Abstract
Multiple sclerosis (MS) is an inflammatory, demyelinating disease of the central nervous system (CNS) with features suggestive of T-cell-mediated pathology. Most prior reports have focused on CD4+ T cells with the underlying assumption that MS is predominantly a CD4+ T helper 1 (Th1)-mediated disease. In this report, we used a novel flow cytometric approach to evaluate autoreactive T-cell responses against a large variety of neuroantigenic targets. We found that both CD4+ and CD8+ T cells targeted against several CNS autoantigens were widely prevalent in patients with MS and healthy individuals. Whereas the distribution of CD4+ responses was similar in different groups, patients with relapsing-remitting MS showed a higher proportion of CNS-specific CD8+ responses. Autoreactive CD4+ T cells from patients with MS exhibited a more differentiated Th1 phenotype compared with healthy subjects. Similarly, CNS-specific CD8+ T-cell responses from patients with MS were functionally distinct from those in healthy individuals. Collectively, these studies reveal the high prevalence of class I-restricted autoreactive CD8+ T-cell responses in MS that has been underappreciated thus far. The results emphasize the need to evaluate both CD4+ and CD8+ T-cell responses in MS and to make both subsets a consideration in the development of novel therapeutic strategies. (Blood. 2004; 103:4222-4231)
Introduction
Multiple sclerosis (MS) is a demyelinating disorder of the central nervous system (CNS) with varied clinical presentations, ranging from relapses and remissions (relapsing-remitting MS or RRMS) to slow accumulation of disability with or without exacerbations (progressive MS).1-4 Progressive MS may be either primary in its presentation (primary progressive [PPMS]) or secondary after a period of RRMS (secondary progressive [SPMS]). Experimental autoimmune encephalomyelitis (EAE) is a well-characterized animal model of MS and is induced by immunization with myelin-associated antigens.5 This demyelinating disease in mice exhibits some features similar to those of MS, including episodic paralysis.6
Whereas EAE is clearly an induced, T-cell-mediated autoimmune disease, the etiology of MS is unclear. Epidemiologic studies suggest that both genetics and environment, particularly inciting viral infections, may play a role in the pathogenesis of MS.1,7,8 The characteristic histologic features1,6 and the presence of T-cell responses to CNS autoantigens in patients with MS9-14 are suggestive of T-cell-mediated pathology.
Most studies in MS and EAE have focused on the role of CD4+ T helper 1 (Th1)-type T cells in the pathogenesis of disease, with the underlying assumption that, like classical EAE, MS is also predominantly mediated and regulated by CD4+ T cells. CNS-reactive CD8+ T-cell responses have been demonstrated in patients with MS.15-21 Both CD8+ and CD4+ T cells contribute to the cellular infiltrate of demyelinating lesions in patients with MS.22 Some studies have shown enrichment and clonal expansion of CD8+ T cells in MS lesions.23-25 However, the antigenic specificity and functional role of these cells are unknown. The overall prevalence of CNS-reactive CD8+ T-cell responses and the breadth of their antigenic reactivity are poorly understood. Several recent studies in EAE suggest that CD8+ T cells may be involved in the pathogenesis of certain models.26-28 CD8+ T cells have also been implicated as regulatory cells in autoimmune demyelination.29-38 These reports emphasize the necessity of dissecting and characterizing the prevalence, specificity, and functional role of both CD4+ and CD8+ T cells in these diseases.
Detection and phenotyping of autoreactive T cells in short-term in vitro assay systems have been a technologic challenge. We have recently adapted a flow cytometric approach to evaluate antigen-specific CD4+ and CD8+ T-cell proliferative responses in patients with MS.39 This approach uses the green fluorescent dye 5 (and 6)-carboxyfluorescien diacetate succinimidyl ester (CFSE), which has been used previously in several systems to visualize dividing cells.40-42 With the use of this system, we have demonstrated the dynamics of CD8+ T-cell responses induced by the drug, glatiramer acetate (GA), in patients with MS.39 In the present study, we used this novel system to evaluate the specificity, prevalence, phenotype, and functional profile of neuroantigen-specific T-cell responses in MS.
Patients, materials, and methods
Human subjects
Eight healthy volunteers and 43 patients with different forms of MS (RRMS, n = 17; PPMS, n = 20; SPMS, n = 6) were recruited at the University of Texas (UT) Southwestern Medical Center (Table 1). At the time of leukapheresis, none of the patients had ever been treated with interferon or glatiramer acetate. A few patients had received steroid therapy, but no treatment was received for at least 3 months preceding the day of leukapheresis; none were suffering from acute relapses.
Characteristics of patients and healthy subjects
Subject no. . | Diagnosis . | Age, y . | Sex . | HLA type: DR/DQ . |
|---|---|---|---|---|
| HS-1 | Healthy subject | 32 | F | 11,15/5,6 |
| HS-2 | Healthy subject | 47 | M | 3,13/2,6 |
| HS-3 | Healthy subject | 33 | F | 4/3,3 |
| HS-4 | Healthy subject | 51 | F | 4,7/2,3 |
| HS-5 | Healthy subject | 22 | M | 13,16/5,6 |
| HS-6 | Healthy subject | 34 | F | 9,15/3,6 |
| HS-7 | Healthy subject | 64 | M | 1,7/2,5 |
| HS-8 | Healthy subject | 29 | M | 7,15/2,6 |
| MS-1 | RRMS | 46 | M | 3,15/2,6 |
| MS-2 | RRMS | 46 | F | 11,15/5,6 |
| MS-3 | RRMS | 39 | F | 4,8/3,4 |
| MS-4 | RRMS | 50 | F | 1,13/3,5 |
| MS-5 | RRMS | 54 | F | 7,15/2,6 |
| MS-6 | RRMS | 30 | F | Not done |
| MS-7 | RRMS | 35 | F | 11,15/3,6 |
| MS-8 | RRMS | 32 | F | Not done |
| MS-9 | RRMS | 45 | F | 3,15/2,6 |
| MS-10 | RRMS | 40 | F | 4,7/2,3 |
| MS-11 | RRMS | 37 | F | 1,3/2,5 |
| MS-12 | RRMS | 50 | F | 12,13/3,6 |
| MS-13 | RRMS | 31 | F | 15,16/5,6 |
| MS-14 | RRMS | 38 | F | 1,13/5,6 |
| MS-15 | RRMS | 33 | F | 4,7/2,3 |
| MS-16 | RRMS | 46 | M | 1,3/2,5 |
| MS-17 | RRMS | 24 | F | 14,15/3,6 |
| MS-18 | PPMS | 46 | M | 3,15/2,6 |
| MS-19 | PPMS | 56 | F | 11,13/3 |
| MS-20 | PPMS | 54 | M | 3,4/2,3 |
| MS-21 | PPMS | 51 | F | 13,15/6 |
| MS-22 | PPMS | 37 | M | 1,7/2,5 |
| MS-23 | PPMS | 48 | M | 15,16/5,6 |
| MS-24 | PPMS | 45 | F | 3,13/2,5 |
| MS-25 | PPMS | 52 | F | 4,15/3,6 |
| MS-26 | PPMS | 39 | F | 7,13/3,6 |
| MS-27 | PPMS | 39 | F | 8,15/3,6 |
| MS-28 | PPMS | 45 | M | 13,/3,6 |
| MS-29 | PPMS | 35 | M | 3,4/2,3 |
| MS-30 | PPMS | 61 | M | 13,15/6 |
| MS-31 | PPMS | 60 | M | 1,15/5,6 |
| MS-32 | PPMS | 56 | M | 8,11/3,4 |
| MS-33 | PPMS | 50 | M | 4,15/3,6 |
| MS-34 | PPMS | 45 | F | 4,13/3,6 |
| MS-35 | PPMS | 55 | M | 13,/3,6 |
| MS-36 | PPMS | 55 | F | 1,13/3,5 |
| MS-37 | PPMS | 47 | M | 4,13/3,6 |
| MS-38 | SPMS | 45 | F | 15/6 |
| MS-39 | SPMS | 51 | F | Not done |
| MS-40 | SPMS | 63 | M | 1,13/3,5 |
| MS-41 | SPMS | 48 | F | 3,15/2,6 |
| MS-42 | SPMS | 54 | M | 1,3/2,5 |
| MS-43 | SPMS | 48 | M | 13,16/3,5 |
Subject no. . | Diagnosis . | Age, y . | Sex . | HLA type: DR/DQ . |
|---|---|---|---|---|
| HS-1 | Healthy subject | 32 | F | 11,15/5,6 |
| HS-2 | Healthy subject | 47 | M | 3,13/2,6 |
| HS-3 | Healthy subject | 33 | F | 4/3,3 |
| HS-4 | Healthy subject | 51 | F | 4,7/2,3 |
| HS-5 | Healthy subject | 22 | M | 13,16/5,6 |
| HS-6 | Healthy subject | 34 | F | 9,15/3,6 |
| HS-7 | Healthy subject | 64 | M | 1,7/2,5 |
| HS-8 | Healthy subject | 29 | M | 7,15/2,6 |
| MS-1 | RRMS | 46 | M | 3,15/2,6 |
| MS-2 | RRMS | 46 | F | 11,15/5,6 |
| MS-3 | RRMS | 39 | F | 4,8/3,4 |
| MS-4 | RRMS | 50 | F | 1,13/3,5 |
| MS-5 | RRMS | 54 | F | 7,15/2,6 |
| MS-6 | RRMS | 30 | F | Not done |
| MS-7 | RRMS | 35 | F | 11,15/3,6 |
| MS-8 | RRMS | 32 | F | Not done |
| MS-9 | RRMS | 45 | F | 3,15/2,6 |
| MS-10 | RRMS | 40 | F | 4,7/2,3 |
| MS-11 | RRMS | 37 | F | 1,3/2,5 |
| MS-12 | RRMS | 50 | F | 12,13/3,6 |
| MS-13 | RRMS | 31 | F | 15,16/5,6 |
| MS-14 | RRMS | 38 | F | 1,13/5,6 |
| MS-15 | RRMS | 33 | F | 4,7/2,3 |
| MS-16 | RRMS | 46 | M | 1,3/2,5 |
| MS-17 | RRMS | 24 | F | 14,15/3,6 |
| MS-18 | PPMS | 46 | M | 3,15/2,6 |
| MS-19 | PPMS | 56 | F | 11,13/3 |
| MS-20 | PPMS | 54 | M | 3,4/2,3 |
| MS-21 | PPMS | 51 | F | 13,15/6 |
| MS-22 | PPMS | 37 | M | 1,7/2,5 |
| MS-23 | PPMS | 48 | M | 15,16/5,6 |
| MS-24 | PPMS | 45 | F | 3,13/2,5 |
| MS-25 | PPMS | 52 | F | 4,15/3,6 |
| MS-26 | PPMS | 39 | F | 7,13/3,6 |
| MS-27 | PPMS | 39 | F | 8,15/3,6 |
| MS-28 | PPMS | 45 | M | 13,/3,6 |
| MS-29 | PPMS | 35 | M | 3,4/2,3 |
| MS-30 | PPMS | 61 | M | 13,15/6 |
| MS-31 | PPMS | 60 | M | 1,15/5,6 |
| MS-32 | PPMS | 56 | M | 8,11/3,4 |
| MS-33 | PPMS | 50 | M | 4,15/3,6 |
| MS-34 | PPMS | 45 | F | 4,13/3,6 |
| MS-35 | PPMS | 55 | M | 13,/3,6 |
| MS-36 | PPMS | 55 | F | 1,13/3,5 |
| MS-37 | PPMS | 47 | M | 4,13/3,6 |
| MS-38 | SPMS | 45 | F | 15/6 |
| MS-39 | SPMS | 51 | F | Not done |
| MS-40 | SPMS | 63 | M | 1,13/3,5 |
| MS-41 | SPMS | 48 | F | 3,15/2,6 |
| MS-42 | SPMS | 54 | M | 1,3/2,5 |
| MS-43 | SPMS | 48 | M | 13,16/3,5 |
Peripheral blood mononuclear cells (PBMCs)
Approval to perform leukapheresis for these studies was obtained from the UT Southwestern institutional review board. Informed consent was provided according to the Declaration of Helsinki. PBMCs, obtained by Ficoll separation, were frozen on the day of collection.14
Antigens
The protein antigens include whole bovine myelin basic protein (MBP; 20 μg/mL; Sigma, St Louis, MO), tetanus toxoid (TT; 20 μg/mL; Accurate Chemical and Scientific, Westbury, NY), and the superantigen, staphylococcal enterotoxin B (SEB; 1 μg/mL; Sigma). In view of the diverse specificity of MS immune responses, we used serial 15-mer peptides (overlapping by 10) spanning 9 entire putative target MS antigens (human sequences) for a total of 530 15-mer peptide epitopes. Peptides were dissolved in dimethyl sulfoxide (DMSO), whereas whole MBP, TT, and SEB were dissolved in phosphate-buffered saline (PBS). Pools of serial peptides were used, as described in other systems.43 No single pool contained more than 45 peptides. Thus, for larger proteins, multiple pools were made, spanning different regions of the protein, as follows: myelin basic protein (MBP; 43 peptides; single pool), proteolipid protein (PLP; 54 peptides; 2 pools), myelin oligodendrocyte glycoprotein (MOG; 52 peptides; 2 pools), myelin-associated glycoprotein (MAG; 124 peptides; 3 pools), S100β glycoprotein (SB; 17 peptides; single pool), oligodendrocyte-myelin glycoprotein (OMGP; 89 peptides; 2 pools), myelin-associated oligodendrocytic basic protein (MOBP; 35 peptides; single pool), αβ-crystallin (CRAB; 33 peptides; single pool), and 2′-3′-cyclic nucleotide 3′-phosphodiesterase (CNP; 83 peptides; 2 pools).44-49 The peptide pools were used at a final optimal concentration of 10 μg/mL for each peptide. In addition, we also used glatiramer acetate (GA; Copaxone; Teva Pharmaceuticals, North Wales, PA) as an antigen for the standardization of this assay.39
CFSE-based proliferation assays
Flow cytometric proliferation assays, using the green fluorescent dye, CFSE, were performed as described.39 Briefly, PBMCs were suspended at 1 × 106/mL in PBS and incubated at 37°C for 7 minutes with 0.25 μM CFSE. Following addition of serum and 2 PBS washes, cells were suspended at 2 × 106/mL in 5% human media (RPMI 1640 supplemented with glutamine, 5% human AB serum, penicillin, and streptomycin) and cultured with various antigens. Background controls consisted of cells cultured in media alone as well as cells cultured with DMSO (as a control for the peptide pools). If more than 1 μL DMSO was added per milliliter of any culture, cells were washed in warm media twice following 18 hours of culture and resuspended in 1 mL media. On day 7 of culture, cells were washed with fluorescence activated cell sorting (FACS) buffer (PBS with 1% bovine serum albumin [BSA] and 0.1% Na-azide) and stained with fluorescently tagged antibodies against CD3 (peridin chlorophyll protein [PerCP]), CD4 (phycoerythrin [PE] or allophycocyanin [APC]) and CD8 (APC or PE) from BD Biosciences, San Diego, CA. Cells were washed and fixed in 1% paraformaldehyde (BD Biosciences). Flow cytometric data (100 000 nongated events) were acquired on a BD FACSCalibur 4-color flow cytometer using BD Cellquest software (both from BD Biosciences). For analysis, BD Paint-A-Gate software (BD Biosciences) was used to gate on CD4+/CD8- or CD8+/CD4-CD3+ T-cell populations. The background proliferation was calculated in the absence of antigen. The Δ proliferating fraction (ΔPF) was calculated by subtracting the mean background proliferation from the proliferating fraction in response to specific antigen. The stimulation index (SI) was calculated by dividing the antigen-induced PF by the background PF. To designate a response as positive, we required both a ΔPF of 1.0% or more and an SI of 2.0 or more. Using both parameters is important, because an SI of 2.0 can be achieved easily with a low background. Similarly, in case of high background, a ΔPF value of 1.0 could represent intra-assay variability. A combination of both criteria is a stringent measure and is based on data from our initial experiments assessing inter-assay reproducibility (not shown).
HLA blockade
These assays were performed as described.39 Purified anti-HLA class I (HLA-A, -B, -C) and anti-class II (HLA-DP, -DQ, -DR) antibodies and their appropriate control antibodies (immunoglobulin G1 [IgG1] and IgG2a; BD Biosciences) were dialyzed in PBS to remove azide. CFSE-stained PBMCs were incubated with 10 μg/mL of the indicated antibodies for 30 minutes before antigens were added.
CFSE-based flow sorting
Proliferating CD4+ or CD8+ T cells were sorted from bulk cultures of CFSE-stained PBMCs using a BD Biosciences FACSVantage SE sorter, as described.39 On populations with adequate yields (> 200 000 cells), a “post-sort” run was performed, revealing more than 90% purity. Sorted cells were collected in 1.5-mL Sarstedt tubes, pelleted, and frozen at -80°C for subsequent molecular analyses.
Generation and testing of T-cell lines and clones
We generated short-term clones (or lines) of CD4+ and CD8+ T cells sorted from CFSE-stained bulk cultures. Single (or multiple), live, proliferating CD4+ and CD8+ T cells were deposited directly into 96-well plates containing media. These cells were stimulated with phytohemagglutinin (PHA; 200 ng/mL; Sigma) and irradiated autologous or allogeneic PBMCs (1 × 105 cells/well), followed by supplementation with recombinant human interleukin 2 (IL-2; 10 U/mL twice a week; Invitrogen, Carlsbad, CA). Following 4 weeks of culture, 3H-thymidine incorporation assays were conducted, as described.39 Cells were stimulated with irradiated autologous PBMCs in the presence of indicated antigens. To assess cloning efficiency, a proportion of the wells were stimulated with concanavalin A (Con A; 5 μg/mL; Sigma). Wells were pulsed with 0.25 μCi/well (0.0093 MBq/well) 3H-Thymidine for the last 18 hours of culture. On day 7, wells were harvested and analyzed by using a Wallac Betaplate liquid scintillation counter (Perkin Elmer Life Sciences, Wellesley, MA).
Quantitative real-time PCR assays
Total RNA was obtained from sorted populations of cells by using the QIAamp RNA mini kit (Qiagen, Valencia, CA) and was reverse transcribed to cDNA using the Ready-To-Go First Strand cDNA kit (Amersham Pharmacia, Piscataway, NJ). Primer pairs were evaluated in qualitative polymerase chain reaction (PCR) assays on control cDNA specimens to ascertain clean products on agarose gels39 (data not shown). Following this, quantitative real-time PCR assays were performed by adding SYBRGreen in the reaction mix, as described.50,51 This dye binds double-stranded DNA products that can be measured in real time at the extension phase of the reaction.50,51 The primer pairs used for the assayed molecules are listed in Table 2 (all 5′-3′; purchased from Invitrogen).
Primers used for PCR assays
Molecule . | Forward primer . | Reverse primer . | Reference . |
|---|---|---|---|
| β-actin | CCTGGACTTCGAGCAAGAGA | ACTTGCGCTCAGGAGGAGCA | Karandikar et al39 |
| GAPDH | GGTATCGTGGAAGGACTCATGAC | ATGCCAGTGAGCTTCCCGTTCAGC | Bertolotto et al52 |
| IFN-γ | TTTAATGCAGGTCATTCAGATGTA | CACTTGGATGAGTTCATGTATTGC | Karandikar et al39 |
| IL-4 | GCCTCACAGAGCAGAAGACT | TCAGCTCGAACACTTTGAAT | Leroy et al53 |
| TNF-α | CGAGTCTGGGCAGGTCTACTTT | AAGCTGTAGGCCCCAGTGAGTT | Karandikar et al39 |
| TGF-β1 | GCCCTGGACACCAACTATTGC | GCTGCACTTGCAGGAGCGCAC | Karandikar et al39 |
| IL-2 | TACAACTGGAGCATTTACTG | GTTTCAGATCCCTTTAGTTC | Leroy et al53 |
| IL-10 | TGCCTGGTCCTCCTGACTGG | GCCTTGCTCTTGTTTTCACA | Leroy et al53 |
| CXCR3 | CACTGCCCTTCTCATTTGGAAACT | GCAAATATAGAGGTCTTGGGGAC | Nanki et al54 |
| CCR5 | TGCTACTCGGGAATCATAAAAACT | TTCTGAACTTCTCCCCGACAAA | Douglas et al55 |
| CCR4 | TTGGACTATGCCATCCAGGC | AATTCCCTCTGGAGAAACCC | van den Berg et al56 |
| IL 5 | CTGATAGCCAATGAGACTCT | TATTATCCACTCGGTGTTCA | Leroy et al53 |
Molecule . | Forward primer . | Reverse primer . | Reference . |
|---|---|---|---|
| β-actin | CCTGGACTTCGAGCAAGAGA | ACTTGCGCTCAGGAGGAGCA | Karandikar et al39 |
| GAPDH | GGTATCGTGGAAGGACTCATGAC | ATGCCAGTGAGCTTCCCGTTCAGC | Bertolotto et al52 |
| IFN-γ | TTTAATGCAGGTCATTCAGATGTA | CACTTGGATGAGTTCATGTATTGC | Karandikar et al39 |
| IL-4 | GCCTCACAGAGCAGAAGACT | TCAGCTCGAACACTTTGAAT | Leroy et al53 |
| TNF-α | CGAGTCTGGGCAGGTCTACTTT | AAGCTGTAGGCCCCAGTGAGTT | Karandikar et al39 |
| TGF-β1 | GCCCTGGACACCAACTATTGC | GCTGCACTTGCAGGAGCGCAC | Karandikar et al39 |
| IL-2 | TACAACTGGAGCATTTACTG | GTTTCAGATCCCTTTAGTTC | Leroy et al53 |
| IL-10 | TGCCTGGTCCTCCTGACTGG | GCCTTGCTCTTGTTTTCACA | Leroy et al53 |
| CXCR3 | CACTGCCCTTCTCATTTGGAAACT | GCAAATATAGAGGTCTTGGGGAC | Nanki et al54 |
| CCR5 | TGCTACTCGGGAATCATAAAAACT | TTCTGAACTTCTCCCCGACAAA | Douglas et al55 |
| CCR4 | TTGGACTATGCCATCCAGGC | AATTCCCTCTGGAGAAACCC | van den Berg et al56 |
| IL 5 | CTGATAGCCAATGAGACTCT | TATTATCCACTCGGTGTTCA | Leroy et al53 |
GAPDH indicates glyceraldehyde-3-phosphate dehydrogenase; IFN-γ, interferon γ; TNF-α, tumor necrosis factor α; CXCR3, CXC chemokine receptor 3; and CCR5, CC chemokine receptor 5.
PCR reactions (25 μL) were performed on the BioRad iCycler thermocycler (BioRad, Hercules, CA) in 1 × PCR buffer, 1.5 mM MgCl2, 0.2 mM deoxynucleotide triphosphate (dNTP), 1.25 U Taq (Amersham Pharmacia), 1.2 μM forward and reverse primers, and a 1:50 000 dilution of SYBRGreen I nucleic acid gel stain 10 000 × (Molecular Probes, Eugene, OR). The following thermocycler conditions were used: 95°C for 10 minutes × 1; 94°C for 45 seconds, 55°C for 1 minute, and 78°C for 1 minutes × 35 cycles; 95°C for 3 minutes and 55°C for 5 minutes × 1. The SYBRGreen fluorescence was read during the extension phase of the reaction. After amplification, melting analysis was performed by heating the reaction mixture from 55°C to 100°C at the rate of 0.5 C/s. The specificity of the product was confirmed by melt curve analyses as well as visualization of product size on agarose gels.
Statistical analyses
Fisher exact tests were used to compare different subject groups for the proportion of responding subjects and proportion of positive responses. Unpaired t tests were used to compare the expression levels of functional molecules in sorted CD4+ and CD8+ T-cell populations between different groups. A P value of less than .05 was considered significant.
Results
Patients with MS harbor heterogeneous CNS-reactive CD4+ and CD8+ T-cell responses
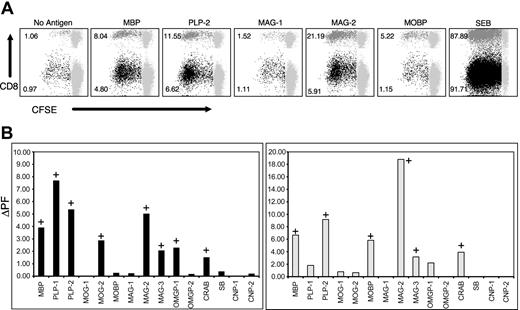
Several studies have demonstrated the presence of neuroantigen-specific T-cell responses in PBMCs obtained from patients with MS and healthy individuals. However, the phenotype of these responding T cells has not been adequately evaluated. To characterize the nature of these responses and to evaluate for potential differences in patients versus healthy individuals, we adapted a CFSE-based flow cytometric proliferation assay, as described.39 CFSE assays were performed on PBMCs from 43 untreated patients with MS (RRMS, n = 17; PPMS, n = 20; SPMS, n = 6) and 8 healthy subjects (Table 1). The sex distribution reflected that of the disease subtypes, with RRMS showing female preponderance and PPMS showing comparable distribution in the 2 sexes. In all subjects, we evaluated proliferative responses against 9 putative target autoantigens by using pools of serial overlapping 15-mer peptides that spanned entire target proteins, including alternatively spliced forms, for a total of 530 peptides separated into 15 distinct pools. 15-mer peptides were used, because they have been shown to induce both CD4+ and CD8+ T-cell responses in other settings.43,57,58 Figure 1 demonstrates the proliferative responses of a patient with MS to the indicated neuroantigenic peptide pools. The dot plots in Figure 1A represent responses to selected antigenic pools, whereas Figure 1B shows CD4+ and CD8+ T-cell responses to all the 15 peptide pools tested. In this patient, we could detect T-cell proliferative responses to 9 of the 15 pools (Figure 1B). Importantly, these responses were not restricted to CD4+ T cells. Significant CD8+ T-cell proliferative responses were observed against several antigens. Whereas both CD4+ and CD8+ T-cell responses were detected against MBP, PLP-mix 2, MAG-mixes 2 and 3, and CRAB, this patient showed isolated CD4+ T-cell responses to PLP-mix 1, MOG-mix 2, and OMGP-mix 1 and isolated CD8+ T-cell responses against MOBP.
CNS-specific CD4+ and CD8+ T-cell responses in a patient with MS. CFSE-based proliferation assays were performed on PBMC specimens from 8 healthy individuals and 43 untreated patients with MS (RRMS, n = 17; PPMS, n = 20; SPMS, n = 6). (A) Selected responses from a patient with MS in the presence (or absence) of indicated antigenic peptide pools. These data represent gated CD3+ T cells that are further gated for CD4+/CD8- or CD8+/CD4- T cells. CFSE staining is shown on the x-axis and CD8 staining on the y-axis. The CD8- populations in the dot plots represent gated CD4+ T cells. The gray populations to the right represent nondividing cells. The numbers next to the darker populations represent the proliferating fraction of CD4+ T cells (black) and CD8+ T cells (dark gray) (ie, the proportion of gated CD4+ T cells or CD8+ T cells that were proliferating on day of analysis). Values for background proliferation are calculated from the “No Antigen” tubes. Δ proliferating fraction (ΔPF) is the difference between specific proliferation and the background, whereas the stimulation index (SI) is the ratio of specific proliferation to the background. A response with a ΔPF of at least 1.00% and an SI of at least 2.0 was considered positive. (B) CD4+ (left) and CD8+ (right) T-cell responses of the same patient to all the indicated antigens, plotted as ΔPF (background subtracted). The positive responses are indicated with a “+” sign.
CNS-specific CD4+ and CD8+ T-cell responses in a patient with MS. CFSE-based proliferation assays were performed on PBMC specimens from 8 healthy individuals and 43 untreated patients with MS (RRMS, n = 17; PPMS, n = 20; SPMS, n = 6). (A) Selected responses from a patient with MS in the presence (or absence) of indicated antigenic peptide pools. These data represent gated CD3+ T cells that are further gated for CD4+/CD8- or CD8+/CD4- T cells. CFSE staining is shown on the x-axis and CD8 staining on the y-axis. The CD8- populations in the dot plots represent gated CD4+ T cells. The gray populations to the right represent nondividing cells. The numbers next to the darker populations represent the proliferating fraction of CD4+ T cells (black) and CD8+ T cells (dark gray) (ie, the proportion of gated CD4+ T cells or CD8+ T cells that were proliferating on day of analysis). Values for background proliferation are calculated from the “No Antigen” tubes. Δ proliferating fraction (ΔPF) is the difference between specific proliferation and the background, whereas the stimulation index (SI) is the ratio of specific proliferation to the background. A response with a ΔPF of at least 1.00% and an SI of at least 2.0 was considered positive. (B) CD4+ (left) and CD8+ (right) T-cell responses of the same patient to all the indicated antigens, plotted as ΔPF (background subtracted). The positive responses are indicated with a “+” sign.
In our initial few experiments, we included a mixture of overlapping 15-mer peptides from the HIV gag protein, as an additional non-neuroantigen-negative control, to which we did not see responses (data not shown). However, to use cells more effectively, we did not perform this additional control in later experiments, because our experimental design has several in-built “peptide mix” controls. None of the subjects responded to all peptide mixes (ie, every subject showed an “absence” of CD4+ and CD8+ T-cell responses to at least 5 of the 15 peptide pools; Figure 2), emphasizing the specificity of the assay. As an example, the patient demonstrated in Figure 1 did not show T-cell responses against MAG-mix 1, OMGP-mix 2, SB, CNP-mix 1, and CNP-mix 2.
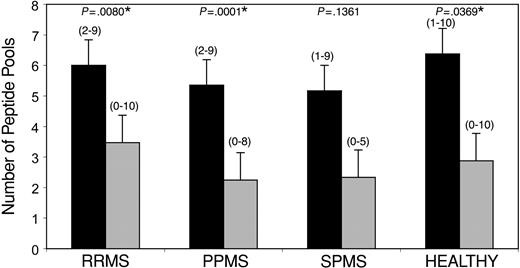
Distribution of CNS-specific CD4+ and CD8+ T-cell responses. CFSE-based proliferation assays were performed on PBMC specimens from 8 healthy individuals and 43 untreated patients with MS (RRMS, n = 17; PPMS, n = 20; SPMS, n = 6). Each subject was tested against 15 distinct pools of CNS peptides, representing 9 distinct CNS target proteins. The y-axis demonstrates the mean number of peptide pools (+ 2 SEM) against which a positive response was observed in the indicated subject groups. Black bars (▪) indicate CD4+ T-cell responses, whereas gray bars ( ) represent CD8+ responses. The ranges are indicated in parentheses. *CD4+ responses to a significantly higher array of peptide pools were observed compared with CD8+ responses (P < .05).
) represent CD8+ responses. The ranges are indicated in parentheses. *CD4+ responses to a significantly higher array of peptide pools were observed compared with CD8+ responses (P < .05).
Distribution of CNS-specific CD4+ and CD8+ T-cell responses. CFSE-based proliferation assays were performed on PBMC specimens from 8 healthy individuals and 43 untreated patients with MS (RRMS, n = 17; PPMS, n = 20; SPMS, n = 6). Each subject was tested against 15 distinct pools of CNS peptides, representing 9 distinct CNS target proteins. The y-axis demonstrates the mean number of peptide pools (+ 2 SEM) against which a positive response was observed in the indicated subject groups. Black bars (▪) indicate CD4+ T-cell responses, whereas gray bars ( ) represent CD8+ responses. The ranges are indicated in parentheses. *CD4+ responses to a significantly higher array of peptide pools were observed compared with CD8+ responses (P < .05).
) represent CD8+ responses. The ranges are indicated in parentheses. *CD4+ responses to a significantly higher array of peptide pools were observed compared with CD8+ responses (P < .05).
Tables 3 and 4 represent an overview of CFSE-based proliferation assays performed on PBMCs from 8 healthy subjects and 43 untreated patients with MS. CD4+ (Table 3) and CD8+ (Table 4) T-cell responses are demonstrated, with numbers indicating the proportion of subjects that showed positive responses to the indicated neuroantigens. Figure 2 demonstrates the mean number of peptide pools (of 15) that generated positive CD4+ versus CD8+ responses in each subject group.
CNS-reactive CD4+ T-cell responses in patients with MS and healthy subjects
Antigen . | RRMS, n (%) . | PPMS, n (%) . | SPMS, n (%) . | Healthy, n (%) . |
|---|---|---|---|---|
| Whole MBP | 7 (41) | 10 (50) | 1 (17) | 5 (63) |
| MBP mix | 10 (59) | 12 (60) | 1 (17) | 7 (88) |
| PLP-mix 1 | 9 (53) | 8 (40) | 3 (50) | 3 (38) |
| PLP-mix 2 | 11 (65) | 9 (45) | 2 (33) | 5 (63) |
| MOG-1 | 7 (41) | 4 (20) | 0 (0) | 2 (25) |
| MOG-2 | 11 (65) | 5 (25) | 2 (33) | 4 (50) |
| MOBP | 8 (47) | 6 (30) | 2 (33) | 4 (50) |
| MAG-1 | 9 (53) | 10 (50) | 4 (67) | 3 (38) |
| MAG-2 | 5 (29) | 7 (35) | 2 (33) | 4 (50) |
| MAG-3 | 3 (18) | 6 (30) | 1 (17) | 2 (25) |
| OMGP-1 | 11 (65) | 12 (60) | 3 (50) | 4 (50) |
| OMGP-2 | 6 (35) | 9 (45) | 3 (50) | 7 (88) |
| CRAB | 2 (12) | 3 (15) | 1 (17) | 0 (0) |
| SB | 0 (0) | 4 (20) | 3 (50) | 1 (13) |
| CNP-1 | 7 (41) | 10 (50) | 4 (67) | 4 (50) |
| CNP-2 | 3 (18) | 2 (10) | 0 (0) | 1 (13) |
| SEB | 17 (100) | 20 (100) | 6 (100) | 8 (100) |
| Total patients | 17/17 (100) | 20/20† (100) | 6/6 (100) | 8/8 (100) |
| Total responses* | 102/255†‡ (40) | 107/300†‡ (36) | 31/90†‡ (34) | 51/120†‡ (43) |
Antigen . | RRMS, n (%) . | PPMS, n (%) . | SPMS, n (%) . | Healthy, n (%) . |
|---|---|---|---|---|
| Whole MBP | 7 (41) | 10 (50) | 1 (17) | 5 (63) |
| MBP mix | 10 (59) | 12 (60) | 1 (17) | 7 (88) |
| PLP-mix 1 | 9 (53) | 8 (40) | 3 (50) | 3 (38) |
| PLP-mix 2 | 11 (65) | 9 (45) | 2 (33) | 5 (63) |
| MOG-1 | 7 (41) | 4 (20) | 0 (0) | 2 (25) |
| MOG-2 | 11 (65) | 5 (25) | 2 (33) | 4 (50) |
| MOBP | 8 (47) | 6 (30) | 2 (33) | 4 (50) |
| MAG-1 | 9 (53) | 10 (50) | 4 (67) | 3 (38) |
| MAG-2 | 5 (29) | 7 (35) | 2 (33) | 4 (50) |
| MAG-3 | 3 (18) | 6 (30) | 1 (17) | 2 (25) |
| OMGP-1 | 11 (65) | 12 (60) | 3 (50) | 4 (50) |
| OMGP-2 | 6 (35) | 9 (45) | 3 (50) | 7 (88) |
| CRAB | 2 (12) | 3 (15) | 1 (17) | 0 (0) |
| SB | 0 (0) | 4 (20) | 3 (50) | 1 (13) |
| CNP-1 | 7 (41) | 10 (50) | 4 (67) | 4 (50) |
| CNP-2 | 3 (18) | 2 (10) | 0 (0) | 1 (13) |
| SEB | 17 (100) | 20 (100) | 6 (100) | 8 (100) |
| Total patients | 17/17 (100) | 20/20† (100) | 6/6 (100) | 8/8 (100) |
| Total responses* | 102/255†‡ (40) | 107/300†‡ (36) | 31/90†‡ (34) | 51/120†‡ (43) |
The table shows the number of subjects from each group exhibiting a positive CD4+ T cell response to the corresponding antigen (percentages are in parentheses). Total patient populations: RRMS, N = 17; PPMS, N = 20; SPMS, N = 6; and healthy subjects, N = 8.
Indicates the total number of positive responses to the peptide pools (of a possible 15 for each subject).
Significantly higher compared with CD8+ T-cell responses in Table 4 (P < .05).
No significant differences in CD4+ responses between different subject groups.
CNS-reactive CD8+ T-cell responses in patients with MS and healthy subjects
Antigen . | RRMS, n (%) . | PPMS, n (%) . | SPMS, n (%) . | Healthy, n (%) . |
|---|---|---|---|---|
| Whole MBP | 5 (29) | 7 (35) | 0 (0) | 1 (13) |
| MBP mix | 7 (41) | 7 (35) | 0 (0) | 1 (13) |
| PLP mix 1 | 6 (35) | 4 (20) | 0 (0) | 2 (25) |
| PLP mix 2 | 7 (41) | 4 (20) | 1 (17) | 2 (25) |
| MOG-1 | 1 (6) | 0 (0) | 1 (17) | 1 (13) |
| MOG-2 | 3 (18) | 2 (10) | 1 (17) | 0 (0) |
| MOBP | 9 (53)† | 3 (15) | 1 (17) | 0 (0) |
| MAG-1 | 6 (35) | 6 (30) | 1 (17) | 3 (38) |
| MAG-2 | 3 (18) | 4 (20) | 1 (17) | 4 (50) |
| MAG-3 | 2 (12) | 1 (5) | 0 (0) | 1 (13) |
| OMGP-1 | 3 (18) | 3 (15) | 1 (17) | 5 (63) |
| OMGP-2 | 3 (18) | 5 (25) | 1 (17) | 1 (13) |
| CRAB | 0 (0) | 4 (20) | 1 (17) | 1 (13) |
| SB | 2 (12) | 1 (5) | 2 (33) | 0 (0) |
| CNP-1 | 5 (29) | 3 (15) | 4 (67) | 2 (25) |
| CNP-2 | 1 (6) | 0 (0) | 0 (0) | 0 (0) |
| SEB | 17 (100) | 20 (100) | 6 (100) | 8 (100) |
| Total patients | 15/17 (88) | 14/20 (70) | 5/6 (83) | 7/8 (88) |
| Total responses* | 63/255‡ (25) | 47/300 (16) | 15/90 (19) | 23/120 (19) |
Antigen . | RRMS, n (%) . | PPMS, n (%) . | SPMS, n (%) . | Healthy, n (%) . |
|---|---|---|---|---|
| Whole MBP | 5 (29) | 7 (35) | 0 (0) | 1 (13) |
| MBP mix | 7 (41) | 7 (35) | 0 (0) | 1 (13) |
| PLP mix 1 | 6 (35) | 4 (20) | 0 (0) | 2 (25) |
| PLP mix 2 | 7 (41) | 4 (20) | 1 (17) | 2 (25) |
| MOG-1 | 1 (6) | 0 (0) | 1 (17) | 1 (13) |
| MOG-2 | 3 (18) | 2 (10) | 1 (17) | 0 (0) |
| MOBP | 9 (53)† | 3 (15) | 1 (17) | 0 (0) |
| MAG-1 | 6 (35) | 6 (30) | 1 (17) | 3 (38) |
| MAG-2 | 3 (18) | 4 (20) | 1 (17) | 4 (50) |
| MAG-3 | 2 (12) | 1 (5) | 0 (0) | 1 (13) |
| OMGP-1 | 3 (18) | 3 (15) | 1 (17) | 5 (63) |
| OMGP-2 | 3 (18) | 5 (25) | 1 (17) | 1 (13) |
| CRAB | 0 (0) | 4 (20) | 1 (17) | 1 (13) |
| SB | 2 (12) | 1 (5) | 2 (33) | 0 (0) |
| CNP-1 | 5 (29) | 3 (15) | 4 (67) | 2 (25) |
| CNP-2 | 1 (6) | 0 (0) | 0 (0) | 0 (0) |
| SEB | 17 (100) | 20 (100) | 6 (100) | 8 (100) |
| Total patients | 15/17 (88) | 14/20 (70) | 5/6 (83) | 7/8 (88) |
| Total responses* | 63/255‡ (25) | 47/300 (16) | 15/90 (19) | 23/120 (19) |
The table shows the number of subjects from each group exhibiting a positive CD8+ T-cell response to the corresponding antigen. Total patient populations: RRMS, N = 17; PPMS, N = 20; SPMS, N = 6; and healthy subjects, N = 8.
Indicates the total number of positive responses to the peptide pools (out of a possible 15 for each subject).
Higher MOBP responses in RRMS, compared with healthy subjects (P < .05).
P < .01, compared with PPMS; P ~ .2, compared with healthy subjects.
As seen in the 2 tables, we observed a very heterogeneous pattern of responses in patients with MS as well as healthy subjects. All subjects (patients with MS and healthy individuals) showed at least 1 positive CNS-specific autoreactive CD4+ T-cell response. When we evaluated the proportion of positive responses of all possible responses to the CNS peptide pools (15 for each subject), a higher prevalence of CD4+ T-cell responses was observed in patients with MS, compared with CD8+ responses (P < .05). This difference was also noted in healthy subjects. There was no significant difference in the prevalence of CD4+ T-cell responses in patients with MS compared with healthy individuals nor were there any significant differences in sex-specific distribution. No pattern of specificity of CD4+ responses to neuroantigenic targets was observed in different forms of the disease (RRMS versus PPMS versus SPMS). Higher prevalence of autoreactive CD4+ T-cell responses in patients with MS and healthy individuals was contributed by CD4+ T-cell responses to a wider array of CNS peptide pools, compared with CD8+ responses (Figure 2). Thus, there tended to be positive CD4+ T-cell responses against a larger number of pools in both patients with MS as well as healthy subjects. Again, no significant differences were seen between the different subject groups.
Interestingly, however, some differences were observed in the patterns of autoreactive CD8+ T-cell responses between different subject groups (Table 4). Overall, there was a trend toward higher prevalence of CD8+ T-cell responses in RRMS, compared with PPMS (P < .05). In addition, we observed that a higher proportion of patients with RRMS harbored CD8+ responses to MOBP, compared with healthy subjects (Table 4; P < .05). Collectively, these studies reveal that CNS-specific CD8+ T-cell responses are more widely prevalent in patients with MS than appreciated thus far.
CFSE-based assay detects HLA-restricted, antigen-specific T-cell responses
As stated earlier, several lines of evidence indicate that the autoreactive CD8+ T-cell responses detected by the CFSE-based assay system are antigen specific and not a result of mere bystander proliferation in response to cytokines secreted by CD4+ T cells. First, not every CD4+ T-cell response was accompanied by a CD8+ response. Second, CD8+ T-cell responses were detected in the absence of CD4+ responses. In addition, to further confirm that the autoreactive CD4+ and CD8+ T-cell proliferation was a response incited by T-cell receptor (TCR)/HLA ligation, we performed assays in the presence of anti-HLA class I and anti-HLA class II antibodies. As expected, blockade of HLA class I molecules resulted in prominent inhibition of the CNS-specific CD8+ T-cell responses, and HLA class II blockade predominantly inhibited CD4+ T-cell responses (Figure 3). In some instances, we also observed cross-inhibition of CD4+ and CD8+ responses by anti-class I and anti-class II antibodies, similar to prior observations.39 But in all cases predominant inhibition of the appropriate response was observed. Taken together, these data suggest that cross-inhibition is most likely due to suppression of T-cell-mediated help to other antigen-specific T cells. Thus, the CFSE assay detects HLA-restricted CD4+ and CD8+ T-cell responses.
Autoreactive CD4+ and CD8+ T-cell responses are HLA restricted. CFSE-based proliferation assays were performed on PBMC specimens from untreated patients with MS in the presence of anti-HLA antibodies or appropriate control immunoglobulin. The dot plots demonstrate CD4+ and CD8+ MOG-specific proliferative responses from one of the patients in the presence of the indicated antibodies. The 2 left panels demonstrate the effect of HLA class I blockade (compared with the IgG1 control), whereas the 2 right panels demonstrate the effect of HLA class II blockade (compared with IgG2a control). CFSE staining is shown on the x-axis and CD8 staining on the y-axis. The numbers represent the proliferating fraction of CD4+ (black) and CD8+ (dark gray) T cells. Anti-class I antibodies predominantly blocked CD8+ T-cell proliferation but also diminished CD4+ proliferation. Anti-class II antibodies had a predominant effect on CD4+ T-cell proliferation but also diminished CD8+ proliferation. These results are representative of HLA blockade data from 35 distinct CD4+ and CD8+ proliferative responses from 13 patients with MS responding to several different neuroantigenic peptide pools (MBP, PLP, MOG, MAG, OMGP, and MOBP).
Autoreactive CD4+ and CD8+ T-cell responses are HLA restricted. CFSE-based proliferation assays were performed on PBMC specimens from untreated patients with MS in the presence of anti-HLA antibodies or appropriate control immunoglobulin. The dot plots demonstrate CD4+ and CD8+ MOG-specific proliferative responses from one of the patients in the presence of the indicated antibodies. The 2 left panels demonstrate the effect of HLA class I blockade (compared with the IgG1 control), whereas the 2 right panels demonstrate the effect of HLA class II blockade (compared with IgG2a control). CFSE staining is shown on the x-axis and CD8 staining on the y-axis. The numbers represent the proliferating fraction of CD4+ (black) and CD8+ (dark gray) T cells. Anti-class I antibodies predominantly blocked CD8+ T-cell proliferation but also diminished CD4+ proliferation. Anti-class II antibodies had a predominant effect on CD4+ T-cell proliferation but also diminished CD8+ proliferation. These results are representative of HLA blockade data from 35 distinct CD4+ and CD8+ proliferative responses from 13 patients with MS responding to several different neuroantigenic peptide pools (MBP, PLP, MOG, MAG, OMGP, and MOBP).
Finally, to test the specificity of the assay, we performed single-cell sorting of antigen-reactive CD4+ and CD8+ T cells to generate T-cell clones. In the experiments in Table 5, CFSE-stained PBMCs were cultured in the presence of the indicated antigens (MBP mix, GA, or TT) to stimulate CD4+ and CD8+ T-cell proliferation.39 On day 7, single, live, proliferating CD4+ and CD8+ T cells were sorted into 96-well plates. These wells were then stimulated with PHA and IL-2 for 3 to 4 weeks, followed by 3H-Thymidine-based proliferation assays. In these assays, for every cell population tested, 12 wells did not receive any antigen (background), 24 wells were stimulated with the original antigen (MBP, GA, TT), 12 wells with a different antigen (GA, MBP, GA, respectively), and 12 wells with Con A (to test for cloning efficiency). With the use of mean background counts (no antigen) + 2 SD as a cutoff for designating positive wells (cutoff CPM values shown in Table 5), the cloning efficiency (proportion of wells positive with Con A stimulation) ranged from 17% (2 of 12 wells) to 75% (9 of 12 wells). In all cases, none of the wells stimulated with irrelevant antigen showed positive proliferation. The proportion of wells showing proliferation to the specific antigen ranged from 17% (4 of 24 wells) to 54% (13 of 24 wells). When this was normalized to the cloning efficiency (assigning a maximum limit of 100%), the calculated specificity of the sorted cells ranged from 69% to 100%.
T cells cloned from CFSE cultures are antigen-specific
Original antigen/sorted cell type . | Cutoff CPM, mean + 2 SD* . | Con A†(cloning efficiency, %) . | Irrelevant antigen (cloning efficiency, %)† . | Same antigen (cloning efficiency, %)† . | Specificity, %‡ . |
|---|---|---|---|---|---|
| MBP mix/CD4+ cells | 17 947.50 | 3/12 (25) | 0/12 (0) | 9/24 (38) | 100 |
| MBP mix/CD8+ cells | 21 033.16 | 9/12 (75) | 0/12 (0) | 13/24 (54) | 72 |
| GA/CD4+ cells | 17 330.29 | 4/12 (33) | 0/12 (0) | 10/24 (42) | 100 |
| GA/CD8+ cells | 16 817.68 | 6/12 (50) | 0/12 (0) | 13/24 (54) | 100 |
| TT/CD4+ cells | 23 222.28 | 8/12 (67) | 0/12 (0) | 11/24 (46) | 69 |
| TT/CD8+ cells | 16 469.92 | 2/12 (17) | 0/12 (0) | 4/24 (17) | 100 |
Original antigen/sorted cell type . | Cutoff CPM, mean + 2 SD* . | Con A†(cloning efficiency, %) . | Irrelevant antigen (cloning efficiency, %)† . | Same antigen (cloning efficiency, %)† . | Specificity, %‡ . |
|---|---|---|---|---|---|
| MBP mix/CD4+ cells | 17 947.50 | 3/12 (25) | 0/12 (0) | 9/24 (38) | 100 |
| MBP mix/CD8+ cells | 21 033.16 | 9/12 (75) | 0/12 (0) | 13/24 (54) | 72 |
| GA/CD4+ cells | 17 330.29 | 4/12 (33) | 0/12 (0) | 10/24 (42) | 100 |
| GA/CD8+ cells | 16 817.68 | 6/12 (50) | 0/12 (0) | 13/24 (54) | 100 |
| TT/CD4+ cells | 23 222.28 | 8/12 (67) | 0/12 (0) | 11/24 (46) | 69 |
| TT/CD8+ cells | 16 469.92 | 2/12 (17) | 0/12 (0) | 4/24 (17) | 100 |
The cutoff to determine positive wells was calculated as mean counts per minute (CPM) of background (no antigen) wells (n = 12) plus 2 SD.
The numbers represent the proportion of wells that showed a proliferative response (CPM > cutoff) to the indicated stimulus. The response to Con A is representative of the cloning efficiency (survival of single-sorted T cells through rounds of PHA/IL-2 stimulation).
This number represents the calculated antigenic specificity of CFSE-sorted T cells (normalized to cloning efficiency; with a maximum value of 100%).
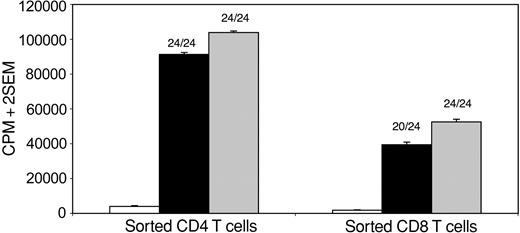
As the cloning efficiency from single-cell sorts was low, we attempted to acquire efficiency closer to 100% by sorting 5, 10, 50, or 100 cells per well. One such experiment is demonstrated in Figure 4. GA-reactive CD4+ (left panel) or CD8+ (right panel) T cells were sorted at 10 cells and 5 cells per well, respectively, followed by expansion with PHA and IL-2. The counts from the 3H-Thymidine-based proliferation assays showed 24 of 24 wells positive with Con A stimulation (100% efficiency). Twenty-four (100%) of 24 CD4+ wells and 20 (83%) of 24 CD8+ wells showed proliferation to GA. The counts from these antigen-stimulated wells approximated those from the Con A-stimulated wells, arguing against the theoretical possibility that only a minority of the 5, 10, 50, or 100 sorted cells were truly antigen specific. Overall, results from these studies show that the CFSE-based 7-day flow cytometric assay system predominantly detects HLA-restricted, antigen-specific CD4+ and CD8+ T-cell proliferation.
Antigenic specificity of sorted CD4+ and CD8+ T cells. CFSE-stained PBMC cultures from a patient with MS were stimulated with GA for 7 days, followed by flow cytometric sorting of proliferating CD4+ or CD8+ T cells into a 96-well plate at 10 cells and 5 cells per well, respectively, followed by expansion with rounds of PHA and IL-2 stimulation for 4 weeks. 3H-Thymidine-based proliferation assays were then performed in the presence of no antigen (□), GA (▪), or Con A ( ) (24 wells per condition). The y-axis represents mean CPM of 24 wells + 2 SEM. Using a cutoff of mean background counts + 2 SD, 24 of 24 Con A-stimulated wells showed positive responses in both cases. Of GA-stimulated wells, 24 (100%) of 24 CD4+ wells and 20 (83%) of 24 CD8+ wells showed positive responses, with counts from positive wells approximating those from Con A-stimulated wells.
) (24 wells per condition). The y-axis represents mean CPM of 24 wells + 2 SEM. Using a cutoff of mean background counts + 2 SD, 24 of 24 Con A-stimulated wells showed positive responses in both cases. Of GA-stimulated wells, 24 (100%) of 24 CD4+ wells and 20 (83%) of 24 CD8+ wells showed positive responses, with counts from positive wells approximating those from Con A-stimulated wells.
Antigenic specificity of sorted CD4+ and CD8+ T cells. CFSE-stained PBMC cultures from a patient with MS were stimulated with GA for 7 days, followed by flow cytometric sorting of proliferating CD4+ or CD8+ T cells into a 96-well plate at 10 cells and 5 cells per well, respectively, followed by expansion with rounds of PHA and IL-2 stimulation for 4 weeks. 3H-Thymidine-based proliferation assays were then performed in the presence of no antigen (□), GA (▪), or Con A ( ) (24 wells per condition). The y-axis represents mean CPM of 24 wells + 2 SEM. Using a cutoff of mean background counts + 2 SD, 24 of 24 Con A-stimulated wells showed positive responses in both cases. Of GA-stimulated wells, 24 (100%) of 24 CD4+ wells and 20 (83%) of 24 CD8+ wells showed positive responses, with counts from positive wells approximating those from Con A-stimulated wells.
) (24 wells per condition). The y-axis represents mean CPM of 24 wells + 2 SEM. Using a cutoff of mean background counts + 2 SD, 24 of 24 Con A-stimulated wells showed positive responses in both cases. Of GA-stimulated wells, 24 (100%) of 24 CD4+ wells and 20 (83%) of 24 CD8+ wells showed positive responses, with counts from positive wells approximating those from Con A-stimulated wells.
Functional differences in CNS-specific T-cell responses between healthy subjects and patients with MS
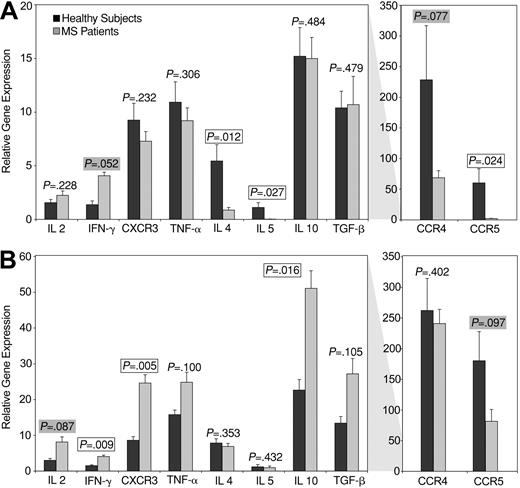
Healthy subjects and patients with MS have been reported to have similar magnitudes of CNS-specific T-cell responses. However, it has been suggested that these responses may be qualitatively distinct in these groups of individuals. Autoreactive T-cell responses in patients with MS are thought to be at a more differentiated memory/effector state, compared with those in healthy subjects.14,59-62 To specifically evaluate the distinct functional attributes of neuroantigen-reactive CD4+ and CD8+ T-cell responses, without inducing biases because of long-term culture, we sorted reactive T-cell populations using the CFSE-based approach.39 RNA from these populations of antigen-specific CD4+ and CD8+ T cells was used to perform real-time PCR assays to quantify the expression of several functional molecules. Figure 5 demonstrates results from these experiments. PCR reactions for GAPDH and β-actin were performed for every specimen and were used for normalization. Two housekeeping genes were used instead of one, because this provides better normalization.63 We used the ΔΔCt method for relative quantification,64 in which GAPDH was used as the control housekeeping gene to calculate a ΔCt value for each cytokine measured. This was followed by normalizing to the Ct values for β-actin (assigned an arbitrary value of 100) by calculating a ΔΔCt.64 Figure 5A demonstrates the mean relative expression of the indicated cytokines in a total of 10 autoreactive CD4+ T-cell populations from healthy subjects and 14 populations from patients with MS (representing responses to 5 different antigenic mixes in 3 healthy subjects and 6 patients with MS). Figure 5B demonstrates cytokine expression in a total of 11 autoreactive CD8+ T-cell populations from 3 healthy subjects and 17 populations from 6 patients with MS. We observed several notable differences in the functional attributes of CNS-specific CD4+ and CD8+ T cells between patients with MS and healthy controls. In the CD4+ T-cell compartment (Figure 4A), autoreactive T cells from patients with MS had significantly lower expression of IL-4, IL-5, CCR5 and CCR4 (borderline) and higher expression of IFN-γ, compared with healthy subjects. Thus, autoreactive CD4+ T cells in patients with MS appeared to be more differentiated toward the “Th1-type.” Autoreactive CD8+ T cells from patients with MS expressed higher levels of CXCR3, IFN-γ, and IL-10 and lower levels of CCR5. In contrast to these molecules, there were no detectable differences in the expression of other molecules analyzed.
Functional profile of autoreactive CD4+ and CD8+ T cells from patients with MS versus healthy subjects. SYBRGreen-based real-time PCR analyses were performed to quantify the expression of indicated molecules in flow-sorted MBP-, PLP-, MOG-, MAG-, or OMGP-specific CD4+ (A) and CD8+ (B) T cells, sorted from healthy subjects (▪) or patients with MS ( ). (A) The data represent a total of 10 autoreactive CD4+ T-cell populations from healthy subjects and 14 autoreactive CD4+ T-cell populations from patients with MS (representing responses to the 5 different CNS antigenic mixes from 3 healthy subjects and 6 patients with MS). (B) The data represent a total of 11 autoreactive CD8+ T-cell populations from 3 healthy subjects and 17 autoreactive CD8+ T-cell populations from 6 patients with MS. The data are normalized to 2 housekeeping genes (GAPDH and β-actin; assigned a value of 100) and are expressed as the mean relative expression (+ 2 SEM) of the indicated molecules in all autoreactive specimens. Significant P values (< .05) are boxed and borderline values (< .10) are highlighted.
). (A) The data represent a total of 10 autoreactive CD4+ T-cell populations from healthy subjects and 14 autoreactive CD4+ T-cell populations from patients with MS (representing responses to the 5 different CNS antigenic mixes from 3 healthy subjects and 6 patients with MS). (B) The data represent a total of 11 autoreactive CD8+ T-cell populations from 3 healthy subjects and 17 autoreactive CD8+ T-cell populations from 6 patients with MS. The data are normalized to 2 housekeeping genes (GAPDH and β-actin; assigned a value of 100) and are expressed as the mean relative expression (+ 2 SEM) of the indicated molecules in all autoreactive specimens. Significant P values (< .05) are boxed and borderline values (< .10) are highlighted.
Functional profile of autoreactive CD4+ and CD8+ T cells from patients with MS versus healthy subjects. SYBRGreen-based real-time PCR analyses were performed to quantify the expression of indicated molecules in flow-sorted MBP-, PLP-, MOG-, MAG-, or OMGP-specific CD4+ (A) and CD8+ (B) T cells, sorted from healthy subjects (▪) or patients with MS ( ). (A) The data represent a total of 10 autoreactive CD4+ T-cell populations from healthy subjects and 14 autoreactive CD4+ T-cell populations from patients with MS (representing responses to the 5 different CNS antigenic mixes from 3 healthy subjects and 6 patients with MS). (B) The data represent a total of 11 autoreactive CD8+ T-cell populations from 3 healthy subjects and 17 autoreactive CD8+ T-cell populations from 6 patients with MS. The data are normalized to 2 housekeeping genes (GAPDH and β-actin; assigned a value of 100) and are expressed as the mean relative expression (+ 2 SEM) of the indicated molecules in all autoreactive specimens. Significant P values (< .05) are boxed and borderline values (< .10) are highlighted.
). (A) The data represent a total of 10 autoreactive CD4+ T-cell populations from healthy subjects and 14 autoreactive CD4+ T-cell populations from patients with MS (representing responses to the 5 different CNS antigenic mixes from 3 healthy subjects and 6 patients with MS). (B) The data represent a total of 11 autoreactive CD8+ T-cell populations from 3 healthy subjects and 17 autoreactive CD8+ T-cell populations from 6 patients with MS. The data are normalized to 2 housekeeping genes (GAPDH and β-actin; assigned a value of 100) and are expressed as the mean relative expression (+ 2 SEM) of the indicated molecules in all autoreactive specimens. Significant P values (< .05) are boxed and borderline values (< .10) are highlighted.
Discussion
Although the etiology of MS is unknown, several features are suggestive of T-cell-mediated pathology.1,6,9-14 A great deal of our understanding about the immune mechanisms that underlie the pathogenesis and regulation of MS derives from studies in EAE, a well-established animal model. Like EAE, the pathology of MS is thought to be mediated and modulated predominantly by CD4+ T cells. Thus, most studies and, as a consequence, therapeutic strategies are focused on the dynamics of CD4+ T-cell responses.
CD8+ T cells have been implicated in the pathogenesis15-21,23-25,27,28,65,66 as well as regulation29-38 of autoimmune demyelination in MS and EAE. However, CNS-specific CD8+ T-cell responses in MS have been largely overlooked or thought to be uncommon. Moreover, readouts from traditional assays (such as 3H-based proliferation assays, ELISA assays, enzyme-linked immunospot [ELISPOT] assays) have been assumed to reflect effector functions of CD4+ T cells. A detailed evaluation of the prevalence and phenotype of CNS-specific autoreactivity has not been performed.
In the current study, we used a recently adapted flow cytometric methodology39,67 to address questions about specificity, phenotype, and functional attributes of CNS-specific T-cell responses in patients with MS, using 15 pools of 530 serial overlapping peptides, representing 9 putative target proteins. Previously, we have used this CFSE-based assay to show the existence of CD8+ T-cell responses to the immunotherapeutic drug, GA, and to study their in vivo dynamics in patients with MS during GA therapy.39 Our results from the current study demonstrate that autoantigen-reactive T cells are also not restricted to the CD4+ T-cell subset. At the very least, it is clear that in vitro proliferation, used as a readout in numerous previous reports, is not merely indicative of a CD4+ T-cell response.
The robustness of the CFSE-based flow cytometric assay in directly evaluating antigen-specific CD4+ and CD8+ T-cell responses is also encouraging. Evaluating the phenotype of autoreactive T cells in human disease has been a technologic challenge. Functional ex vivo flow cytometry systems have been successfully used to study human virus-specific immune responses,57,68,69 providing a direct enumeration of antiviral precursor frequencies. However, in several attempts by us and others, such approaches have proven to be insufficiently sensitive to allow reliable and reproducible detection of autoreactive T-cell responses, most likely owing to their relatively low precursor frequency or low T-cell receptor avidity. Thus, it is essential to develop sensitive flow cytometric technology to effectively detect and study autoantigen-specific T cells. The CFSE-based assay provides a system that can now be used to interrogate proliferating T cells for their phenotype and other functional characteristics. Using this assay system, we found that CNS-specific HLA class I-restricted CD8+ T-cell responses were widely prevalent in patients with MS. By sorting the proliferating cells from a short-term culture (day 7), we found that a large proportion of these were specific for the stimulating antigen and not a mere bystander phenomenon. Indeed, a recent report using a similar assay system with longer peptides for stimulation reported robust detection of CD4+ T-cell responses without the detection of CD8+ T-cell responses,67 strongly arguing against bystander proliferation.
Overall, we found a higher prevalence of CNS-reactive CD4+ T-cell responses, against a wider array of CNS peptide pools, compared with CD8+ T-cell responses. This was true of not only patients with MS but also healthy subjects. Potentially, this may be due to the use of 15-mer (versus 9-mer) peptides in our assay system, leading to underestimation of CD8+ responses. However, prior studies have shown robust detection of CD4+ and CD8+ T-cell responses using peptides of varying lengths (including 15-mer and 18-mer peptides) even in 6-hour ex vivo assay systems70 ; thus, 15-mer peptides are probably a satisfactory intermediate for screening purposes.
Using pools of serial peptides, representing entire target proteins, without focusing on “dominant” epitopes, we did not detect significant differences in the prevalence or antigenic specificity of CD4+ responses in patients with MS versus healthy subjects. It is still a formal possibility that we may observe differences when these responses are dissected down to specific epitopes. However, it is fair to say that our findings corroborate with many prior studies that have failed to observe an obvious difference in the magnitude of CNS-specific reactivity between patients with MS and healthy subjects. It may be interesting to modify the current assay system by adding cytokines or costimulatory modifiers that might be able to delineate differences in patients with MS versus healthy individuals.
We found some statistical differences in the distribution of autoreactive CD8+ T-cell responses between the different subject groups. Overall, a higher prevalence of autoreactive CD8+ T-cell responses was noted in patients with RRMS. In addition, compared with healthy subjects, a higher proportion of patients with RRMS showed CD8+ T-cell responses to MOBP, which is a known encephalitogenic target protein.71,72 The significance of these findings is uncertain in this study of limited numbers of subjects. However, these results suggest that differences may exist in the specificity, prevalence, and functional role of CNS-specific CD8+ T-cell responses in different subtypes of MS. Further evaluations would be required to determine whether these observations hold up in larger studies and to delineate the pathophysiologic significance of these differences.
Overall, we did not observe any obvious pattern of antigenic reactivity in different forms of MS. Although MBP-specific reactivity was the most prevalent, it appears that the specificity of CNS-reactive T cells is quite varied from patient to patient. This may be reflective of several factors such as different inciting events, varied HLA haplotypes, and differential dynamics of epitope spreading in each individual. Thus, it may be essential to target “antigen-specific” therapeutic strategies to an individualized set of antigens in patients with MS, on the basis of the T-cell responses present in a given individual, rather than focus on putative dominant epitopes.
Although the distribution of CNS-specific T-cell responses was similar in patients versus healthy subjects, the functional attributes of these cells were quite distinct. Such a dichotomy has been proposed in prior studies, wherein the CNS-targeted T cells from patients with MS are thought to be more differentiated or “memory-like” compared with those from healthy subjects, suggesting that the cells may have experienced antigen in vivo.14,59-61 Such a hypothesis is corroborated by the current study, because patients with MS appeared to harbor differentiated Th1 type autoreactive CD4+ T cells, whereas those from healthy individuals showed a Th0/Th2-type, anti-inflammatory cytokine pattern (including significantly higher levels of CCR4, IL-4, and IL-5).
We found higher CCR5 expression in autoreactive CD4+ and CD8+ T cells from healthy subjects, compared with patients with MS. CCR5 is thought to represent an important receptor in mediating CNS trafficking of cells. High CCR5 expression has been reported in patients with MS, particularly in cerebral spinal fluid (CSF)/CNS studies.73-77 Thus, it is possible that our observations are reflective of cells from peripheral blood that did not traffic to the CNS or were cycling. Larger studies will probably be required to definitively address this issue.
In autoreactive CD8+ T cells, we found what appears to be a mixed functional profile. Higher IFN-γ and CXCR3 expression was accompanied by higher IL-10 expression in patients with MS. The roles of these different cytokines and chemokine receptors in CD8+ T-cell biology are not as well understood, especially in the autoimmune setting. Such a functional profile may be compatible with pathogenic and/or immunoregulatory functions; thus, further studies are required to delineate the functional attributes of individual CD8+ T-cell clones in this disease. Recent studies have shown enhanced TNF-α production by enriched populations of bulk CD8+ T cells from patients with SPMS, with borderline expression in RRMS.65 Our observations corroborate these findings, with borderline significance of TNF-α expression (P = .100) in CNS-specific CD8+ T cells.
Overall, these studies demonstrate that CNS-specific CD8+ T-cell responses are heterogeneous and may play important roles in the pathogenesis and regulation of human MS. In addition, CD8+ T cells of other reactivity, such as those that respond to GA, may also contribute to disease regulation, as suggested by our previous studies.39 Whereas patients with MS have widespread CNS-specific CD8+ T-cell responses, they appear to require GA therapy to restore GA-specific CD8 responses to the levels found in healthy subjects, with potential regulatory benefit.39 Thus, a complex pathogenic and regulatory balance may exist within the CD8+ T-cell subset, as has been characterized in CD4+ T cells. We believe that these findings strengthen the need for dissecting the functional roles of these T-cell subsets and to consider developing therapeutic strategies that target and modulate such class I-restricted CD8+ T-cell responses.
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2003-11-4025.
Supported in part by US Public Health Service NIH grants AI49990 and AI53439 (N.J.K); National Multiple Sclerosis Society grants RG3380-A-1 and JF2118-A-2 (N.J.K.); The Wadsworth Foundation, Seattle, WA (N.J.K.); the University of Texas Southwestern President's Research Council Distinguished Young Researcher Award (N.J.K.); the Multiple Sclerosis Society of Great Britain and Northern Ireland grant 589-00 (D.A.P.); NIH grants NS37513, AI47133, and NS044250 (M.K.R.); and the National Multiple Sclerosis Society grant RG2969-B-7 (M.K.R.). N.J.K. is a Harry Weaver Neuroscience Scholar of the National Multiple Sclerosis Society.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ellen Vitetta for helpful discussions and review of the manuscript; Becky Price, RN, for performing the leukapheresis; and Bonnie Darnell and Andrew Benagh for technical assistance with the cell sorting experiments.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal