Abstract
Although most multiple myeloma (MM) cases are characterized by the detection of a monoclonal immunoglobulin in the serum, about 15% of the patients present only immunoglobulin light chains, detected either in the urine or serum or both. These patients are designated as having light-chain (LC) MM. Using fiber-fluorescent in situ hybridization, and in contrast to patients and myeloma cell lines secreting heavy chains (who presented a legitimate functional IgH rearrangement in every case), LC MM never displayed a functional IgH recombination. Interestingly, most LC MM cases presented one IgH allele with a germline configuration (including the DJ region), the second allele being usually involved in an illegitimate recombination. Of note, most of these translocations occurred close to (or at) switch regions, even though in some cases, breakpoints involving nonswitch regions were observed. Thus, this study clearly showed that LC MM is due to the absence of legitimate IgH rearrangement at the DNA level, reflecting possible abnormalities in the IgH gene recombinations during B-cell maturation. Furthermore, it showed that this defect did not prevent the activation of the switch process because most of 14q32 translocations observed in LC MM occurred at switch regions.
Introduction
Multiple myeloma (MM) is characterized by the accumulation of malignant plasma cells within the bone marrow. The normal counterpart of these malignant cells remains unknown. However, molecular studies have shown that these malignant cells are heavily mutated, without intraclonal variation,1 and display a postswitch isotype in almost every case. Thus, the best candidate for the “myeloma stem cell” is a postgerminal center cell. This hypothesis is supported by the molecular analysis of the IgH gene. As in other late B-cell malignancies, the IgH gene is illegitimately rearranged in almost 100% of human myeloma cell lines (HMCLs),2 through translocations with various chromosomal partners,3-6 but especially with 3 different genes, specifically, FGFR3 or MMSET at 4p16,7 CCND1 at 11q13,8 and c-maf at 16q23.9 In contrast to most of the less mature B-cell malignancies, in which IgH breaks occur within the JH region, in MM the breakpoints are scattered in the whole constant domain. In most cases, these translocations involve switch regions, supporting the hypothesis of molecular errors occurring during the switch process, thus further supporting the hypothesis of a postgerminal origin.2 The molecular analysis of these translocations has shown that they mostly occurred on the nonproductive IgH allele.10 However, illegitimate IgH rearrangements occurring outside of the switch regions have been described, supporting the hypothesis of other mechanisms, such as somatic mutations or “genomic instability.”11
In about 15% of the patients, no complete monoclonal immunoglobulin can be detected in the serum, but these patients produce large amounts of light chains. These patients are commonly referred to as having light-chain (LC) MM. The reasons for this apparent lack of heavy-chain formation are not clearly understood. Several hypotheses can be considered, including the presence of nonfunctional rearrangements of the IgH gene, the production of aberrant IgH mRNA, instability of such mRNA, or the incapacity for heavy-chain secretion. Very few published studies have addressed this issue. The most important one has been reported by Szczepanski et al,12 and showed that in most of the 10 patients analyzed by molecular techniques, the IgH constant region was not, or aberrantly, rearranged at the DNA level. However, in our large experience on IgH rearrangements in patients with MM, we have shown that illegitimate IgH rearrangements were at least as frequent in LC MM as in other common MMs.13 Thus, the IgH gene would be able to be illegitimately rearranged, generating translocations as common MM, but would not be able to generate productive rearrangements.
To further characterize the IgH configuration in patients with LC MM, we used a special fluorescence in situ hybridization (FISH) technique on DNA fibers (known as the fiber-FISH technique) to understand the relationships between illegitimate IgH rearrangements and the absence of detectable immunoglobulin heavy chains in patients with LC MM. Using a combination of adequately chosen, differentially labeled probes, it produces a “bar code” aspect, that is, a succession of green and red signals.
Patients, materials, and methods
Cell lines and patients
To establish the germline bar code of the IgH constant region, we selected 2 non-B cell lines (K562, a chronic myeloid leukemia cell line, and HL60, a promyelocytic leukemia cell line), and 1 immature B-cell line (NALM6, a pre-B cell line), with a germline IgH constant domain. All 3 cell lines were obtained from American Type Culture Collection (Rockville, MD). We then selected 5 HMCLs derived from patients with an LC MM (for a review, see Drexler and Matsuo14 ). The OPM2 HMCL derives from a patient with a λ LC MM and is known to present a t(4;14). The SKMM1 HMCL derives from a λ LC MM and presents a t(14;20). The ANBL6 HMCL derives from a λ LC MM patient and displays a t(14;16). The 2 other HMCLs (MDN and XG5) have been established in our laboratory and derive from a κ and a λ LC MM patient, respectively. Both have been shown to present a t(11;14). We have also selected 11 patients with an LC MM (6 with a κ and 5 with a λ subtype), for whom frozen cells were available. Eight of these patients were analyzed at diagnosis, and 3 were at relapse, but also presented an LC MM at diagnosis. Four of these patients presented a t(11;14), 4 patients displayed an illegitimate IgH rearrangement with an unknown chromosomal partner, 1 patient was shown to have a t(4;14), and the 2 latter patients did not present any illegitimate IgH rearrangement. For these 11 patients, malignant plasma cells were purified using anti-CD138–coated microbeads, according to the manufacturer's instructions (Miltenyi Biotec, Paris, France) before freezing in dimethyl sulfoxide (DMSO). This step enabled us to obtain a plasma cell purity greater than 95%.
To further analyze the pertinence of the results, we have selected 2 HMCLs (U266 and LP1) and 6 patients presenting either a “common” MM, that is, secreting a full monoclonal immunoglobulin (the 2 HMCLs and 5 patients) or a nonsecretory MM (NS MM), that is, without secretion of any immunoglobulin component (1 patient). The HMCLs and the patients were chosen to display chromosomal features close to the LC MM population. One HMCL (U266) and 2 patients presented a t(11;14), 1 HMCL (LP1) and 2 patients presented a t(4;14), and 2 patients presented an illegitimate IgH rearrangement with an unknown chromosomal partner.
Probes
The IgH gene was analyzed using the following probes: cosmids Ig6 and Ig10 were provided by Terry Rabbitts (Medical Research Council, Cambridge, United Kingdom), and cosmid U2-2, cosmid 3/64, plasmid α2, and plasmid γ4 were provided by Ed Schuuring (Department of Pathology, University of Leiden, the Netherlands). All these probes have been previously reported.15-17 Briefly, the U2-2 cosmid maps to the DH and JH region; the 3/64 cosmid maps to the JH, Cμ, and Cδ regions; the α2 plasmid maps to the α2 region; and the γ4 plasmid maps to the Cγ4 region. The Ig6 cosmid has been mapped to the Cγ3 and Cγ1 regions, whereas the Ig10 cosmid is specific for the Cϵ and Cα2 regions. Chromosome 11q13 region was analyzed using 2 probes; the 6.22 cosmid (containing the CCND1 gene) was provided by Ed Schuuring. To obtain a probe specific for the BCL1 major translocation cluster (MTC) region, we screened the RPCI-11 PAC library (Research Genetics, Huntsville, AL) with an MTC-specific probe and identified PAC 799B16. Chromosome 4p16 region was analyzed with a P1 artificial chromosome (PAC) probe, specific for the FGFR3 locus, telomeric to the 4p16 breakpoints (named FGFR3 probe), kindly provided by Leif Bergsagel (Weill School of Medicine, New York, NY), and previously reported.7 Translocation involving the c-maf gene at 16q23 was analyzed using a c-maf–specific probe previously reported.9,13 Finally, the t(14;20) translocation involving the MAFB gene was characterized using the RPCI11-80L13 PAC probe.18
FISH techniques
We used 2 types of FISH techniques: “common-FISH,” on either chromosomes or interphase nuclei, and fiber-FISH on extended DNA fibers. Probes for common-FISH were labeled with SpectrumGreen-dUTP or SpectrumOrange-dUTP (Vysis, Downers Grove, IL) by nick-translation. The same probes were labeled with either biotin-dUTP or digoxigenin-dUTP (Life Technologies, Gaithersburg, MD) when used in fiber-FISH experiments. In both techniques, 60 ng of each probe was coprecipitated with 1 μg human Cot1-DNA as a competitor and resuspended in 10 μL Hybrisol VII (Q-Biogen, Ilkirch, France). After denaturation for 10 minutes at 75° C, the probes and the competitor were allowed to preanneal at 37° C for 15 to 30 minutes. During this time, the slides bearing either plasma cells or DNA fibers were denatured for 2 minutes at 73° C in 70% formamide/2 × standard sodium citrate (SSC), pH 7, and dehydrated during 1 minute in each cold ethanol solution (70%, 85%, and 100%). Slides were then placed on a slide warmer to allow rapid ethanol evaporation. Probes were then dropped on the cells or DNA fibers, covered with a 22 × 22-mm coverslip, and hybridized overnight at 37° C. The following day, the coverslip was carefully removed, and the slides with nuclei and chromosomes were washed in 2 × SSC, pH 7, at 73° C for 4 minutes to remove the nonhybridized probes. Slides were then rinsed in 2 × SSC/0.1% Triton, and 10μL antifade with DAPI (4,6-diamino-2-phenylindole; Vectashield, Vector Laboratories, Burlingame, CA) was dropped on the cells before they were covered with a coverslip.
In FISH experiments on DNA fibers with biotin- and digoxigenin-labeled probes, fibers were washed 3 times in 2 × SSC at 37° C for 5 minutes, and once in TNT buffer (1 × Tris [tris(hydroxymethyl) aminomethane] NaCl/0.05% Tween 20) at room temperature for 5 minutes. Fibers were then covered with 100 μL TNB buffer (1 × Tris NaCl/0.5% blocking reagent) at 37° C for 15 minutes, to saturate the nonspecific sites, and then washed for 5 minutes in TNT buffer at room temperature. We then incubated the fibers with a 4-step revelation protocol. Each antibody was diluted in TNB buffer according to the manufacturer's instructions. First, slides were incubated with Texas red–avidin (Vector Laboratories), second with goat biotinylated antiavidin (Vector Laboratories) and mouse fluorescein isothiocyanate (FITC)–antidigoxigenin (Jackson ImmunoResearch Laboratories, Bar Harbor, ME), third with Texas red–avidin and rabbit FITC-antimouse (Jackson ImmunoResearch), and fourth with goat FITC-antirabbit (Jackson ImmunoResearch). For each step, 100 μL of antibody diluted in TNB buffer was dropped on the slides and covered with a coverslip. Incubations were performed at 37° C for 30 minutes, and slides were washed 3 times in TNT buffer for 5 minutes at room temperature after each antibody incubation.
Fiber preparation
We used a technique derived from that described by Heiskanen et al.19 We prepared 100-μL 1.2% low-melting-point agarose plugs containing 106 cells. Plugs were then incubated overnight in a lithium dodecyl sulfate solution (1% dodecyl sulfate/0.01 M Tris HCl/EDTA [ethylenediaminetetraacetic acid 0.1M) at 37° C to lyse the cells. DNA fibers were then released by two 15-minute incubations in a 0.02% N-laurylsarcosin solution (0.02% N-lauryl-sarcosin/0.002 M Tris HCl/0.1M EDTA). Finally, the plugs were washed several times in Tris-EDTA (20:1) at room temperature and were stored in this buffer at 4° C until use, even several months later. To prepare slides with DNA fibers, plugs were cut into 8 equal parts. Each one eighth of plug was placed on a slide coated with poly-L-lysine, covered with 15 μL distilled water. The slides were then placed in a 1000-W microwave for 15 seconds to melt the agarose. Fibers were then mechanically stretched using the edge of another slide. Finally, the fibers were fixed by UV irradiation for 7 minutes.
Results
Establishment of the germline CH bar code
The use of an adequately chosen combination of green and red probes generated a fluorescent “bar code.” We first established the bar code of the IgH constant domain on nonmyeloma cell lines, known to lack any rearrangement of this region. The combination of the U2-2, 3/64, Ig6, and Ig10 probes covered the entire constant region, except 2 small gaps: one 33-kilobase (kb) gap between the Cδ and Cγ3 loci, and another 51-kb gap between the Cα1 and Cψγ loci (Figure 1A). At least 50 fibers of each cell line were analyzed for all the probes. Even though interindividual variations in stretching were observed, the intraindividual variability was low (< 10% variation). Consequently, all the fibers analyzed in this study were prepared by the same person (M.-L.C.). The U2-2 probe covered the most 3′ DH segments and all 6 JH loci. The 3/64 probe covered the Cμ and Cδ loci, and overlapped U2-2 on a few kilobases. The Ig6 probe has been described to contain the Cγ3 locus and the surrounding regions, but not other CH loci. Using fiber-FISH, the probe generated 2 distinct signals, each covering about twice the size of the insert, because it cross-hybridized on all Cγ loci (including the Cψγ locus) and the surrounding regions, reflecting the evolutionary duplications observed in the IgH gene. Similarly, the Ig10 probe generated 2 similar images, 2 stretches mapping to the ψϵ-α1 region and the ϵ-α2 region, respectively, plus 2 shorter signals (about 5 kb) located 3′ to the γ3 and ψγ loci, respectively. Finally, the γ4 probe generated 5 short signals, corresponding to the 4 γ genes, plus the ψγ locus. Of note, another bar code was observed in about half of the NALM6 fibers. In these fibers, a sixth γ signal was observed, within the second evolutionary duplicated unit. In this case, the region hybridized by the Ig6 probe was longer. The combination of the Ig10 and γ4 probes was in favor of the insertion/duplication of a γ region between γ2 and γ4 loci. This specific configuration was also observed in 7 patients of this series.
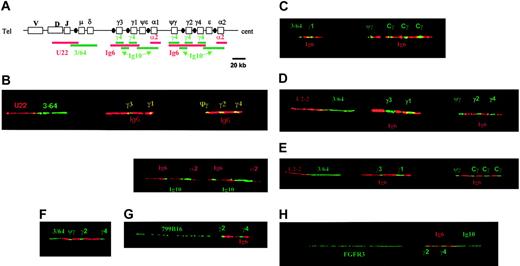
Bar code representation and results of probe studies. (A) This figure is a schematic representation of the bar code obtained with the combination of all the probes. Because of the evolutionary duplication of the γ, ϵ, and α regions, all the probes specific for these regions generated 2 stretches. Also, because of the conservation of the γ loci, the γ4 probe hybridized to all these γ loci. Finally, the Ig10 probe generated 4 signals (instead of 2), 2 signals corresponding to the ϵ and α loci, and 2 other smaller signals, located on the telomeric side of the normal hybridized region. Of note, one of the smaller γ signals is located on the 3′ side of ψγ, enabling us to differentiate this ψγ signal from an extra γ signal (polymorphism). The yellow signals correspond to the superimposition of green and red signals. (B) The first image represents a nonrearranged IgH allele from the HL60 leukemia cell line, hybridized with the U2-2, 3/64, Ig6, and γ4 probes. The second image represents the same allele hybridized with the Ig6, Ig10, and α2 probes. (C) This fiber presents a legitimate switch (μ-γ1, patient 16). The U2-2 probe is not visible because of the large size of the deleted regions. The 3/64 probe generates only a small green signal on the 5′ side of γ1. This patient presents an extra γ signal within the centromeric γ stretch (polymorphism). (D) A nonrearranged fiber observed in patient no. 6, hybridized with the U2-2, 3/64, Ig6, and γ4 probes. (E) This fiber, hybridized with the U2-2, 3/64, Ig6, and γ4 probes, is also in germline configuration (patient no. 8). Of note, this fiber presents an extra signal with the γ4 probe, corresponding to a polymorphism. (F) This fiber (from the SKMM1 HMCL) has been hybridized with the U2-2, 3/64, Ig6, and γ4 probes and shows a nonfunctional rearrangement with deletion of the DNA located between the 3/64 and ψγ regions. (G) A typical fiber from patient no. 1, presenting a t(11;14), and hybridized with the Ig6, γ4, and 799B16 (specific for 11q13) probes. This fiber clearly shows a CCND1-IgH rearrangement involving the Sγ2 region. (H) This fiber (from the OPM2 HMCL) has been hybridized with the Ig6, Ig10, γ4, and FGFR3 probes, showing a typical FGFR3-IgH rearrangement involving the Sγ2 region.
Bar code representation and results of probe studies. (A) This figure is a schematic representation of the bar code obtained with the combination of all the probes. Because of the evolutionary duplication of the γ, ϵ, and α regions, all the probes specific for these regions generated 2 stretches. Also, because of the conservation of the γ loci, the γ4 probe hybridized to all these γ loci. Finally, the Ig10 probe generated 4 signals (instead of 2), 2 signals corresponding to the ϵ and α loci, and 2 other smaller signals, located on the telomeric side of the normal hybridized region. Of note, one of the smaller γ signals is located on the 3′ side of ψγ, enabling us to differentiate this ψγ signal from an extra γ signal (polymorphism). The yellow signals correspond to the superimposition of green and red signals. (B) The first image represents a nonrearranged IgH allele from the HL60 leukemia cell line, hybridized with the U2-2, 3/64, Ig6, and γ4 probes. The second image represents the same allele hybridized with the Ig6, Ig10, and α2 probes. (C) This fiber presents a legitimate switch (μ-γ1, patient 16). The U2-2 probe is not visible because of the large size of the deleted regions. The 3/64 probe generates only a small green signal on the 5′ side of γ1. This patient presents an extra γ signal within the centromeric γ stretch (polymorphism). (D) A nonrearranged fiber observed in patient no. 6, hybridized with the U2-2, 3/64, Ig6, and γ4 probes. (E) This fiber, hybridized with the U2-2, 3/64, Ig6, and γ4 probes, is also in germline configuration (patient no. 8). Of note, this fiber presents an extra signal with the γ4 probe, corresponding to a polymorphism. (F) This fiber (from the SKMM1 HMCL) has been hybridized with the U2-2, 3/64, Ig6, and γ4 probes and shows a nonfunctional rearrangement with deletion of the DNA located between the 3/64 and ψγ regions. (G) A typical fiber from patient no. 1, presenting a t(11;14), and hybridized with the Ig6, γ4, and 799B16 (specific for 11q13) probes. This fiber clearly shows a CCND1-IgH rearrangement involving the Sγ2 region. (H) This fiber (from the OPM2 HMCL) has been hybridized with the Ig6, Ig10, γ4, and FGFR3 probes, showing a typical FGFR3-IgH rearrangement involving the Sγ2 region.
LC MM patients and HMCL analyses
We then analyzed the 11 LC MM patients and the 5 LC HMCLs using all these probes. At least 50 fibers were analyzed in each HMCL and patient. The results are summarized in Table 1.
OPM2 HMCL. Fiber-FISH experiments revealed 2 types of fibers, in agreement with the metaphase FISH data showing a t(4;14), and an insertion of IgH sequences in the 8q24 region. One fiber contained the γ1 and α1 regions, and a second fiber contained the γ2 region and the gDNA 3′ to this locus. Hybridization experiments combining the IgH and the FGFR3 probes showed that the der(14)t(4;14) corresponded to the second fiber. The distance between the γ2 locus and the FGFR3 probe was 47 kb (in agreement with published data). The second image corresponded to the insertion in the 8q24 region. No productive rearrangement was observed.
MDN HMCL. Two types of fibers were observed, one containing a truncated 3/64 probe and then a germline constant domain, and a second fiber containing the ψγ locus and the 3′ constant regions in germline configuration. Metaphase FISH experiments revealed a t(11;14), plus a cytogenetically normal chromosome 14. Fiber-FISH cohybridization experiments with the 11q13-specific probes failed to show any colocalization, probably because of a more centromeric breakpoint on chromosome 11. Thus, the fiber corresponding to the der(14)t(11;14) could not be determined. Nevertheless, no legitimate rearrangement was present in this HMCL.
XG5 HMCL. Two types of fibers were found, in agreement with the metaphase FISH results (showing a t(11;14) and a cytogenetically normal chromosome 14). The first fiber contained no U2-2, a truncated 3/64, and then a germline CH domain, except an interstitial deletion of Cα1 locus. The second fiber corresponded to the der(14)t(11;14) and was highly rearranged; the 799B16 11q13 probe was truncated (54 kb instead of 110 kb), fused to a short 3/64 probe, and then the region 3′ to α1 (with deletion of the DNA located between Cμ-Cδ and Cψγ). No legitimate switch was observed.
SKMM1 HMCL. Metaphase FISH experiments showed a t(14;20), a cytogenetically normal chromosome 14, and a der(8) bearing 2 insertions of IgH sequences. Fiber-FISH revealed 3 types of fibers. The first fiber contained a truncated 3/64 probe and then a germline CH domain. The second fiber contained also a truncated 3/64 probe, juxtaposed to the ψγ locus, and then a germline CH domain, resembling a legitimate switch involving the ψγ locus. The third fiber corresponded to the der(20)t(14;20), the ψγ locus being juxtaposed with a truncated RPCI11-80L13 PAC probe (normally located about 600 kb centromeric to MAFB on chromosome 20). No productive IgH rearrangement was observed.
ANBL6 HMCL. Metaphase FISH showed a t(14;16) and a cytogenetically normal chromosome 14. These data were confirmed by fiber-FISH, revealing 2 types of fibers. The first fiber contained a truncated 3/64 probe and then a germline CH domain. The second fiber contained also a truncated 3/64 probe, juxtaposed to the ψγ locus, and then a germline CH domain, resembling a legitimate switch involving the ψγ locus, as in the SKMM1 HMCL. No legitimate switch was observed. Hybridization with the c-maf probe failed to identify which fiber was involved in the t(14;16).
Patients. In 9 of 11 patients with LC MM, one CH allele presented a germline configuration (patients no. 3-11; Figure 1B). This germline CH allele cannot correspond to contaminating nonplasma cells. In all the cases, plasma cells represent more than 95% of the cells. Moreover, an equal number of germline and rearranged fibers was observed in all the cases, confirming the plasma cell origin of these fibers. In 7 patients presenting a germline CH allele (patients no. 3-9), the second allele was involved in an illegitimate IgH rearrangement (“Patients and HMCL secreting a complete monoclonal immunoglobulin do present legitimate CH rearrangements in all cases”). In patients no. 10 and 11 (patients lacking an illegitimate IgH rearrangement by interphase FISH), the second allele presented a nonfunctional CH rearrangement. In the 2 other patients (patients no. 1 and 2), one allele was involved in a 14q32 translocation. In patient no. 1, the second allele was in germline configuration between the 2 α loci, but all the 5′ CH sequences have been lost. In patient no. 2, the second allele presented an apparently legitimate Cμ-Cγ2 rearrangement; the 3/64 probe was truncated and physically associated with the 3′γ2 region.
Patients and HMCL secreting a complete monoclonal immunoglobulin do present legitimate CH rearrangements in all cases
We then analyzed 2 HMCLs and 6 patients presenting either a common MM (2 HMCLs and 5 patients) or an NS MM (1 patient). One HMCL (U266) and 2 patients presented a t(11;14), one HMCL (LP1) and 2 patients presented a t(4;14), and 2 patients presented an illegitimate IgH rearrangement with an unknown chromosomal partner.
U266 HMCL. Metaphase FISH showed an insertion of IgH sequences at 11q13 and a nonrearranged IgH locus. These data were in agreement with fiber-FISH results, showing a localization of the 6.22 probe immediately 5′ to the α1 locus, and a second fiber combining a truncated 3/64 probe and a truncated Ig10 probe, corresponding to the Sμ-Sϵ legitimate rearrangement (in agreement with the IgE isotype).
LP1 HMCL. Fiber-FISH experiments revealed 2 types of fibers. The first fiber presented a Sμ-Sγ1 legitimate rearrangement (in agreement with the IgG isotype). The second fiber corresponded to the der(14)t(4;14), the FGFR3 probe being located 5′ to the ψγ locus.
Patients. The 5 patients presenting a common MM (3 IgG and 2 IgA) all presented a legitimately rearranged fiber (corresponding to the immunoglobulin isotype), plus an illegitimately rearranged (translocated) fiber corresponding to the der(14). Finally, the patient presenting the NS MM displayed 2 types of fibers, 1 bearing the t(11;14), with a breakpoint 5′ to γ1, and a second fiber bearing a nonrearranged U2-2 probe fused to the ψγ region.
Translocation breakpoints
We then analyzed the location of the IgH breakpoints on the (der)14 for patients and HMCLs presenting a 14q32 translocation. Nine patients or HMCLs presented a t(11;14), 5 others displayed a t(4;14), and 8 presented a 14q32 translocation with another chromosomal partner (including 1 with c-maf and 1 with MAFB). A mechanism involving the switch process was observed in 13 cases, but other mechanisms must be proposed in the 9 other cases (Table 1). Of note, a breakpoint located immediately 5′ to the ψγ locus was observed in 4 cases (MDN, SKMM1, LP1 HMCLs, and patient no. 15). Because the Ig10 probe generated an extra signal on the 3′ side of the ψγ region, this locus could be unambiguously differentiated from other γ loci, confirming the breakpoints on the ψγ 5′ side. In patient no. 5, who presented a t(11;14), the translocated fiber displayed a deletion of the region located between the δ and γ1 loci, but with nonrearranged U2-2 and 3/64 probes (signing a nonrearranged D-J region). The 799B16 probe is fused to U2-2. In patient 6 (14q32 translocation with an unknown chromosomal partner), the second fiber presented an apparently legitimate μ-ψγ rearrangement. Patients no. 7 and 8 (both presenting a 14q32 translocation with an unknown chromosomal partner) displayed similar configurations: nonrearranged U2-2 and 3/64 probes fused to the ψγ locus. Of note, whereas the colocalization of the FGFR3 and IgH probes was observed in all 5 cases with t(4;14), colocalization of the CCND1 and IgH probes was seen in only 5 of the 9 cases with t(11;14), probably because of the scattering of the 11q13 breakpoints over a much larger distance.
Discussion
Unlike most B-cell neoplasms, MM is characterized by complex rearrangements involving the IgH gene, particularly at the constant locus. According to the mature phenotype of malignant plasma cells, most patients present a monoclonal immunoglobulin in the serum. Similarly to normal plasma cells, myeloma cells secrete most frequently an IgG or an IgA, rarely IgD, IgM, or IgE. The ability to produce mature IgG or an IgA presupposes several IgH rearrangements at the DNA level. Like in other B cells, VDJ recombinations occur during the initial differentiation steps within the bone marrow. Unlike less mature B cells, plasma cells have undergone class switching, enabling the formation of an IgG or an IgA instead of an IgM. This switch process involves complex, not totally resolved, mechanisms, leading to the recombination of 2 switch regions, usually the Sμ and another more 3′ switch (S) region. This process usually occurs on one allele. In most cases of MM analyzed so far, the second allele is involved in a translocation with various chromosomal partners. This situation is observed in 60% to 70% of the patients.3-6 Most of the translocation breakpoints cloned so far involved switch regions, even though other locations have been recently reported.11
However, in about 15% of patients with MM, no complete immunoglobulin is detected in the serum or the urine. In contrast, in most of these patients, large amounts of monoclonal light chains are detected in the urine and to a lesser extent in the serum. These so-called light-chain MM (LC MM) patients have in two thirds of the cases a κ LC type, whereas the remaining one third of the patients produce λ LC (a proportion similar to that observed in normal plasma cells). The mechanisms leading to this peculiar type of MM are not fully understood. Several hypotheses have been proposed: (1) abnormalities at the DNA level preventing the formation of a productive (legitimate) rearrangement; (2) instability of the IgH mRNA or protein; or (3) abnormalities in the heavy/light-chain assembly process. A recent study strongly suggested that the absence of heavy chain was related to defects in DNA rearrangements.12 Our current study further extends the understanding of the molecular bases of LC MM. With the exception of one patient, we have shown that no legitimate rearrangement of the constant domain occurred in 10 patients and 5 HMCLs. In the other patient (no. 2), a seemingly legitimate μ-γ2 rearrangement was observed. In this case, the absence of IgG2 might be related to nonfunctional VDJ rearrangements or out-of-frame recombinations. In the other 10 patients and 5 HMCLs, the fiber-FISH analysis failed to identify any productive rearrangement. Of note, in 9 of these patients, one fiber displayed an apparently fully germinal configuration, including the U2-2 and 3/64 probes, meaning that no DJ recombination occurred on this allele. Because the U2-2 probe covers most of the JH and DH regions, physiologic VDJ rearrangements remove most the probe targets, making it sometimes invisible. In contrast, in these 9 patients, the U2-2 probe presented a normal size, ruling out any classical VDJ recombination. One hypothesis could have been a contamination by nonplasma cells. This hypothesis can be easily ruled out because the percentage of plasma cells before freezing was always over 95%. Moreover, the analysis of patients with common MM (secreting a complete monoclonal immunoglobulin) showed that these patients do present 2 types of fibers, one legitimately rearranged and another one usually involved in a translocation. In these patients, an equal number of these 2 types of fibers was always observed, with very occasionally a nonrearranged IgH allele (probably corresponding to the few contaminating nonplasma cells).
This situation has been previously observed by Szczepanski et al.12 Discrepant data have been published regarding the physiologic IgH rearrangements. One study suggested that IgH rearrangements (especially those involving the VDJ regions) occur on both alleles, even though only one is functional.20 Another study suggested that VDJ rearrangements were observed mainly on one allele.21 In these patients, IgH rearrangements were observed only on the translocated allele. The current understanding of B-cell maturation until the plasma cell stage supposes the occurrence of functional (legitimate) VDJ rearrangements within the bone marrow, to “authorize” the B cell to pursue its differentiation within peripheral lymphoid organs. A likely hypothesis would be to suppose the occurrence of functional IgH rearrangements on the second (usually translocated) allele, enabling the B-cell survival and differentiation until the plasma cell stage, and then an illegitimate rearrangement producing the translocation. Interestingly, the 2 patients lacking any illegitimate IgH rearrangement (patients no. 10 and 11) presented one allele in germline configuration, and one allele with a nonfunctional recombination. However, we cannot exclude the possibility that this second allele underwent a first legitimate (functional) recombination, and then a secondary nonfunctional rearrangement.
In patients and HMCLs secreting an intact monoclonal immunoglobulin, we have shown that one allele presented a functional (legitimate) rearrangement, whereas the second allele was involved in a translocation. Thus, our results clearly demonstrate that the absence of heavy-chain secretion in LC MM is related to the absence of functional IgH rearrangement at the DNA level. These data are further strengthened by our recent study using gene expression profiling, showing that LC MM presents neither the IgG nor the IgA signature.22 Six patients in the present study have also been analyzed by gene expression profiling and did not express any heavy-chain RNA. Of note, the only patient with an NS MM in this current series presented a similar configuration, 1 allele involved in a t(11;14) and the second one presenting a nonfunctional rearrangement. Based on these similarities between LC and NS MM, common molecular abnormalities may relate these 2 entities. Furthermore, as previously reported, both types of MM present an abnormally high incidence of t(11;14).23 The differences may be due to the IgL (κ and λ) configuration, functionally rearranged in LC MM, and possibly nonfunctionally rearranged in NS MM.
Because the fiber-FISH technique allows a direct visualization of the entire IgH locus, it was possible to analyze the breakpoint location in cases harboring a 14q32 translocation, enabling an approach to the mechanisms involved in these illegitimate rearrangements. Using several sets of probes, we attempted to locate the breakpoints on each chromosomal partner. We showed that the t(4;14) involved a switch region in 4 of the 5 cases, confirming previous studies showing Iμ-MMSET transcripts in most t(4;14).24 We detected an Iμ-MMSET transcript in these 4 patients or HMCLs, but not in the LP1 HMCL (data not shown). Interestingly, this fifth case (the LP1 HMCL) presented a breakpoint located at the 5′ side of ψγ. Of note, 3 other cases (harboring other 14q32 translocations) presented a breakpoint in this region. This breakpoint has also been described in 2 other HMCLs in the literature, suggesting the presence of a nonrandom hotspot.25 So far, no switch region has been described upstream from ψγ. However, because of the recurrence of breakpoints in this region, and because of the similarities between the ψγ and other γ regions, the hypothesis of a (nonclassical?) switch region might be raised. The t(11;14) involved a classical switch region in 6 of 9 cases. A seventh case was one of the HMCLs with a ψγ breakpoint. In the XG5 HMCL, the breakpoint was located within the 3/64 cosmid probe, but not at the Sμ site. Finally, the ninth patient with t(11;14) (patient no. 5) presented a breakpoint in the VD region. These 2 latter cases suggested the involvement of nonswitch mechanisms, such as somatic hypermutation. Finally, in the 8 other cases with nonrandom chromosomal partners, a switch breakpoint was identified in 3 cases (plus 2 cases with ψγ breakpoints). In the 3 last patients, the breakpoints did not involve switch regions and were located in the VD region in at least 2 of them. From these data, and hypothesizing the presence of a nonclassical switch region upstream from ψγ, switch-induced translocations were observed in 17 of 22 cases with a 14q32 translocation. In the other 5 cases, other mechanisms should be considered.
In conclusion, most LC MM patients' plasma cells present abnormalities in the IgH rearrangements, at the DNA level, explaining their inability to produce immunoglobulin heavy chains. In most cases, one IgH allele presents a germline configuration (at least for the D, J, and C domains), whereas the second allele is involved in a translocation. These results are in contrast to those observed in classical MM, in which one allele presents a functional rearrangement, whereas the second allele is usually involved in a translocation. Interestingly, the switch process appears to have been activated in both types of MM, because most of these translocations occurred at switch regions. Our data also showed a novel breakpoint hotspot, on the 5′ side from ψγ, raising the hypothesis of a (nonclassical?) switch region.
Appendix
Members of the Intergroupe Francophone du Myélome:
France: Dr Fillol, Rhumatologie, Agen; Dr Rispal, Médecine Interne, Agen; Dr Gaspard, Oncologie Médicale, Albi; Dr Frenkiel, Médecine Interne, Alençon; Dr Garidi, Maladies du Sang, Amiens; Dr Salle, Médecine Interne, Amiens; Dr Dib, Maladies du Sang, Angers; Dr Martin, Hématologie, Annecy; Dr Dingremont, Médecine Interne, Auch; Dr Lepeu, Hématologie, Avignon; Dr Renoux, Maladies du Sang, Bayonne; Dr Voillat, Hématologie, Besançon; Dr Rodon, Médecine Interne, Blois; Prof Casassus, Hématologie, Bobigny; Dr Eghbali, Hématologie, Bordeaux; Dr Fitoussi, Onco-Hématologie, Bordeaux; Prof Marit, Maladies du Sang, Bordeaux; Dr Agape, Hématologie, Boulogne/Mer; Dr Orfeuvre, Médecine Interne, Bourg/Bresse; Prof Berthou, Hématologie, Brest; Dr Talarmin, Médecine Interne, Brest; Dr Levaltier, Hématologie, Caen; Dr Peny, Oncologie, Caen; Dr Lassoued, Rhumatologie, Cahors; Dr Morlock, Rhumatologie, Carcassonne; Dr Pitie, Médecine Interne, Castres; Dr Salles, Hématologie, Chalon/Saône; Dr Blanc, Hématologie, Chambéry; Dr Zannetti, Hématologie, Cholet; Prof Nédellec, Hématologie, Clamart; Dr Fouilhoux, Hématologie, Clermont-Ferrand; Dr Audhuy, Onco-Hématologie, Colmar; Dr Zylberait, Onco-Hématologie, Compiègne; Dr Guy, Hématologie, Dijon; Dr Maillefert, Rhumatologie, Dijon; Dr Valenza, Médecine Interne, Draguignan; Dr Wetterwald, Hématologie, Dunkerque; Prof Sotto, Dr Pégourié,Hématologie, Grenoble; Dr Campos, Médecine B, Hadueneau; Dr Tiab, Médecine Interne, La Roche/Yon; Dr Fleck, Oncologie, La Rochelle; Dr Jacomy, Médecine Interne, Laval; Dr Zarnitsky, Médecine, Le Havre; Dr Dugay, Médecine Interne, Le Mans; Dr Voog, Onco-Hématologie, Le Mans; Dr Dervite, Hématologie, Lens; Prof Rose, Onco-Hématologie, Lille; Prof Facon, Maladies du Sang, Lille; Dr Moreau, Médecine Interne, Lorient; Dr Troncy, Hématologie, Lyon; Dr Sebban, Hématologie, Lyon-Sud; Dr Belhabri, Onco-Hématologie, Macon; Prof Sébahoun, Hématologie, Marseille; Dr Stoppa, Hématologie, Marseille; Dr Nezri, Médecine Interne, Martigues; Dr Gandon, Médecine Interne, Mazamet; Dr Dorvaux, Hématologie, Metz; Dr Carreiro, Dr Redon, Médecine Interne, Montauban; Dr Chait, Hématologie, Montfermeil; Dr Denizon, Hématologie, Montluçon; Dr Eisenmann, Hématologie, Mulhouse; Dr Hulin, Hématologie, Nancy; Dr Ramée, Hématologie, Nantes; Prof Harousseau, Prof Moreau, Hématologie, Nantes; Prof Euller-Ziegler, Rhumatologie, Nice; Prof Fuzibet, Médecine Interne, Nice; Prof Cassuto, Hématologie, Nice; Prof Thyss, Onco-Hématologie, Nice; Dr D'Harlac, Médecine Interne, Niort; Dr Lucas, Oncologie, Orléans; Dr Decaudin, Hématologie, Paris; Dr Rio, Hématologie, Paris; Dr Garderet, Maladies du Sang, Paris; Dr Merlet, Médecine Interne, Pau; Dr Vallantin, Hématologie, Perpignan; Dr Bouabdallah, Hématologie, Bordeaux; Dr Azaïs, Rhumatologie, Poitiers; Dr Renaud, Hématologie, Poitiers; Dr Vilque, Hématologie, Quimper; Dr Kolb, Hématologie, Reims; Dr Gagneux, Rhumatologie, Reims; Prof Grosbois, Médecine Interne, Rennes; Dr Dauriac, Hématologie, Rennes; Dr Gouttebel, Médecine Interne, Roanne; Dr Marre, Oncologie, Rodez; Dr Plantier, Hématologie, Roubaix; Dr Le Loet, Rhumatologie, Rouen; Prof Monconduit, Rhumatologie, Rouen; Dr Maigre, Hématologie, Saumur; Dr Morice, Hématologie, St-Brieuc; Dr Janvier, Hématologie, St-Cloud; Dr Jaubert, Hématologie, St-Etienne; Dr Collet, Rhumatologie, St-Etienne; Dr Azagury, Médecine Interne, St-Germain/Laye; Dr Schlaifer, Oncologie, Tarbes; Dr De Jaureguiberry, Médecine Interne, Toulon; Prof Attal, Dr Huynh, Hématologie; Toulouse, Dr Benbouker, Onco-Hématologie, Tours; Dr Anglaret, Hématologie, Valence; Dr Simon, Hématologie, Valenciennes; Dr Jardel, Médecine Interne, Vannes; and Dr Brault, Hématologie, Villejuif.
Belgium: Prof Feremans, Hématologie, Bruxelles; Prof Bron, Hématologie, Bruxelles; Dr André, Hématologie, Charleroi; Dr Prijck, Hématologie, Liège; and Prof Bosly, Dr Doyen, Hématologie, Yvoir.
Switzerland: Dr Muller, Hématologie, Aarau; Dr Bernimoulin, Hématologie, Basel; Dr Zenhausern, Hématologie, Berne; Prof Matthes, Hématologie, Genève; and Prof Leyvraz, Hématologie, Lausanne.
Prepublished online as Blood First Edition Paper, January 8, 2004; DOI 10.1182/blood-2003-07-2501.
Supported by grants from the Ligue contre le Cancer and from a Programme Hospitalier de Recherche Clinique. F.M. and M.L.C. contributed equally to this work.
A complete list of members of the Intergroupe Francophone du Myélome appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Rafael Fonseca for the revision of this manuscript.