donors. These differences may explain why a GVL effect was not seen with acute GVHD.
The incidence of grades 3 and 4 GVHD was higher in patients 10 years or older (14.3%) compared with that in patients younger than 10 years (4.6%, P = .044). Increasing patient age has been reported as a risk factor for the development of acute GVHD.19-22 In those studies, pediatric patients often were grouped as those younger than 18 to 20 years, and assessment of GVHD included grade 2 and grades 3 and 4 acute GVHD. Because patients in studies with grades 1 or 2 acute GVHD had improved DFS compared with those with none or severe GVHD, identification of patients at risk for developing severe acute GVHD may justify more intensive GVHD prophylaxis for older patients. We observed that DFS in recipients of grafts disparate at a single HLA locus was no different than DFS in recipients of HLA-identical grafts, the number of disparate grafts were too few for statistical comparison. These results are, however, consistent with previous reports showing that recipients of grafts disparate for one HLA locus have similar outcomes to recipients of HLA-identical grafts.23
In contrast to our experience with patients who underwent autologous BM transplantation,24 patients aged birth to 2 years at diagnosis did not have worse outcome than patients older than 2 years. Because both autologous and allogeneic BM transplantation arms were given identical conditioning regimens, we hypothesize that GVL may have compensated for a higher relapse rate because of the increased clearance of busulfan known to occur in infants.25,26
Recipients of intensive-timing induction therapy had better DFS compared with recipients of standard-timing therapy, which confirmed our previous reports.1,2 The mechanism by which intensive-timing therapy results in improved DFS after BM transplantation is unclear. Recipients of intensive-timing therapy had improved RFS compared with recipients of standard-timing therapy, but these differences were not significant. Recipients of intensive-timing therapy also had lower, but not statistically significant, incidence of grades 3 and 4 acute GVHD. We hypothesize that the higher incidence of severe GVHD and higher relapse rates seen in recipients of standard-timing induction therapy may account for the improved DFS seen in patients who received intensive-timing induction therapy.
This study identified potentially controllable variables such as intensive-timing induction therapy and GVHD prophylaxis that may affect outcome after BM transplantation. Our results suggest that it is reasonable to use only methotrexate for GVHD prophylaxis for patients younger than 10 years so that the GVL effect is not compromised. Older patients, who are at greater risk for developing grade 3 or 4 GVHD, may benefit from more intensive prophylaxis. The data from this study provide the justification for further clinical and laboratory investigations to optimize the GVL effect in pediatric patients with AML.
Appendix
This list provides the name of each CCG institution, its location, the name of the principal investigator, and the grant number (where applicable): C.S. Mott Children's Hospital, Ann Arbor, MI, Raymond Hutchinson (grant no. CA 02971); Memorial Miller Children's Hospital at LBMMC, Long Beach, CA, W. Roberts; Miller Children's Hospital/Harbor-UCLA, Long Beach, CA, W. Roberts; UCSF School of Medicine, San Francisco, CA, Katherine Matthay (grant no. CA 17829); UCLA School of Medicine, Los Angeles, CA, Stephen Feig (grant no. CA 27678); University of Wisconsin–Childrens Hosp Madison, Madison, WI, Yousif (Joe); Matloub; University of Iowa Hospitals & Clinics, Iowa City, IA, Raymond Tannous (grant no. CA 29314); Children's Hospital and Regional Medical Center, Seattle, WA, J. Geyer (grant no. CA 10382); The Children's Hospital–Denver, CO, Denver, CO, Linda Stork; Rainbow Babies and Childrens Hospital, Cleveland, OH, Eric Kodish; Mayo Clinic and Foundation, Rochester, MN, Carola Arndt; Children's National Medical Center–D.C., Washington, DC, Patricia Dinndorf; IWK Health Centre, Halifax, NS, Dorothy Barnard; Saint John Regional Hospital, Saint John, NF, Dorothy Barnard; University of North Carolina at Chapel Hill, Chapel Hill, NC, Stuart Gold; Childrens Hospital Los Angeles, Los Angeles, CA, Paul Gaynon (grant no. CA 02649); United Hospitals Medical Center, New Jersey, NJ, Richard Drachtman; University of Medicine and Dentistry of New Jersey, New Brunswick, NJ, Richard Drachtman; Cooper Hospital/University Medical Center, Camden, NJ, Richard Drachtman; Childrens Hospital of Columbus, Columbus, OH, Frederick Ruymann (grant no. CA 03750); The Childrens Mercy Hospital, Kansas City, MO, Maxine Hetherington; Columbia Presbyterian College of Phys & Surgeons, New York, NY, Linda Granowetter; University of Nebraska Medical Center, Omaha, NE, Peter Coccia; Children's Hospital of Pittsburgh, Pittsburgh, PA, A. Ritchey (grant no. CA 36015); Vanderbilt Children's Hospital, Nashville, TN, James Whitlock; University of Chicago Medical Center, Chicago, IL, James Nachman (grant no. CA 61833); Doernbecher Childrens Hospital–Oregon HSU, Portland, OR, H. Nicholson (grant no. CA 26044); Kaiser Permanente–Northwest Region, Portland, OR, H. Nicholson; M.D. Anderson Cancer Center, Houston, TX, Joann Ater; University of Minnesota Cancer Center, Minneapolis, MN, Joseph Neglia (grant no. CA 07306); Princess Margaret Hospital for Children, Perth, WA, David Baker (grant no. CA 79726); Childrens Hospital of Philadelphia, Philadelphia, PA, Beverly Lange (grant no. CA 11796); New York University Medical Center, New York, NY, Aaron Rausen (grant no. CA 79753); Memorial Sloan Kettering Cancer Center, New York, NY, Peter Steinherz (grant no. CA 42764); Childrens Hospital of Orange County, Orange, CA, Violet Shen (grant no. CA 69274); Indiana University–Riley Childrens Hospital, Indianapolis, IN, Robert Fallon; Primary Childrens Medical Center, Salt Lake City, UT, Elizabeth Raetz; British Columbia Cancer Agency, British Columbia, BC, Paul Rogers (grant no. CA 29013); British Columbia's Children's Hospital, Vancouver, BC, Paul Rogers; Childrens Hospital Medical Center Cincinnati, Cincinnati, OH, Robert Wells (grant no. CA 26126); David Grant USAF Medical Center, Travis AFB, CA, Peter Chenaille; Western Reserve Care System–Tod Childrens Hosp, Youngstown, OH, Aly Mageed; Raymond Blank Children's Hospital, Des Moines, IA, Stephen Elliott; Janeway Child Health Center, St. John's, NF, John (Jack); Hand; Monmouth Medical Center, Long Branch, NJ, Peri Kamalakar; Newark Beth Israel Medical Center, Newark, NJ, Peri Kamalakar; Wright State University, Emmett Broxson; Children's Medical Center Dayton, Dayton, OH, Emmett Broxson; USAF Medical Center–Wright Patterson AFB, Within OH., OH, Emmett Broxson; Lutheran General Childrens Medical Center, Park Ridge, IL, Jong-Hyo Kwon; Women's and Children's Hospital in San Antonio, San Antonio, TX, Jaime Estrada; Southwest Texas Methodist Hospital, San Antonio, TX, Jaime Estrada; Brookdale Hospital Medical Center, Brooklyn, NY, Kusum Viswanathan; A.B. Chandler Medical Center–University of Kentucky, Lexington, KY, Martha Greenwood; Michigan State University, East Lansing, MI, Renuka Gera; Children's Hospital Central California, Fresno, CA, Vonda Crouse; Albany Medical Center, Albany, NY, Jennifer Pearce; University of Illinois–Rockford, Rockford, IL, Torrey Mitchell; Mary Bridge Hospital, Tacoma, WA, William Thomas; Duluth Clinic, Duluth, MN, Robert Niedringhaus; Baystate Medical Center, Springfield, MA, David Steele; Atlantic Health System, Summit, NJ, Michelle Miller; Morristown Memorial Hospital, Morristown, NJ, Michelle Miller; South Carolina Cancer Center, Columbia, SC, Ronnie Neuberg; Children's Hospital of Austin, Austin, TX, Sharon Lockhart; Dakota Clinic, Fargo, ND, Janet Tillisch; New York Hospital-Cornell Univ Medical Center, New York, NY, Patricia Giardina; Children's Healthcare of Atlanta at Scottish Rite, Atlanta, GA, P. Davis; Northside Hospital, Within Georgia state, GA, P. Davis; William Beaumont Hospital, Royal Oak, MI, Charles Main; Sunrise Childrens Hospital, Sunrise Hosp & Medical Center, Las Vegas, NV, Ronald Oseas; St. Mary's Hospital Medical Center (Dean Medical Center); Madison, WI, MeritCare Hospital, Fargo, ND, Nathan Kobrinsky; East Tennessee Childrens Hospital, Knoxville, TN, Ray Pais; Fort Sanders Presbyterian, Knoxville, TN, Ray Pais; Southwest Cancer Center–Texas Tech/Lubbock, Lubbock, TX, John Iacuone; Texas Tech Regional Academic Health Center, El Paso, TX, John Iacuone; Children's Hem/Onc Team @ Covenant Children's Hosp, Lubbock, TX, John Iacuone; Providence Memorial Hospital–El Paso, El Paso, TX, John Iacuone; Gundersen Lutheran, La Crosse, WI, Robert Ettinger; Christiana Hospital, Wilmington, DE, Gregory Griffin; Christiana Care Health Services/A.I. duPont Inst., Wilmington, DE, Gregory Griffin; Childrens Hospital Medical Center-Akron, Ohio, Akron, OH, Jeffrey Hord; Akron City Hospital, Akron, OH, Jeffrey Hord; DeVos Children's Hospital, Grand Rapids, MI, David Freyer; Cedars-Sinai Medical Center, Los Angeles, CA, Carole Hurvitz; Metro-Health Medical Center, Cleveland, OH; Saskatoon Cancer Center, Saskatoon, SK, Kaiser Ali; Southern California Permanente Medical Group, Downey, CA, Willye Powell; Medical College of Georgia Childrens Medical Center, Augusta, GA, Roger Vega; Penn State Children's Hospital, Hershey Medical Center, Hershey, PA, John Neely; Henry Ford Hospital, Detroit, MI, Hassan Yaish; Presbyterian/St Lukes Medical Center and CHOA, Denver, CO, Patricia Cullen; Childhood Hem/Onc Associates and Memorial Hospital, Colorado Springs, CO, Patricia Cullen; Memorial Hospital, Colorado Springs, CO, Patricia Cullen; Texas Tech UHSC–Amarillo, Amarillo, TX, Trib Vats; Marshfield Clinic, Marshfield, WI, H. Nickerson; Geisinger Medical Center, Danville, PA, Narayan Shah; Kosair Childrens Hospital, Louisville, KY, Salvatore Bertolone; Kalamazoo Center for Medical Studies, Kalamazoo, MI, Leonard Mattano, Jr.; Phoenix Childrens Hospital, Phoenix, AZ, Paul Baranko; Dakota Midwest Cancer Institute, Sioux Falls, SD, Marwan Hanna; Loyola University Medical Center, Maywood, IL, Ricarchito Manera; Albert Einstein Medical Center, Philadelphia, PA; Allan Blair Cancer Centre, Regina, SK, Ten Goh; University of Illinois, Chicago, IL, Helen Johnstone; Children's Health Care–Minneapolis, Minneapolis, MN, Maura O'Leary; Mercy Children's Hospital, Toledo, OH, Rama Jasty; Clarian Health, Indianapolis, IN; Childrens Hospital-King's Daughters, Norfolk, VA, Rebecca Byrd; Loma Linda University Medical Center, Loma Linda, CA, Antranik Bedros; Southern Illinois University School of Medicine, Springfield, IL, Gregory Brandt; Georgetown University Medical Center, Washington, DC, Aziza Shad; Brooklyn Hospital Center, Brooklyn, NY, Swayamprabha Sadanandan; Children's Hospitals and Clinics–St. Paul, St. Paul, MN, Christopher Moertel; Sinai Hospital of Baltimore, Baltimore, MD, Joseph Wiley; University of Manitoba, Manitoba, MB, Rochelle Yanofsky; CancerCare Manitoba, Winnipeg, MB, Rochelle Yanofsky; Childrens Hospital of Winnipeg, Winnipeg, Manitoba, MB, Rochelle Yanofsky; SUNY Health Science Center at Brooklyn, Brooklyn, NY, Sreedhar Rao; University of Virginia Childrens Medical Center, Charlottesville, VA; Quain & Ramstad Clinic, Bismarck, ND; Montefiore Medical Center, Bronx, NY, Eva Radel; Women's and Children's Pavillion at USC; Connecticut Children's Medical Center, Farmington, CT, Arnold Altman; Childrens Hospital Oakland, Oakland, CA, James Feusner; Staten Island University Hospital, Staten Island, NY, Arlene Redner; North Shore University Hospital–Cornell U Medical Ct, Manhasset, NY, Arlene Redner; New York Medical College, Valhalla, NY, Fevzi Ozkaynak; Kaiser Permanente Medical Group, Inc., Northern CA, Oakland, CA, Kenneth Leung; Tulane University Medical School, New Orleans, LA; Group Health Cooperative of Puget Sound, Seattle, WA, Philip Herzog; and Deaconess Medical Center, Spokane, WA, Frank Reynolds.
Prepublished online as Blood First Edition Paper, January 29, 2004; DOI 10.1182/blood-2003-08-2705.
Supported by grants from the Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
Contributing Children's Cancer Group investigators, institutions, and grant numbers are given in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal