Abstract
Neonates respond suboptimally to many vaccines. The reasons for this defect are unclear, but suboptimal antigen presentation by dendritic cells has been suggested as one possibility. In this report we describe an in vitro system that allows the generation of large numbers of resting murine neonatal dendritic cells facilitating their study. Using this system, we show a clear reduction in the ability of neonatal dendritic cells to present soluble ovalbumin, while the capacity to present ovalbumin peptide is intact. This suggests a specific defect in cross-presentation of exogenous antigen via the major histocompatibility complex (MHC) class I pathway. Deficient cross-presentation may contribute to the suboptimal CD8 T-cell response to vaccines in neonates. (Blood. 2004;103:4240-4242)
Introduction
The induction of antigen-specific CD8+ cytotoxic T lymphocytes (CTLs) by vaccines is reduced in neonates compared with adults.1,2 However, this deficit can be overcome if the antigen is introduced into or produced within the cytoplasm of neonatal dendritic cells (DCs).2,3 Neonates also generate CTLs if the antigen is given as processed peptide.3 This suggests a defect in the neonates' ability to process and present exogenous antigens via the major histocompatibility complex (MHC) class I pathway, which is known as cross-presentation.4,5 The pathways for cross-presentation of soluble antigens versus cell-associated antigens are known to be different.6,7 Cross-presentation by neonatal DCs of soluble antigens has not been investigated. As this is relevant to the design of vaccines for neonates, we wished to establish an in vitro system amenable to experimental manipulation. Using this system, we set out to test our primary hypothesis that cross-presentation of soluble antigens by neonatal DCs is less efficient than that by adult DCs.
Study design
DCs were isolated directly ex vivo from neonatal (5-day-old) or adult mouse spleens by positive selection using anti-CD11c magnetic beads.3 To generate DCs in vitro, adult bone marrow and neonatal (< 24 hours) liver from C57BL/6 mice were used as the richest source of hematopoetic precursor cells.8 Single-cell suspensions of these organs were cultured in medium containing granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN) or Flt-3 ligand (Flt-L; Amgen, Thousand Oaks, CA), as described.9 When indicated, DCs were stimulated with polyinosinic-polycytidylic acid (pI:C; 50 μg/mL; Amersham, Arlington Heights, IL) or lipopolysaccharide (LPS; 100 ng/mL Salmonella minnesota Re595; Sigma-Aldrich, St Louis, MO). CD8+ T cells were isolated from the spleens of ovalbumin (OVA) T-cell receptor (TCR)-transgenic (OTI) mice10 on a C57BL/6 background by positive selection using anti-CD8 magnetic beads after depletion of CD11c+ cells. OVA-specific (Sigma; grade VI, 99% pure, 50 μg/mL) and OVA peptide-specific (SIINFEKL; UBR Peptide; 10 μM) T-cell priming was detected in vitro as described.11 Flow cytometry was performed as described.3 All mice were bred under specific pathogen-free conditions and used under the University of Washington's institutional animal care and use committee-approved protocols.
Results and discussion
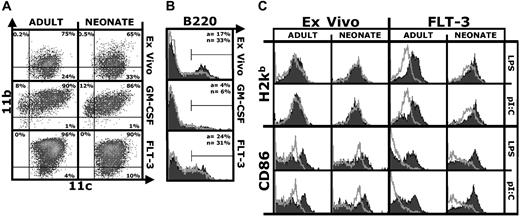
We first characterized ex vivo, GM-CSF, or Flt-3L DCs (defined as CD11c+ cells) from neonatal and adult mice by flow cytometry. Overall, neonatal and adult DCs displayed a similar surface phenotype (Figure 1A), although as expected the phenotype of the 3 DC populations differed. As previously reported, CD11c+/B220+ plasmacytoid DCs were present in a higher percentage in the neonatal spleen12 ; a similar trend was observed for Flt-3L DCs (Figure 1B). We also analyzed DCs for the expression of markers relevant to presentation of antigen to CD8+ T cells. Neonatal or adult ex vivo DCs cultured short term without further stimulation shared high MHC class I and CD86 expression (Figure 1C), indicative of an activated phenotype.13 CD86 but not MHC class I expression was further up-regulated by stimulation with LPS or pI:C (Figure 1C), confirming that the regulation of MHC class I surface expression differs from that of CD86.14 Unstimulated ex vivo neonatal DCs expressed more CD86 than adult DCs, but similar amounts after activation. Compared with ex vivo DCs, Flt-3L DCs had a resting phenotype—they expressed low amounts of MHC class I and CD86. Both adult and neonatal Flt-3L DCs up-regulated MHC class I and CD86 in response to LPS or pI:C (Figure 1C). Results with resting and stimulated neonatal and adult GM-CSF DCs were similar (not shown). Overall, there was little or no difference between neonatal and adult DCs in MHC class I, CD86 (Figure 1), CD80, and CD40 (not shown) expression.
Similar surface phenotype of adult and neonatal DCs. (A) CD11b/CD11c density plot of DCs isolated from spleens of adult or neonatal mice (ex vivo) or derived by culturing precursors in GM-CSF or Flt-3L. Acquisition gates were set to exclude debris and dead cells, and quadrants for analysis were set based on control isotype-stained samples. Percentage of positive cells is indicated for each quadrant. (B) B220 expression on neonatal (black filled) or adult (gray line) DCs. Percentage of positive cells for adults (a) or neonates (n) is indicated. (C) H2Kb (MHC-I) and CD86 expression on ex vivo or Flt-3L neonatal and adult DCs after short-term culture (18 hours) without (gray line) or with (black filled) LPS (100 ng/mL) or pI:C (50 μg/mL). The figures show one representative experiment of 3.
Similar surface phenotype of adult and neonatal DCs. (A) CD11b/CD11c density plot of DCs isolated from spleens of adult or neonatal mice (ex vivo) or derived by culturing precursors in GM-CSF or Flt-3L. Acquisition gates were set to exclude debris and dead cells, and quadrants for analysis were set based on control isotype-stained samples. Percentage of positive cells is indicated for each quadrant. (B) B220 expression on neonatal (black filled) or adult (gray line) DCs. Percentage of positive cells for adults (a) or neonates (n) is indicated. (C) H2Kb (MHC-I) and CD86 expression on ex vivo or Flt-3L neonatal and adult DCs after short-term culture (18 hours) without (gray line) or with (black filled) LPS (100 ng/mL) or pI:C (50 μg/mL). The figures show one representative experiment of 3.
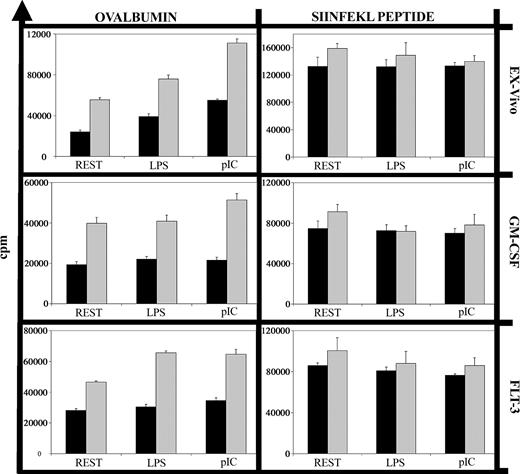
To analyze cross-presentation of soluble antigen, we compared DCs in their capacity to stimulate OVA-specific (OTI) CD8+ T cells from adult mice, using OVA peptide as a positive control for direct presentation not requiring antigen processing. While both neonatal and adult DCs induced the proliferation of OTI cells after loading with OVA peptide, neonatal DCs displayed a clearly reduced capacity to process and present soluble OVA (Figure 2). This did not reflect a difference in the optimal concentration of soluble OVA, since preliminary experiments demonstrated that 50 μg/mL induced maximal proliferation of OTI cells cultured with adult or neonatal DCs (data not shown). This deficit was evident with ex vivo, Flt-3L, and GM-CSF neonatal DCs. Cross-presentation of cell-associated antigen by neonatal DCs and of cell-associated or soluble antigens by adult DCs can be enhanced by inflammatory stimuli.15-18 In our system, the proinflammatory stimuli LPS and pI:C enhanced cross-presentation of soluble OVA by adult but not neonatal DCs (Figure 2).
Deficient cross-presentation of soluble ovalbumin by neonatal DCs. Neonatal (▪) and adult (▦) DCs (1 × 104 cells) were incubated with soluble ovalbumin (50 μg/mL) or the MHC class I-restricted ovalbumin peptide SIINFEKL (10 μM) along with nothing (REST), LPS (100 ng/mL), or pI:C (50 μg/mL) for 18 hours. After 3 washes, purified adult OT1 CD8+ T cells (1 × 105 cells) were added, and after 48 hours cultures were pulsed with 5 μCi (0.185 MBq) 3H-thymidine for 18 hours. Background proliferation of DCs alone, T cells alone, or DCs plus T cells but without ovalbumin or peptide was always less than 1000 counts per minute (cpm). Error bars indicate the standard error of the mean (SEM) of triplicate samples for this experiment. The graphs show one representative experiment of 3.
Deficient cross-presentation of soluble ovalbumin by neonatal DCs. Neonatal (▪) and adult (▦) DCs (1 × 104 cells) were incubated with soluble ovalbumin (50 μg/mL) or the MHC class I-restricted ovalbumin peptide SIINFEKL (10 μM) along with nothing (REST), LPS (100 ng/mL), or pI:C (50 μg/mL) for 18 hours. After 3 washes, purified adult OT1 CD8+ T cells (1 × 105 cells) were added, and after 48 hours cultures were pulsed with 5 μCi (0.185 MBq) 3H-thymidine for 18 hours. Background proliferation of DCs alone, T cells alone, or DCs plus T cells but without ovalbumin or peptide was always less than 1000 counts per minute (cpm). Error bars indicate the standard error of the mean (SEM) of triplicate samples for this experiment. The graphs show one representative experiment of 3.
Uptake of fluorescein isothiocyanate (FITC)-labeled OVA was equally efficient in neonatal and adult DCs (data not shown) as previously shown,3 and thus is unlikely to be the limiting factor in cross-presentation for neonatal DCs. Peptide presentation on the cell surface also appears intact, as the proliferation of OTI T cells was induced equally by neonatal and adult DCs loaded with OVA peptide (Figure 2, peptide; Dadaglio et al3 ). Thus, the difference between the neonatal and adult DCs in their ability to cross-present soluble antigen must lie in the processing and/or transport pathways between phagosome and cytosol.19,20 For adult in vitro-derived DCs, soluble OVA is known to be sequestered intracellulary after uptake, and to enter the cross-presentation pathway only after receiving other signals.21 The exact nature of these signals is unknown, and may differ between adult and neonatal DCs. It remains to be determined where in these complex pathways the defect in the neonatal DC lies: in the pathway of antigen processing, signal transduction, or both. Our data show a clear reduction in cross-presentation of soluble antigen in neonatal DCs. This defect was not overcome by inflammatory stimuli, and may contribute to the neonate's reduced generation of CTLs in response to vaccines.
Prepublished online as Blood First Edition Paper, February 24, 2004; DOI 10.1182/blood-2003-11-3805.
Supported by a grant from the National Institutes of Health (NIH; no. HD18184 to C.B.W.), a Pfizer postdoctoral fellowship grant in infectious diseases (T.R.K.), and a grant from the National Institute of Child Health and Human Development (NICHD; no. K12-H000850 [S.S.W.]).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Flt-3L was generously provided by Amgen.