Abstract
One Japanese pedigree of familial essential thrombocythemia (FET) inherited in an autosomal-dominant manner is presented. A unique point mutation, serine 505 to asparagine 505 (Ser505Asn), was identified in the transmembrane domain of the c-MPL gene in all of the 8 members with thrombocythemia, but in none of the other 8 unaffected members in this FET family. The Ba/F3 cells expressing the mutant Asn505 acquired interleukin 3 (IL-3)-independent survival capacity, whereas those expressing wild-type Ser505 did not. The autonomous phosphorylation of Mek1/2 and Stat5b was observed in the mutant Ba/F3 cells in the absence of IL-3. The former was also found in platelets derived from the affected individual in the absence of thrombopoietin. These results show that the Asn505 is an activating mutation with respect to the intracellular signaling and survival of the cells. This is the first report of FET deriving from a dominant-positive activating mutation of the c-MPL gene. (Blood. 2004;103: 4198-4200)
Introduction
Familial essential thrombocythemia (FET) is a rare hereditary chronic myeloproliferative disorder, which is characterized by autonomously activated megakaryocytopoiesis with the excessive production of platelets. The c-MPL gene and its ligand, thrombopoietin (TPO), regulate the proliferation and differentiation of megakaryocytes and platelets. The germline mutations in the promoter region of the TPO gene, which produce the aberrantly stable TPO mRNA resulting in FET, have been reported.1,2 Germline mutations in the c-MPL gene have been also reported to cause congenital amegakaryocytic thrombocytopenia,3-6 which is characterized by defective proliferation of megakaryocytes in bone marrow. In the present study, we found one Japanese pedigree of FET in which affected members showed autosomal-dominant inheritance, and investigated the underlying molecular mechanisms.
Study design
Mutation analysis of c-MPL and TPO genes
We amplified all of the exons and splice sites of TPO and c-MPL genes and then performed direct sequencing using an automated sequencer (model ABI377; Applied Biosystems, Foster City, CA) to find the germline mutations in gDNAs derived from peripheral blood mononuclear cells.7 Primer sequences are given in the Supplemental Materials: see the Supplemental Table link at the top of the online article on the Blood website.
Cell survival assay using stably transfected Ba/F3 cells with wild and mutant types of c-MPL
The Humplpas12 plasmid carrying a full length of the wild-type c-MPL cDNA was provided by Amgen (Thousand Oaks, CA). The mutant c-MPL cDNA was generated by reverse transcription-polymerase chain reaction (RT-PCR) using platelet-derived RNA of patient `b' in Figure 1A. The coding region of each c-MPL cDNA fragment was ligated into the pCI-Neo expression vector (Promega, Madison, WI) and transfected into Ba/F3 cells by electroporation (1040 μF and 0.3 kV). The resistant clones against 1 mg/mL G418 were selected and maintained in RPMI 1640 with 10% fetal bovine serum (FBS), 10% supernatant from WEHI cells, as a source of interleukin 3 (IL-3). Immunoblotting was performed using anti-c-Mpl polyclonal antibody (Upstate Biotechnology, Lake Placid, NY), as previously reported.8 Methylthiotetrazole (MTT) assays were used to evaluate viable cell numbers in the presence and absence of IL-3.9
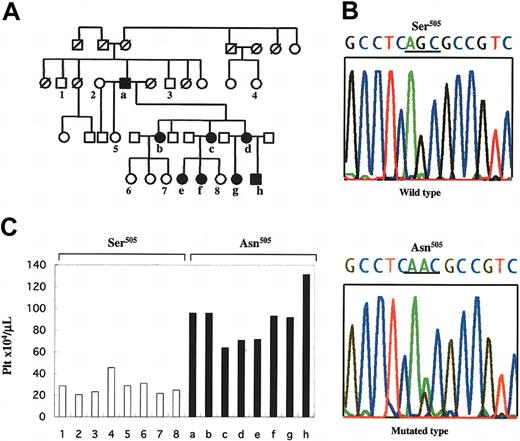
Results of study of family affected with FET. (A) Pedigree of FET. Members of the family, designated as `a' through `h' and `1' through `8', were examined clinically; solid symbols (a-h) indicate ET-affected members, and numbers 1-8 indicate unaffected members. Square symbols denote men; circles, women; and symbols with a slash, deceased members. This pedigree shows the autosomal-dominant inheritance of disease penetration. (B) Sequence of the Asn505 mutation of the c-MPL gene detected in ET-affected members of the family. Upper sequence indicates the wild type, and the lower sequence indicates the point mutation detected in the affected members. The nucleotide change from guanine to adenine caused an amino acid substitution from Ser505 to Asn505. (C) Platelet counts for the family members and the association with the status of the Asn505 mutation of the c-MPL gene. ▪ indicates platelet counts of the members carrying the Asn505 mutation of the c-MPL gene; □, platelet counts of the members with wild-type alleles of the homozygous Ser505, showing that the Asn505 mutation is unique in affected members; 1-8 and a-h correspond to the designations in panel A.
Results of study of family affected with FET. (A) Pedigree of FET. Members of the family, designated as `a' through `h' and `1' through `8', were examined clinically; solid symbols (a-h) indicate ET-affected members, and numbers 1-8 indicate unaffected members. Square symbols denote men; circles, women; and symbols with a slash, deceased members. This pedigree shows the autosomal-dominant inheritance of disease penetration. (B) Sequence of the Asn505 mutation of the c-MPL gene detected in ET-affected members of the family. Upper sequence indicates the wild type, and the lower sequence indicates the point mutation detected in the affected members. The nucleotide change from guanine to adenine caused an amino acid substitution from Ser505 to Asn505. (C) Platelet counts for the family members and the association with the status of the Asn505 mutation of the c-MPL gene. ▪ indicates platelet counts of the members carrying the Asn505 mutation of the c-MPL gene; □, platelet counts of the members with wild-type alleles of the homozygous Ser505, showing that the Asn505 mutation is unique in affected members; 1-8 and a-h correspond to the designations in panel A.
Phosphorylation analysis of the downstream signaling in Mek1/2 and Stat5b status
Exponentially growing cells were cultured at 2 × 107/mL in the absence of IL-3 for 6 hours, followed by incubation for 90 minutes with or without 10% WEHI cells' supernatant. The cells were dissolved in lysis buffer containing a protease-inhibitor cocktail (Complete mini; Roche Diagnostics, Mannheim, Germany). Anti-phospho-Mek1/2 and anti-whole-Mek1/2 (Phospho Plus ser 217/221 antibody kit; Cell Signaling Technology, Beverly, MA) were used to evaluate the phosphorylation status using immunoblot analysis. Immunoprecipitation was performed using Stat5b antibody (c-17) followed by immunoblotting with anti-p-Tyr antibody (PY-99; Santa Cruz Biotechnology, Santa Cruz, CA) for the detection of the phosphorylation status of Stat5b. The platelets isolated from patient `a' shown in Figure 1A and one healthy volunteer were stimulated with 200 ng/mL recombinant human TPO (IM-25A; Invitrogen, Carlsbad, CA) for 10 minutes at 37°C10 and evaluated for the phosphorylation status of Mek1/2. Study approval was obtained from the institutional review board of the Nagoya City University Graduate School of Medical Science. Informed consent was provided according to the Declaration of Helsinki.
Results and discussion
We used the revised criteria of the Polycythemia Vera Study Group for the diagnosis of essential thrombocythemia (ET), which include a platelet count more than 600 × 109/L (600 000/μL).11 Eight of 16 members examined in 3 generations of this pedigree were found to be affected with ET in an autosomal-dominant manner (Figure 1A). The individuals with FET showed normocellular and normoplastic marrows except for increased megakaryocytes.
To clarify the genetic alterations responsible for this hereditary thrombocythemia, we investigated if germline mutations in TPO and c-MPL genes were present in the affected members. Interestingly, a unique variant of c-MPL was identified in all of the 8 affected members but not in any of the 8 unaffected members, whereas no unique nucleotide variants could be detected in TPO. This unique variant presented as a heterozygous G>A nucleotide substitution at position 1073 in exon 10 of c-MPL (GenBank accession no. U68161), which results in an amino acid exchange from serine to asparagine (Ser505Asn; GenBank accession no. NP_005364; Figure 1B-C). RT-PCR followed by direct sequencing confirmed that the mutant c-MPL mRNA encoding Asn505 was expressed in the platelets (data not shown), suggesting that this mutant c-Mpl plays a crucial role in the pathogenesis of FET.
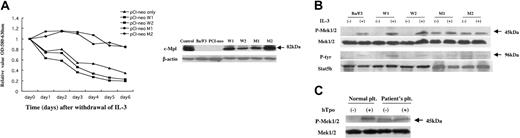
To investigate the mechanisms contributing to the cellular growth and survival of the mutant c-MPL, we performed an MTT assay using stably transfected Ba/F3 cell clones containing wild-type or mutant c-MPL. Although the mock and Ba/F3 cells expressing wild-type c-Mpl are completely dependent on the presence of IL-3 and die immediately after withdrawal of IL-3, Ba/F3 cells expressing mutant c-Mpl could survive for at least 6 days even in the absence of IL-3 (Figure 2A). This suggests that the mutant c-Mpl (Asn505) provides megakaryocytic progenitors with a cell survival capacity.
MTT and Western blotting analyses. (A) (Left) MTT assay of mutant-type c-Mpl. W1 and W2 are the transfectants with the wild-type allele (Ser505), and M1 and M2 are those with the mutant type (Asn505). The x-axis indicates time of culture after withdrawal of IL-3. The y-axis shows the relative value of cell viability (OD, 580-630 nm). Half the culture medium was exchanged with IL-3-free medium every other day. Mutant cells (Asn505) exhibited survival capacity in the absence of IL-3 (each experiment was repeated 3 times). (Right) Western blot with the c-Mpl antibody. Arrow shows the c-Mpl products (82 kDa). The FDCP2 cells transfected with the wild-type c-MPL were used as a positive control. The lysate of Ba/F3 cells and the plasmid PCI-neo carrying no foreign gene were analyzed as negative controls. The same filter was stripped and blotted with β-actin antibody. (B) Western blot with the Mek1/2 antibody of Ba/F3 transfectants and immunoprecipitation-Western blot with p-Tyr and Stat5b antibodies. Phosphorylated Mek1/2 signals (45 kDa) were detected in M1 and M2 after 6 hours culture with the IL-3-free medium. The same filter was stripped and blotted with anti-Mek1/2 antibody. The phosphorylated Stat5b signals of 96-kDa products were observed in M1 and M2 in the IL-3-free condition. The same filters were stripped and blotted with anti-Stat5b antibody. This experiment was repeated twice and the results were reproducible. (C) Western blot with the Mek1/2 antibody of the platelets from the ET-affected member. The constitutively phosphorylated Mek1/2 was detected in the platelets of the ET-affected individual (patient `a' in Figure 1A) even in the absence of the stimulation of TPO.
MTT and Western blotting analyses. (A) (Left) MTT assay of mutant-type c-Mpl. W1 and W2 are the transfectants with the wild-type allele (Ser505), and M1 and M2 are those with the mutant type (Asn505). The x-axis indicates time of culture after withdrawal of IL-3. The y-axis shows the relative value of cell viability (OD, 580-630 nm). Half the culture medium was exchanged with IL-3-free medium every other day. Mutant cells (Asn505) exhibited survival capacity in the absence of IL-3 (each experiment was repeated 3 times). (Right) Western blot with the c-Mpl antibody. Arrow shows the c-Mpl products (82 kDa). The FDCP2 cells transfected with the wild-type c-MPL were used as a positive control. The lysate of Ba/F3 cells and the plasmid PCI-neo carrying no foreign gene were analyzed as negative controls. The same filter was stripped and blotted with β-actin antibody. (B) Western blot with the Mek1/2 antibody of Ba/F3 transfectants and immunoprecipitation-Western blot with p-Tyr and Stat5b antibodies. Phosphorylated Mek1/2 signals (45 kDa) were detected in M1 and M2 after 6 hours culture with the IL-3-free medium. The same filter was stripped and blotted with anti-Mek1/2 antibody. The phosphorylated Stat5b signals of 96-kDa products were observed in M1 and M2 in the IL-3-free condition. The same filters were stripped and blotted with anti-Stat5b antibody. This experiment was repeated twice and the results were reproducible. (C) Western blot with the Mek1/2 antibody of the platelets from the ET-affected member. The constitutively phosphorylated Mek1/2 was detected in the platelets of the ET-affected individual (patient `a' in Figure 1A) even in the absence of the stimulation of TPO.
Next, because the downstream signaling pathways including Shc-Ras-Raf-Mek1/2-Mapk (Erk) and Jak-Stat cascades have been reported to be associated with the Tpo-c-Mpl system in megakaryopoiesis,12 we examined the phosphorylation status of Mek1/2 and Stat5b in the c-Mpl-expressing Ba/F3 cells in the presence and absence of IL-3 to determine if the mutant c-Mpl can activate those signaling molecules. In wild-type c-Mpl-expressing cells, tyrosine phosphorylation of Mek1/2 and Stat5b was detected only in the presence of IL-3, whereas mutant c-Mpl-expressing cells harbored constitutively phosphorylated Mek1/2 even in the absence of IL-3. Constitutive but faint phosphorylation of Stat5b was also detected in mutant c-Mpl-expressing cells, although it was never seen in wild-type c-Mpl-expressing cells in the absence of IL-3 (Figure 2B). Moreover, constitutive phosphorylation of Mek1/2 was observed in the platelets of the ET-affected individual even in the absence of TPO stimulation (Figure 2C). These observations show that mutant c-Mpl with Asn505 activates the downstream signals involved in the Tpo-c-Mpl pathway with factor independence in vitro and in vivo, suggesting that the mutant c-Mpl-expressing megakaryocytes acquire promoted cell survival capacity in bone marrow, leading to the excessive production of platelets. The mechanism underlying how c-Mpl with Asn505 induces constitutive phosphorylation of Mek1/2 and Stat5b remains unknown. However, it is assumed that the dimerization of c-Mpl molecules, which is believed to be a requisite step for activation of c-Mpl, may be constitutive as a result of this specific mutation, as in the cases of mutations of c-kit and Neu at their transmembrane domains.9,13 Further investigation is needed to elucidate the mechanism.
The same Asn505 mutation of c-MPL, which had been prepared artificially by PCR-driven random mutagenesis in vitro, was previously reported by Onishi et al to be an activating mutation that manifests higher sensitivity to very low doses of TPO and tumorigenicity in mice.14 However, we have not yet found any ET-affected individuals in this family who have developed malignancies including leukemia.
Finally, we attempted to analyze whether the same mutation, Asn505, is found in the sporadic ET cases. Platelet-derived RNAs of 19 sporadic ET patients were screened for the c-MPL mutations, but none were found (data not shown). This is consistent with the study of Horikawa et al who demonstrated that the c-Mpl-mediated signaling pathway is not constitutively activated in platelets derived from sporadic ET patients in the absence of TPO.15 Also, the somatic mutations of the c-MPL gene have not been identified in sporadic ET patients yet.16 This indicates that the FET pedigree reported here is an exceptional case, but one that provides novel insights into ET and the operation of the Tpo-c-Mpl system.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-10-3471.
Supported by grants from the Ministry of Education Science, Sports and Culture, Japan (S.I., A.W., and R.U.), grants-in aid for research from Nagoya City University (H.K.), and by the Ministry of Education, Culture, Sports, Science and Technology “Honors Scholarship for privately financed International Student” (J.D.).
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal