Abstract
Dendritic cells (DCs) show a remarkable functional plasticity in the recognition of Aspergillus fumigatus and orchestrate the antifungal immune resistance in the lungs. Here, we show that thymosin α 1, a naturally occurring thymic peptide, induces functional maturation and interleukin-12 production by fungus-pulsed DCs through the p38 mitogen-activated protein kinase/nuclear factor (NF)-κB-dependent pathway. This occurs by signaling through the myeloid differentiation factor 88-dependent pathway, involving distinct Toll-like receptors. In vivo, the synthetic peptide activates T-helper (Th) cell 1-dependent antifungal immunity, accelerates myeloid cell recovery, and protects highly susceptible mice that received hematopoietic transplants from aspergillosis. By revealing the unexpected activity of an old molecule, our finding provides the rationale for its therapeutic utility and qualify the synthetic peptide as a candidate adjuvant promoting the coordinated activation of the innate and adaptive Th immunity to the fungus. (Blood. 2004;103: 4232-4239)
Introduction
Invasive aspergillosis (IA), characterized by hyphal invasion, destruction of pulmonary tissue, and dissemination to other organs, is the leading cause of both nosocomial pneumonia and death in allogeneic bone marrow transplantation (BMT) with an estimated infection rate of 5% to 10% and an associated mortality rate of 90% to 100%.1-3 The most important risk factor for IA has historically been neutropenia, such that reconstitution with myeloid progenitors offered protection against IA in a murine model of allogeneic BMT.4 However, recent studies on the epidemiology of IA in BM transplant recipients indicated a reduced neutropenia-related infection and an increase “late-onset” infection, in concomitance with the occurrence of graft-versus-host disease.3 These findings, together with the occurrence in patients without neutropenia,1 attest to the importance of specific defects in both the innate and adaptive immune effector mechanisms in the pathogenesis of the disease.3,5
Clinical and experimental evidence point to the crucial role of a T-cell helper 1 (Th1) reactivity in the control of IA.5-8 Dendritic cells (DCs) instruct Th1 priming to the fungus in vivo and in vitro.9-11 Evidence indicates that the ability of pulmonary DCs to instruct the appropriate T-cell responses to fungal antigens may be affected by local immunoregulatory events, including signaling through Toll-like receptors (TLRs).10-13 This evidence is consistent with a certain degree of flexibility of the immune recognition pathways to Aspergillus antigens and allergens, such that an allergen could be converted to a potential protective antigen, provided deoxycytidylate-phosphate-deoxyguanylate (CpG) as adjuvant.11 The model has brought DCs to center stage as promising targets for intervention for immunotherapy and vaccine development14 and has shifted the emphasis from the “antigen” toward the “adjuvant.”15,16 Thus, the promise of a fungal vaccine will demand for an adjuvant capable of both stimulating the appropriate type of response best tailored to combating the infection and being effective in conditions of immunosuppression.
Thymosin α 1 is a naturally occurring thymic peptide first described and characterized by Goldstein et al.17 In the form of a synthetic 28-amino acid peptide, thymosin α 1 is in clinical trials worldwide for the treatment of some viral infections, either as monotherapy or in combination with interferon α.18,19 Additional indications are some immunodeficiencies,20 malignancies,21 and HIV/AIDS.18 The mechanism of action of the synthetic polypeptide is not completely understood, but it is thought to be related to its immunomodulating activities, centered primarily on the augmentation of T-cell function. Because of its immunomodulatory function on cells on the innate immune system,22 including the ability to activate mitogen-activated protein kinases (MAPKs)23 and gene expression24 on macrophages, we hypothesized that thymosin α 1 could act as an adjuvant capable of activating DCs for Th1 priming to Aspergillus. In this article, we present evidence that thymosin α 1 activates DCs for antifungal Th1 priming in vitro and in vivo by signaling through distinct TLRs and the MyD88 adaptor protein.
Materials and methods
Animals
Female, 8- to 10-week-old, BALB/c and C57BL6 mice were from Charles River (Calco, Italy). Nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice were from Jackson Labs (Bar Harbor, ME). Breeding pairs of homozygous TLR2-, TLR9- and MyD88-deficient mice, raised on C57BL6 background, and of homozygous interferon γ (IFN-γ)- and interleukin 4 (IL-4)-deficient mice, raised on BALB/c background, were bred under specific pathogen-free conditions at the breeding facilities of the University of Perugia, Perugia, Italy.
Microorganism, culture conditions, infection, and treatments
The strain of A fumigatus and the culture conditions were as described elsewhere.7,10 For infection, mice were intranasally injected for 3 consecutive days with a suspension of 2 × 107 conidia/20 μL saline. For the quantification of fungal growth in the lungs, the chitin assay was used as described.7,10 The chitin content was expressed as micrograms of glucosamine per organ. The glucosamine content of lungs from uninfected mice was used as a negative control (range, 0.80-2.25 μg glucosamine/organ). For histologic analysis, lungs were excised and immediately fixed in formalin. Sections (3-4 μm) of paraffin-embedded tissues were stained with the periodic acid-Schiff procedure as described.7 Thymosin α 1 and the scrambled polypeptide (both from SciClone Pharmaceuticals, San Mateo, CA) were supplied as purified (the endotoxin levels were < 0.03 pg/mL, by a standard limulus lysate assay) sterile lyophilized acetylated polypeptides. The sequences were as follows: Ac-Ser-Asp-Ala-Ala-Val-Asp-Thr-Ser-Ser-Glu-IIe-Thr-Thr-Lys-Asp-Leu-Lys-Glu-Lys-Lys-Glu-Val-Glu-Glu-Ala-Glu-Asn-O (Thymosin α 1) and Ac-Ala-Lys-Ser-Asp-Val-Lys-Ala-Glu-Thr-Ser-Ser-Glu-Ile-Asp-Thr-Thr-Glu-Leu-Asp-Glu-Lys-Val-Glu-Val-Lys-Ala-Asn-Glu-OH (sThymosin α 1). The lyophilized powders were reconstituted in sterile water. Treatments were as follows: in mice that received BM transplants, thymosin α 1 at different doses intraperitoneally, sthymosin α 1 400 μg/kg intraperitoneally, and human recombinant granulocyte colony-stimulating factor (G-CSF; Granocyte 34; Aventis Pharma, Antony Cedex, France) 250 μg/kg intravenously were given daily beginning the day of the BM infusion, in concomitance with the infection and continuing for additional 3 days. Amphotericin B (A-2942; Sigma, St Louis, MO) was given daily for 3 days in concomitance with the infection, at the dose of 4000 μg/kg intraperitoneally. In preliminary experiments, this dose was found to cure IA in cyclophosphamide-treated mice (data not shown). Cyclophosphamide (Sigma), 150 mg/kg intraperitoneally, was given a day before the infection. In cyclophosphamide-treated mice, 400 μg/kg thymosin α 1 was given intraperitoneally for 5 consecutive days beginning the day of the infection; neutrophil depletion was obtained by treatment with 1 mg Gr-1-neutralizing RB6-8C5 antibody intravenously, a day before and after the infection. In preliminary experiments, lower amounts of antibodies did not completely ablate the highly represented population of Gr1+F480- neutrophils in the lungs of NOD/SCID mice (twice as much present than in wild-type mice, ie, from 7%-15%) or in the peripheral blood (a mean of 24.8 × 105 cells/mL; ie, > 70% of white blood cells, the remaining being represented by monocytes [about 27%] and < 2% by lymphocytes). The treatment dramatically reduced the number of lung neutrophils (see footnote of Table 1) but not that of DCs (between 5 and 6 × 105 CD11c+, major histocompatibility complex [MHC] class II+, F480- cells before and after treatment). Control mice received an equivalent amount of purified rat IgG2b (catalog no. 02-9288; Zymed Laboratories, South San Francisco, CA). Fluorescence activated cell sorting (FACS) analysis of lung cells a day after treatment with cyclophosphamide revealed a profound and long-lasting (for up to 5 days) leukopenia (from 35% of CD4+ T cells in controls to < 5% in treated mice; from 20% to 4% of CD8+ T cells; from 19% to 7% of B220+ cells, and from 7% to < 2% Gr1+ F480- cells). The percentages of F480+ cells (about 20%) and that of CD11c+, MHC class II+, F480- DCs (< 3%) were unaffected by treatment.
Neutrophils and Th1 cells are required for the therapeutic efficacy of thymosin α 1
Group . | Mice* . | Treatment† . | Neutrophils/lung, × 103‡ . | Chitin content‡ . | MST, d‡ . |
|---|---|---|---|---|---|
| 1 | BMT | None | 71 ± 8 | 44 ± 6 | 4.5 |
| 2 | BMT | G-CSF | 544 ± 28§ | 25 ± 4§ | 10§ |
| 3 | BALB/c | None | 54 ± 4 | 38 ± 4 | 5.5 |
| 4 | BALB/c | Thymosin α 1 | 714 ± 12§ | 18 ± 2 | > 60§ |
| 5 | NOD/SCID | None | 194 ± 19 | 68 ± 12 | 6 |
| 6 | NOD/SCID | Thymosin α 1 | 491 ± 22§ | 31 ± 7§ | 15§ |
| 7 | NOD/SCID | Thymosin α 1 + neutrophil depletion | 69 ± 14§ | 59 ± 10 | 5.5 |
| 8 | IFN-γ deficient | None | 84 ± 10 | 49 ± 5 | 7.5 |
| 9 | IFN-γ deficient | Thymosin α 1 | 681 ± 44§ | 25 ± 4§ | 18§ |
| 10 | IL-4 deficient | None | 88 ± 8 | 32 ± 5 | 22 |
| 11 | IL-4 deficient | Thymosin α 1 | 704 ± 51§ | 0.9 ± 0.3§ | > 60§ |
Group . | Mice* . | Treatment† . | Neutrophils/lung, × 103‡ . | Chitin content‡ . | MST, d‡ . |
|---|---|---|---|---|---|
| 1 | BMT | None | 71 ± 8 | 44 ± 6 | 4.5 |
| 2 | BMT | G-CSF | 544 ± 28§ | 25 ± 4§ | 10§ |
| 3 | BALB/c | None | 54 ± 4 | 38 ± 4 | 5.5 |
| 4 | BALB/c | Thymosin α 1 | 714 ± 12§ | 18 ± 2 | > 60§ |
| 5 | NOD/SCID | None | 194 ± 19 | 68 ± 12 | 6 |
| 6 | NOD/SCID | Thymosin α 1 | 491 ± 22§ | 31 ± 7§ | 15§ |
| 7 | NOD/SCID | Thymosin α 1 + neutrophil depletion | 69 ± 14§ | 59 ± 10 | 5.5 |
| 8 | IFN-γ deficient | None | 84 ± 10 | 49 ± 5 | 7.5 |
| 9 | IFN-γ deficient | Thymosin α 1 | 681 ± 44§ | 25 ± 4§ | 18§ |
| 10 | IL-4 deficient | None | 88 ± 8 | 32 ± 5 | 22 |
| 11 | IL-4 deficient | Thymosin α 1 | 704 ± 51§ | 0.9 ± 0.3§ | > 60§ |
Mice that received BM transplants (groups 1 and 2) or treated with cyclophosphamide (groups 3-11) were infected with 2 × 107Aspergillus conidia intranasally for 3 consecutive days.
In mice that received BM transplants, G-CSF (250 μg/kg intravenously) was given daily, beginning the day of the BM infusion, in concomitance with the infection and continuing for an additional 3 days. In cyclophosphamide-treated mice, thymosin α 1 (400 μg/kg intraperitoneal) was given for 5 consecutive days, beginning the day of the infection; neutrophil depletion was obtained by treatment with 1 mg RB6-8C5 antibody intravenously a day before and after the infection.
Lungs were assessed for the presence of fungal cells (chitin content, expressed as microgram glucosamine per lung) and neutrophils (FACS analysis) a day (untreated mice, None) or 3 days (treated mice) after the last Aspergillus inoculation. Values are the mean ± SE for the neutrophil and chitin columns. MST indicates median survival time.
P < .05, treated versus untreated mice. Each type of intact mice survived the infection and efficiently controlled the fungal growth.
TLR ligands
Zymosan from Saccharomyces cerevisiae, lipoteichoic acid (LTA) from Staphylococcus aureus, lipopolysaccharide (LPS) from Salmonella minnesota Re 595, and Poly(I:C) (polyinosine-polycytidylic acid) were purchased from Sigma. CpG oligonucleotides 1826 and 2006 of proven immunostimulatory sequences were obtained as described.11
Generation of mice that received BM transplants
DC isolation and culture
Blood CD1c+ myeloid DCs (MDCs) were generated from CD14+ mononuclear cells by magnetic cell sorting (MiniMACs; Miltenyi Biotec SRL, Bologna, Italy) and cultured for 5 days in Iscoves modified medium, containing 10% fetal bovine serum, 50 μM 2-mercaptoethanol, sodium pyruvate (1 mM), 2 mM l-glutamine, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; 10 mM), and 50 μg/mL gentamycin in the presence of 50 ng/mL rHuman granulocyte-macrophage colony-stimulating factor (GM-CSF; Schering-Plough, Milano, Italy), and 200 U/mL rHuman IL-4 (Peprotech, Inalco, Milano, Italy).26 Immature MDCs were cultured for 24 hours with 1000 ng/mL trimeric human CD40 ligand-leucine-zipper fusion protein (Immunex, Seattle, WA) to obtain mature DCs. CD123+ plasmacytoid DCs (PDCs) were isolated by using the BDCA-4 isolation kit (Miltenyi Biotec). Purity of CD123+ cells was more than 96%. For mature PDCs, immature DCs were cultured with the trimeric human CD40 ligand as above and 10 ng/mL IL-3 (R&D System, Milano Italy). FACS analysis revealed that PDCs were CD123bright, CD4+, CD45RA+, and CD11c- as opposed to MDCs characterized as being CD1a+, CD11c+, CD11b+, CD4+, CD14low, and CD8-. The expression of HLA class II, CD80, and CD86 was high in both immature and mature DCs. Murine lung CD11c+ DCs (between 5% and 7% positive for CD8α and between 30% and 35% positive for Gr-1) were isolated by magnetic cell sorting.10 For phagocytosis, DCs were pre-exposed to 100 ng/mL thymosin α 1 for 60 minutes and subsequently incubated at 37°C with Aspergillus conidia for an additional 60 minutes.10 Percentage of internalization was calculated as described.10 Photographs were taken by using a high Resolution Microscopy Colour Camera AxioCam, using the AxioVision Software Rel. 3.1 (Carl Zeiss, Milano, Italy). For functional maturation and cytokine determination, purified DCs were resuspended in Iscoves medium (with no serum but with polymixin B, to avoid nonspecific activation by serum components and endotoxin) and pulsed with 100 ng/mL thymosin α 1 for 24 hours either alone or together with TLR ligands or unopsonized Aspergillus conidia.
Phenotypic analysis
Cell surface phenotype was assessed by reacting samples with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated rat antimouse antibodies from PharMingen (San Diego, CA). Unrelated histotype-matched antibodies were used as control. Analysis was performed on a FACScan (Becton Dickinson, Mountain View, CA).
Antifungal effector activity
For phagocytosis, bronchoalveolar macrophages and peripheral neutrophils were pre-exposed to 100 ng/mL thymosin α 1 for 60 minutes and incubated at 37°C with unopsonized Aspergillus conidia for 60 minutes.10 For the conidiocidal activity, the number of colony-forming units and the percentage of colony-forming unit inhibition (mean ± SE), referred to as conidiocidal activity, were determined as described.10
Assay with HEK293-transfected cells
The human embryonic kidney cell line HEK293, wild type or stably transfected with human TLR2, TLR9, and TLR4/CD14m,27 was cultured in low-glucose Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (FCS), HEPES (10 nM), l-glutamine (2 μg/mL), gentamycin (50 μg/mL) (Invitrogen Srl Life Technologies, Milano, Italy). Transfectants were additionally supplemented with puromycin (100 μg/mL; Sigma). For stimulation experiments, cells were cultured at a density of 3 to 5 × 105 cells/wells in 12-well tissue culture plates overnight. Cells were washed and stimulated with 100 ng/mL thymosin α 1 either alone or together with TLR ligands for 5 hours before the assessment of IL-8 production in the supernatants.
Cytokine and spot enzyme-linked immunosorbent (ELISPOT) assay
The levels of tumor necrosis factor α (TNF-α), IL-10, IL-12 p70, IFN-α, and IL-8 in culture supernatants were determined by Kit enzyme-linked immunosorbent assays (ELISAs; Endogen Human Elisa Kits, S.r.l.; R&D Systems and Euroclone, Milan, Italy). The detection limits (picogram per milliliter) of the assays were less than 3 (human) and less than 32 (murine) for TNF-α, less than 12 (murine) and less than 5 (human) for IL-10, less than 16 (murine) and less than 3 (human) for IL-12 p70, less than 25 (human) for IL-8, and less than 3 (human) for IFN-α. For enumeration of cytokine-producing cells, an ELISPOT assay was used on purified CD4+ T cells and DCs from lungs, as described.10
Proliferation assay by flow cytometric analysis
Proliferation of lung CD4+ T lymphocytes stimulated with 10 μg/mL concanavalin A (Con A) or heat-inactivated conidia in the presence of lung DCs was assessed by labeling with CFSE (56-carboxyfluorescein diacetate succinimidyl ester; Molecular Probes, Eugene, OR), as described.11
Reverse transcriptase (RT)-PCR
Total RNA was extracted (TRIZOL; Invitrogen) from immature DCs pretreated with 100 ng/mL thymosin α 1 for 60 minutes followed by the exposure to unosponized Aspergillus conidia for 60 minutes, as suggested by initial experiments. Synthesis and polymerase chain reaction (PCR) of cDNA were done as described.10 The forward and reverse PCR primers and the cycles used for murine and human TLRs and hypoxanthine phosphoribosyl-transferase (HPRT) were as described.28 The synthesized PCR products were separated by electrophoresis on 2% agarose gel and visualized by ethidium bromide staining.
Analysis of p38 and NF-κB activation
The activation of p38 and nuclear factor-κB (NF-κb on lung DCs exposed for 20 minutes (as suggested by initial experiments) at 37°C to Aspergillus conidia and/or 100 ng/mL thymosin α 1 was done as described.29 Blots of cell lysates were incubated with rabbit polyclonal antibodies (Abs) recognizing either the unphosphorylated form of p38 MAPK or the double-phosphorylated (Thr-180/Tyr-182) p38 MAPK (Cell Signaling Technology, Celbio, Milano, Italy), or Abs specific for the Rel A, 65-kDa DNA binding subunit of human NF-κb (Zymed Laboratories), followed by horseradish peroxidase-conjugated goat antirabbit IgG (Cell Signaling Technology), as per the manufacturer's instructions. Blots were developed with the enhanced chemiluminescence detection kit (Amersham Pharmacia Biothech, Cologno Monzese, Milano, Italy). Bands were visualized after exposure of the blots to a Kodak RX film. To ensure similar protein loading in each lane, the phospho blots were stripped, and the membranes were reprobed with Abs against p38 and NF-κB.
Statistical analysis
Survival data were analyzed by using the Mann-Whitney U test. Student t test was used to determine the significance of values in experimental group (significance was defined as P < .05). In vivo groups consisted of 6 animals.
Results
Thymosin α 1 activates DCs
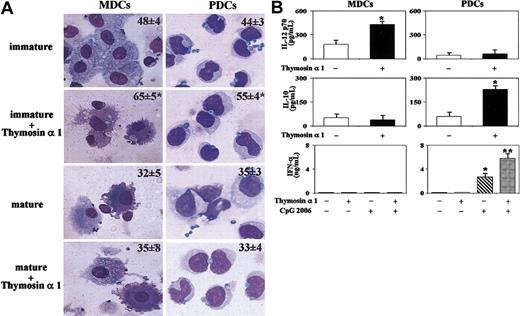
Previous studies have shown that murine DCs phagocytose Aspergillus in vitro and at the site of infection.10 We show here that thymosin α 1 but not the scrambled peptide (sThymosin α 1) activates lung DCs for phagocytosis of unopsonized conidia (more than hyphae), costimulatory antigen expression, and cytokine production (Figure 1A). In line with previous findings,10 Aspergillus conidia alone did not represent a sufficient stimulus to induce the activation of DCs, but the combined exposure to thymosin α 1 remarkably increased the expression of MHC class II antigens, CD86, and CD40 molecules (Figure 1B) and the frequency of IL-12 p70-producing DCs. Interestingly, IL-12 p70-producing DCs were also increased by thymosin alone (Figure 1C).
Thymosin α 1 activates murine DCs on exposure to A fumigatus Murine lung CD11c+ DCs were pretreated with thymosin α 1 or the scrambled peptide (sThymosin α1) before the exposure to conidia or hyphae for the assessment of (A) phagocytosis, (B) costimulatory molecule expression, and (C) frequency of IL-12 p70-producing cells. (A) Assessment of phagocytosis (the data are the means ± SEs of 3 independent experiments and expressed as the percentage of internalization [numbers within figures]; *P < .05, thymosin-exposed versus unexposed cells). Cells were stained with Diff-Quik stain (Carlo Erba Reagents, Milan, Italy). Original magnification, ×100. (B) Costimulatory molecule expression by FACS analysis (data from one representative experiment of 3). Black histograms represent cells stained with an irrelevant antibody. The numbers refer to the median fluorescence intensity. (C) Frequency of IL-12 p70-producing cells (the values are the mean ± SE per 105 cells of samples from 3-5 experiments, calculated by ELISPOT assay). *P < .05, exposed versus unexposed (None) or conidia-exposed cells.
Thymosin α 1 activates murine DCs on exposure to A fumigatus Murine lung CD11c+ DCs were pretreated with thymosin α 1 or the scrambled peptide (sThymosin α1) before the exposure to conidia or hyphae for the assessment of (A) phagocytosis, (B) costimulatory molecule expression, and (C) frequency of IL-12 p70-producing cells. (A) Assessment of phagocytosis (the data are the means ± SEs of 3 independent experiments and expressed as the percentage of internalization [numbers within figures]; *P < .05, thymosin-exposed versus unexposed cells). Cells were stained with Diff-Quik stain (Carlo Erba Reagents, Milan, Italy). Original magnification, ×100. (B) Costimulatory molecule expression by FACS analysis (data from one representative experiment of 3). Black histograms represent cells stained with an irrelevant antibody. The numbers refer to the median fluorescence intensity. (C) Frequency of IL-12 p70-producing cells (the values are the mean ± SE per 105 cells of samples from 3-5 experiments, calculated by ELISPOT assay). *P < .05, exposed versus unexposed (None) or conidia-exposed cells.
The assessment of the actual cytokine production in supernatants of DCs confirmed the higher production of IL-12 p70 along with TNF-α in response to conidia and thymosin α 1 as compared with conidia or thymosin α 1 alone [from 0.75 ± 0.15 (conidia alone) or 1.4 ± 0.37 [thymosin alone] to 2.6 ± 0.51 [in combination] ng/mL of IL-12 p70, and from 147 ± 32 [conidia alone] or 289 ± 60 [thymosin alone] to 681 ± 97 [in combination] pg/mL TNF-α). The scrambled peptide failed to up-regulate class II antigens and costimulatory molecule expression and to induce cytokine production by DCs in response to conidia (data not shown).
Thymosin α 1 also activated human MDC and PDC subsets. Both immature and mature DC subsets phagocytose conidia. Thymosin α 1 increased the phagocytic activity of immature DCs, affected the DC morphology, as more cytoplasmic projections were detected in immature MDCs (Figure 2A) and up-regulated the HLA class II antigens and costimulatory molecule expression in response to conidia (data not shown). In terms of cytokine production, thymosin α 1 significantly increased the release of IL-12 p70 in response to conidia (Figure 2B) and to zymosan (data not shown) by immature MDCs and that of IL-10 in response to conidia by immature PDCs (Figure 2B). The production IFN-α, known to be produced by immature PDCs,30 was also assessed and found not to be released by either DC subset in response to conidia and/or thymosin α 1. However, it was produced by PDCs in response to the TLR9 ligand CpG and significantly potentiated by thymosin α 1 (Figure 2B). Together, these data point to a novel, previously undefined, immunoregulatory role for thymosin α 1 in the activation and functioning of DCs.
Thymosin α 1 activates human DCs on exposure to A fumigatus MDCs and PDCs were obtained from blood mononuclear cells. For phagocytosis (A), see legend to Figure 1. The data are the means ± SEs of 3 independent experiments and expressed as the percentage of internalization (numbers within figures). *P < .05, thymosin-exposed versus unexposed cells. Cells were stained with Diff-Quik stain (Carlo Erba Reagents, Milan, Italy). Original magnification, ×100. (B) Cytokines were determined in 24-hour culture supernatants of DCs stimulated (+) or not (-) with thymosin α 1 in the presence of conidia or CpG 2006 (3 μg/mL). Bars indicate the standard errors. *P < .05, cytokine production in thymosin-exposed versus unexposed cells. **P < .05, thymosin + CpG versus CpG alone.
Thymosin α 1 activates human DCs on exposure to A fumigatus MDCs and PDCs were obtained from blood mononuclear cells. For phagocytosis (A), see legend to Figure 1. The data are the means ± SEs of 3 independent experiments and expressed as the percentage of internalization (numbers within figures). *P < .05, thymosin-exposed versus unexposed cells. Cells were stained with Diff-Quik stain (Carlo Erba Reagents, Milan, Italy). Original magnification, ×100. (B) Cytokines were determined in 24-hour culture supernatants of DCs stimulated (+) or not (-) with thymosin α 1 in the presence of conidia or CpG 2006 (3 μg/mL). Bars indicate the standard errors. *P < .05, cytokine production in thymosin-exposed versus unexposed cells. **P < .05, thymosin + CpG versus CpG alone.
Thymosin α 1 activates the MyD88-dependent pathway through TLR signaling
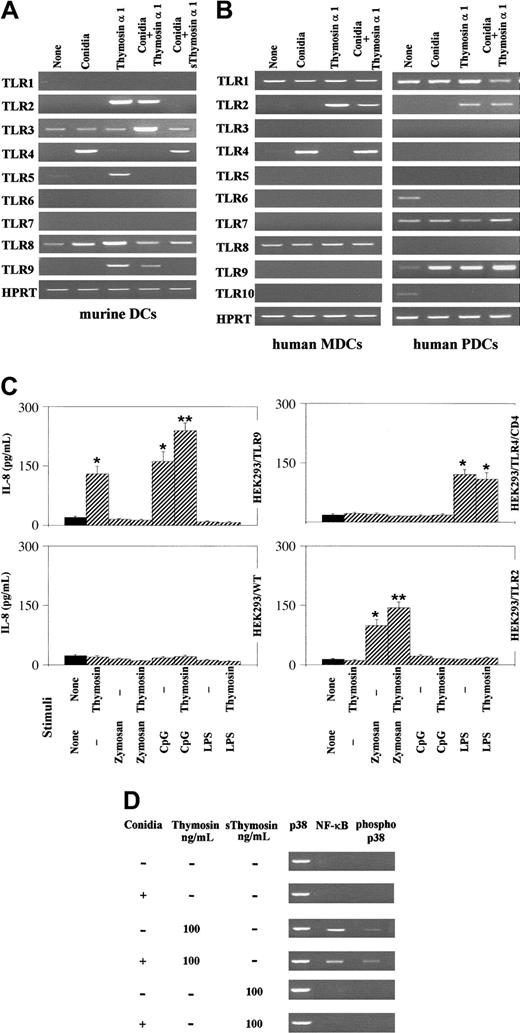
TLRs signaling occurs in response to Aspergillus and mediates functional responses to the fungus.11-13 Thymosin α 1 strongly activated the expression of TLR2, TLR5, and TLR9 on murine DCs, as opposed to conidia that poorly or not at all activated TLR expression, with the exception of TLR4. TLR2 and TLR9 were still activated on the combined exposure to conidia and thymosin α 1, whereas this was not the case for TLR5 whose expression was instead inhibited. Importantly, the scrambled peptide failed to activate TLR2 and TLR9 expression in response to conidia (Figure 3A) or alone (data not shown). The pattern of TLR expression was not different with and without polymixin B (data not shown). Similar to murine DCs, conidia induced the expression of TLR4 on human MDCs and thymosin α 1, alone or together with conidia, induced TLR2 expression on both DC subsets and TLR9 on PDCs (Figure 3B). No TLRs expression was induced on incubation of cells with the diluent alone (Figure 3A-B).
Thymosin α 1 activates TLR-dependent signaling. Murine lung DCs (A) and human DC subsets (B), pretreated with thymosin α 1 or the scrambled peptide (sThymosin α 1), were exposed to Aspergillus conidia and assessed for TLR expression by RT-PCR. cDNA levels were normalized against the HPRT gene. None indicates cells exposed to the diluent alone. (C) Production of IL-8 by wild-type or TLR-transfected HEK293 cells stimulated with thymosin α 1 and/or zymosan (10 μg/mL), CpG 2006 (3 μg/mL), and LPS (1 mg/mL). *P < .05, stimulated versus unstimulated (None) cells. **P < .05, CpG or zymosan + thymosin versus each single agent alone. Bars indicate the standard errors. (D) Activation of NF-κB and p38 on murine lung DCs exposed to Aspergillus conidia, thymosin α 1, or the scrambled peptide, either alone or in combination. The assay was done by probing the cell lysates with specific antiphospho-38 and anti-NF-κB antibodies. Shown are the data from 1 representative experiment of 3.
Thymosin α 1 activates TLR-dependent signaling. Murine lung DCs (A) and human DC subsets (B), pretreated with thymosin α 1 or the scrambled peptide (sThymosin α 1), were exposed to Aspergillus conidia and assessed for TLR expression by RT-PCR. cDNA levels were normalized against the HPRT gene. None indicates cells exposed to the diluent alone. (C) Production of IL-8 by wild-type or TLR-transfected HEK293 cells stimulated with thymosin α 1 and/or zymosan (10 μg/mL), CpG 2006 (3 μg/mL), and LPS (1 mg/mL). *P < .05, stimulated versus unstimulated (None) cells. **P < .05, CpG or zymosan + thymosin versus each single agent alone. Bars indicate the standard errors. (D) Activation of NF-κB and p38 on murine lung DCs exposed to Aspergillus conidia, thymosin α 1, or the scrambled peptide, either alone or in combination. The assay was done by probing the cell lysates with specific antiphospho-38 and anti-NF-κB antibodies. Shown are the data from 1 representative experiment of 3.
To get further insights into the ability of thymosin α 1 to activate TLR-dependent signaling, HEK293 cells transfected with TLR2, TLR9, and TLR4/CD14 were assessed for IL-8 production in response to thymosin α 1 alone or together with the relevant TLR ligands. The results show that thymosin α 1 significantly increased the production of IL-8 by TLR9-transfected cells either alone or in response to CpG; it did not stimulate the production of IL-8 by TLR2-transfected cells alone but slightly increased the production in response to zymosan, and it did not induce IL-8 by TLR4/CD14-transfected cells either alone or in response to LPS (Figure 3C). Therefore, thymosin α 1 appears to be able to signal directly through TLR9 and to potentiate TLR2 signaling by the relevant ligand.
As NF-κB and p38 MAPK activation are early events in triggering TLR-induced gene expression,31,32 and thymosin α 1 activates MAPK-transduction pathways,23 the nuclear translocation of NF-κB as well as p38 phosphorylation in murine DCs was also assessed. Conidia alone failed to induce NF-κB activation and p38 phosphorylation that were instead induced on treatment with thymosin α 1, but not with the scrambled peptide, either alone or combined with conidia (Figure 3D).
The SN50 (an inhibitor of NF-κB nuclear translocation)33 or SB202190 (an inhibitor of p38 MAPK)34 inhibitors ablated the effect of thymosin α 1 on DCs in terms of IL-12 p70 production (from 1.6 ± 0.62 ng/mL of the control to undetectable levels [< 16 pg/mL] in the presence of both inhibitors).
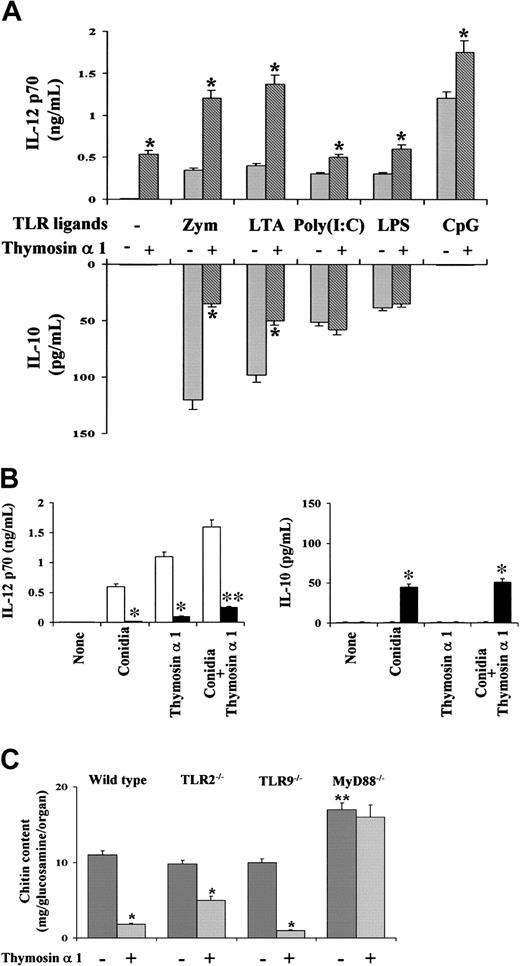
Further studies showed that thymosin α 1 affected the ability of murine DCs to produce IL-12 p70 and IL-10 in response to known microbial TLR ligands.31,32,35 Thymosin α 1 did not affect the cytokine production in response to Poly(I:C) and LPS. However, it significantly increased (at about 4-fold) the production of IL-12 p70 and decreased that of IL-10 to stimulation with the TLR2 ligands, such as zymosan and LTA. The production of IL-12 p70 was also increased to stimulation with the TLR9 ligand CpG, although to a lesser extent (Figure 4A). Among others,31,32 the myeloid differentiation factor 88 (MyD88) is an adaptor protein essential for the activation of NF-κB and MAPK and the production of IL-12 p70 on signaling by TLRs. We found here that the IL-12 p70 production was dramatically ablated in MyD88-deficient mice in response to conidia and/or thymosin α 1. Interestingly, IL-10 production was observed in response to conidia in MyD88-deficient mice but was not modified by thymosin α 1 (Figure 4B). Therefore, it appears that the MyD88-dependent pathway plays an essential role in the ability of thymosin α 1 to modulate IL-12 p70 production by DCs.
Thymosin α 1 activates the MyD88-dependent pathway of TLR signaling. (A) Production of IL-12 p70 and IL-10 by murine lung DCs on exposure to TLR ligands and/or thymosin α 1. Zym (zymosan, 10 μg/mL), LTA (1 μg/mL), Poly(I:C) (10 μg/mL), LPS (1 mg/mL), and unmethylated CpG 1826 (CpG, 2 μM) are shown. *P < .05, cytokine production in the presence (+) or not (-) of thymosin α 1. Bars indicate the standard errors. (B) Production of IL-12 p70 and IL-10 from lung DCs from wild-type (□) or MyD88-deficient (▪) mice, on exposure to conidia and/or thymosin α 1. Bars indicate the standard errors. *P < .01, cytokine production in MyD88-deficient versus wild-type mice. (C) Mice were intranasally infected with Aspergillus conidia and treated with thymosin α 1 for 5 consecutive days, beginning the day of the infection. The fungal growth (chitin content of the lungs) was assessed a day after treatment. Bars indicate the standard errors. *P < .05, treated versus untreated mice. **P < .05, MyD88-deficient versus wild-type mice.
Thymosin α 1 activates the MyD88-dependent pathway of TLR signaling. (A) Production of IL-12 p70 and IL-10 by murine lung DCs on exposure to TLR ligands and/or thymosin α 1. Zym (zymosan, 10 μg/mL), LTA (1 μg/mL), Poly(I:C) (10 μg/mL), LPS (1 mg/mL), and unmethylated CpG 1826 (CpG, 2 μM) are shown. *P < .05, cytokine production in the presence (+) or not (-) of thymosin α 1. Bars indicate the standard errors. (B) Production of IL-12 p70 and IL-10 from lung DCs from wild-type (□) or MyD88-deficient (▪) mice, on exposure to conidia and/or thymosin α 1. Bars indicate the standard errors. *P < .01, cytokine production in MyD88-deficient versus wild-type mice. (C) Mice were intranasally infected with Aspergillus conidia and treated with thymosin α 1 for 5 consecutive days, beginning the day of the infection. The fungal growth (chitin content of the lungs) was assessed a day after treatment. Bars indicate the standard errors. *P < .05, treated versus untreated mice. **P < .05, MyD88-deficient versus wild-type mice.
To assess whether the MyD88-dependent pathway could play an essential role also in vivo, we resorted to an experimental model of IA, in which wild-type, TLR2-, TRL9-, and MyD88-deficient mice were intranasally infected with Aspergillus conidia, treated with thymosin α 1, and assessed for the local fungal growth. The fungal growth in TLR2- and TLR9-deficient mice was comparable to that of wild-type mice; it was similarly impaired on thymosin treatment in wild-type, TLR9-, and, to a lesser extent, TLR2-deficient mice; however, it was not impaired in MyD88-deficient mice and could not be restored by thymosin α 1 (Figure 4C). Together, these data provide evidence that, despite a degree of redundancy in the TLR usage, the MyD88-dependent signaling pathway is essential in the activity of thymosin α 1 in vitro and in vivo.
Thymosin α 1 protects mice that received BM transplants from IA
On the basis of the earlier findings, we assessed whether treatment with thymosin α 1 would increase antifungal resistance in mice that received BM transplants with IA.25 Treatment with thymosin α 1, but not with the scrambled peptide, cured the mice from the infection, as revealed by the increased survival after the infection that paralleled the reduced fungal growth in the lungs. The effect on protection was dose dependent, full protection (> 60-day survival) was achieved in mice treated with 200 and 400 μg/kg thymosin α 1 and was superior to that of amphotericin B. Interestingly, thymosin α 1 appeared to increase the therapeutic efficacy of amphotericin B, as indicated by the increased survival and decreased fungal burden of mice treated with both agents (Figure 5A). Thymosin α 1 also decreased lung pathology. Lung sections from infected mice showed the presence of numerous Aspergillus hyphae infiltrating the lung parenchyma, with severe signs of bronchial wall damage and necrosis and scarce inflammatory cell recruitment (Figure 5B). These features were not observed in thymosin-treated mice, whose lungs were characterized by healing infiltrates of inflammatory cells with no evidence of fungal growth and bronchial wall destruction (Figure 5C). These data point to the therapeutic efficacy of thymosin α 1 in IA and suggest the benefit of the association with antifungals known to have a reduced activity in BMT settings.3
Thymosin α 1 protects mice that received BM transplants from invasive aspergillosis. Mice that received BM transplants were intranasally infected with Aspergillus conidia a week after the BM infusion. Thymosin α 1 or the scrambled peptide (group 7) was given intraperitoneally daily beginning the day of the BM infusion, in concomitance with the infection and continuing for additional 3 days. Amphotericin B was given intraperitoneally for 3 days, in concomitance with the infection. (A) MST indicates median survival times (days), calculated from the beginning of the infection. The numbers refer to animals that died over total injected. The fungal growth was assessed at 1 (control) or 4 days after the last conidia inoculation. Bars indicate the standard errors. *P < .05, treated versus untreated mice. **P < .05, combined treatment versus each single treatment. (B-C) Periodic acid-Schiff-stained sections from lungs of infected mice that received BM transplants either untreated (panel B at 1 day after infection) or treated with thymosin α 1 (panel C at 4 days after infection). Numerous Aspergillus hyphae (arrows) infiltrating the lung parenchyma, with severe signs of bronchial wall damage and necrosis and scarce inflammatory cell recruitment are observed in the lungs of untreated mice, as opposed to that observed in thymosin-treated mice, whose lungs were characterized by healing infiltrates of inflammatory cells with no evidence of bronchial wall damage and fungal growth. Magnification × 400 in both panels.
Thymosin α 1 protects mice that received BM transplants from invasive aspergillosis. Mice that received BM transplants were intranasally infected with Aspergillus conidia a week after the BM infusion. Thymosin α 1 or the scrambled peptide (group 7) was given intraperitoneally daily beginning the day of the BM infusion, in concomitance with the infection and continuing for additional 3 days. Amphotericin B was given intraperitoneally for 3 days, in concomitance with the infection. (A) MST indicates median survival times (days), calculated from the beginning of the infection. The numbers refer to animals that died over total injected. The fungal growth was assessed at 1 (control) or 4 days after the last conidia inoculation. Bars indicate the standard errors. *P < .05, treated versus untreated mice. **P < .05, combined treatment versus each single treatment. (B-C) Periodic acid-Schiff-stained sections from lungs of infected mice that received BM transplants either untreated (panel B at 1 day after infection) or treated with thymosin α 1 (panel C at 4 days after infection). Numerous Aspergillus hyphae (arrows) infiltrating the lung parenchyma, with severe signs of bronchial wall damage and necrosis and scarce inflammatory cell recruitment are observed in the lungs of untreated mice, as opposed to that observed in thymosin-treated mice, whose lungs were characterized by healing infiltrates of inflammatory cells with no evidence of bronchial wall damage and fungal growth. Magnification × 400 in both panels.
Thymosin α 1 accelerates myeloid and Th1 cell recovery in mice with IA
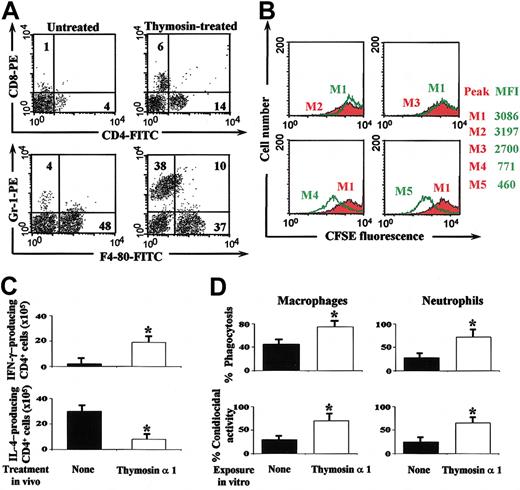
To evaluate whether thymosin α 1 would activate protective IFN-γ-producing Th1 cells in mice that received BM transplants with IA,7,8,10 we assessed cell recovery by FACS analysis together with the antigen-specific lymphoproliferation, pattern of local cytokine production, and antifungal activity of effector phagocytes. Although the absolute number of circulating lymphocytes and neutrophils significantly increased after thymosin treatment (data not shown), a cytofluorimetric analysis was performed on lung cells, as blood neutrophil levels do not predict susceptibility to aspergillosis.4 The numbers of CD4+ cells, CD8+ cells, and neutrophils were significantly increased in mice that receive BM transplants on treatment with thymosin α 1 (Figure 6A). Recovered lung CD4+ T lymphocytes were functionally active as indicated by the antigen-specific proliferation (Figure 6B) and the IFN-γ production. The frequency of IFN-γ-producing cells was higher and that producing IL-4 lower in mice treated with thymosin α 1 as compared with untreated mice (Figure 6C). On assessing the level of antifungal activity of effector phagocytes it was found that the conidiocidal activity of both macrophages and neutrophils was higher in thymosin-treated than untreated mice (data not shown). The finding that the phagocytosis and killing of conidia by alveolar macrophages and circulating neutrophils from uninfected mice were also significantly potentiated in vitro in the presence of thymosin α 1 (Figure 6D) suggests that thymosin α 1 not only promotes DC maturation but will also activate local effector cells for prompt phagocytosis and killing of the fungus.
Thymosin α 1 accelerates functional Th1 cell recovery in BM-transplanted mice with IA. Mice that received BM transplants were infected and treated as described in the legend to Figure 5. (A) The numbers refer to the percentage of positive cells in the lungs on FACS analysis. (B) Lung CD4+ T lymphocytes were CFSE-stained (M1) and stimulated with Con A(M4) or heat-inactivated Aspergillus (M5) in the presence of lung DCs for 5 days before FACS analysis. M2 and M3, CD4+ T cells without and with DCs alone, respectively. Shown are the results from 1 representative experiment of 3. The numbers refer to the median fluorescence intensity, MFI. (C) Lung CD4+ T cells producing cytokines were enumerated by ELISPOT assays. *P < .05, thymosin-treated versus untreated (none) mice. (A-C) The assays were done 4 days after the last conidia inoculation. Bars indicate the standard errors. (D) Bronchoalveolar macrophages and peripheral neutrophils from uninfected mice were pre-exposed to thymosin α 1 and then to conidia before the assessment of the phagocytic and conidiocidal activity. Values are the mean ± SE of samples taken from 3 to 5 experiments. *P < .05, thymosin-exposed versus unexposed (None) cells.
Thymosin α 1 accelerates functional Th1 cell recovery in BM-transplanted mice with IA. Mice that received BM transplants were infected and treated as described in the legend to Figure 5. (A) The numbers refer to the percentage of positive cells in the lungs on FACS analysis. (B) Lung CD4+ T lymphocytes were CFSE-stained (M1) and stimulated with Con A(M4) or heat-inactivated Aspergillus (M5) in the presence of lung DCs for 5 days before FACS analysis. M2 and M3, CD4+ T cells without and with DCs alone, respectively. Shown are the results from 1 representative experiment of 3. The numbers refer to the median fluorescence intensity, MFI. (C) Lung CD4+ T cells producing cytokines were enumerated by ELISPOT assays. *P < .05, thymosin-treated versus untreated (none) mice. (A-C) The assays were done 4 days after the last conidia inoculation. Bars indicate the standard errors. (D) Bronchoalveolar macrophages and peripheral neutrophils from uninfected mice were pre-exposed to thymosin α 1 and then to conidia before the assessment of the phagocytic and conidiocidal activity. Values are the mean ± SE of samples taken from 3 to 5 experiments. *P < .05, thymosin-exposed versus unexposed (None) cells.
However, recovery from neutropenia alone, as by treatment with a dose of G-CSF known to accelerate neutrophil recovery in mice,4 was not sufficient to mediate a degree of antifungal resistance comparable to that obtained with thymosin α 1, as mice that receive BM transplants were unable to survive the infection (Table 1). Similarly, despite a significant neutrophil recovery and the ablation of resistance on neutrophil depletion, thymosin α 1 had a limited therapeutic efficacy in mice devoid of T cells or IFN-γ-producing Th1 cells. In contrast, the optimal therapeutic efficacy of thymosin α 1 was achieved in the presence of an heightened antifungal Th1 reactivity, such as that occurring in IL-4-deficient mice (Table 1). Therefore, although neutrophils play an essential role in mediating antifungal resistance in the absence of an adaptive Th1-dependent immunity, the achievement of a state of full protection to the fungus, as that obtained by treatment with thymosin α 1, appears to rely on the coordinated action between innate effector phagocytes and protective Th1 cells.
Discussion
The results of our study identify a novel immunoregulatory activity of thymosin α 1 that may have important theoretical and therapeutic implications. Thymosin α 1 promoted the production of IL-12 p70 in DCs through the MyD88-dependent pathway, which is known to lead to production of Th1-promoting cytokines. The production of IL-12 p70 occurred to a different degree between the different DC subsets, a finding in line with the observation that, unlike murine PDCs that produce IL-12 p70 in response to TLR9 stimulation,36 human PDCs are known to produce little IL-12 p70.26 However, human PDCs produced IL-10 more than IL-12 p70 in response to conidia alone or together with thymosin α 1 and produced IFN-α in response to CpG and, more, in response to CpG and thymosin α 1. In TLR-transfected cells, thymosin α 1 directly activated TLR9 but not TLR2 signaling, the last being potentiated in response to the relevant ligand. Preliminary experiments seem to indicate a role for MD2, known to potentiate TLR2 signaling,37 in the TLR2 activating ability of thymosin α 1 (data not shown). It appears, therefore, that thymosin α 1 activates TLR signaling either directly or indirectly. However, whether TLR9 activation occurs in the endoplasmic reticulum of PDCs, as recently reported28 and whether intermediate molecules are licensed by thymosin α 1 in vivo to increase functional expression of TLRs are as yet unsolved issues. In this regard, it is worth mentioning that the 28-residue peptide thymosin α 1 may undergo conformational changes depending on the environment.38 Whatever the case will be, it is conceivable that thymosin α 1 may exploit the TLR2-dependent pathway on MDCs for IL-12 p70 production and the TLR9-dependent on PDCs for IFN-α production. However, the production of IL-10 by PDCs in response to thymosin α 1 is also of interest. Much evidence indicates that the TLR signaling pathways differ from one other and elicit different biologic responses.30,31 Our data would suggest that the production of IL-10 in response to distinct TLR ligands and to conidia mainly occurs in a MyD88-independent manner. Thymosin α 1 did not modify the production of IL-10 in MyD88-deficient mice, a finding confirming the essential role of the MyD88-dependent pathway in the regulation of IL-10 production by thymosin α 1. However, an activity of thymosin α 1 on additional signaling pathways cannot be excluded. Regardless, as IL-10 production by DCs is an essential component of memory protective antifungal immunity,39 balancing the IL-12/IL-10 production on DCs and/or different DC subsets may represent the very essence of adjuvanticity of thymosin α 1 in aspergillosis.
The effects of thymosin α 1 on DCs are consistent with its antiapoptotic activity40 as well as with its ability to induce prostaglandins41 and to share structural homology with human IFN-α,42 both agents known to induce DCs maturation.15 Given that DCs are central in the balancing act between immunopathology, immunity, and autoimmunity,43 and that PDCs signaling through TLR9 are present in the thymus,44 the ability to modulate DC functioning qualifies thymosin α 1 as a novel endogenous regulator of the innate and adaptive immune systems acting through TLR exploitation. From a conceptual point of view, this may provide an explanation for the, as yet elusive, function of naturally occurring thymosin α 1 that is produced in vivo by lysosomal asparaginyl endopeptidase cleavage of prothymosin α in diverse mammalian tissues45 and also the rationale for the therapeutic prescription of thymosin α 1 in some viral infections, in which PDCs producing IFN-α, for which TLR9 is essentially required,46 are considered to play a central role.47,48 It is interesting that PDCs also participate in immune responses after hematopoietic cell transplantation,49 a finding that may explain, among others,50 the beneficial effect of thymosin α 1 in the immunoreconstitution in mice that received BM transplants.
TLRs activate the innate immune system not only to assist the adaptive immune system but also for direct antimicrobial effector activity.31,32 The fact that thymosin α 1 not only activated murine and human DCs for Th1 priming to A fumigatus, but also effector neutrophils to an antifungal state, further points to the beneficial effect of thymosin α 1 in fungal infections.22 However, in line with the notion that the recovery from neutropenia is not sufficient to confer protection against IA in mice that received BM transplants,4 an action on neutrophils alone is not sufficient to mediate the curative effect of thymosin α 1.
In conclusion, the unique nature of Aspergillus, a saprophytic fungus colonizing immunocompromised hosts, demands for a better understanding of immunologic mechanisms required to both oppose fungal infectivity and optimize the efficacy of antifungal therapy. This study shows that the deliberate targeting of cells and pathways of cell-mediated immunity may increase resistance to Aspergillus and qualifies thymosin α 1 as a promising candidate adjuvant programming the appropriate Th reactivity to the fungus through TLR exploitation.
Prepublished online as Blood First Edition Paper, February 24, 2004; DOI 10.1182/blood-2003-11-4036.
Supported by the National Research Project on AIDS (contract no. 50D.27 and project no. 1AF/F) from Istituto Superiore di Sanità (ISS), and by the Basic Research Funds (no. 01p4B5-006 and project no. 120.5/RF00.121) from the Ministry for Education, University and Research (MIUR), Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Lara Bellocchio for superb editorial assistance; Dr Paolo Mosci for the histology; Drs Giorgio Trinchieri and Elisabeth Beth from Schering-Plough, Dardilly, France, for providing us with breeding pairs of TLR9-, TLR2-, and MyD88-deficient mice; Dr Manfred Kopf from Swiss Federal Institute of Technology, Zurich, Switzerland, for providing us with breeding pairs of IFN-γ- and IL-4-deficient mice; and Dr Terje Espevik from the Norwegian University of Science and Technology, Trondheim, Norway, for the generous supply of transfected HEK cell lines through the courtesy of Prof Giò Teti (University of Messina, Italy).

![Figure 1. Thymosin α 1 activates murine DCs on exposure to A fumigatus Murine lung CD11c+ DCs were pretreated with thymosin α 1 or the scrambled peptide (sThymosin α1) before the exposure to conidia or hyphae for the assessment of (A) phagocytosis, (B) costimulatory molecule expression, and (C) frequency of IL-12 p70-producing cells. (A) Assessment of phagocytosis (the data are the means ± SEs of 3 independent experiments and expressed as the percentage of internalization [numbers within figures]; *P < .05, thymosin-exposed versus unexposed cells). Cells were stained with Diff-Quik stain (Carlo Erba Reagents, Milan, Italy). Original magnification, ×100. (B) Costimulatory molecule expression by FACS analysis (data from one representative experiment of 3). Black histograms represent cells stained with an irrelevant antibody. The numbers refer to the median fluorescence intensity. (C) Frequency of IL-12 p70-producing cells (the values are the mean ± SE per 105 cells of samples from 3-5 experiments, calculated by ELISPOT assay). *P < .05, exposed versus unexposed (None) or conidia-exposed cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/11/10.1182_blood-2003-11-4036/6/m_zh80110462050001.jpeg?Expires=1770777600&Signature=GYhPGQaySyO~PLCTRFHP1Z-WSpR-l6i3TH7W-iNb6S24DABgcd6F4OWIDRnaYTt98ilqezSWAa~yGbB51FcSGfDHhqxbV1VjB36aiYjxVtunj67MPmw-jMHigMq6vWjteS99zUSM0OjE8sPo8~KeqrcrcuGtpP6-ES4pN8KM5aendSIv0HGlUEY1ozuHumJxC4rePkXtPuOzMZLIN~ElYHhEx~zyBDY0m7BWfCRIBSVyIgYZWw3o1FPKY1RgYUKlm1ISQGJvUdoznCG0iUV-Far42-yzg12NmYtCJ4JtDjSk2RaH0qD-cQRaU1XlxcTkRKYyPRqT98YQe4-oQn7ddQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal