Abstract
The finding that chronic lymphocytic leukemia (CLL) consists of 2 clinical subsets, distinguished by the incidence of somatic mutations in the immunoglobulin (Ig) variable region (V) genes, has clearly linked prognosis to biology. Antigen encounter by the cell of origin is indicated in both subsets by selective but distinct expression of V genes, with evidence for continuing stimulation after transformation. The key to distinctive tumor behavior likely relates to the differential ability of the B-cell receptor (BCR) to respond. Both subsets may be undergoing low-level signaling in vivo, although analysis of blood cells limits knowledge of critical events in the tissue microenvironment. Analysis of signal competence in vitro reveals that unmutated CLL generally continues to respond, whereas mutated CLL is anergized. Differential responsiveness may reflect the increased ability of post-germinal center B cells to be triggered by antigen, leading to long-term anergy. This could minimize cell division in mutated CLL and account for prognostic differences. Unifying features of CLL include low responsiveness, expression of CD25, and production of immunosuppressive cytokines. These properties are reminiscent of regulatory T cells and suggest that the cell of origin of CLL might be a regulatory B cell. Continuing regulatory activity, mediated via autoantigen, could suppress Ig production and lead to disease-associated hypogammaglobulinemia. (Blood. 2004;103:4389-4395)
Introduction
The key molecule for a B cell is immunoglobulin (Ig), a receptor with no fixed ligand, but individually constructed so that each B cell can recognize a particular antigen. Genetic recombinations and somatic mutations in the Ig genes occur during maturation of the B cell en route to production of high-affinity antibody. The outcome is antibody-mediated protection against the wide range of infectious organisms, but the price is development of B-cell tumors. An advantage for the investigator is that the status of the Ig genes in tumor cells reveals the clonal history of the cell of origin, together with evidence of continuing environmental influences.
Over several years, the accumulation of variable (V) region gene sequences has allowed a biologic classification of B-cell tumors to emerge. For tumors of mature B cells, the point of differentiation reached by the cell of origin can be assigned broadly as: first, pre-germinal center (GC), with no somatic mutations; second, located in a GC site with mutations and ongoing mutational activity; third, post-GC with a stable pattern of mutations.1 Isotype switch events can also be tracked.
Until recently, clinical application of this knowledge had been confined largely to using probes for specific Ig sequences to assess minimal residual disease. This situation changed dramatically in 1999 in relation to the most common adult B-cell tumor, chronic lymphocytic leukemia (CLL). CLL had previously been considered as a single entity with a variable clinical course, but Ig gene analysis showed it consists of 2 subsets,2 distinguished by the incidence of somatic mutations in the Ig V genes. Importantly, the prognosis of patients in the 2 subsets was markedly different, with the prognosis of those bearing unmutated V genes being worse.3,4 This widely confirmed correlation immediately linked prognosis to biology. Although each B-cell tumor has a different V-gene sequence, it indicated that within each subset there is a common feature that determines malignant behavior. It is now timely to focus on the nature of the B-cell receptor (BCR) in the 2 subsets and to consider how the interactions of this key molecule with environmental stimuli could contribute to differential disease progression. A second question is whether new knowledge of the biology of the tumor cells can help the long quest for the cell of origin.
Origin and activation of the CLL subsets
By comparison with normal B-cell differentiation, unmutated CLL (U-CLL), of poorer prognosis, is derived from a naive B cell, and mutated CLL (M-CLL) from a memory B cell. This is based simply on the fact that memory B cells have mutated V genes which confer higher affinity for antigen. However, as in human society, naivity may not mean complete lack of experience, and the frequent presence of activation markers such as CD23, CD25, CD69, and CD71, is suggestive of continuing environmental stimulation.5 If antigen had been encountered by the cell of origin of U-CLL, it was evidently insufficient to induce maturation in a GC site. In contrast, M-CLL apparently arises from a B cell which has seeded a GC where it has undergone somatic mutation, and presumably antigen selection, prior to exit. Posttransformation stimulation of the M-CLL cells is also apparently continuing, leading to an activated phenotype similar to that of U-CLL. The environmental stimulus is unknown, but, even if it occurs via the BCR, it need not be the initiating antigen, but could be a cross-reacting antigen, possibly an autoantigen (Figure 1).
Origin and features of the 2 subsets of chronic lymphocytic leukemia. The development of unmutated CLL (U-CLL) is likely to be from a naive B cell that has encountered antigen but with insufficient stimulus to form a germinal center (GC). This subset has a poorer prognosis, displays a preference for V1-69 genes, and frequently expresses ZAP-70 and activation-induced cytidine deaminase (AID). In contrast, mutated CLL (M-CLL) develops from a cell that, following antigen encounter, has undergone somatic mutation and presumably antigen selection in the GC. The final neoplastic event is likely to have occurred after exit from the GC. This subset has a superior prognosis and displays a preference for V4-34 genes, but infrequently expresses ZAP-70 or AICD.
Origin and features of the 2 subsets of chronic lymphocytic leukemia. The development of unmutated CLL (U-CLL) is likely to be from a naive B cell that has encountered antigen but with insufficient stimulus to form a germinal center (GC). This subset has a poorer prognosis, displays a preference for V1-69 genes, and frequently expresses ZAP-70 and activation-induced cytidine deaminase (AID). In contrast, mutated CLL (M-CLL) develops from a cell that, following antigen encounter, has undergone somatic mutation and presumably antigen selection in the GC. The final neoplastic event is likely to have occurred after exit from the GC. This subset has a superior prognosis and displays a preference for V4-34 genes, but infrequently expresses ZAP-70 or AICD.
Surprisingly, gene expression profiles have suggested that both subsets resemble memory B cells.6,7 However, the limited differences between naive and memory B-cell profiles,8 and the evidence of continuing stimulation, may complicate assignment. Two molecules with influence on the BCR tend to be more highly expressed in U-CLL than M-CLL. The first, activation-induced cytidine deaminase (AID), an enzyme required for somatic mutation and isotype switching,9 is up-regulated, at least at the mRNAlevel.10,11 While there is evidence that AID expression could be confined to a small proportion of the clone,12 it appears to be functional, since U-CLL cases can generate isotype-switched transcripts and protein.11 With regard to somatic mutation, a role for AID in the subset defined as “unmutated” would be unexpected. However, although the level of somatic mutation is by definition less than 2%, there can be a very low level of ongoing somatic mutation in some cases.13 Similar ongoing mutational activity has also been reported in M-CLL, which is commonly AID negative.13 The significance of this mutational activity is difficult to assess, since levels are considerably less than those observed in GC lymphomas.13 It is reminiscent of the low levels of mutational activity detectable in T-independent normal B-cell responses in mouse models.14 In U-CLL, this low level appears not to distinguish prognosis from that of cases with truly unmutated cases, indicating a similarity in the biology of the cells. The second gene up-regulated in U-CLL is zeta-associated protein 70 (ZAP-70), a receptor-associated protein tyrosine kinase usually found in T cells. High levels of ZAP-70 protein are detectable in the majority of cases of U-CLL.15,16 The features of continuing mutational and isotype switch activity, together with up-regulation of genes associated with cell signaling, support the concept that cases of U-CLL are able to receive signals via the BCR.
Superantigenic or antigenic drive on CLL subsets
Superantigens, commonly derived from pathogens, bind to framework regions of certain Ig variable regions and drive expansion of the Ig-expressing B cells.17 Evidence for the possibility of a superantigenic drive on the BCR of the cell of origin of a malignancy therefore lies in the biased usage of certain V genes by B-cell tumors. In CLL, this is seen in both subsets, with the most marked being overexpression of V1-69 in U-CLL and of V4-34 in M-CLL.3,18 An influence of antigen is also seen in selective usage of the D3-3 second reading frame, together with JH6, in the V1-69-expressing subset.19 Bias could reflect a narrowing B-cell repertoire in the older patient group, but we have shown that there is no increased usage of V1-69 with age,20 and the selective sequence characteristics of V 1-69-encoded sequences are not age related but, as suspected,19 appear CLL specific.
In contrast, we did observe an increased number of B cells expressing V4-34-encoded Ig in older healthy subjects. A rise in V4-34-encoded serum Ig is known to follow infection with certain herpes viruses.21 It is possible that the expanded V4-34-expressing B cells represent a response to cytomegalovirus (CMV) reactivation, known to perturb the T-cell repertoire in the older age group and in CLL.22 While there is no indication that CMV is involved in the transforming events leading to CLL, the V-gene profile of emergent B-cell tumors may reflect the available repertoire, and this could explain the bias toward the V4-34 gene in M-CLL. Bias in usage of the V3-21 gene has been reported in both U-CLL and M-CLL, often in combination with λ light chains and selected Vλ genes.22,23 Curiously, the incidence of the V3-21 gene in CLL appears higher in certain geographic regions,23 and this could reflect either a different pathogenesis or variable bias in the aging repertoire. The origin and features of U-CLL and M-CLL in relation to the site of somatic mutation and antigen selection, and the features characteristic of the 2 subsets, are summarized in Figure 1.
BCR engagement determines the fate of B cells
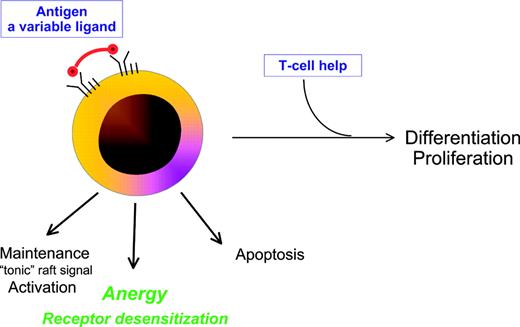
The BCR is the key to B-cell behavior, and is unusual in having no unique ligand. The level of BCR engagement therefore varies, being modulated by antigenic valency, epitope density, and epitope organization. The threshold required for effective signaling, and the downstream outcome, also differ according to the stage of differentiation. Naive B cells require higher concentrations of antigen than memory B cells to initiate responses.24 As expected from their biologic role in enhancing secondary immune reponses, memory B cells also respond more rapidly to T-cell help.25 In fact, the initial strength of engagement may determine whether response is T-cell independent or requires T-cell help. Provision of T-cell help to a mature B cell is critical for generating high-affinity antibody. If present, proliferation and differentiation can proceed. If not, options include apoptosis or anergy (Figure 2).
Multiplicity of potential outcomes following encounter of a B cell with antigen. Antigen, the ligand for the B-cell receptor, varies in properties that influence outcome including avidity, molecular form, concentration, and time of exposure. In the presence of CD40L-expressing Th cells, differentiation and proliferation can occur. In the absence of T-cell help, possible fates of the B cell include activation/maintenance, apoptosis, or anergy with receptor desensitization.
Multiplicity of potential outcomes following encounter of a B cell with antigen. Antigen, the ligand for the B-cell receptor, varies in properties that influence outcome including avidity, molecular form, concentration, and time of exposure. In the presence of CD40L-expressing Th cells, differentiation and proliferation can occur. In the absence of T-cell help, possible fates of the B cell include activation/maintenance, apoptosis, or anergy with receptor desensitization.
The BCR includes membrane Ig noncovalently associated in a 1:1 ratio with an Ig-α/Ig-β dimer responsible for signal transduction. It appears to form oligomeric complexes, inside which signals can be processed and amplified.26 Expression of surface Ig (sIg) is essential for survival of B cells in the periphery27 and sIg evidently provides a maintenance “tonic” signal either via natural oligomers, or via an exogenous interaction (Figure 2). Perturbation of the complexes by antigen targets the activated BCR into membrane rafts which then signal the cell to respond.28 In transgenic models, binding of low-affinity multivalent antigen to the BCR in the absence of T-cell help fails to induce proliferation and differentiation and leads instead to anergy.29 This is accompanied by reduced expression of sIg and inability of the BCR to respond to subsequent signals. The mechanism of desensitization of the BCR apparently involves uncoupling of the Ig-α/Ig-β subunits from the sIg, and this state can persist for a long period.29
BCR status in CLL subsets
Following engagement of the BCR, receptor aggregation induces phosphorylation of the immunoreceptor tyrosine-based activation motifs (ITAMs) in the Ig-α/Ig-β sequences, by receptor-associated Src-family tyrosine kinases.28 This leads to recruitment of other kinases including Syk, which itself becomes phosphorylated, prior to activation of intracellular signaling cascades. Using phosphorylation of Syk as a membrane-proximal indicator of signal transduction, we and others have shown that the majority (66% in our recent dataset) of U-CLL cases are able to signal via sIgM.30,31 Phosphorylation of the highly expressed ZAP-70 in U-CLL has also been observed following engagement of the BCR, and the subsequent association of this protein with sIg argues for involvement in BCR signaling.31 Signal competence is consistent with the observation that genes associated with signal transduction are expressed in U-CLL.6,7 In contrast, the majority of cases of M-CLL (63% currently), fail to signal via sIgM in vitro.30,31 Strikingly, desensitized cells that fail to respond to ligation of sIgM can signal normally if sIgM is bypassed,30 again in parallel with transgenic models of anergy.29 For CLL, we have found that approximately 50% of M-CLL cases unable to signal via sIgM were able to signal via sIgD. A further small subset was competent to signal only via Ig-α.30 The ability to signal via other molecules in the BCR indicates that the downstream pathways of signal transduction are operative in CLL. Failure to signal is therefore a membrane-proximal event characteristic of anergic cells. As described in defined models of anergy, it is likely to be due to uncoupling of the sIg from the Ig-α/Ig-β dimer which desensitizes the IgM-mediated signal pathway.
Factors associated with BCR signal anergy
A possible explanation for failure to signal via the BCR would be a mutation in one of the associated molecules, which could lead to constitutive low-level stimulation and consequent anergy. There is evidence for mutations in GC or post-GC tumors, which may be linked to mutational activity in the V genes.32 In CLL, there is some controversy on this point, with a report of mutations in Ig-β (CD79b),33 but no detection of mutations in another study.34 Overexpression of an alternative transcript of Ig-β in CLL that lacks the extracellular domain has also been reported.34-36 This splice variant is a feature of normal activated B cells,37 and can both prevent cell-surface expression of the BCR38 and inhibit signaling.36 One possibility is that the low or absent expression of Ig-β in CLL reflects the activation status of the cell, rather than being a CLL-related feature. There is no evident difference in expression of CD79b between U-CLL and M-CLL, consistent with both having indicators of activation. An alternative intriguing explanation proposed for reduced expression of both sIg and CD79b in CLL is that there may be a posttranscriptional defect in assembly or trafficking of the BCR from the endoplasmic reticulum to the cell surface.35 An anergic profile could also result from mutations in the transmembrane sequence of sIgM required for interaction with Ig-α (CD79a).39 To assess this, we analyzed 10 signal-incompetent cases and all had normal sequence, extending previous data from cases of familial CLL.40 So far therefore a mutational change has not been found to account for the anergic profile characteristic of M-CLL.
The distinctive anergic status evident in the majority of M-CLL, and in a proportion of U-CLL, is more likely the result of prior signal events which have rendered the cell resistant at the membrane to further stimulation. As might be expected, but was not evident in our previous small cohort,30 expression of sIgM is slightly but significantly lower in signal-incompetent cases, possibly due to long-term down-regulation (Louise Neville, C. Ian Mockridge, Kathleen N. Potter, Stuart Lanham, Islay Wheatley, Graham K. Packham, and F.K.S., manuscript in preparation, February 2004). However, sIgM is still detectable in nonsignalers, and can undergo capping and endocytosis, again mirroring the anergic status induced in transgenic models41 (see also Paolo Ghia, Paolo Circosta, Cristina Scielzo, Antonella Vallario, Annalise Camporeale, Luisa Granziero, and F.C.C., manuscript in preparation, February 2004). Presumably antigen presentation would be normal. Anergy appears to be a stable state, and we were unable to induce signaling in these cases by hyper cross-linking using a secondary antibody. This suggests that competence cannot be restored by simple aggregation.
Among M-CLL, there is a hierarchy of anergic status, with the largest group showing sIgM signal incompetence which can be bypassed via sIgD. Differential destabilizing effects on sIgM or sIgD may be due to isotype-specific sequences in the transmembrane domain which interacts with Ig-α/Ig-β.26 It appears that the interaction with IgD may be more difficult to disrupt. Models indicate that negative signals are dominant and therefore these cells would fail to respond to antigen,41 but this has not yet been established for CLL. Signal competence of the BCR is not completely concordant with the 2 subsets of CLL. However, within the M-CLL subset, there is an influence of CD38+ expression on signal competence.30 Therefore, the 2 poor prognostic factors, unmutated V genes and CD38 expression, are associated with signal competence. This might suggest that signals mediated by the BCR lead to an increased mitotic rate. It would be consistent with the presence of shorter telomeres in U-CLL.5
Events in tissues
All physiologic events that involve B cells, from antigen (Ag) encounter/presentation to B-cell activation, proliferation, and differentiation, occur in specialized anatomical microenvironments where B cells are brought into intimate contact with T and accessory cells. Classically, human T-cell-dependent responses take place in the GC of secondary lymphoid follicles in the presence of follicular dendritic cells (FDCs) and Ag-specific CD4+ T cells.42 It is in this site that somatic mutation of Ig V genes, antigen selection, and isotype switch events occur. However, these activities may not be completely confined to the GC, since somatic mutations can accumulate outside this site.43 Malignant B cells from several chronic B-cell malignancies are often highly dependent on the microenvironment, and tumors such as follicular lymphoma inhabit organized lymphoid tissue.44 Several can also retain the ability to undergo somatic mutation and isotype switch events posttransformation.1
For CLL, the temptation to investigate the readily available tumor cells in the peripheral blood (PB) has perhaps obscured the question as to what extent this compartment can be used as a paradigm to dissect the development and natural history of the disease. Three points need to be taken into account. First, virtually all circulating CLL cells are in the G0/early G1 phase of the cell cycle.45 However, the investigation of telomere length and telomerase activity in CLL cells indicates that a considerable number of cell divisions have occurred within the leukemic clones and that U-CLL has apparently undergone more divisions than M-CLL.46 These findings lead to the questions of where and to what extent CLL cells proliferate and how the proliferative compartment nourishes the accumulation compartment.
Second, most studies of cellular behavior have been performed using blood cells of patients with CLL, often purified to remove T cells or accessory cells. It is clear that these blood cells undergo apoptosis when cultured in vitro, unless they are exposed to stromal cells or to combinations of cytokines.47 This indicates that the in vivo accumulation of apoptosis-resistant lymphocytes is favored by the microenvironment. Third, CLL clones show a phenotypic heterogeneity as demonstrated by the bimodal expression of CD38.48 In individual CD38 bimodal patients, the comparison of PB with bone marrow (BM) reveals that the CD38+ subpopulation is more highly represented in infiltrated BM.48 These data suggest that the interaction with the BM environment may influence expression of CD38 and raise the question of which are the mechanisms that control CLL cell trafficking between blood and tissue.
CLL proliferative compartment: the pseudofollicles
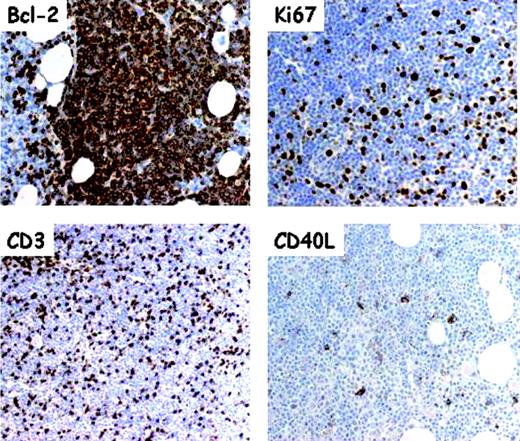
The CLL proliferating compartment is represented by pseudofollicles, vaguely nodular areas without mantles that are observed in lymph nodes and BM and represent the histopathologic hallmark of CLL. Immunohistochemical studies reveal that pseudofollicles are focal scattered aggregates of Ki67+ large tumor cells which express CD5 and differ from reactive GC B cells by being CD10-, Bcl-6-, and Bcl-2+.49,50 As in reactive GC, pseudofollicular cells express survivin,51 a member of the family of inhibitor of apoptosis proteins (IAPs)52 that integrates apoptosis and proliferation.53
Pseudofollicles are not simply a collection of proliferating monoclonal B lymphocytes (Figure 3). They also host a number of bystander nontumor cells. A striking presence of CD3+ T cells can be observed.54 Most belong to the CD4+ subset and are in close physical contact with proliferating Ki67+ CLL cells.55 Several CD4+ T cells within pseudofollicles express CD40L, implying that they are in an activated state. Further, some FDCs may be detected within pseudofollicles, above all in the early phase of BM involvement.56 It is unclear when, where, and through which stimulation CD4+ T cells that gather in CLL pseudofollicles acquire the expression of CD40L. Another debated issue concerns which mechanism drives activated CD4+ cells to preferentially locate in pseudofollicles. One possible explanation is that proliferating CLL cells themselves are able to recruit activated CD4+ T cells as they constitutively express and produce the T-cell-attracting chemokines CCL17 and CCL22.55
Serial section analysis of pseudofollicles in B-CLL bone marrow. Frozen tissue sections of a bone marrow trephine biopsy with heavy CLL infiltration were cut on slides covered with adhesive. All sections were subjected, before immunostaining, to antigen retrieval and developed using a sensitive avidinstreptavidin-peroxidase technique and standardized procedures. The monoclonal antibodies included anti-Bcl-2 (Ab124; Dako, Glostrup, Denmark), anti-Ki-67 (MIB-1; Dako), CD3 (Dako), and anti-CD40L (clone TRAP1; Pharmingen-Becton Dickinson, San Jose, CA). Virtually all cells are intensely Bcl-2+; pseudofollicles contain numerous Ki-67+ (ie, proliferating) elements that are interspersed with numerous CD3+ T cells, some being CD40L+. Original magnification for Bcl-2 and Ki67, × 250; for CD3 and CD40L, × 100.
Serial section analysis of pseudofollicles in B-CLL bone marrow. Frozen tissue sections of a bone marrow trephine biopsy with heavy CLL infiltration were cut on slides covered with adhesive. All sections were subjected, before immunostaining, to antigen retrieval and developed using a sensitive avidinstreptavidin-peroxidase technique and standardized procedures. The monoclonal antibodies included anti-Bcl-2 (Ab124; Dako, Glostrup, Denmark), anti-Ki-67 (MIB-1; Dako), CD3 (Dako), and anti-CD40L (clone TRAP1; Pharmingen-Becton Dickinson, San Jose, CA). Virtually all cells are intensely Bcl-2+; pseudofollicles contain numerous Ki-67+ (ie, proliferating) elements that are interspersed with numerous CD3+ T cells, some being CD40L+. Original magnification for Bcl-2 and Ki67, × 250; for CD3 and CD40L, × 100.
A remarkable difference between cells proliferating in tissue pseudofollicles and cells accumulating in the peripheral blood (PB) is that circulating CLL cells do not usually constitutively express CCL17, CCL22, and survivin. However, CD40 cross-linking of resting PB CLL cells induces the RNA expression of both chemokines and the secretion of CCL22.55 Adding interleukin 4 (IL-4) to the in vitro system induces also the release of CCL17. Further, CD40 stimulation in vitro induces the expression of survivin.51 The fact that PB CLL can be induced by CD40L to express survivin and T-cell-chemoattracting chemokines indicates that some PB malignant cells retain the capacity to respond to the proliferative and antiapoptotic microenvironmental signals provided by tissue bystander cells through cellular contacts.
The role of T cells in CLL
The issue of T cells in CLL is far from being settled. Rather surprisingly for this prototype of B-cell tumors, the absolute number of T cells is increased and the repertoire of both CD4+ and CD8+ T cells obtained from PB shows a marked oligoclonality.57 No information on the repertoire of tissue T cells is available and the attractive hypothesis that oligoclonality may reflect a tumor-specific or tumor-induced T-cell response is tempered by the observation that a substantial degree of oligoclonality is detected also in PB T cells from healthy elderly controls. At least some of the oligoclonal CD8+ population is likely to be directed against antigens of CMV.22 The possibility that some T-cell subpopulations might initially hinder the disease progression but ultimately fail, as natural killer (NK) T cells appear to do in the progression of monoclonal gammopathy of undetermined significance (MGUS) to overt multiple myeloma,58 is attractive but still unproven.
Considering that in vivo the physiologic stimulus provided by CD40L is available to malignant B cells within proliferation centers and gives CLL cells the capacity to chemoattract activated CD4+ T cells, it follows that T cells through their interactions with the malignant clone may influence the proliferation of CLL cell populations. In vitro findings match in vivo immunohistologic evidence and indicate that T lymphocytes may have a role in a microenvironment favorable to CLL progression. The stimulation of CD40 rescues blood CLL cells from apoptosis and induces their proliferation.59-61 CLL-T-cell interactions lead to production by both cell types of several cytokines, such as IL-4, interferon α (IFN-α), and IFN-γ,47 that may be involved in negative autocrine circuits able to inhibit CLL cell apoptosis. Whether the up-regulation of Bcl-2 protein is due to cytokine influence or to other environmental stimuli is still debated. Preliminary data (Paolo Ghia, Paolo Circosta, Cristina Scielzo, Antonella Vallario, Annalise Camporeale, Luisa Granziero, and F.C.C., manuscript in preparation, February 2004), based on in vitro cell responses to CD40L, suggest the existence of 2 functional subsets of patients with CLL, CD40L responders and CD40L nonresponders. As these 2 subsets differ in terms of lymphocyte doubling time, progression rate, and need for treatment, several interrelated crucial questions concerning the role of T cells in CLL need to be formally addressed. The first is whether T-cell influence and activity differ in U-CLL as compared with M-CLL. The second is whether the leukemic cell response to microenvironmental physiologic signals such as CD40 ligation persists and somehow controls disease progression. A further question is whether the accumulation over time of secondary genetic lesions favors the independence of leukemic cells from microenvironment control.
CLL cell trafficking and interactions with stromal cells
The precise trafficking rules and routes of CLL cells are unknown. PB CLL cells express specific sets of chemokine receptors such as CXCR3 and CXCR5 and respond to specific chemokines produced by microenvironmental elements.62,63 They also have a pattern of integrin expression that favors their interaction with the microenvironment.64-67 One possibility is that accessory cells operating in different microenvironments are involved in the trafficking and tissue retention of CLL. These cells also participate in the inhibition of CLL cell apoptosis.
Further, the PB of patients with CLL contains cells that in vitro can differentiate into adherent “nurselike” cells. Blood-derived nurse cells utilize a mechanism dependent on stromal cell-derived factor-1 (SDF-1, CXCL12), a CXC chemokine constitutively secreted by BM stromal cells68,69 that binds to CXCR4 (CD184), a chemokine receptor consistently overexpressed by CLL cells.70,71 The interaction of CXCR4 with SDF-1 not only protects the attached CLL cells from apoptosis,70 but also allows their spontaneous migration beneath BM stromal cells, suggesting a mechanism utilized to infiltrate the BM.
A new pathway that favors the extended survival of CLL and depends on contacts between CLL cells and FDCs has recently been identified. The CLL/FDC interactions are mediated at least in part by CD44 and induce the expression of the Bcl2-related antiapoptotic protein Mcl-1.72 In vitro cultures demonstrate that the survival of CLL cells is extended by their direct physical contact with BM stromal cells.64,65 β1 and β2 integrins favor the binding of CLL cells to a host of imperfectly defined BM stromal cells64,65 and α4/β1 allow the interaction of CLL cells with the activated endothelium.67 Another receptor/ligand system of interest is represented by CD100, which is uniformly expressed on the membrane of CLL cells, and its high-affinity receptor Plexin-B1, which is expressed by BM stromal cells, FDCs, and activated T lymphocytes. CD100+ CLL cells exposed in vitro to Plexin-B1 increase their proliferative activity and have an extended life span.73
By and large it appears that circulating CLL cells may re-enter the tissues and participate in environmental conditions that promote survival and proliferation. It is unclear whether M-CLL and U-CLL differ in the number, frequency, and mechanism of selection of circulating cells that propagate the disease by fueling the tissue events. It can be predicted that U-CLL has a more aggressive course because unmutated cells are more easily recruited into tissues where they undergo more division and thus have an increased risk of acquiring more genetic abnormalities.
What is the function of the cell of origin in CLL?
The picture emerging of CLL is of both subsets being stimulated in tissue sites by persistent endogenous antigen likely to be of low affinity. The response of the majority of U-CLL, derived from a pre-GC cell, is signal reception, although possible partial anergy cannot be ruled out. For M-CLL, derived from a more responsive memory B cell generated via a conventional GC route, triggering by endogenous antigen drives the cell into an anergic state. In both subsets, the default pathway awaiting normal B cells that fail to differentiate further is blocked by antiapoptotic mechanisms. The question arises as to the nature of the antigen. There is evidence that at least some of the IgM expressed by CLL cells has autoantibody activity.74,75 For those cases derived from the V1-69 gene, rheumatoid factor activity has been detected.76 Candidate antigen therefore include autoantigens, but antigens derived from endogenous organisms cannot be excluded. Engagement with antigen, and cell division, are likely to occur in tissue sites, and the proportion of the circulating clone with evidence for recent division is low, especially in M-CLL. However, an activated phenotype can persist in blood cells of both subsets, and both are dependent for survival on contact with stroma. The picture is of a dynamic and heterogeneous clone distributed among the lymphoid tissues, and undergoing stroma-dependent signals for activation and occasional division. Studies of the turnover of tumor cells in vivo should illuminate this point (N. Chiorazzi, personal communication, October 2003).
A feature of CLL that has not yet been completely explained is the profound immunosuppression, especially in the antibody response. Although serum Ig falls slowly as disease progresses, the ability to mount a primary antibody response declines early in disease.77 This is suggestive of a specific suppression rather than a consequence of simple occupation of the bone marrow and lymphoid tissue by tumor cells. It is evident in both subsets of CLL, indicating a shared factor independent of differential clinical progression, although clearly with clinical significance for patient management. It is known that CLL cells produce immunosuppressive cytokines, including transforming growth factor β (TGFβ) and IL-10,78 especially when activated. There is also an intriguing observation that CLL cells inhibit spontaneous Ig production by autologous bone marrow cells.79 The mechanism suggested involves Fas ligand (CD95L), known to be expressed by CLL cells, interacting with CD95-bearing, Ig-secreting cells.
Do CLL cells have immunoregulatory activity?
A unifying hypothesis could be that CLL cells have immunoregulatory activity that results in suppression of antibody responses. B cells capable of suppressing Th1 responses have been identified in mouse models.80 In a preclinical model of autoimmunity, where the mediators of disease are the Th1 cells, agonistic anti-CD40 activates inhibitory B cells which then protect against disease.80 The B cells produce IL-10, which suppresses proliferation of antigen-specific Th1 cells and down-regulates costimulatory molecules on antigen-presenting cells. The immunosuppressive power of the B cells is reminiscent of CD4+CD25+ regulatory T cells, which can also act via IL-10 and TGFβ.81 Other features common to CLL cells and regulatory T cells include expression of CD25 and CD26.82 The latter is an enzyme that appears to mediate immunosuppression by degrading cell-surface-bound chemoattractants, thereby reducing essential cell-cell interactions.83 If CLL represents a tumor of immunoregulatory B cells, it would explain the disease-associated immunosuppression and the accompanying dys-regulation of T-cell function activity. The mechanism of action of immunoregulatory T cells is still unclear, and that of B cells lags behind. However, availability of large numbers of CLL cells should make investigation of this hypothesis possible.
Concluding comments
The remarkable difference in clinical behavior between U-CLL and M-CLL is likely to involve BCR signal competence. Naive B cells are less easily anergized than post-germinal center B cells, and that may account for the difference between the subsets. In most cases of M-CLL, the threshold required for desensitization has been reached, and the consequent anergic state may be prolonged. Clearly if response is turned off there can be no BCR-mediated signal for cell division and this must be at least a partial explanation for the less aggressive nature of M-CLL. However, expression of sIg is retained, possibly as a maintenance signal known to be required for normal B cells.27 Effects on apoptosis are more difficult to assess, since environmental factors will have a major influence on survival.47 An important factor is T-cell help, including CD40L, which determines the fate of normal B cells at this point. One problem for investigators is the difficulty of extrapolating from studies of CLL cells obtained from blood, separated from tissue sites where critical events are likely to be occurring.
The biologic picture emerging for CLL is of tumor cells interacting with (super)antigen, but failing to differentiate further, either due to blocks or to lack of T-cell help. This interaction could be critical in maintaining production of immunosuppressive cytokines, possibly reflecting a regulatory function of the cell of origin. Antiapoptotic mechanisms inhibit the default pathway to death awaiting normal B cells. For U-CLL, signal reception may stimulate division and contribute to malignant behavior. For M-CLL, the response is to down-regulate the BCR and reduce signal reception, minimizing division and leading to lethargy. Signal-competent U-CLL is the main therapeutic target and the focus of attention will be on the (super)antigenic drivers and on the environmental interactions that contribute to the malignant behavior of this subset.
Prepublished online as Blood First Edition Paper, February 12, 2004; DOI 10.1182/blood-2003-12-4312.
Supported by Tenovus and the Leukaemia Research Fund, United Kingdom, and by the Associazione Italiana per la Ricerca sul Cancro (AIRC), MURST, and Ministero della Salute-Programmi Speciali.
We wish to thank Drs Graham Packham, Paolo Ghia, Kathy Potter, Stuart Lanham, and Louise Neville for invaluable contributions. We also thank Ian Mockridge for FACS data and Islay Wheatley for sequence analysis. The help and collaboration of Professor Marco Chilosi, University of Verona, in obtaining the immunohistochemistry figure are gratefully acknowledged.