Abstract
Natural interferon (IFN)-producing cells (IPCs) recognize certain viruses and DNA containing deoxycytidylate-phosphatedeoxyguanylate (CpG) motifs through the toll-like receptor (TLR) 9, resulting in secretion of IFN-α, interleukin 12 (IL-12), and proinflammatory chemokines. Human IPCs are found mainly in inflamed lymph nodes, where they are presumably recruited from the blood to activate both innate and adaptive responses to microbial infections. Demonstrating IPC recruitment and function in murine infection models has been difficult because multiple antibodies are required to distinguish IPCs from other immune cells and very few IPCs can be recovered from lymph nodes. Here we describe a monoclonal antibody (mAb) that exclusively detects murine IPCs in all lymphoid organs under both normal and inflammatory conditions. Using this antibody, we demonstrate that IPCs are normally present in the T-cell zone of lymph nodes and spleen and that inoculation of peripheral tissues with inflammatory stimuli triggers recruitment of IPC into sentinel lymph nodes, whether the stimuli are able to directly stimulate IPCs through TLR or not. Remarkably, we show that incubation of IPCs with the antibody in vitro or administration of the antibody in vivo dramatically reduce secretion of IFN-α in response to CpG DNA without causing IPC depletion. Thus, the antibody identifies an IPC-specific surface molecule that, when engaged, inhibits IFN-α secretion. (Blood. 2004;103:4201-4206)
Introduction
Natural interferon-producing cells (IPCs) were originally identified in human blood as a small subset of leukocytes that secrete high levels of type I interferon (IFN) (ie, IFN-α and -β) when incubated in vitro with a variety of DNA and RNA viruses, including herpes simplex virus type 1 (HSV-1), cytomegalovirus (CMV), Sendai virus, and influenza virus.1-5 More recently, it was shown that human and murine IPCs also respond to single-stranded oligodeoxynucleotides (ODNs) containing deoxycytidylate-phosphate-deoxyguanylate (CpG) motifs,6-9 which mimic unmethylated CG dinucleotides found in microbial DNA.10 Recognition of CpG ODNs and certain viruses by IPCs is mediated by Toll-like receptor (TLR) 9.11 In addition to IFN-α and -β, IPCs secrete interleukin 12 (IL-12)12-14 and proinflammatory chemokines in response to viruses and CpG ODNs.15-17 Together, these cytokines and chemokines recruite and activate natural killer (NK) cells and T cells as well as modulating the antigen-presenting function of dendritic cells (DCs).18 Furthermore, IPCs themselves function as antigen-presenting cells that expand memory T cells and induce T helper 1 (Th1) differentiation.17-20 Thus, IPCs may provide a first line of host defense against viral infections by activating both innate and adaptive responses in vivo.
To interact with and activate NK cells, T cells, and antigen-presenting cells, IPCs must migrate from the blood into lymph nodes during an immune response in vivo. Consistent with this hypothesis, human IPCs are particularly abundant in inflamed lymph nodes of individuals affected by chronic infections or autoimmune diseases, especially around high endothelial venules (HEVs).21-23 Moreover, IPCs express homing molecules and chemokine receptors that can direct their migration from the blood into inflamed lymph nodes through HEVs.5,15,24,25 In mice, footpad immunization with HSV-1 induces a modest influx of IPCs in draining lymph nodes.26 To date, however, there is no experimental evidence that IPCs accumulate in lymph nodes as a direct result of inflammation. Demonstrating IPC accumulation in mouse lymph nodes is difficult due to the low numbers of IPCs, their complex phenotype (CD11c+, Ly-6C+, Gr-1+, B220+, CD11b-, CD8a+/-),13,24,27 and the lack of an antibody that directly detects them in both normal and inflammatory conditions.
Here we describe a monoclonal antibody (mAb) that exclusively recognizes murine IPCs in all lymphoid organs under both normal and inflammatory conditions. Using this antibody, we demonstrate recruitment of murine IPCs into lymph nodes draining inflammations caused by inoculation of bacterial products in peripheral tissues. Most important, we show that either incubation of IPCs with the antibody in vitro or administration of the antibody in vivo dramatically reduce secretion of IFN-α in response to CpG DNA without causing IPC depletion. Thus, the antibody identifies an IPC-specific cell-surface molecule that regulates secretion of IFN-α.
Materials and methods
Isolation and culture of IPCs and DCs
Bone marrow-derived IPCs and myeloid DCs were prepared by culturing bone marrow cells of 129/SvJ mice (Jackson Laboratories, Bar Harbor, ME) with FLT3 ligand (FLT3-L) and granulocyte-macrophage colony-stimulating factor (GM-CSF), respectively, as previously described.19 CD11c+ cells were isolated from spleen of approximately 8- to 12-week-old 129/SvJ mice by digestion with collagenase followed by magnetic sorting.19
Generation of mAb 440c
Wistar/CRL rats were immunized subcutaneously in hind footpads with 107 purified bone marrow-derived IPCs and CpG oligodeoxynucleotide (ODN) 1826 (100 μg) as adjuvant at day 0, day 4, and day 7. At day 8, popliteal lymph nodes were fused with SP2/0 myeloma. We selected hybridoma supernatants that stained bone marrow-derived IPCs and fractions of primary CD11c+ spleen cells, but not bone marrow-derived myeloid DCs. We further screened for hybridoma supernatants that only stained CD11c+/Ly-6C+/CD11b- splenocytes. The IPC-specific mAb 440c presented here is a rat IgG2b.
Flow cytometric analysis and immunohistochemistry
Spleen, thymus, lymph node, and liver cell suspensions were prepared by mechanical disruption of tissues and digestion with collagenase. Liver was perfused with phosphate-buffered saline (PBS) prior to disruption. Liver cells were further purified on a 30% Percoll gradient. Blood, bone marrow, and spleen cells were analyzed after red blood cell lysis. Cells were stained with 440c (rat IgG2b) and 1 or 2 of the following antibodies: anti-B220 (rat IgG2a, fluorescein isothiocyanate [FITC], or biotin, RA3-6B2); anti-CD19 (rat IgG2a, FITC, ID3); anti-CD3ϵ (hamster IgG, FITC, 145-2C11); anti-CD11b (rat IgG2a, biotin, 3A33); pan NK cell (rat IgM, allophycocyanin [APC], DX5); CD8α (rat IgG2a, FITC, 53-6.7); anti-CD11c (hamster IgG, phycoerythrin [PE], or biotin, HL3); and anti-Ly-6C (rat IgG2c, PE, HK1.4, and rat IgM, FITC, AL-21). mAb 440c was detected with mouse antirat IgG2b (FITC or biotin). Biotinylated antibodies were detected with streptavidin-APC. FcR block (mouse antimouse CD16/32, Ly17.1, 17.2; Caltag, Burlingame, CA) was used to prevent nonspecific binding of antibodies to Fc receptors. All antibodies other than 440c were purchased from BD Biosciences (San Diego, CA) and Southern Biotechnology Associates (SBA; Birmingham, AL). In some experiments, stainings were perfomed on splenocytes derived from mice that had been injected intraperitoneally with murine cytomegalovirus (MCMV; 104 /multiplicity of infection [MOI] per mouse) or subcutaneously with CpG ODN 2216 (100 μg/mouse). Stainings were also performed on splenocytes activated in vitro with CpG ODN 1826 and 2216 (3 μg/mL) or HSV-1 (1 MOI/cell).
For detection of 440c by immunohistochemistry, fresh-frozen sections of lymph nodes and spleens were blocked with normal rabbit serum (1:20; Dako, Carpinteria, CA) and sequentially treated with mAb 440c, biotinylated goat antirat IgG (Vector Laboratories, Burlingame, CA), streptavidin-FITC (SBA), immunoperoxidase (HRP)-conjugated anti-FITC (Dako) and aminoethylcarbazole (AEC) as chromogen. Sections were counterstained with Meyer haematoxylin. In serial histologic sections, anti-CD3 (rat IgG2a, CT-CD3; Caltag Laboratories, Burlingame, CA), anti-B220 (rat IgG2a, RA3-6B2; Caltag Laboratories), and anti-IgD (rat IgG2a; Caltag Laboratories) were detected using goat antirat IgG (Vector Laboratories), streptavidine-HRP (Stravigen MultiLink; Biogenex Laboratories, San Ramon, CA), and AEC as chromogen. Negative controls were stained with an irrelevant rat mAb (rat anti-HHV8 ORF73; Advanced Biotechnologies, Columbia, MD).
Sorting of IPCs and DCs and assessment of IFN-α secretion
CD11c+ cells were purified from spleens and stained with either mAb 440c or a combination of antibodies specific for CD11c (biotin, HL3), Gr-1 (anti-Gr-1 encompasses Ly-6C and Ly-6G, PE, RB6-8C6), and CD11b (FITC, MI/70). 440c+, 440c-, CD11c+/Gr-1+/CD11b-, and CD11c+/Gr-1-/CD11b+/- cells were sorted on a MoFlow cell sorter (Cytomation, Fort Collins, CO). Sorted cells (106/mL, 100 000/well) were stimulated in vitro for 24 hours with CpG ODN 2216 (3 μg/mL) or MCMV (1 MOI). IFN-α released into culture supernatants was quantitated by enzyme-linked immunosorbent assay (ELISA; PBL Biomedical Laboratories, New Brunswick, NJ).
Induction of lymph node inflammation
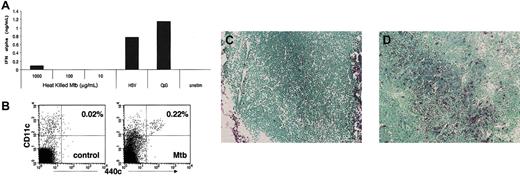
Mice were inoculated in the left hind footpads and legs with heat-killed Mycobacterium tuberculosis (Mtb) suspended in RPMI (∼500-700 μg/mouse) or HSV-1 KOS strain (5 × 105 plaque forming units/mouse). Bilateral popliteal and inguinal nodes were excised, digested with collagenase, and analyzed by flow cytometry. To determine whether Mtb directly activates IPCs, we incubated bone marrow-derived IPCs with various concentrations of Mtb and determined IFN-α released into culture supernatants by ELISA.
Blockade of IFN-α responses of IPCs. In vitro blockade: IPCs were sorted from spleens and cultured for 24 hours in medium alone or with varying concentrations of CpG ODN 2216 (3 μg/mL). Cells were cultured on 96-well plates coated with mAb 440c or control rat IgG2b (Sigma, St Louis, MO). One day later, culture supernatants were collected and tested for IFN-α and IL-12 by ELISA. In vivo blockade: Mice were injected subcutaneously with 100 μg CpG ODN 2216 or PBS and intraperitoneally with 200 μg mAb 440c, or control rat IgG2b (Sigma). One day later, serum was collected and IFN-α levels were determined by ELISA. To determine if mAb 440c depletes IPCs, 129/SvJ mice were treated with 2 intraperitoneal injections each of 200 μg mAb 440c at a 24-hour interval. Splenocytes were isolated 24 hours after the last injection, stained with anti-CD11c, anti-Gr-1, and anti-CD11b mAbs, and subjected to flow cytometric analysis.
Depletion of IPCs by 440c-based immunotoxin. Nonadherent cells were harvested from bone marrow cultures treated with FLT3-L15 and characterized for expression of 440c, CD11c, and B220 by flow cytometry. Cells were incubated in vitro with biotinylated 440c or biotinylated control antibody (rat IgG; BD Biosciences) for 15 minutes on ice, washed, and incubated with avidinylated saporin (Advanced Targeting Systems, San Diego, CA) for 15 minutes on ice. Cells were washed and cultured in media containing FLT3-L for 36 hours. Cells were then harvested and analyzed by flow cytometry.
Results
Mab 440c selectively recognizes murine IPCs
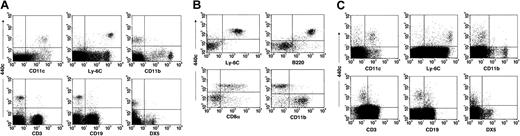
To establish a murine IPC-specific mAb, we immunized rats with IPCs grown from bone marrow precursors and screened for mAbs that stained cultured IPCs and subsets of CD11c+ spleen cells (data not shown). Antibodies recognizing bone marrow-derived myeloid DCs were excluded. The mAb 440c stained subsets of CD11c+ and Ly-6C+ splenocytes, whereas it did not recognize myeloid DCs, T, B, or NK cells (Figure 1A). Within CD11c+ cells purified from spleen, mAb 440c recognized Ly-6C+/B220+/CD8a+/- cells, but not CD11b+ myeloid DCs (Figure 1B). mAb 440c also exclusively detected CD11c+/Ly-6C+ cells within splenocytes isolated from mice injected in vivo with MCMV (Figure 1C), CpG ODN 2216, or activated in vitro with CpG ODN or HSV-1 (data not shown). Thus, mAb 440c selectively identifies cells with the phenotype of murine IPCs.
Cellular distribution of 440c antigen within murine splenocytes. (A) In total splenocytes, mAb 440c recognizes subsets of CD11c+ and Ly-6C+ cells, but not CD11b+, CD3+, CD19+, or DX5+ cells. (B) Within CD11c-enriched splenocytes, mAb 440c recognizes a Ly-6C+/B220+/CD8αhi/lo/CD11b- subpopulation. Plots represent gated CD11c+ cells. (C) mAb 440c recognizes only CD11c+/Ly-6C+ cells within splenocytes derived from mice that have been injected intraperitoneally with MCMV.
Cellular distribution of 440c antigen within murine splenocytes. (A) In total splenocytes, mAb 440c recognizes subsets of CD11c+ and Ly-6C+ cells, but not CD11b+, CD3+, CD19+, or DX5+ cells. (B) Within CD11c-enriched splenocytes, mAb 440c recognizes a Ly-6C+/B220+/CD8αhi/lo/CD11b- subpopulation. Plots represent gated CD11c+ cells. (C) mAb 440c recognizes only CD11c+/Ly-6C+ cells within splenocytes derived from mice that have been injected intraperitoneally with MCMV.
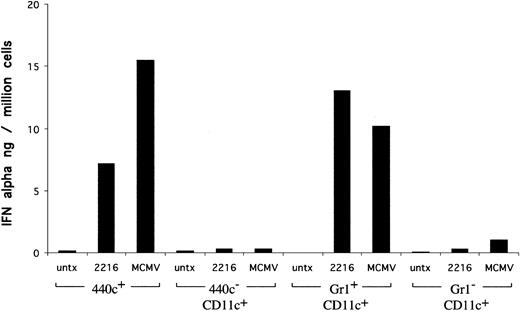
To provide functional evidence that 440c+ cells correspond to IPCs, we sorted 440c+ and 440c- cells from CD11c+ splenocytes, stimulated them in vitro with CpG ODN and MCMV, and measured IFN-α secretion. As controls, we determined IFN-α responses of IPCs and conventional DCs that had been sorted using the classical markers (CD11c+/Gr-1+/CD11b- and CD11c+/Gr-1-/CD11b+/-, respectively). As shown in Figure 2, 440c+ cells secreted markedly higher levels of IFN-α than did 440c- cells. Importantly, IFN-α responses of 440c+ cells were comparable to those of CD11c+/Gr-1+/CD11b- IPCs. Altogether, these results demonstrate that mAb 440c selectively recognizes murine IPCs.
Comparison of IFN-α responses of 440c+, 440c-, CD11c+/Gr-1+/CD11b-, and CD11c+/Gr-1-/CD11b+/- splenocytes to in vitro stimulation with CpG and MCMV. 440c+, 440c-, Gr-1+/CD11b-, and Gr-1-/CD11b+/- were sorted from CD11c+-enriched splenocytes and stimulated in vitro with MCMV or CpG ODN 2216 for 24 hours. IFN-α was measured in culture supernatants by ELISA.
Comparison of IFN-α responses of 440c+, 440c-, CD11c+/Gr-1+/CD11b-, and CD11c+/Gr-1-/CD11b+/- splenocytes to in vitro stimulation with CpG and MCMV. 440c+, 440c-, Gr-1+/CD11b-, and Gr-1-/CD11b+/- were sorted from CD11c+-enriched splenocytes and stimulated in vitro with MCMV or CpG ODN 2216 for 24 hours. IFN-α was measured in culture supernatants by ELISA.
Murine IPCs are located within the T-cell zone and at its border with mantle-zone B cells
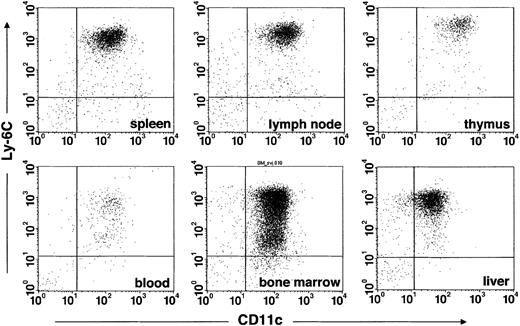
To investigate the tissue distribution of IPCs, we analyzed expression of 440c, CD11c, and Ly-6C in cell suspensions obtained from primary and secondary lymphoid organs as well as liver. mAb 440 detected IPCs in lymph nodes, spleen, thymus, bone marrow, blood, and liver (Figure 3). In general, 440c+ cells coexpressed CD11c and Ly-6C (Figure 3A). Only 440c+ cells isolated from bone marrow and blood included an additional CD11c+/Ly-6Clow population. These cells were B220+ and NK1.1- (data not shown). Because expression of Ly-6C is induced by IFN-α,28,29 440c+/CD11c+/Ly-6Clowcells may be IPC precursors that have not yet fully acquired the capacity to produce IFN-α.
mAb 440c identifies IPCs in all immune organs and liver. Total cell populations from 129SvJ spleen, peripheral lymph nodes, thymus, bone marrow, blood, and liver were stained with 440c, Ly-6C, and CD11c. Plots represent gated 440c+ cells. Equal numbers of events were collected from spleen, thymus, lymph nodes, bone marrow, and liver.
mAb 440c identifies IPCs in all immune organs and liver. Total cell populations from 129SvJ spleen, peripheral lymph nodes, thymus, bone marrow, blood, and liver were stained with 440c, Ly-6C, and CD11c. Plots represent gated 440c+ cells. Equal numbers of events were collected from spleen, thymus, lymph nodes, bone marrow, and liver.
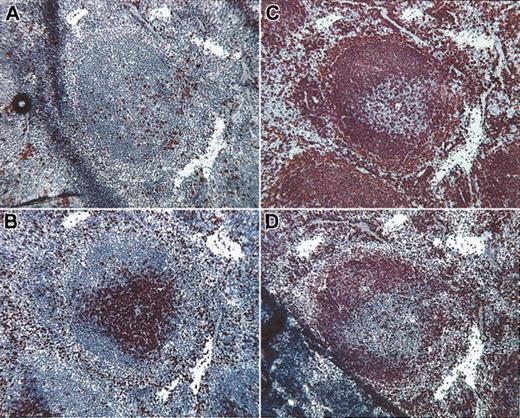
To localize IPCs within normal lymphoid organs, we performed immunohistochemistry using the 440c mAb. In the spleen, IPCs appeared as single scattered cells or aggregates in the periarteriolar lymphoid sheets (PALS) and at the border of PALS with mantle-zone B cells (Figure 4). In lymph nodes, very few IPCs were detectable in the T-cell zone of the paracortical area (Figure 5). IPC numbers varied considerably among the mouse strains analyzed. IPCs were readily detectable in 129/SvJ mice, whereas C57Bl/6 and B10.BR mice had consistently lower numbers of IPCs (data not shown). Human IPCs are located in the T-cell region of lymph nodes in the vicinity of HEVs and in the splenic PALS.21-23 Thus, the tissue distribution of IPCs is equivalent in mice and humans.
Location of IPCs within normal spleen. (A) Abundant 440c+ IPCs are evident in the periarteriolar sheet (PALS) and at the border of PALS with mantle-zone B cells. Serial histologic sections were stained with anti-CD3 (B), anti-B220 (C), and anti-IgD (D) to identify the T-cell and the B-cell zones in the white pulp. Rare IPCs are also visible in the red pulp. Original magnification, × 10.
Location of IPCs within normal spleen. (A) Abundant 440c+ IPCs are evident in the periarteriolar sheet (PALS) and at the border of PALS with mantle-zone B cells. Serial histologic sections were stained with anti-CD3 (B), anti-B220 (C), and anti-IgD (D) to identify the T-cell and the B-cell zones in the white pulp. Rare IPCs are also visible in the red pulp. Original magnification, × 10.
Accumulation of IPCs in inflamed lymph nodes. (A) Heat-killed Mtb does not trigger secretion of IFN-α by IPCs in vitro. Bone marrow-derived IPCs were incubated with varying concentrations of heat-killed Mtb and IFN-α response was measured by ELISA. Controls include stimulation with HSV-1 (1 MOI), CpG ODN 2216 (3 μg/mL), and no stimulation. (B) 440c+/CD11c+ IPCs in lymph nodes draining either the site of inflammation (right plot) or the controlateral site (left plot). The percent of IPCs is indicated. Equal numbers of events are shown in each plot. (C-D) IPCs in histologic sections of lymph nodes draining either the site of inflammation (C) or the controlateral site (D). 129/SvJ mice were inoculated with heat-killed Mtb in the left hind leg and footpad twice at a 48-hour interval. At 72 hours, inguinal and popliteal lymph nodes were separately harvested from the left and right sides of the mouse. Total cell populations were stained with 440c and CD11c (original magnification, × 10). Lymph node sections were analyzed by immunohistochemistry.
Accumulation of IPCs in inflamed lymph nodes. (A) Heat-killed Mtb does not trigger secretion of IFN-α by IPCs in vitro. Bone marrow-derived IPCs were incubated with varying concentrations of heat-killed Mtb and IFN-α response was measured by ELISA. Controls include stimulation with HSV-1 (1 MOI), CpG ODN 2216 (3 μg/mL), and no stimulation. (B) 440c+/CD11c+ IPCs in lymph nodes draining either the site of inflammation (right plot) or the controlateral site (left plot). The percent of IPCs is indicated. Equal numbers of events are shown in each plot. (C-D) IPCs in histologic sections of lymph nodes draining either the site of inflammation (C) or the controlateral site (D). 129/SvJ mice were inoculated with heat-killed Mtb in the left hind leg and footpad twice at a 48-hour interval. At 72 hours, inguinal and popliteal lymph nodes were separately harvested from the left and right sides of the mouse. Total cell populations were stained with 440c and CD11c (original magnification, × 10). Lymph node sections were analyzed by immunohistochemistry.
Inflammation drives recruitment of murine IPCs into sentinel lymph nodes
To determine whether IPCs are recruited into inflamed lymph nodes, we compared 2 models of inflammation. First, mice were inoculated twice with high doses of heat-killed mycobacteria tuberculosis (Mtb), which induces strong inflammation by engaging TLR2 on macrophages.30,31 Heat-killed Mtb does not directly stimulate IPCs, as these cells do not express TLR2 (Figure 5A).6-9 Second, mice were infected once with live HSV-1, which stimulates IPCs through TLR911 and induces a modest influx of IPCs into lymph nodes after footpad injection.26 Inoculations were performed in the left footpad to generate ipsilateral popliteal and inguinal lymphadenitis. After 48 to 72 hours, bilateral popliteal and inguinal nodes were excised for flow cytometric and immunohistochemical analysis. In the Mtb model we observed a significantly higher proportion of IPCs in reactive lymph nodes in comparison to controlateral nodes (Figure 5B). Infiltrating IPCs were mostly present in the T-cell zone (Figure 5C-D). IPC infiltration was less pronounced in the HSV-1 model (data not shown). We also observed a significant variability depending on the mouse strain. In 129/SvJ mice, we consistently observed a 5- to 10-fold increase in the relative percent of IPCs in inflamed lymph nodes (Figure 5B,D); less dramatic increases were detected in other strains (data not shown). However, since the total cell number in lymph nodes expanded 2- to 4-fold during inflammation, even a small increase in the percent of IPCs reflects a considerable increase in the total number of IPCs. In conclusion, these results demonstrate that inflammation is the stimulus that drives the recruitment of IPCs into sentinel lymph nodes, whether the pathogenic stimuli are able to directly stimulate IPCs through TLR (ie, HSV-1) or not (ie, heat-inactivated mycobacterium tuberculosis). IPC recruitment varies depending on the strength of inflammatory stimulus and the mouse strain.
mAb 440c blocks secretion of IFN-α by murine IPCs
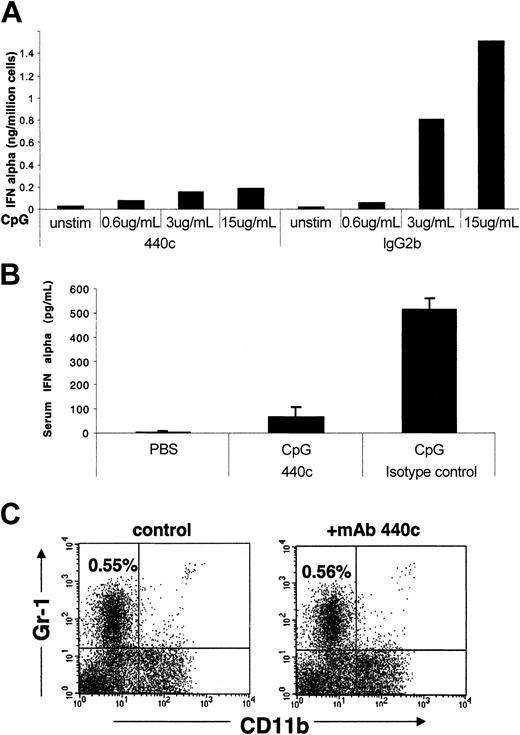
To determine whether the cell-surface molecule recognized by the mAb 440c is directly involved in IPC function, IPCs were sorted from the spleen using the conventional markers CD11c and Gr-1, and cultured in vitro with CpG ODN on plates coated with either mAb 440c or control rat IgG2b. Measurement of IFN-α and IL-12 responses showed that mAb 440c significantly reduced secretion of IFN-α (Figure 6A) but not IL-12 (data not shown). To corroborate this result in vivo, we simultaneously injected CpG ODN subcutaneously and either mAb 440c or control rat IgG2b intraperitoneally, and measured serum levels of IFN-α. As shown in Figure 6B, administration of a single dose of mAb 440c almost completely abolished the IFN-α response to CpG ODN in vivo. Notably, administration of mAb 440c did not cause depletion of IPCs (Figure 6C). We conclude that blockade of IFN-α response by mAb 440c is due to the engagement of a cell-surface molecule selectively expressed on IPCs that inhibits IFN-α response to CpG ODN.
IFN-α response of IPCs to CpG after in vitro or in vivo treatment with mAb 440c. (A) In vitro cross-linking of IPCs with mAb 440c blocks IFN-α secretion. IPCs were sorted from the spleen and cultured with CpG 2216 ODN on plates coated with mAb 440c or control rat IgG2b. After 24 hours, IFN-α was measured in culture supernatants by ELISA. Representative data of one of 3 separate experiments are shown. (B) In vivo treatment of mice with mAb 440c reduces serum levels of IFN-α. 129/SvJ mice were simultaneously treated subcutaneously with CpG 2216 ODN or PBS and intraperitoneally with mAb 440c or control rat IgG2b. After 16 hours, serum levels of IFN-α were detected by ELISA. Results are expressed as the mean of 3 mice per group with standard deviation indicated. Representative data of one of 3 independent experiment are shown. (C) In vivo treatment with mAb 440c does not deplete IPCs. 129/SvJ mice were treated with 2 intraperitoneal injections each of 200 μg mAb 440c at a 24-hour interval. Splenocytes were isolated 24 hours after the last injection and subjected to flow cytometric analysis. Plots show expression of Gr-1 and CD11b on gated CD11c+ cells. The percent of CD11c+/Gr-1+/CD11b-IPCs is indicated. Results are representative of 3 separate experiments.
IFN-α response of IPCs to CpG after in vitro or in vivo treatment with mAb 440c. (A) In vitro cross-linking of IPCs with mAb 440c blocks IFN-α secretion. IPCs were sorted from the spleen and cultured with CpG 2216 ODN on plates coated with mAb 440c or control rat IgG2b. After 24 hours, IFN-α was measured in culture supernatants by ELISA. Representative data of one of 3 separate experiments are shown. (B) In vivo treatment of mice with mAb 440c reduces serum levels of IFN-α. 129/SvJ mice were simultaneously treated subcutaneously with CpG 2216 ODN or PBS and intraperitoneally with mAb 440c or control rat IgG2b. After 16 hours, serum levels of IFN-α were detected by ELISA. Results are expressed as the mean of 3 mice per group with standard deviation indicated. Representative data of one of 3 independent experiment are shown. (C) In vivo treatment with mAb 440c does not deplete IPCs. 129/SvJ mice were treated with 2 intraperitoneal injections each of 200 μg mAb 440c at a 24-hour interval. Splenocytes were isolated 24 hours after the last injection and subjected to flow cytometric analysis. Plots show expression of Gr-1 and CD11b on gated CD11c+ cells. The percent of CD11c+/Gr-1+/CD11b-IPCs is indicated. Results are representative of 3 separate experiments.
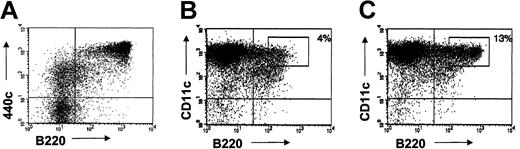
Although mAb 440c does not deplete IPCs, such depletion may be achieved by conjugating 440c with toxins, such as saporin.32 We tested this possibility on IPCs cultured in vitro from bone marrow cells with FLT3-L. Cultured CD11c+ B220hi IPCs, which correspond to fully developed IPCs, expressed high levels of 440c (Figure 7A-B). A minor subset of CD11c+ B220- cells displayed low levels of 440c antigen and may correspond to a developmental intermediate of IPCs. Upon treatment of bone marrow-derived IPCs with biotinylated 440c mAb followed by an avidin-saporin conjugate, approximately 70% of CD11c+ B220hi 440cbright IPCs were depleted from culture within 36 hours (Figure 7C-D). Analysis of IPC depletion at later time points was not reliable as IPCs survived poorly in vitro in the absence of stromal cells and, therefore, IPC numbers were reduced not only in 440c-treated cultures but also in control cultures. Nevertheless, these results indicate that mAb 440c conjugated with a toxin represents a useful tool to achieve IPC depletion.
Sequential treatment with mAb 440c and saporin depletes IPCs in vitro. (A) Expression of 440c on bone marrow cells cultured in FLT3-L for 10 days. CD11c- cells were excluded from the gate. 440c is highly expressed on B220+ cells, which represent fully developed IPCs. (B-C) Depletion of CD11c+/B220+ cells from FLT3-L-derived bone marrow IPCs by treatment with 440c-saporin. Cells were stained on ice with biotinylated-440c (B) or a biotinylated control antibody (C), followed by avidin-saporin, cultured for an additional 36 hours, and analyzed for residual presence of CD11c+/B220+ cells. The percentage of gated cells is indicated. Results are representative of 3 separate experiments.
Sequential treatment with mAb 440c and saporin depletes IPCs in vitro. (A) Expression of 440c on bone marrow cells cultured in FLT3-L for 10 days. CD11c- cells were excluded from the gate. 440c is highly expressed on B220+ cells, which represent fully developed IPCs. (B-C) Depletion of CD11c+/B220+ cells from FLT3-L-derived bone marrow IPCs by treatment with 440c-saporin. Cells were stained on ice with biotinylated-440c (B) or a biotinylated control antibody (C), followed by avidin-saporin, cultured for an additional 36 hours, and analyzed for residual presence of CD11c+/B220+ cells. The percentage of gated cells is indicated. Results are representative of 3 separate experiments.
Discussion
In this study, we identified a mAb that selectively recognizes murine IPCs. Another murine IPC-specific mAb, called 120G8, was recently characterized.33 mAb 120G8 selectively recognizes IPCs within resting leukocyte populations, but also recognizes B cells and DCs upon activation. In contrast, mAb 440c is specific for IPCs not only under physiologic conditions, but also after activation of immune cells in vitro and in vivo with microbial components or viruses. Thus, mAb 440c provides a unique tool to precisely identify and localize IPCs in murine tissues in both normal and inflammatory conditions. Using this antibody, we demonstrated the presence of IPCs in blood and in primary and secondary lymphoid organs, corroborating previous studies performed using a combination of different mAbs.13 In addition, we observed the presence of IPCs in the liver, suggesting the involvement of IPCs in hepatic immune responses.
Importantly, we found that IPCs are located in the T-cell zone of the lymph nodes and in the PALS of the spleen, paralleling the localization previously described for human IPCs. The presence of IPCs in the T-cell areas of secondary lymphoid organs strongly supports a role of IPCs in regulating adaptive T-cell responses. Furthermore, we found IPCs at the border between the T-cell zone and mantle-zone B cells, suggesting that IPCs may provide help to B cells or modulate T-cell help to B cells. Consistent with this, it was recently shown that human IPCs promote differentiation of B cells into IgM-secreting plasma cells through sequential secretion of IFN-α and IL-6.34 Previous studies performed with anti-IFN-α antibodies detected cells producing IFN-α in the marginal zone of mouse spleen.35,36 However, our antibody did not detect IPCs in this area, and hence the nature of these cells that produce IFN-α remains to be determined. They may correspond to DCs that have been converted into high interferon producers by viral infection.37 In general, 440c+ IPCs have the CD11c+/Ly-6C+/B220+/CD11b- phenotype originally described for IPCs. We also detected a 440c+ cell population in bone marrow and blood that expresses low levels of Ly-6C. As expression of Ly-6C is induced by IFN-α,28,29 these cells may well represent IPC precursors that have not yet fully developed their IFN-α production potential.
For many years pathologists have known IPCs as cells with plasma cell-like morphology or “plasmacytoid monocytes.”21-23 These cells are particularly abundant around HEVs in the lymph nodes of patients with certain chronic lymphadenitis.22,23 This notion, together with the expression of L-selectin and receptors for proinflammatory chemokines, has suggested that IPCs may be recruited from the blood to the lymph nodes during inflammatory conditions. In this study, we provided experimental evidence for this hypothesis. Using the mAb 440c, we detected a significant increase of IPCs in lymph nodes draining a local infection as compared with control lymph nodes. High doses of Mtb were more effective than HSV-1 in promoting IPC recruitment, although heat-killed Mtb is unable to directly stimulate IPCs through TLRs. Thus, massive IPC accumulation like that observed in the lymph nodes of patients with inflammatory diseases most likely requires strong and/or prolonged inflammatory stimuli, whether they are able to directly stimulate IPCs through TLRs or not. Intriguingly, the number of IPCs detected under both normal and inflammatory conditions greatly varied depending on the mouse strain. 129/SvJ mice harbor relatively high numbers of IPCs that are clearly recruited to draining lymph nodes following inflammation. In other strains, fairly small numbers of IPCs were detected and recruitment was less evident following inflammation. Thus, IPC differentiation and migration may be influenced by genetic factors. Recently, it was shown that certain tumors release chemokines that attract IPCs and that tumor-associated IPCs induce tolerogenic T-cell responses.38 mAb 440c provides an important tool to investigate IPC recruitment and function in murine tumor models.
The most remarkable characteristic of mAb 440c is its ability to block secretion of IFN-α in response to CpG ODN in vitro and in vivo without depleting IPCs. Thus, mAb 440c identifies a novel cell-surface molecule that, upon engagement, negatively regulates the secretion of type I IFN by IPCs. It is noteworthy that human IPCs express a C-type lectin, called BDCA-2, which is also exclusively found on IPCs and blocks secretion of IFN-α by triggering intracellular calcium flux.39 Thus, it is tempting to speculate that mAb 440c may detect the murine homolog of BDCA-2. This possibility is further supported by the observation that the 440c cell-surface molecule is internalized upon ligation with the specific mAb, as is BDCA-2 (data not shown). It has been proposed that excessive secretion of type I IFN by IPCs in response to microbes may contribute to the pathogenesis of autoimmune responses.34,40,41 Consistent with this, patients with systemic lupus erithematosus (SLE) have infiltrating IPCs in skin lesions42 and high levels of IFN-α in their serum.43 In addition, treatment of virally infected patients with IFN-α may trigger SLE-like syndromes and, in particular, the appearance of autoantibodies. mAb 440c will allow testing the function of IPCs in experimental models of infections and autoimmune diseases by directly modulating IFN-α responses of IPCs or by depleting IPCs in conjunction with a toxin.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, December 24, 2003; DOI 10.1182/blood-2003-09-3108.
We are grateful to Francesca Gentili for excellent technical assistance; Fondazione Beretta, Brescia, Italy, for support to Francesca Gentili; Susan Gilfillan for her advice on different aspects of this work and critical reading of the manuscript; the Alvin J. Siteman Cancer Center at the Washington University School of Medicine in St Louis, MO; and Bill Eades for the use of the High Speed Cell Sorter Core.