Abstract
This phase 2 pilot study was conducted to determine the efficacy and safety of imatinib mesylate in patients with c-kit–positive acute myeloid leukemia (AML) refractory to or not eligible for chemotherapy. Twenty-one patients were enrolled and received imatinib 600 mg orally once daily. Five responses were seen primarily in patients, starting with relatively low blast counts in bone marrow (BM) and peripheral blood (PB): 2 patients who were considered refractory on chemotherapy on the basis of persistence of blasts in PB and BM met the criteria for complete hematologic remission, 1 patient had no evidence of leukemia, and 2 patients achieved a minor response. Treatment with imatinib demonstrated a good safety profile and was well tolerated. Western blot analysis and immunohistochemistry demonstrated c-Kit activation in primary AML cells. Further, imatinib treatment of primary AML cells inhibited c-Kit tyrosine-phosphorylation. Genomic DNA-sequencing of c-KIT showed no mutations in exons 2, 8, 10, 11, 12, and 17. Although some of the responses derived from relatively small reductions in leukemic blasts and may be attributable, in part, to prior chemotherapy, these cases suggest that imatinib has interesting clinical activity in a subset of patients with c-kit–positive AML. Further clinical trials are warranted to explore the clinical potential of imatinib in AML and to identify the underlying molecular mechanism.
Introduction
The outcome for patients with acute myelogenous leukemia (AML) depends primarily on biologic characteristics of the disease, including karyotype, age of the patient, and intensity of postremission therapy.1 Standard chemotherapy using daunorubicin and cytarabine or their analogs induces complete remissions in 70% to 80% of patients younger than age 60 years.1 The 5-year overall survival rate for this patient population is approximately 40%.2 However, a comparison of the outcomes achieved in multicenter trials, including patients older and younger than age 60 years, indicates that older age is consistently associated with poorer complete remission rates and shorter overall survival times.3 A recent review by the Eastern Cooperative Oncology Group (ECOG) of the outcome for more than 1400 patients with AML revealed that the 5-year overall survival rate was 6% to 15% for patients aged 55 years and older.4-6 Because the median age of patients with AML at diagnosis is approximately 65 to 70 years, it is clear that most patients with AML still die of their disease.7,8
A similar outcome is seen in patients with relapsed or refractory AML.9-15 This situation for patients with relapsed/refractory AML can be improved with allogeneic peripheral blood stem cell transplantation (PBSC-T).16 However, because of age-related morbidity, mortality, and donor limitations, only a minority of these patients are eligible for allogeneic PBSC-T.
Therefore, there is a general agreement that alternative approaches, including immunomodulation, antibody-based therapies, inhibition of angiogenesis, and, in particular, inhibition of intracellular signals that promote proliferation and/or block differentiation, are warranted for patients who are elderly and for patients who have relapsed or refractory AML.17-23
More than 70% of patients with AML have blast cells that express c-kit, a receptor tyrosine kinase for the ligand stem cell factor (SCF).24,25 C-kit is essential for the maintenance of normal hematopoiesis and also plays a role in the biologic aspects of some human malignancies, including gastrointestinal stromal tumors (GISTs),26-28 small cell lung cancer,29-33 breast cancer,34 and AML.24,35 In AML, addition of SCF results in proliferation of AML blasts, and c-kit has been found activated in the absence of exogenous SCF in a proportion of cases.24,36,37 Also acquired mutations in the KIT gene are found with low frequency.38,39 In AML blasts, inhibition of c-kit in vitro results in caspase-3 activation and in cleavage of poly (adenosine diphosphate-ribose) polymerase (PARP).40 In addition, Mesters et al41 published a case study showing that treatment with a receptor tyrosine kinase inhibitor targeting c-kit (SU5416) resulted in stable remission in a patient with refractory AML. More recently, our group has published the first report of complete hematologic remission achieved with imatinib mesylate in a patient with refractory, secondary c-kit–positive AML.42 Imatinib mesylate (formerly STI-571; Glivec/Gleevec) is a 2-phenylaminopyrimidine compound designed to specifically interact with the adenosine triphosphate (ATP) binding site of protein tyrosine kinases (PTKs). Imatinib is highly selective and inhibits Abl, the chimeric Bcr-Abl fusion protein, platelet-derived growth factor receptor (PDGF-R) α and β, and c-kit.43 Recently, this compound has shown remarkable clinical efficacy with minimal side effects in bcr-abl–positive leukemias44,45 and in GISTs with c-kit– or PDGF-Rα–activating mutations.46
On the basis of the information outlined earlier, a phase 2 pilot study was conducted in adult patients with c-kit–positive AML. The primary objective of the study was to determine the rate of hematologic responses. The secondary objectives were to determine the duration of hematologic response, measure overall survival, evaluate improvement in symptoms, and determine the safety profile of imatinib in this patient population. In addition, we sought to gain molecular information on c-kit signaling in leukemic blasts from patients included in this trial.
Patients, materials, and methods
Subjects
Male or female patients (aged ≥ 18 years) with c-kit–positive AML refractory to or not eligible for standard chemotherapy were eligible to enter the trial. The following inclusion criteria were required: at least 30% blasts in peripheral blood (PB) and/or bone marrow (BM) at initial diagnosis (prior to start of standard chemotherapy), refractory to 2 cycles of prior standard chemotherapy or not eligible for standard chemotherapy because of age (> 75 years) or concomitant disease, and at least 30% (CD117) positivity in the blast population (“blast gate”) determined by fluorescence activated cell sorting (FACS) analysis (before starting imatinib).
Patients were required to be free of marked liver or kidney disease as indicated by levels of serum aspartate aminotransferase (AST) and serum alanine aminotransferase (ALT) not higher than 2.5 times the upper-normal limit (UNL) or, in the case of patients with suspected leukemic liver involvement, not higher than 5 times UNL. Total bilirubin level and serum creatinine could not be higher than 2.5 times UNL. All patients were required to have an ECOG performance status of 2 or less. Women of childbearing potential were only enrolled if they had a negative pregnancy test before starting imatinib and agreed to use an effective method of birth control during the study and for 3 months after stopping treatment.
Patients were not included in the study if they had received anthracycline, mitoxantrone, etoposide, or moderate- to high-dose cytosine arabinoside for AML treatment within 14 days prior to the start of imatinib. Other exclusion criteria included hematopoietic stem cell transplantation in the previous 6 weeks, any other investigational treatment within the previous 28 days, grade 3 and 4 cardiac disease, or any serious concomitant medical condition. The study was carried out in accordance with the Declaration of Helsinki. Each patient gave written informed consent, and local ethics committee approval was obtained.
Study design and treatment regimen
This was a single-center, open-label, single-arm trial. Patients received imatinib 600 mg orally once daily with meals for up to 6 months. After a minimum of 4 weeks of treatment the dose could be increased to 800 mg daily (400 mg twice daily) in patients who showed no evidence of hematologic response, as demonstrated by a progressive rise in leukemic blasts (documented by 2 samples taken at least 2 weeks apart). Patients who responded hematologically by a reduction (or at least stabilization) of elevated blast counts, but did not achieve a complete hematologic response (CHR) after 25 weeks of treatment or relapsed after having achieved a CHR, could have the dose of imatinib increased to 800 mg daily (400 mg twice daily).
Hematologic and nonhematologic toxicity was assessed according to the National Cancer Institute/National Institutes of Health (NCI/NIH) Common Toxicity Criteria. Dose modifications were required in cases of nonhematologic or hematologic toxicity. If a patient experienced a grade 3/4 nonhematologic toxicity or a grade 2 nonhematologic toxicity lasting for longer than 2 days, imatinib was stopped until the toxicity had resolved to grade 1 or less when treatment was restarted at the same daily dose. If grade 2 toxicity recurred, imatinib was again discontinued until the toxicity resolved to grade 1 or less and restarted at a lower dose. If there was a further episode of toxicity at lower doses, the dose was further reduced.
No dose reductions were required for grades 1 to 4 neutropenia or thrombocytopenia during the first 28 days of treatment. After 28 days of treatment, if a patient had grade 4 cytopenia that persisted for at least 2 weeks, a BM aspirate was performed to assess the cellularity and percentage of blasts. If BM cellularity was less than 10% and blasts less than 10%, the daily dose of imatinib was reduced from 600 mg to 400 mg or from 800 mg to 600 mg. If BM cellularity was more than 10% and/or blasts more than 10%, treatment was continued at 600 mg or 800 mg per day, and if the cytopenia persisted for at least another 2 weeks, the patient was reassessed. If the repeated BM was hypocellular and blasts 10% or less, imatinib was held until absolute neutrophil count (ANC) was at least 1.0 × 109/L and platelets were more than 20 × 109/L at which time imatinib was resumed at 300 mg or 400 mg per day. If blasts recurred or increased in the PB or BM during periods of dose reduction or treatment breaks, dose escalation could be considered (at the discretion of the investigator), depending on the individual clinical circumstances. Treatment toxicity was evaluated by patient interview and laboratory tests at each visit.
Concomitant therapies could be used if they were considered necessary for the supportive care and safety of the patients. However, anticancer drugs, including chemotherapeutic and biologic agents, were not permitted nor were any other investigational drugs. Two exceptions to this procedure were the use of intrathecal chemotherapy as treatment or maintenance for central nervous system leukemia and the use of hydroxyurea (5 mg/m2 per day for up to 7 days) at any time during the first 28 days on the study.
Granulocyte colony-stimulating factor (G-CSF) was permitted by protocol. Patients 1, 6, 9, 11, and 16 received G-CSF during imatinib therapy. Patient 9 who achieved no leukemic evidence started G-CSF 6 months after the start of imatinib because of granulocytopenia.
Efficacy assessments
Patients were evaluated for hematologic response at specified intervals. BM aspirations and biopsies were performed at screening and at weeks 4, 25, 37, and 49 and every 6 months thereafter and whenever it was clinically necessary. PB samples were obtained twice weekly for the first 4 weeks, every 2 weeks after week 5, and every 4 weeks after week 17. In addition, FACS analysis of PB and BM samples was performed to evaluate for c-kit–positive and c-kit–negative blast populations.
The primary efficacy parameters were sustained hematologic response lasting at least 4 weeks: CHR, no leukemic evidence (NLE) (blood and marrow) with no recovery of PB cells, and minor response (MR). CHR was defined according to conventional criteria as a blast count of less than 5% with no circulating PB blasts, a neutrophil count of at least 1.5 × 109/L and a platelet count 100 × 109/L or greater, and no evidence of extramedullary involvement. NLE was defined as all of the following: blast count less than 5% in BM with no circulating PB blasts; a neutrophil count 1.0 × 109/L or greater and a platelet count 20 × 109/L or greater, platelet-transfusion independence, and no evidence of bleeding; no evidence of extramedullary involvement. MR was defined as a percentage of c-kit–positive blasts (measured by FACS analysis) in PB less than 50% value at start of imatinib treatment lasting 4 weeks or more. Secondary efficacy parameters assessed were overall survival time, duration of hematologic response, and cancer-related symptoms.
Reagents and antibodies
SCF was purchased from CellConcepts (Umkirch, Germany). Imatinib mesylate for laboratory studies was kindly provided by Dr Buchdunger (Novartis, Basel, Switzerland). A 10 mM stock solution in dimethyl sulfoxide (DMSO) was prepared and stored at –20° C. Stock solution was diluted in cell culture medium and added directly to cells. Antibodies for Western blot analysis used in these studies include anti–c-kit antibody (Santa Cruz, Heidelberg, Germany) and antiphospho–c-kit antibody, antiphospho-Akt antibody, and anti-Akt antibody (Cell Signaling Technology, Frankfurt, Germany).
Immunohistochemical analyses
Immunohistochemical (IHC) staining was performed on slides of paraffin-embedded BM biopsies with the use of the polyclonal p145 antihuman c-Kit (CD117) antibody (Dako, Hamburg, Germany), the PDGF-R α and β antibodies (R&D Systems, Wiesbaden, Germany), the antiphospho–c-Kit (Tyr 719) antibody, and the antiphospho-Akt antibody (all Cell Signaling Technology) by the peroxidase/diaminobenzidine (DAB) method. Immunoreactivity on leukemic blasts in bone marrow biopsies was scored from 0 to 12 according to the scoring system developed by Remmele and Stegner,47 taking into account the percentage of receptor-positive (RP) blasts and their staining intensity (SI) as outlined in Table 1.
Immunoreactivity score (IRS) of leukemic blasts
Scoring points . | Receptor-positive (RP) blasts, % . | Staining intensity (SI) . |
|---|---|---|
| 0 | Negative | Negative |
| 1 | < 10 | Weakly positive |
| 2 | 10-50 | Moderately positive |
| 3 | 51-80 | Strongly positive |
| 4 | > 80 | — |
Scoring points . | Receptor-positive (RP) blasts, % . | Staining intensity (SI) . |
|---|---|---|
| 0 | Negative | Negative |
| 1 | < 10 | Weakly positive |
| 2 | 10-50 | Moderately positive |
| 3 | 51-80 | Strongly positive |
| 4 | > 80 | — |
Immunoreactivity score (IRS, 0-2) = RP (0-4) × SI (0-3).
— indicates none defined.
Isolation of primary AML blasts and cell culture techniques
Heparin-treated BM samples and/or 20 mL heparin-treated PB samples were obtained from patients enrolled in this study. In addition, 5 PB samples from patients with AML treated in a parallel protocol were obtained. Mononuclear cells (MNCs), including AML blasts, were isolated by means of Ficoll-Hypaque (Seromed, Berlin, Germany) density-gradient centrifugation. AML blasts were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.3, 50 μM β-mercaptoethanol, 2 mM L-glutamine, penicillin G (100 U/mL)/streptomycin (100 μg/mL), and growth factors as indicated.
Protein extract preparation
RPMI medium (1 × 107 or 2 × 106 cells/mL) plus 0.5% FCS were treated either with SCF (100 ng/mL), imatinib mesylate (1 μM), or with both compounds for 15 minutes at 37° C. Cells were washed twice in cold phosphate-buffered saline (PBS) and pelleted by centrifugation. Preparation of cellular lysates was performed as described.48 Supernatants were shock frozen and stored at –80° C. Protein concentration was measured by using the Bradford assay.
Western blot analysis
Protein lysates were subjected to sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) and blotted onto nitrocellulose membrane (Amersham, Freiburg, Germany) as previously described.48 To verify application of equal amounts of protein, blots were stripped and reprobed.
Mutation analysis
For mutation analysis, genomic DNA from PB was extracted by using the QIAmp DNA Blood Midi-Kit (Qiagen, Hilden, Germany), and RNA was isolated using RNeasy Kit (Qiagen). In addition, in some patients genomic DNA was extracted from BM smears of patients as described.42 C-KIT exons 2, 8, 10, 11, 12, and 17 were amplified by standard polymerase chain reaction (PCR) and sequenced, as described previously.42,49 For cDNA amplification and sequencing the following primers, covering the coding region of exon 2 to 21 of c-KIT, were used according to standard protocols: primer/f, GATCCCATCGCAGCTACCGC; primer/r, AAGAAGAGATCATTCCTGGAG. In addition, the following primers were used: primer1b/f, TGACTGTCGCTGTAAAGATG; primer1b/r, GTAGTCGAGCGTTTCCTTTA; primer4/f, AGGACTTGAGGTTTATTCCTG; primer4/r, CCAGATGAGTTTAGTGTCTGC; primer9/f, TATCCCAAGTCTGAGAATGAA; primer9/r, AAAAGATCACCATAGCAACAA.
Statistical methods
The population for analysis consisted of patients who had received at least one dose of imatinib and for whom response status could be determined (intent-to-treat [ITT] population). Analyses were performed after 25 weeks of treatment. Cancer-related symptoms and the severity of these symptoms were presented by visit on the basis of frequency tables. The paired t test was used to evaluate the relative effects of imatinib on c-kit+ blast populations (1993 GraphPad Software, San Diego, CA).
Results
Patients and treatment
A total of 21 patients were enrolled from November 2001, and efficacy and safety data for analysis were collected through to January 2003. All patients started treatment at a daily dose of 600 mg. Baseline demographic and disease characteristics for the 21 patients are summarized in Table 2, and Table 3 shows French-American-British (FAB) subtypes and cytogenetics for each patient.
Baseline demographic and disease characteristics
Age, y | |
| Mean ± SD | 63.5 ± 14.1 |
| Median | 66.0 |
| Range | 21.0-82.0 |
| Younger than 50 (%) | 2 (9.5) |
| At least 50 (%) | 19 (90.5) |
| Sex, no. (%) | |
| Male | 9 (46.9%) |
| Female | 12 (57.1%) |
| ECOG performance status, no. (%) | |
| 0 | 6 (28.6) |
| 1 | 8 (38.1) |
| 2 | 7 (33.3) |
| Duration of disease, mo/d | |
| Mean ± SD | 15.9 ± 24.94/479.5 ± 748.4 |
| Median | 9.2/278.0 |
| Range | 0.2-112.2/5.0-3380.0 |
| Disease characteristics, no. (%) | |
| Not eligible for CT | 3 (14) |
| Refractory | 5 (24) |
| Relapsed | 13 (62) |
| Interval between last dose of induction/consolidation CT to start of imatinib | |
| Median, d | 158 |
| Range, d | 21-616 |
| Previous CT cycles, no. (%) | |
| None | 3 (14) |
| 1-2 | 10 (48) |
| At least 3 | 8 (38) |
| Previous allogeneic PBSC-T, no. (%) | 4 (19) |
| PB blast count, × 109/L (median/range) | 0.87 (0-59.9) |
| BM blast cell infiltration (%) | 50.5 (2-90) |
| WBC, × 109/L (median/range) | 4.4 (1.0-76.9) |
| Hb, mg/dL (median/range) | 9.1 (6.6-12.5) |
| PLT, × 109/L (median/range) | 46 (10-2400) |
Age, y | |
| Mean ± SD | 63.5 ± 14.1 |
| Median | 66.0 |
| Range | 21.0-82.0 |
| Younger than 50 (%) | 2 (9.5) |
| At least 50 (%) | 19 (90.5) |
| Sex, no. (%) | |
| Male | 9 (46.9%) |
| Female | 12 (57.1%) |
| ECOG performance status, no. (%) | |
| 0 | 6 (28.6) |
| 1 | 8 (38.1) |
| 2 | 7 (33.3) |
| Duration of disease, mo/d | |
| Mean ± SD | 15.9 ± 24.94/479.5 ± 748.4 |
| Median | 9.2/278.0 |
| Range | 0.2-112.2/5.0-3380.0 |
| Disease characteristics, no. (%) | |
| Not eligible for CT | 3 (14) |
| Refractory | 5 (24) |
| Relapsed | 13 (62) |
| Interval between last dose of induction/consolidation CT to start of imatinib | |
| Median, d | 158 |
| Range, d | 21-616 |
| Previous CT cycles, no. (%) | |
| None | 3 (14) |
| 1-2 | 10 (48) |
| At least 3 | 8 (38) |
| Previous allogeneic PBSC-T, no. (%) | 4 (19) |
| PB blast count, × 109/L (median/range) | 0.87 (0-59.9) |
| BM blast cell infiltration (%) | 50.5 (2-90) |
| WBC, × 109/L (median/range) | 4.4 (1.0-76.9) |
| Hb, mg/dL (median/range) | 9.1 (6.6-12.5) |
| PLT, × 109/L (median/range) | 46 (10-2400) |
PBSC-T indicates peripheral blood stem cell transplantation; PB, peripheral blood; BM, bone marrow; WBC, white blood count; Hb, hemoglobin; PLT, platelets; CT, chemotherapy; ECOG, Eastern Cooperative Oncology Group.
FAB subtypes and cytogenetics of each patient at baseline
Patient no. . | FAB subtype . | Disease status at start of imatinib . | Cytogenetic alterations involving the PDGF-R gene locus . | Risk classification*/cytogenetic analysis . |
|---|---|---|---|---|
| 1 | M4 | 3rd relapse | Negative | Intermediate/46,XY |
| 2 | M2 | 1st relapse | Negative | Intermediate/del(12)(p) |
| 3 | ND | 2nd relapse | Negative | Poor/t(11;19)(q23;p13) |
| 4 | ND | Refractory | Negative | Poor/complex abnormalities; del(3)(q) |
| 5 | M2 | 1st relapse | Negative | Intermediate/del(20(q) |
| 6 | M2 | 1st relapse | Negative | Intermediate/45,X |
| 7 | M1 | Refractory | Negative | Intermediate/46,XY |
| 8 | M5b | Refractory | Negative | Intermediate/trisomy 21 |
| 9 | M2 | 1st relapse | Negative | Poor/complex abnormalities; -7 |
| 10 | Sec AML | Refractory | Negative | Intermediate/46,XY |
| 11 | ND | 1st relapse | Negative | Intermediate/46,XX |
| 12 | M4 | Chemonaive | Negative | Intermediate/46,XX |
| 13 | ND | 1st relapse | Negative | Intermediate/46,XX |
| 14 | M5a | Chemonaive | Negative | Intermediate/46,XX, del(20)(q) |
| 15 | M5a | 1st relapse | Negative | Poor/del(5)(q) |
| 16 | ND | 1st relapse | ND | ND |
| 17 | M0 | Refractory | Negative | Poor/-7; inv(3)(qq) |
| 18 | M5a | 1st relapse | Negative | Poor/complex abnormalities; -7 |
| 19 | M0 | 1st relapse | Negative | Poor/complex abnormalities; -7;-3 |
| 20 | ND | 1st relapse | Negative | Poor/complex abnormalities; -5 |
| 21 | ND | Chemonaive | Negative | Intermediate/46,XY |
Patient no. . | FAB subtype . | Disease status at start of imatinib . | Cytogenetic alterations involving the PDGF-R gene locus . | Risk classification*/cytogenetic analysis . |
|---|---|---|---|---|
| 1 | M4 | 3rd relapse | Negative | Intermediate/46,XY |
| 2 | M2 | 1st relapse | Negative | Intermediate/del(12)(p) |
| 3 | ND | 2nd relapse | Negative | Poor/t(11;19)(q23;p13) |
| 4 | ND | Refractory | Negative | Poor/complex abnormalities; del(3)(q) |
| 5 | M2 | 1st relapse | Negative | Intermediate/del(20(q) |
| 6 | M2 | 1st relapse | Negative | Intermediate/45,X |
| 7 | M1 | Refractory | Negative | Intermediate/46,XY |
| 8 | M5b | Refractory | Negative | Intermediate/trisomy 21 |
| 9 | M2 | 1st relapse | Negative | Poor/complex abnormalities; -7 |
| 10 | Sec AML | Refractory | Negative | Intermediate/46,XY |
| 11 | ND | 1st relapse | Negative | Intermediate/46,XX |
| 12 | M4 | Chemonaive | Negative | Intermediate/46,XX |
| 13 | ND | 1st relapse | Negative | Intermediate/46,XX |
| 14 | M5a | Chemonaive | Negative | Intermediate/46,XX, del(20)(q) |
| 15 | M5a | 1st relapse | Negative | Poor/del(5)(q) |
| 16 | ND | 1st relapse | ND | ND |
| 17 | M0 | Refractory | Negative | Poor/-7; inv(3)(qq) |
| 18 | M5a | 1st relapse | Negative | Poor/complex abnormalities; -7 |
| 19 | M0 | 1st relapse | Negative | Poor/complex abnormalities; -7;-3 |
| 20 | ND | 1st relapse | Negative | Poor/complex abnormalities; -5 |
| 21 | ND | Chemonaive | Negative | Intermediate/46,XY |
PN indicates patient number; FAB, French-American-British classification group; ND, not determined; and refractory, failure of initial therapy.
According to risk group definition of the HOVON/SAKK (Dutch-Belgian Cooperative Hemato-Oncology/Swiss Cancer study) group.50
Expression and activation of c-kit receptors in AML blasts from PB and BM
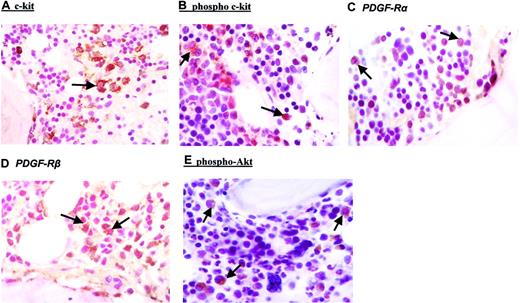
To investigate whether AML blasts from patients included in this study display activated c-kit receptors, expression and tyrosine phosphorylation of c-kit was investigated. By IHC, in all cases, expression of c-kit receptors could be confirmed in AML blasts (Figure 1A; Table 4). FACS analysis using an anti-CD117 antibody showed a median percentage of c-kit–positive blasts in PB of 43.5% and in BM of 54.5% (Table 4). In general, results obtained in PB correlated well with results from BM analysis (Table 4). Next, we addressed the question whether c-kit is activated in AML blasts from patients included in this study. Activation of c-kit promotes dimerization and autophosphorylation of the receptor at specific tyrosine residues. With the use of an anti-phospho (Tyr 719) c-kit antibody, IHC performed on BM biopsies exhibited positive staining in all patients investigated. A representative result is demonstrated in Figure 1B. To assess the degree of membrane expression of phosphorylated c-kit receptors in leukemic blasts, the IRS was evaluated for all patients (Table 4). Median IRS using the anti-phospho (Tyr 719) c-kit antibody was 4, indicating moderate staining intensity.
Immunohistochemical analysis of leukemic blasts from bone marrow biopsies. Bone marrow biopsies were taken before start of imatinib therapy. Immunohistochemistry was performed by applying the peroxidase/diaminobenzidine (DAB) method for anti–c-kit (A), antiphosphotyrosine c-kit (B), anti–PDGF-Rα (C), anti–PDGF-Rβ (D), and antiphospho-Akt (E). The specificity of positive phospho-Akt staining was controlled by the use of a specific blocking peptide (data not shown). Arrows indicate positive staining of AML blasts. Each figure shows a representative analysis of a total of 20. Original magnification, × 100.
Immunohistochemical analysis of leukemic blasts from bone marrow biopsies. Bone marrow biopsies were taken before start of imatinib therapy. Immunohistochemistry was performed by applying the peroxidase/diaminobenzidine (DAB) method for anti–c-kit (A), antiphosphotyrosine c-kit (B), anti–PDGF-Rα (C), anti–PDGF-Rβ (D), and antiphospho-Akt (E). The specificity of positive phospho-Akt staining was controlled by the use of a specific blocking peptide (data not shown). Arrows indicate positive staining of AML blasts. Each figure shows a representative analysis of a total of 20. Original magnification, × 100.
Characteristics of c-Kit and PDGF-Rα/β expression in peripheral blood and bone marrow at baseline
PN . | % c-kit+ blasts PB . | % c-kit+ blasts BM . | c-kit (IRS) . | phospho-c-kit (IRS) . | phospho-akt . | PDGFRα (IRS) . | PDGFRβ (IRS) . |
|---|---|---|---|---|---|---|---|
| 1 | ND | 42 | 3 × 3 (9) | 3 × 3 (9) | + | 2 × 1 (2) | 2 × 1 (2) |
| 2 | 22 | 32 | 4 × 3 (12) | 4 × 2 (8) | (+) | 4 × 1 (4) | 4 × 1 (4) |
| 3 | 20 | 86 | 4 × 3 (12) | 3 × 2 (6) | (+) | 2 × 2 (4) | 4 × 3 (12) |
| 4 | 31 | 27 | 3 × 3 (9) | 3 × 2 (6) | (+) | 3 × 2 (6) | 3 × 2 (6) |
| 5 | 43 | 46 | 3 × 2 (6) | 3 × 1 (3) | (+) | 3 × 3 (9) | 3 × 3 (9) |
| 6 | 81 | 92 | 3 × 2 (6) | 2 × 2 (4) | ++ | 2 × 2 (4) | 3 × 2 (6) |
| 7 | 71 | 71 | 4 × 3 (12) | 2 × 2 (4) | (+) | 3 × 2 (6) | 4 × 2 (8) |
| 8 | ND | 94 | 2 × 3 (6) | 2 × 3 (6) | +++ | 2 × 3 (6) | 4 × 3 (12) |
| 9 | 94 | 90 | 4 × 3 (12) | 4 × 3 (12) | ++ | 3 × 2 (6) | 4 × 2 (8) |
| 10 | 41 | 41 | 4 × 3 (12) | 4 × 3 (12) | ++ | 3 × 2 (6) | 4 × 3 (9) |
| 11 | ND | 96 | 2 × 2 (4) | 2 × 2 (4) | ND | 2 × 2 (4) | 2 × 2 (4) |
| 12 | 75 | 80 | 2 × 3 (6) | 2 × 2 (4) | (+) | 2 × 1 (2) | 3 × 3 (9) |
| 13 | 44 | 50 | ND | ND | ND | ND | ND |
| 14 | ND | 59 | 2 × 2 (4) | 2 × 2 (4) | (+) | 3 × 2 (6) | 3 × 2 (6) |
| 15 | 88 | 84 | 1 × 3 (3) | 1 × 3 (3) | (+) | 3 × 2 (6) | 3 × 3 (9) |
| 16 | 74 | ND | 2 × 1 (1) | 2 × 1 (2) | ((+)) | 2 × 2 (4) | 2 × 2 (4) |
| 17 | 37 | 43 | 1 × 3 (3) | 1 × 2 (2) | +++ | 3 × 3 (9) | 4 × 3 (12) |
| 18 | 26 | 43 | 2 × 2 (4) | 2 × 1 (2) | ++ | 2 × 1 (2) | 2 × 2 (4) |
| 19 | 5 | 31 | 1 × 2 (2) | 2 × 2 (4) | ND | 2 × 2 (6) | 2 × 2 (6) |
| 20 | 22 | 40 | 1 × 2 (2) | 1 × 1 (1) | ND | 2 × 3 (6) | 2 × 1 (2) |
| 21 | 65 | 63 | 1 × 1 (1) | 1 × 1 (1) | + | 2 × 2 (4) | 2 × 2 (4) |
| Median | 43.5 | 54.5 | 6 (IRS) | 4 (IRS) | NA | 6 (IRS) | 6 (IRS) |
PN . | % c-kit+ blasts PB . | % c-kit+ blasts BM . | c-kit (IRS) . | phospho-c-kit (IRS) . | phospho-akt . | PDGFRα (IRS) . | PDGFRβ (IRS) . |
|---|---|---|---|---|---|---|---|
| 1 | ND | 42 | 3 × 3 (9) | 3 × 3 (9) | + | 2 × 1 (2) | 2 × 1 (2) |
| 2 | 22 | 32 | 4 × 3 (12) | 4 × 2 (8) | (+) | 4 × 1 (4) | 4 × 1 (4) |
| 3 | 20 | 86 | 4 × 3 (12) | 3 × 2 (6) | (+) | 2 × 2 (4) | 4 × 3 (12) |
| 4 | 31 | 27 | 3 × 3 (9) | 3 × 2 (6) | (+) | 3 × 2 (6) | 3 × 2 (6) |
| 5 | 43 | 46 | 3 × 2 (6) | 3 × 1 (3) | (+) | 3 × 3 (9) | 3 × 3 (9) |
| 6 | 81 | 92 | 3 × 2 (6) | 2 × 2 (4) | ++ | 2 × 2 (4) | 3 × 2 (6) |
| 7 | 71 | 71 | 4 × 3 (12) | 2 × 2 (4) | (+) | 3 × 2 (6) | 4 × 2 (8) |
| 8 | ND | 94 | 2 × 3 (6) | 2 × 3 (6) | +++ | 2 × 3 (6) | 4 × 3 (12) |
| 9 | 94 | 90 | 4 × 3 (12) | 4 × 3 (12) | ++ | 3 × 2 (6) | 4 × 2 (8) |
| 10 | 41 | 41 | 4 × 3 (12) | 4 × 3 (12) | ++ | 3 × 2 (6) | 4 × 3 (9) |
| 11 | ND | 96 | 2 × 2 (4) | 2 × 2 (4) | ND | 2 × 2 (4) | 2 × 2 (4) |
| 12 | 75 | 80 | 2 × 3 (6) | 2 × 2 (4) | (+) | 2 × 1 (2) | 3 × 3 (9) |
| 13 | 44 | 50 | ND | ND | ND | ND | ND |
| 14 | ND | 59 | 2 × 2 (4) | 2 × 2 (4) | (+) | 3 × 2 (6) | 3 × 2 (6) |
| 15 | 88 | 84 | 1 × 3 (3) | 1 × 3 (3) | (+) | 3 × 2 (6) | 3 × 3 (9) |
| 16 | 74 | ND | 2 × 1 (1) | 2 × 1 (2) | ((+)) | 2 × 2 (4) | 2 × 2 (4) |
| 17 | 37 | 43 | 1 × 3 (3) | 1 × 2 (2) | +++ | 3 × 3 (9) | 4 × 3 (12) |
| 18 | 26 | 43 | 2 × 2 (4) | 2 × 1 (2) | ++ | 2 × 1 (2) | 2 × 2 (4) |
| 19 | 5 | 31 | 1 × 2 (2) | 2 × 2 (4) | ND | 2 × 2 (6) | 2 × 2 (6) |
| 20 | 22 | 40 | 1 × 2 (2) | 1 × 1 (1) | ND | 2 × 3 (6) | 2 × 1 (2) |
| 21 | 65 | 63 | 1 × 1 (1) | 1 × 1 (1) | + | 2 × 2 (4) | 2 × 2 (4) |
| Median | 43.5 | 54.5 | 6 (IRS) | 4 (IRS) | NA | 6 (IRS) | 6 (IRS) |
FACS was used to determine percentage of c-kit+ blasts in PB and BM. IHC was used to determine IRS of c-kit, phospho-c-kit, phospho-akt, PDGFRα, and PDGFRβ.
Immunoreactivity on leukemic blasts in bone marrow biopsies was scored from 0 to 12, taking into account the percentage of receptor-positive blasts and their staining intensity [IRS (0-12) = RP (0-4) × SI (0-3)].
In normal hematopoietic cells as lymphocytes, plasma cells and normoblasts the IRS was 0 for c-kit, phospho-c-kit and phospho-Akt.
PN indicates patient number; FACS, fluorescence activated cell sorter; IRS, immunoreactivity score; IHC, immunohistochemistry; ND, not determined; NA, not available; symbols ((+)), (+), +, ++, +++ indicate very low, low, moderate, high, and very high staining signals obtained by IHC analysis, respectively.
Downstream pathways implicated in c-kit–mediated signal transduction include phosphatidylinositol 3′-kinase (PI-3K)/Akt activation, Ras-Raf-MAP (mitogen-activated protein) kinase cascade and the JAK/STAT (Janus kinase/signal transducer and activator of transcription) pathway. With the use of an antiphospho-Akt antibody, activation of the PI-3K signaling pathway was detected in most leukemic blasts from all cases as well as in healthy BM cells (Table 4). A representative result is depicted in Figure 1E.
In addition to c-kit, imatinib also inhibits activation of PDGF-R. Therefore, expression of PDGF-R α and β chains was examined. IHC analysis of BM biopsies (Figure 1C-D, respectively) indicated moderate expression of both receptor chains on AML blasts. Median IRS was 6 for both the α and β chain, respectively (Table 4).
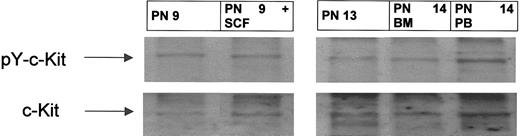
Because positive staining obtained by IHC using the antiphospho (Tyr719) c-kit antibody may show some degree of cross-reactivity, Western blotting was performed in parallel in 8 patients using PB MNCs highly enriched in leukemic blasts. This analysis revealed a major c-kit receptor–specific band of an apparent molecular mass of 160 kDa, which precisely corresponded to the major band observed using the antiphospho–c-kit (Tyr 719) antibody (Figure 2). This result confirms that c-kit is present in its tyrosine-phosphorylated form in AML blasts examined. Taken together, these results indicate that c-kit receptors are at least partially activated in AML blasts from patients included in this trial.
c-kit is tyrosine phosphorylated in peripheral blood and bone marrow blasts from patients with AML. BM aspirates and peripheral blood sampling were performed at screening visits before start of imatinib therapy. MNCs were separated, and whole cell lysates were prepared as described in “Patients, materials, and methods.” Western blot analysis was performed by using an antiphosphotyrosine c-kit antibody. To control for equal loading, the blot was stripped and reprobed with anti–c-kit antibody. Shown are representative results from 3 patients (PN 9, 13, and 14). AML blasts obtained from PN 9 were incubated with and without SCF (100 ng/mL) for 15 minutes. Arrows indicate the position of the 160-kD isoform of c-kit.
c-kit is tyrosine phosphorylated in peripheral blood and bone marrow blasts from patients with AML. BM aspirates and peripheral blood sampling were performed at screening visits before start of imatinib therapy. MNCs were separated, and whole cell lysates were prepared as described in “Patients, materials, and methods.” Western blot analysis was performed by using an antiphosphotyrosine c-kit antibody. To control for equal loading, the blot was stripped and reprobed with anti–c-kit antibody. Shown are representative results from 3 patients (PN 9, 13, and 14). AML blasts obtained from PN 9 were incubated with and without SCF (100 ng/mL) for 15 minutes. Arrows indicate the position of the 160-kD isoform of c-kit.
Imatinib inhibits c-kit signal transduction in primary c-kit–positive AML blasts
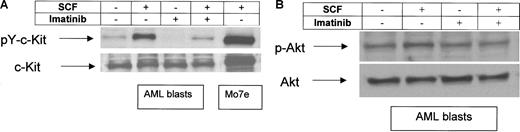
The ability of imatinib to inhibit the initial events in c-kit signaling in vitro was investigated. As shown by Western blot analysis, c-kit receptors from freshly isolated c-kit–positive AML blasts were found to be activated in the absence of SCF stimulation (Figure 3A). Incubation with SCF for 15 minutes resulted in marked up-regulation of c-kit tyrosine phosphorylation. The presence of imatinib completely inhibited tyrosine phosphorylation of c-kit receptors from untreated AML blasts and strongly down-regulated this event in SCF-treated AML cells (Figure 3A).
Effects of imatinib on c-kit tyrosine-phosphorylation and SCF-induced phosphorylation of Akt. PB was taken before start with imatinib. MNCs were separated and treated with SCF (100 ng/mL), imatinib (1 μM), and in combination for 15 minutes. As a control, the SCF-dependent leukemic cell line Mo7e was used. Whole cell lysates were prepared and immunoblotted using an antiphosphotyrosine c-kit antibody (A) or an antiphospho-Akt antibody (B). To control equal loading, the blot was stripped and reprobed with anti–c-kit (A) or Akt (B). Shown is a representative result of a total of 5. Arrows indicate the position of the 160-kD isoform of c-kit (A) and of Akt (B), respectively.
Effects of imatinib on c-kit tyrosine-phosphorylation and SCF-induced phosphorylation of Akt. PB was taken before start with imatinib. MNCs were separated and treated with SCF (100 ng/mL), imatinib (1 μM), and in combination for 15 minutes. As a control, the SCF-dependent leukemic cell line Mo7e was used. Whole cell lysates were prepared and immunoblotted using an antiphosphotyrosine c-kit antibody (A) or an antiphospho-Akt antibody (B). To control equal loading, the blot was stripped and reprobed with anti–c-kit (A) or Akt (B). Shown is a representative result of a total of 5. Arrows indicate the position of the 160-kD isoform of c-kit (A) and of Akt (B), respectively.
To examine an SCF-mediated signaling event downstream of c-kit activation, Akt phosphorylation was analyzed in the presence and absence of imatinib. As shown in Figure 3B, imatinib modestly down-regulates Akt phosphorylation following SCF stimulation but not constitutive Akt activation. Together, these results demonstrate that in primary c-kit–positive blasts suppression of c-kit signal transduction can be achieved by using imatinib at a concentration which corresponds to steady-state trough concentrations achieved at an oral dose of at least 400 mg.51
Hematologic response: efficacy of imatinib in vivo therapy
Efficacy analysis included all 21 patients. Among these patients, 18 had received previous standard AML chemotherapy regimens and 2 of these 18 patients (PNs 4 and 7) had received chemotherapy in relatively close proximity of imatinib (day 31 and day 24 after start of chemotherapy, respectively). For the remaining patients, the interval between last dose of standard chemotherapy and start of imatinib was between 48 days and 616 days. Three patients were chemonaive but not eligible for standard chemotherapy because of age and/or concomitant disease. For control of PB blast counts, one patient (PN 8) had received hydroxyurea until day –5 prior to start of imatinib.
In 5 patients, treatment with imatinib resulted in a hematologic response on at least one occasion (Table 5). Values represent the best response observed at any time during therapy: 2 patients had a CHR, 1 patient met the criteria for NLE, and 2 patients achieved a sustained MR. Table 5 also shows percentage of leukemic blasts in BM and absolute PB blast counts prior to start of imatinib. From this analysis it is obvious that hematologic responses were seen in patients starting with a relatively low blast count in PB and BM.
Hematologic responses and leukemic blasts in BM and PB prior to start of imatinib
Response . | N = 21 . | BM blast infiltration prior to start of imatinib as evaluated by BM cytology, % . | Maximal absolute PB blast count prior to start of imatinib, per μL . |
|---|---|---|---|
| Complete hematologic response (CHR) | 2 (PNs 4, 7) | PN 4: 5-10; PN 7: 2 | PN 4: 144; PN 7: 384 |
| No leukemic evidence (NLE) | 1 (PNs 9) | PN 9: 5 | PN 9: 1808 |
| Minor response (MR) | 2 (PNs 5, 18) | PN 5: 13 | PN 5: 1080 |
| PN 18: 62 | PN 18: 686 | ||
| Total (%) | 5 (24) | — | — |
Response . | N = 21 . | BM blast infiltration prior to start of imatinib as evaluated by BM cytology, % . | Maximal absolute PB blast count prior to start of imatinib, per μL . |
|---|---|---|---|
| Complete hematologic response (CHR) | 2 (PNs 4, 7) | PN 4: 5-10; PN 7: 2 | PN 4: 144; PN 7: 384 |
| No leukemic evidence (NLE) | 1 (PNs 9) | PN 9: 5 | PN 9: 1808 |
| Minor response (MR) | 2 (PNs 5, 18) | PN 5: 13 | PN 5: 1080 |
| PN 18: 62 | PN 18: 686 | ||
| Total (%) | 5 (24) | — | — |
PN indicates patient number; — indicates no total.
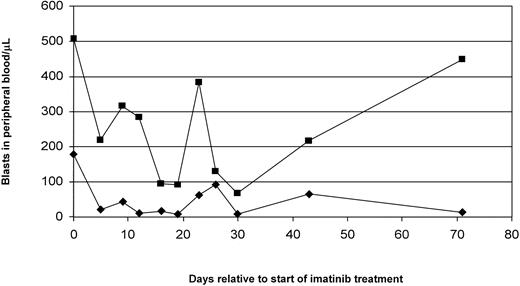
In addition, 2 patients (PNs 14, 16) showed a transient reduction in their PB blast counts more than 50% but did not reach the criteria of a minor response (Figure 4C and data not shown). Figures 4 and 5 give detailed information on the clinical courses of patients achieving CHR, NLE, reduction in PB blast count, and MR, respectively. The 2 patients achieving CHR (PN 4 and 7) were both considered refractory on chemotherapy. They showed persistence of low numbers of blasts in PB and BM during hematopoietic reconstitution (Table 5; Figure 4A; data not shown). In this situation, imatinib was started at day 31 (PN 4) and at day 24 (PN 7) after initiation of chemotherapy, respectively.
Peripheral blood blast counts before and during imatinib therapy in 3 representative patients achieving CHR, NLE, and more than 50% reduction in blast count. (A) This 73-year-old man (PN 7) was diagnosed with AML M1. Because of age and concomitant disease he received modified induction chemotherapy (5 + 2) consisting of cytosine arabinoside 200 mg/m2 for 5 days and idarubicin 12 mg/m2 for 2 days. On day 18 after start of chemotherapy, the white blood count was 0.9 × 109/L with 20% blasts. As blasts persisted, BM cytology (2% blasts) and biopsy (5% blasts) was performed and raised the question of persistence of a small population of leukemic blasts. Because FACS analysis of BM MNCs revealed persistence of a cell population (15%) featuring the initial AML immunophenotype, the patient was considered refractory, and imatinib 600 mg/d was commenced on day 24 after start of chemotherapy. Intermittent presence of blasts in peripheral blood was documented until day 24 of imatinib therapy. On day 35 of imatinib therapy the patient had a white blood count of 8.0 × 109/L with 3.9 × 109/L neutrophils and 0% blasts. The platelet count was 139 × 109/L, and a bone marrow aspirate showed remission with 0.5% blasts. Temporally, the recovery of hematopoiesis is consistent with the time course anticipated if chemotherapy had been successful. However, in patients who achieve remission with chemotherapy persisting blasts in PB and detected by BM FACS analysis are not expected. The IRS for c-kit was 12, and 71% of blasts in BM were c-kit–positive by FACS analysis (Table 4). (B) This is a 52-year-old woman (PN 9) who was diagnosed with secondary AML M2 deriving from MDS/RAEB (myelodysplasia/refractory anemia with excess blasts). Because of numerous serious complications during the first cycle of induction chemotherapy, she then underwent matched unrelated donor stem cell transplantation. Her posttransplantation course was complicated by acute graft-versus-host disease (GVHD) grade 3 and chronic extensive GVHD. Thirteen months after stem cell transplantation she experienced a cytogenetic relapse, and the immunosuppressive regimen was discontinued. However, 19 months later there was hematologic relapse. Therefore, she was started on imatinib 600 mg/d. Four days prior to start of imatinib her white blood count was 7.6 × 109/L with 15% blasts. Platelet count was 149 × 109/L. A bone marrow aspirate showed 5% blasts, and cytogenetics revealed multiple aberrations involving chromosomes 1, 3, 7, 9, 10, and 19. No metaphases deriving from the sex-mismatched donor could be identified. On treatment with imatinib, a steady decrease of PB blasts was observed. BM aspiration obtained on day 38 already showed 0% blasts, and cytogenetics revealed partial reappearance (3 of 32) of donor type metaphases. Treatment with imatinib was continued, and on day 140 the patient cleared PB blasts. The IRS for c-kit was 12, and 90% of blasts in BM were c-kit–positive by FACS analysis (Table 4). (C) This is a 80-year-old woman (PN 14) diagnosed with AML M5a deriving from MDS. The patient presented with a WBC of 14.0 × 109/L with 30% blasts; platelet count was 8 × 109/L with a hemoglobin of 10.2 g%. A bone marrow aspirate showed 40% blasts. Because of her age and concomitant disease this patient was treated with imatinib as upfront therapy. Significant reduction of PB blasts was rapidly achieved on imatinib treatment. However, after 31 days the patient decided to stop experimental therapy and asked for hospice care. The IRS for c-kit was 4, and 59% of blasts in BM were c-kit–positive by FACS analysis (Table 4).
Peripheral blood blast counts before and during imatinib therapy in 3 representative patients achieving CHR, NLE, and more than 50% reduction in blast count. (A) This 73-year-old man (PN 7) was diagnosed with AML M1. Because of age and concomitant disease he received modified induction chemotherapy (5 + 2) consisting of cytosine arabinoside 200 mg/m2 for 5 days and idarubicin 12 mg/m2 for 2 days. On day 18 after start of chemotherapy, the white blood count was 0.9 × 109/L with 20% blasts. As blasts persisted, BM cytology (2% blasts) and biopsy (5% blasts) was performed and raised the question of persistence of a small population of leukemic blasts. Because FACS analysis of BM MNCs revealed persistence of a cell population (15%) featuring the initial AML immunophenotype, the patient was considered refractory, and imatinib 600 mg/d was commenced on day 24 after start of chemotherapy. Intermittent presence of blasts in peripheral blood was documented until day 24 of imatinib therapy. On day 35 of imatinib therapy the patient had a white blood count of 8.0 × 109/L with 3.9 × 109/L neutrophils and 0% blasts. The platelet count was 139 × 109/L, and a bone marrow aspirate showed remission with 0.5% blasts. Temporally, the recovery of hematopoiesis is consistent with the time course anticipated if chemotherapy had been successful. However, in patients who achieve remission with chemotherapy persisting blasts in PB and detected by BM FACS analysis are not expected. The IRS for c-kit was 12, and 71% of blasts in BM were c-kit–positive by FACS analysis (Table 4). (B) This is a 52-year-old woman (PN 9) who was diagnosed with secondary AML M2 deriving from MDS/RAEB (myelodysplasia/refractory anemia with excess blasts). Because of numerous serious complications during the first cycle of induction chemotherapy, she then underwent matched unrelated donor stem cell transplantation. Her posttransplantation course was complicated by acute graft-versus-host disease (GVHD) grade 3 and chronic extensive GVHD. Thirteen months after stem cell transplantation she experienced a cytogenetic relapse, and the immunosuppressive regimen was discontinued. However, 19 months later there was hematologic relapse. Therefore, she was started on imatinib 600 mg/d. Four days prior to start of imatinib her white blood count was 7.6 × 109/L with 15% blasts. Platelet count was 149 × 109/L. A bone marrow aspirate showed 5% blasts, and cytogenetics revealed multiple aberrations involving chromosomes 1, 3, 7, 9, 10, and 19. No metaphases deriving from the sex-mismatched donor could be identified. On treatment with imatinib, a steady decrease of PB blasts was observed. BM aspiration obtained on day 38 already showed 0% blasts, and cytogenetics revealed partial reappearance (3 of 32) of donor type metaphases. Treatment with imatinib was continued, and on day 140 the patient cleared PB blasts. The IRS for c-kit was 12, and 90% of blasts in BM were c-kit–positive by FACS analysis (Table 4). (C) This is a 80-year-old woman (PN 14) diagnosed with AML M5a deriving from MDS. The patient presented with a WBC of 14.0 × 109/L with 30% blasts; platelet count was 8 × 109/L with a hemoglobin of 10.2 g%. A bone marrow aspirate showed 40% blasts. Because of her age and concomitant disease this patient was treated with imatinib as upfront therapy. Significant reduction of PB blasts was rapidly achieved on imatinib treatment. However, after 31 days the patient decided to stop experimental therapy and asked for hospice care. The IRS for c-kit was 4, and 59% of blasts in BM were c-kit–positive by FACS analysis (Table 4).
Effect of imatinib on the expansion of c-kit–positive or c-kit–negative blast populations in peripheral blood. Values are derived from CD117 (c-kit)–FACS analysis of PB blast populations performed at time points indicated. Shown is a representative patient (PN 18) achieving a MR (> 50% reduction in c-kit–positive blast levels lasting ≥ 28 days) on imatinib treatment. ♦ indicates c-kit–positive PB blasts; ▪, c-kit–negative PB blasts.
Effect of imatinib on the expansion of c-kit–positive or c-kit–negative blast populations in peripheral blood. Values are derived from CD117 (c-kit)–FACS analysis of PB blast populations performed at time points indicated. Shown is a representative patient (PN 18) achieving a MR (> 50% reduction in c-kit–positive blast levels lasting ≥ 28 days) on imatinib treatment. ♦ indicates c-kit–positive PB blasts; ▪, c-kit–negative PB blasts.
Patients were allowed to receive hydroxyurea for up to 7 days within the first 28 days on study. Five patients received combination therapy because of high PB blast counts (PN 13, 19, 20, 21) or because of thrombocytosis (PN 4). Imatinib plus hydroxyurea resulted in resolution of thrombocytosis in patient 4 and in a significant reduction in PB blast counts from 23.4 × 109/L at screening to 4.0 × 109/L on day 28 in patient 21; patients 13, 19, and 20 were refractory. However, for efficacy analysis, patient 21 was not counted as a responder (Table 5) because of concomitant treatment with hydroxyurea.
Time to response was 28 days and 38 days for patients achieving a complete response, 138 days for the patient with NLE, and 33 days and 30 days for 2 patients with a MR. Median time to response was 30 days. The estimated median duration of response according to Kaplan-Meier analysis was 113 days (data not shown). Duration of CR ranged from 113 days to 224+ days. Duration of NLE was 52+ days, and the duration of MR was 41 days and 80 days, respectively.
At baseline few patients had cancer-related symptoms. One patient (4.8%) had fever, bone pain, and arthralgia (grade 1); 2 patients (9.5%) had abdominal discomfort (grade 1/2), and 4 patients (19.0%) had night sweats (grade 1/2). During the course of the study, the incidence of abdominal discomfort did not change, but fever, night sweats, bone pain, and arthralgia all improved. At the individual end, 2 patients had abdominal discomfort but the other 19 (90.5%) were free of symptoms. However, symptom improvement was likely be influenced by other medications.
Pharmacodynamic effects of imatinib on c-kit–positive AML blast cell populations
To elucidate the effects of imatinib on c-kit–positive and c-kit–negative blast populations, FACS analysis of AML blasts using a CD117-specific monoclonal antibody was performed at regular intervals throughout the course of imatinib treatment in all patients. Data shown in Figure 5 were obtained in a representative patient (PN 18), who at the start of imatinib therapy had quantitatively comparable populations of c-kit–positive and –negative blasts. Treatment with imatinib led to a rapid decrease in c-kit–positive PB blasts, and this pharmacodynamic effect was maintained with prolonged treatment until day 71 (Figure 5). At the same time, c-kit–negative blast levels were also reduced by imatinib (Figure 5). However, the relative decrease was more pronounced for c-kit–positive blasts (Figure 5). This difference between relative reductions in c-kit–positive and c-kit–negative populations was statistically significant (P = .024 [paired t test]; data not shown), suggesting that c-kit–positive blasts are preferentially sensitive to inhibition by imatinib. For the entire group of patients displaying a more than 50% reduction in PB blast counts at day 28 ± 2 days, the following results were obtained: in 1 patient this analysis was not informative because blast cell populations were more than 90% c-kit–positive at the start of imatinib, in 1 patient this analysis was not done, and in another 3 patients complete clearance of PB blasts was rapidly observed on days 5, 14, and 28. Interestingly, in another patient (PN 5) a similar result as presented in Figure 5 was obtained (data not shown).
Taken together, these results indicate a pharmacodynamic effect of imatinib on c-kit–positive blasts. Regarding the decrease observed in c-kit–negative populations, several molecular mechanisms will be discussed.
DNA-sequence analysis of c-KIT
In AML featuring inv(16) in-frame deletion plus insertion mutations have been identified in exon 8 of c-KIT.52 Rarely, mutations were detected in exon 10.52 Additional activating mutations of c-KIT were described in exons 2, 11, and 17, associated with myeloproliferative disorders, GIST, and mastocytosis, respectively.38 Recently, a novel c-KIT–activating mutation, Asn822Lys, in exon 17 was identified in AML cells.53 In this study, genomic sequence analysis of exons 2, 8, 10, 11, 12, and 17 was performed (Table 6). Within these exons, no alterations or activating mutations of c-KIT were detected in patients included (Table 6). However, cDNA sequencing revealed dominance of the Gly-Asn-Asn-Lys– (GNNK–) isoform of c-KIT in 6 of 6 patients investigated (Table 6). The GNNK– isoform is characterized by deletion of a 12-bp segment corresponding to amino acids 510 to 513 and represents an alternatively spliced isoform that has been shown to exist at varying levels in a range of tumor cell lines and blasts from patients with AML.55,56 The GNNK– isoform displays remarkably different signaling characteristics and is likely to contribute to the neoplastic process.57,58
DNA mutation analysis of c-KIT
PN . | Exon 2 . | Exon 8 . | cDNA sequence corresponding to exon 9 . | Exon 10 . | Exon 11 . | Exon 12 . | Exon 17 . |
|---|---|---|---|---|---|---|---|
| 1 | ND | ND | ND | ND | ND | ND | ND |
| 2 | WT | WT | ND | Polymorphism1 | WT | WT | WT |
| 3 | WT | WT | ND | WT | WT | WT | WT |
| 4 | WT | WT | Del 12 bp* | WT | WT | WT | WT |
| 5 | WT | WT | ND | WT | WT | WT | WT |
| 6 | ND | WT | ND | Polymorphism1 | WT | WT | WT |
| 7 | ND | WT | ND | WT | WT | WT | WT |
| 8 | WT | WT | ND | Polymorphism2 | WT | WT | WT |
| 9 | WT | WT | Del 12 bp* | WT | WT | WT | WT |
| 10 | ND | WT | Del 12 bp* | Polymorphism1 | WT | WT | WT |
| 11 | ND | WT | ND | WT | WT | WT | Polymorphism3 |
| 12 | WT | WT | ND | WT | WT | WT | WT |
| 13 | ND | ND | ND | WT | WT | WT | WT |
| 14 | ND | WT | ND | Polymorphism1 | WT | WT | WT |
| 15 | WT† | WT† | Del 12 bp* | WT | WT† | WT† | WT |
| 16 | ND | ND | ND | ND | ND | ND | ND |
| 17 | WT | WT | ND | WT | WT | WT | WT |
| 18 | ND | ND | ND | ND | ND | ND | ND |
| 19 | ND | ND | ND | ND | ND | ND | ND |
| 20 | ND | WT† | Del 12 bp* | WT* | WT† | WT† | ND |
| 21 | ND | WT† | Del 12 bp* | ND | ND | ND | ND |
PN . | Exon 2 . | Exon 8 . | cDNA sequence corresponding to exon 9 . | Exon 10 . | Exon 11 . | Exon 12 . | Exon 17 . |
|---|---|---|---|---|---|---|---|
| 1 | ND | ND | ND | ND | ND | ND | ND |
| 2 | WT | WT | ND | Polymorphism1 | WT | WT | WT |
| 3 | WT | WT | ND | WT | WT | WT | WT |
| 4 | WT | WT | Del 12 bp* | WT | WT | WT | WT |
| 5 | WT | WT | ND | WT | WT | WT | WT |
| 6 | ND | WT | ND | Polymorphism1 | WT | WT | WT |
| 7 | ND | WT | ND | WT | WT | WT | WT |
| 8 | WT | WT | ND | Polymorphism2 | WT | WT | WT |
| 9 | WT | WT | Del 12 bp* | WT | WT | WT | WT |
| 10 | ND | WT | Del 12 bp* | Polymorphism1 | WT | WT | WT |
| 11 | ND | WT | ND | WT | WT | WT | Polymorphism3 |
| 12 | WT | WT | ND | WT | WT | WT | WT |
| 13 | ND | ND | ND | WT | WT | WT | WT |
| 14 | ND | WT | ND | Polymorphism1 | WT | WT | WT |
| 15 | WT† | WT† | Del 12 bp* | WT | WT† | WT† | WT |
| 16 | ND | ND | ND | ND | ND | ND | ND |
| 17 | WT | WT | ND | WT | WT | WT | WT |
| 18 | ND | ND | ND | ND | ND | ND | ND |
| 19 | ND | ND | ND | ND | ND | ND | ND |
| 20 | ND | WT† | Del 12 bp* | WT* | WT† | WT† | ND |
| 21 | ND | WT† | Del 12 bp* | ND | ND | ND | ND |
Additionally, the following c-KIT exons were determined as WT by cDNA-sequencing: PN 4, exons 4, 5, 6, 7, 8, 14, 15, and 16; PN 9, exons 6, 7, 10, 11, 12, 13, and 14; PN 10, exons 4, 5, 6, 7, 8, 10, 11, 12, 14, 15, and 16; PN 15, exons 3, 4, 5, 6, 7, 10, 13, 14, 15, and 16; PN 16, exons 14 and 15; PN 19, exons 14, 15, and 16; PN 20, exons 4, 5, 6, 7, 13, 14, 15, and 16; PN 21, exons 4, 5, 6, 7, 14, 15, and 16.
ND, indicates not determined; WT, wild type, Polymorphism1 , ATG↔CTG54 ; Polymorphism2 , AAA↔AAG, Polymorphism3 , ATC↔ATT.
Deletion of a 12-bp segment encoding amino acids 510 to 513 (GNNK), representing an isoform of c-kit.56
Determined by cDNA-sequencing.
Safety profile
All 21 patients experienced at least one adverse event (AE). The most frequently reported adverse reactions with suspected relationship to treatment were edema (66.6%), gastrointestinal disorders (nausea [19.1%], vomiting [38.1%], diarrhea [19.1%], constipation [4.8%]), and headache (23.8%). The majority of all adverse events were mild to moderate (83.2%).
Prior to the start of imatinib, 10 and 11 patients fulfilled criteria of grade 3/4 neutropenia and grade 3/4 thrombocytopenia, respectively. During treatment with imatinib the following hematologic abnormalities were observed. Grade 1/2 and grade 3/4 neutropenia was reported in 2 and 7 patients, respectively. One patient experienced grade 1/2 and 9 patients grade 3/4 anemia, whereas grade 1/2 and grade 3/4 thrombocytopenia were reported in 1 and 8 patients, respectively.
Serious AEs with a suspected relationship to study medication were reported in 4 patients (19.1%). They included hematologic events such as neutropenia (1 patient) and thrombocytopenia (1 patient), dizziness (1 patient), and a complex condition, including hepatomegaly and ascites (1 patient). Eleven patients died during the course of the study. Seven of the deaths were due to progression of the underlying disease, and the other 4 were due to serious AEs that were not causally related to treatment.
Discussion
This pilot phase 2 study was conducted to determine whether imatinib monotherapy could induce hematologic responses in patients with c-kit–positive AML refractory to or ineligible for standard chemotherapy. Using chemotherapeutic agents, remission rates from 7% up to 68% are reported for patients with relapsed or refractory AML.9-15 These rates are primarily dependent on age, duration of first complete remission, and cytogenetic characteristics. C-kit has been shown to be critical for survival and proliferation of AML cells. Pharmacologic inhibition of c-kit by other components has been reported to result in induction of apoptosis in AML blasts and in clinical remissions.40,41 In this relatively small cohort of patients, imatinib mesylate treatment resulted in a hematologic response in 5 patients. Median duration of response was 113 days. Two patients experienced a CHR, and NLE was seen in one patient. Furthermore, MR concerning PB blast counts was reported in another 2 patients. The 2 patients achieving CHR had chemotherapy in relatively close proximity to imatinib. In these 2 patients, treatment with imatinib was started during hematopoietic reconstitution. Thus, we cannot exclude the possibility that decreases in blasts may be attributable, in part, to prior therapy. In addition, in patient 4, hydroxyurea may account for some of the responses observed. As shown in Figure 4 and Table 5, responses were generally observed in patients with low blast counts. Counting small numbers of blasts is difficult, and the reported numbers may have some variability in this situation.
Recently, Cortes et al59 reported the results of a trial exploring the effects of imatinib in 8 patients with AML and in 10 patients with myelodysplastic syndrome (MDS). None of these patients showed a hematologic response, although 2 patients had a significant reduction in spleen size (from 10 cm below costal margin [BCM] to 0 cm BCM and from 7 cm BCM to 1 cm BCM). However, imatinib was given at 400 mg/d, which is suboptimal for acute leukemia as shown by the results from the phase 2 trial in chronic myelogenous leukemia (CML) blast crisis.60,61 In acute-phase CML, none of the 32 patients treated with imatinib at 400 mg/d achieved a CHR, whereas at 600 mg/d 10% of patients achieved a CHR. Further, preliminary evidence for a clinically interesting activity of imatinib in AML has also been provided by several recent case reports.62-65
In the current trial, patients were selected on the basis of presence of c-kit receptors on at least a portion of AML blasts. Molecular analysis, including IHC and Western blotting, provided evidence that c-kit receptors were active in AML blasts from patients included in this study. Furthermore, imatinib was shown to inhibit the early signaling events of c-kit in AML blasts. In primary AML blasts cultured in medium supplemented with SCF, inhibition of c-kit signaling with imatinib results in induction of apoptosis (T.K., unpublished observations, April 2003).
The density of c-Kit receptors expressed on AML blasts was evaluated by IHC and flow cytometry (Table 4). There did not appear to be a close correlation between these parameters and clinical sensitivity. It has been shown previously that the proliferative response of primary AML cells to SCF is very heterogenous with a stimulation index of 0.7 to 68.5.66 Interestingly, in that analysis, the proliferation index did not correlate with number of SCF receptors.66 Therefore, it is conceivable that the therapeutic potential of a c-kit inhibitor is primarily determined by the in vivo proliferative response of AML blasts to SCF rather than by the number of c-kit receptors expressed. In this context, it is of note that a proliferative response to SCF in vitro has been observed in AML blasts negative for c-kit by flow cytometry analysis.67 This identifies that very low numbers of c-kit receptors (below the detection threshold of FACS analysis) are sufficient for a biologic response to SCF. Thus, the molecular basis for susceptibility to the actions of c-kit inhibition may also be present in so-called “c-kit–negative” cells. This may account for the decrease in c-kit–negative blasts seen during imatinib therapy (Figure 5). In addition, maturation of c-kit–negative AML blasts, thereby generating a c-kit–positive phenotype, has been described recently.67
Before starting imatinib, activation of c-kit was found in all patients studied. There were no sequence aberrations in c-KIT exons which have recently been described as harboring mutations in a subset of AML cases. However, cDNA sequencing revealed dominance of the GNNK– isoform of c-KIT which harbors a deletion of 4 amino acids (GNNK) in the juxtamembrane region of the extracellular receptor domain. The GNNK– isoform strongly promoted anchorage-independent growth, loss of contact inhibition, and tumorigenicity in nude mice.57 In addition, it has been shown to result in remarkably more rapid and extensive tyrosine autophosphorylation, faster internalization, and more MAP and Src kinase phosphorylation.57,58
Activation of c-Kit may derive from autocrine and/or paracrine mechanisms within the hematopoietic microenvironment.68 Because of the dominance of the GNNK– isoform, it is tempting to speculate that this results in high levels of c-kit activation for prolonged periods of time. Interactions between AML cells and bone marrow stromal cells have been described to play an important role in inhibition of apoptosis and in increased proliferation observed in AML clonogenic cells.66,69-73 Thus, we assume that reductions in leukemic blasts observed are due to suppression of c-kit signaling. However, the data obtained did not detect an obvious correlation between expression and/or activation of c-kit on leukemic blasts and clinical response to imatinib. Moreover, in view of the molecular heterogeneity in AML, it is not likely that c-kit is the sole driver of proliferation in most cases of AML. For this cohort of patients, we cannot rule out that inhibition of PDGFR signaling may have also played a role in clinical response to imatinib. Further investigations are warranted to better define the spectrum of patients sensitive to inhibition of c-kit tyrosine kinase.
The safety profile of imatinib in the current study was generally similar to that observed in earlier phase 2 clinical trials in CML.45,60,61 Imatinib was well tolerated by these high-risk patients with AML. However, episodes of severe cytopenias were frequent and required close monitoring with frequent transfusions of platelets and red blood cells. Overall grade 3/4 hematologic toxicity was in the same range or below that observed in CML accelerated or blastic phase.45,60,61 In total, 7 patients experienced grade 3 or 4 neutropenia, 9 patients had anemia, and 8 patients had thrombocytopenia. The 2 most frequent AEs considered to be treatment related were edema and vomiting.
In conclusion, in this heterogenous cohort of patients, low tumor burden and start of imatinib during hematopoietic reconstitution in a refractory situation after chemotherapy was associated with response. Some of the responses derived from relatively small reductions in leukemic blasts in BM and/or PB. Nevertheless, within the limits discussed earlier, the cases described here illustrate that tyrosine kinase inhibition of c-kit and/or PDGFR using imatinib may have potential therapeutic use in AML. However, this observation needs to be further explored in clinical trials. This further exploration also implies the need to validate the therapeutic target of this approach.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-06-2071.
Supported in part by a research grant from Novartis Pharma.
Presented in part at the 44th annual meeting of the American Society of Hematology, Philadelphia, PA, December 6-10, 2002.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank B. Carius and K. Kunz for expert technical assistance and P. Meinhardt and I. El-Kohly for excellent study management. We are grateful to Rico Hylla for assistance in documentation and statistical evaluation. We also thank F. Leigh and S. Neville who are medical writers with ACUMED for their assistance in drafting an earlier version of the manuscript. We are indebted to our patients and their families and to Dr Elke Jäger and Dr Walter Aulitzky for their collaboration.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal