Abstract
Imatinib mesylate, an inhibitor of the Bcr-Abl tyrosine kinase, has modest activity in refractory/relapsed Philadelphia chromosome (Ph)-positive acute lymphocytic leukemia (ALL). Use of concurrent chemotherapy and imatinib mesylate in newly diagnosed Ph-positive ALL was explored. There were 20 patients who received hyper-CVAD (cyclophosphamide, vincristine, Adriamycin, and dexamethasone) and imatinib mesylate followed by imatinib mesylate-based consolidation/maintenance therapy. Of these patients, 11 had de novo disease, 4 were primary failures after induction (without imatinib mesylate), and 5 were in complete remission (CR) after induction (without imatinib mesylate). All 15 patients treated for active disease achieved CR. Within a median of 3.5 months in first CR, 10 patients underwent allogeneic stem cell transplantation (SCT). One patient relapsed after matched related SCT. The other 9 patients remained alive in CR with median follow-up of 12 months after SCT (range, 1+ to 17+ months). Among 10 patients ineligible for (no donor or older age) or refusing allogeneic SCT, 1 patient relapsed after one year. There were 5 patients who remained alive in continuous CR for a median of 20 months (range, 4+ to 24+ months), with 2 older patients dying in CR at 15 and 16 months of comorbid conditions. Molecular CRs were achieved in both groups (SCT or no SCT). Outcome with hyper-CVAD and imatinib mesylate appears better than with prior regimens; continued accrual and longer follow-up of the current cohort is needed. (Blood. 2004;103:4396-4407)
Introduction
Philadelphia chromosome (Ph)-positive acute lymphocytic leukemia (ALL) is characterized by a reciprocal translocation between the long arms of chromosomes 9 and 22 [t(9;22)(q34;q11)], leading to the formation of the Bcr-Abl fusion protein. This translocation occurs in about 3% to 5% of children and 20% to 30% of adults with ALL, and is associated with a very poor prognosis.1,2 Modern chemotherapy regimens for adult Ph-negative ALL result in complete response (CR) rates of 90% to 95% and long-term overall survival (OS) rates of 35% to 40%.3-7 Despite achievement of similarly high CR rates of 80% to 90% with these chemotherapy regimens, most patients with Ph-positive ALL relapse and die from the disease.8-10 The 5-year survival rates are less than 10% for adults. Children with Ph-positive ALL treated with chemotherapy alone have higher survival rates of 25% to 30%, but worse outcomes are seen with leukocyte counts higher than 100 × 109/L and/or failure to respond to glucocorticoid therapy.11,12 Allogeneic stem cell transplantation (SCT) in first CR can increase the long-term event-free survival (EFS) rate to 28% to 40% for adults and to 60% to 75% for children.11,13-18
The unique biologic behavior of Ph-positive ALL is attributed to molecular changes resulting from the Abl proto-oncogene from chromosome 9 fusing within the 5′ half of the breakpoint cluster region (bcr) sequences on chromosome 22.19,20 The chimeric Bcr-Abl oncogene leads to fusion proteins of different sizes depending on the location of the Bcr breakpoint. If the break within the first intron of BCR (m-bcr region) splices the first exon of the BCR gene to the second exon of the ABL gene (e1a2), the 190 kDa fusion oncoprotein is generated. This oncoprotein (p190bcr-abl) occurs in 60% to 80% of patients with Ph-positive ALL; the other 20% to 40% are characterized by the larger p210bcr-abl fusion oncoprotein typical of chronic myelogenous leukemia (CML). The latter is formed when exon b2 or exon b3 of the BCR gene (M-bcr region) is coupled to ABL exon 2 (b2a2 or b3a2 junction). Gleissner et al21 reported an association of the p210bcr-abl subtype with older age and trend toward worse 3-year survival rates, although most studies have not demonstrated differences in clinical presentation or outcome with either p190bcr-abl or p210bcr-abl ALL.21-24
These Bcr-Abl molecular abnormalities may be sufficient for leukemic transformation as demonstrated in animal models showing a p210bcr-abl tyrosine kinase-dependent development of a CML-like disease.25 The mechanism of leukemogenesis appears related to the constitutively activated and dysregulated tyrosine kinase activity that results in downstream effects on multiple signal transduction pathways controlling cellular proliferation and apoptosis.26 Imatinib mesylate (Gleevec, STI571; Novartis Pharmaceuticals Corporation) is a selective inhibitor of the Bcr-Abl tyrosine kinase, platelet-derived growth factor (PDGF) receptor tyrosine kinase, and the c-kit receptor kinase.27 In vitro, imatinib mesylate inhibited the growth of Bcr-Abl-positive cells by inducing apoptosis and suppressing proliferation.28-30 In clinical trials, imatinib mesylate demonstrated significant anti-CML activity and is now a standard frontline treatment in newly diagnosed Ph-positive CML.31-39 Single-agent imatinib mesylate therapy for previously treated relapsed or refractory Ph-positive ALL and CML in lymphoid blast phase was associated with objective response rates of 40% to 50%; true CR rates (lasting for 4 weeks or more) were 5% to 7% with the median survival of 2 to 5 months. 39-41
Mechanisms of resistance such as Bcr-Abl amplification or mutations in the Bcr-Abl tyrosine kinase have been identified in previously treated Ph-positive ALL patients salvaged with single-agent imatinib mesylate.42,43 These results suggest that use of imatinib mesylate as a single agent in Ph-positive ALL is unlikely to be sufficient for long-term disease control. In vitro studies suggest synergistic activity of imatinib mesylate with chemotherapy agents such as anthracyclines, vincristine, and cytarabine (ara-C).44,45 This report summarizes the experience with hyper-CVAD (cyclophosphamide, vincristine, Adriamycin, and dexamethasone),46 a dose-intensive chemotherapy regimen used at our institution for adult ALL since 1992, and imatinib mesylate for patients with de novo Ph-positive ALL.
Patients and methods
Eligibility
Adults (age 15 years or older) with Ph-positive ALL, newly diagnosed or minimally treated, were entered on the institutional review board-approved study after informed consent was obtained according to institutional guidelines. Patients previously treated with induction chemotherapy without imatinib mesylate (either failing after 1 course or in CR after up to 2 courses of therapy without imatinib mesylate) were eligible. Entry criteria included Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2, adequate renal and hepatic functions (serum creatinine < 176.8 μM [2 mg/dL] and bilirubin < 34.2 μM [2 mg/dL]), and adequate cardiac status (New York Heart Association class III-IV excluded). Patients with uncontrolled serious infections or active secondary malignancy that was thought to shorten survival to less than one year were not eligible. No investigational antileukemic therapy could have been administered within the prior 7 days. Patients must have been willing to practice contraception.
Therapy
Therapy was given with 8 induction-consolidation courses alternating hyper-CVAD with high-dose methotrexate (MTX) and cytarabine (ara-C), concurrently with imatinib mesylate.
Briefly, the treatment regimen was as follows. Imatinib mesylate was given at the standard dose of 400 mg orally daily on days 1 to 14 of each of the intensive chemotherapy courses, without resumption until the next course to allow recovery from myelosuppression. Odd courses (nos. 1, 3, 5, and 7) were hyper-CVAD: hyper-fractionated cyclophosphamide (CTX) given 300 mg/m2 intravenously over 2 hours every 12 hours for 6 doses on days 1 to 3 with 600 mg/m2 Mesna per day intravenously via continuous infusion on days 1 to 3 beginning 1 hour prior to CTX and completed by 12 hours after the last dose of CTX; 2 mg vincristine intravenously on days 4 and 11; 50 mg/m2 doxorubicin (Adriamycin) intravenously over 24 hours via central venous catheter on day 4 (given over 48 hours in patients with reduced ejection fractions < 50%); and 40 mg dexamethasone daily either orally or intravenously on days 1 to 4 and days 11 to 14. Granulocyte colony-stimulating factor (G-CSF, 10 μg/kg [rounded]) was initiated approximately 24 hours after completion of chemotherapy until the absolute neutrophil count (ANC) was higher than 1 × 109/L.
The first course was accompanied by appropriate intravenous hydration and alkalinization (eg, dextrose water or one-half normal saline with 75 to 100 milliequivalents of sodium acetate per liter to run at 50 to 100 mL/h) and allopurinol to reduce the incidence of tumor lysis syndrome. Oral sodium bicarbonate supplemented the intravenous formulation on days 1 to 3. Rasburicase could be substituted for allopurinol in cases with high white blood cell count at presentation.
Even courses (nos. 2, 4, 6, and 8) included high-dose MTX and ara-C: 1 g/m2 MTX intravenously over 24 hours on day 1; and 3 g/m2 (1 g/m2 in patients aged 60 or older) ara-C over 2 hours every 12 hours for 4 doses on days 2 and 3 were given. Intravenous alkalinization was used to promote excretion of MTX in all courses (as for course 1 at a rate of 100 to 125 mL/h). Calcium leucovorin was given at a dose of 50 mg intravenously starting 12 hours after the completion of MTX and continued at a dose of 15 mg intravenously every 6 hours for 8 doses until MTX blood levels were less than 0.1 μM. An algorithm of additional leucovorin rescue (50 mg intravenously every 6 hours) was followed if MTX blood levels were elevated at 0 hour (at end of infusion and confirmed on repeat sample), more than 20 μM; 24 hours, more than 1 μM; and 48 hours, more than 0.1 μM. Oral acetazolamide was used if the urine pH was less than 7.0 to promote excretion. G-CSF was repeated as for the odd courses.
Central nervous system (CNS) prophylaxis included alternating intrathecal therapy with 12 mg MTX (6 mg only if via ommaya) on day 2 and 100 mg ara-C on day 7 or 8 of each course for either a total of 6 or 8 intrathecal treatments, depending on risk for CNS relapse (based on serum lactate dehydrogenase [LDH] level > 1400 U/L and/or proliferative index %S + G2M ≥ 14%).47 Therapy for active CNS leukemia at presentation included an increase in frequency of the alternating intrathecal therapy during the induction course to twice weekly until the CSF cell count normalized and the cytologic examination was negative for evidence of malignant cells. Intrathecal therapy was then administered weekly for 4 weeks; then the prophylactic schedule (2 intrathecals per course) was resumed for the remaining courses of intensive chemotherapy. No prophylactic cranial irradiation (XRT) was administered. Therapeutic XRT was given if indicated for CNS disease at presentation (eg, cranial nerve palsies with XRT to the base of the skull).
All de novo (n = 11) or primary refractory (n = 4) patients began imatinib mesylate treatment with course 1, whereas 5 previously treated patients started protocol therapy with course 2, since they had received hyper-CVAD or anthracycline-based chemotherapy (without imatinib mesylate) as induction therapy. Eligible patients in first CR were referred for allogeneic SCT as soon as feasible after treatment was initiated.
Following completion of the 8 courses of intensive chemotherapy, maintenance therapy was planned for 13 months and consisted of 600 mg imatinib mesylate orally daily, 2 mg vincristine intravenously every month, and 200 mg prednisone daily for 5 days every month (commenced the day of the vincristine infusion). Higher doses of imatinib mesylate were given owing to the known dose-response phenomenon observed in the studies of imatinib mesylate in CML and the absence of concurrent myelosuppressive therapy.31 Maintenance therapy was interrupted during months 6 and 13 with hyper-CVAD and imatinib mesylate intensifications given as with prior courses of intensive chemotherapy.
Dose reductions included the following: (1) ara-C decreased to 1 g/m2 for age 60 years or older, creatinine level higher than 2 g/dL, or MTX level at 0 hour (repeated) higher than 20 μM; (2) vincristine to 1 mg for total bilirubin higher than 2 g/dL; (3) vincristine eliminated if above total bilirubin higher than 3 g/dL or grades 3 to 4 peripheral neuropathy or ileus; (4) doxorubicin decreased by 50% for bilirubin 2 to 3 g/dL, by 75% for bilirubin 3 to 5 g/dL, and eliminated for bilirubin higher than 5 g/dL; (5) MTX decreased by 50% for calculated creatinine clearance 10 to 50 mL/min, with decrease by 25% to 50% for delayed excretion, nephrotoxicity, or grade 3 or greater mucositis with prior courses. Imatinib mesylate was reduced to 300 mg for grades 3 to 4 hepatotoxicity during intensive chemotherapy courses (400 mg if during maintenance).
Supportive care
Oral prophylactic antibiotic therapy included either a quinolone (500 mg ciprofloxacin twice daily or 500 mg levofloxacin daily) or trimethoprim-sulfamethoxazole one double strength twice daily for antibacterial coverage, 200 mg fluconazole daily for antifungal coverage, and 200 mg acyclovir twice daily (or 500 mg valacyclovir daily) for mucositis prophylaxis with antiviral coverage. Hematologic profiles were obtained at least weekly. Appropriate transfusion support was provided with packed red blood cells given for symptomatic and/or severe anemia. Platelet transfusions were given prophylactically for platelet counts lower than 10 to 15 × 109/L or therapeutically for platelet count lower than 30 to 50 × 109/L with hemorrhage. All blood products were irradiated. Neutropenic febrile episodes generally resulted in hospitalization and initiation of broad spectrum parenteral antibiotics.
Assessments
Pretreatment evaluations included history and physical examination, complete blood count with differential, Sequential Multiple Analysis-12, and bone marrow aspiration for histology, flow cytometry, and cytogenetic studies. Cytogenetic analysis was performed by standard techniques, with bone marrow specimens examined on direct or short-term (24-hour) cultures.48 Fluorescent in-situ hybridization (FISH) and quantitative real-time polymerase chain reaction (RT-PCR) assays for bcr-abl were performed on marrow samples of patients who were in complete remission when feasible by methods detailed previously.49 RT-PCR was confirmed by nested PCR when negative.
Marrow aspirations were repeated approximately day 14 and day 21 of course 1 in patients with active disease at study entry, with the latter sample assessed for cytogenetics, FISH, and quantitative RT-PCR. Complete blood counts were performed at least weekly during the intensive courses of chemotherapy. Analyses of renal and hepatic function were performed prior to each cycle of chemotherapy. Bone marrow aspirations were repeated every 2 to 3 courses.
Baseline cardiac function was assessed by echocardiogram or multi-gated radionuclide ventriculography (MUGA) in patients with cardiac risk factors, and repeated in cases where clinical symptoms were reported or in preparation for allogeneic SCT. The presence or absence of CNS disease was evaluated in all patients concurrently with administration of CNS prophylaxis with methotrexate (approximately day 2 of the first course). Cerebrospinal fluid was assessed by cell count with differential and cytologic evaluation.
Response criteria
Complete remission (CR) was defined as 5% or less blasts in a normocellular or hypercellular marrow with a granulocyte count of 1.0 × 109/L or higher and platelet count of 100 × 109/L or higher. Complete resolution of extramedullary disease was required for CR. Molecular CR was defined as achievement of RT-PCR negativity in addition to hematologic criteria for CR. Other response outcomes were defined as induction death (if occurring after start of therapy without meeting the definition of CR or resistant disease) and resistant disease (if the patient survived the induction treatment period but the leukemia persisted or regrew). Relapse was defined as disease recurrence at any site after achieving CR.
Statistical considerations
The characteristics and treatment results of the de novo patients were compared with the historical control group of patients with Ph-positive ALL treated with either VAD (vincristine, doxorubicin, and dexamethasone) regimens50 from 1983 until 1991, or with hyper-CVAD with or without rituximab from 1992 until 2000.46,51 Differences in response rates by pretreatment characteristics among subgroups were analyzed by χ2 or Fisher exact tests.52 Survival was measured from the date of initiation of therapy until death from any cause. Disease-free survival was measured from the date of CR until documented relapse. Survival and disease-free survival curves were plotted according to the methods of Kaplan and Meier, with differences among them analyzed by the log-rank test.53 Toxicity was evaluated according to the National Cancer Institute Expanded Common Toxicity Criteria (version 2.0).
Results
Study group
From April 2001 to March 2003, 20 patients with newly diagnosed Ph-positive ALL were entered in the study. Of the patients, 11 (55%) presented with de novo disease, 4 (20%) were refractory to standard induction chemotherapy, and 5 (25%) entered the study in CR after one course of induction chemotherapy (all but 1 with minimal residual disease detectable by FISH and/or PCR). The p190bcr-abl was expressed in 50% of the patients, with p210bcr-abl in the remainder. The median age of the group was 42 years (range, 17 to 75 years); 70% were males. Characteristics of this group were compared with 31 de novo Ph-positive ALL patients treated with VAD50 and with 50 patients treated with the hyper-CVAD regimen with or without rituximab46,51 (Table 1). Patients in the hyper-CVAD and imatinib mesylate group had a lower incidence of elevated β2 microglobulin and LDH levels; however, they had a higher incidence of adverse features such as CNS disease and lower albumin levels compared with patients treated with prior regimens.
Characteristics and response of Philadelphia-positive ALL study groups
. | No. (%) . | . | . | . | ||
|---|---|---|---|---|---|---|
| Parameter . | VAD . | Hyper-CVAD . | Hyper-CVAD + imatinib mesylate . | P . | ||
| No. treated | ||||||
| Total | 31 | 50 | 20 | — | ||
| Status at study entry | ||||||
| Active disease | 31 | 50 | 15 (75) | — | ||
| In CR | — | — | 5 (25) | — | ||
| Age, y [median, range] | ||||||
| 60 or older | 8 (26) | 15 (30) | 4 (20) | NS | ||
| [47, 19-73] | [44, 16-79] | [42, 19-75] | ||||
| Performance, ECOG | ||||||
| 0 | 7 (23) | 3 (6) | 4 (20) | .07 | ||
| 1 to 2 | 23 (74) | 45 (90) | 16 (80) | |||
| Hemoglobin, g/L | ||||||
| Less than 100 | 14 (45) | 35 (70) | 13 (65) | .08 | ||
| WBC, × 109/L | ||||||
| 30 or higher | 15 (48) | 22 (44) | 4 (20) | NS | ||
| Platelet count, × 109/L | ||||||
| Lower than 50 | 15 (48) | 29 (58) | 7 (35) | NS | ||
| Albumin, g/L | ||||||
| Lower than 30 | 4 (13) | 7 (14) | 7 (35) | .08 | ||
| LDH, U/L | ||||||
| More than 620 | 30 (97) | 46 (92) | 12 (60) | < .01 | ||
| CNS disease | ||||||
| Yes | — | 1 (2) | 3 (15) | .02 | ||
| Karyotype | ||||||
| Ph or complex Ph | 12 (39) | 17 (34) | 8 (40) | NS | ||
| Ph, −5, −7 | 6 (19) | 4 (8) | 1 (5) | |||
| Ph and others (+8, +21) | 13 (42) | 28 (58) | 11 (55) | |||
| B2 microglobulin, mg % | 13/19 | 19/39 | 4/18 | .03 | ||
| More than 3 | (68) | (49) | (22) | |||
| Response | ||||||
| CR | 19 (61) | 47 (94) | 15/15 | < .01 | ||
| PR | 4 (13) | — | — | |||
| Resistant | 8 (26) | 3 (6) | — | |||
| CR at start | — | — | 5 (25) | |||
| Death | — | — | — | |||
| Courses to CR | ||||||
| 1 | 16 (84) | 33 (70) | 14 (93) | NS | ||
| 2 | 3 (16) | 6 (13) | 1 (7) | |||
| More than 2 | — | 8 (17) | — | |||
| Median days to response [range] | ||||||
| CR | 24 [19-42] | 20 [11-32] | 19 [15-22] | NS | ||
| ANC 109/L or higher | 22 [6-27] | 23 [15-30] | 20 [17-28] | |||
| PLT 100 × 109/L or higher | 24 [20-48] | 25 [18-107] | 21 [17-44] | |||
| Incidence during induction* | ||||||
| FUO | 10 (22) | 31 (41) | 4 (25) | NS | ||
| Infections | 8 (17) | 27 (36) | 5 (31) | |||
| Pneumonia | 4 | 9 | 3 | |||
| Sepsis/bacteremia | 2 | 12 | — | |||
| Fungemia | 1 | 1 | 1 | |||
| Other (minor) | 1 | 5 | 1 | |||
. | No. (%) . | . | . | . | ||
|---|---|---|---|---|---|---|
| Parameter . | VAD . | Hyper-CVAD . | Hyper-CVAD + imatinib mesylate . | P . | ||
| No. treated | ||||||
| Total | 31 | 50 | 20 | — | ||
| Status at study entry | ||||||
| Active disease | 31 | 50 | 15 (75) | — | ||
| In CR | — | — | 5 (25) | — | ||
| Age, y [median, range] | ||||||
| 60 or older | 8 (26) | 15 (30) | 4 (20) | NS | ||
| [47, 19-73] | [44, 16-79] | [42, 19-75] | ||||
| Performance, ECOG | ||||||
| 0 | 7 (23) | 3 (6) | 4 (20) | .07 | ||
| 1 to 2 | 23 (74) | 45 (90) | 16 (80) | |||
| Hemoglobin, g/L | ||||||
| Less than 100 | 14 (45) | 35 (70) | 13 (65) | .08 | ||
| WBC, × 109/L | ||||||
| 30 or higher | 15 (48) | 22 (44) | 4 (20) | NS | ||
| Platelet count, × 109/L | ||||||
| Lower than 50 | 15 (48) | 29 (58) | 7 (35) | NS | ||
| Albumin, g/L | ||||||
| Lower than 30 | 4 (13) | 7 (14) | 7 (35) | .08 | ||
| LDH, U/L | ||||||
| More than 620 | 30 (97) | 46 (92) | 12 (60) | < .01 | ||
| CNS disease | ||||||
| Yes | — | 1 (2) | 3 (15) | .02 | ||
| Karyotype | ||||||
| Ph or complex Ph | 12 (39) | 17 (34) | 8 (40) | NS | ||
| Ph, −5, −7 | 6 (19) | 4 (8) | 1 (5) | |||
| Ph and others (+8, +21) | 13 (42) | 28 (58) | 11 (55) | |||
| B2 microglobulin, mg % | 13/19 | 19/39 | 4/18 | .03 | ||
| More than 3 | (68) | (49) | (22) | |||
| Response | ||||||
| CR | 19 (61) | 47 (94) | 15/15 | < .01 | ||
| PR | 4 (13) | — | — | |||
| Resistant | 8 (26) | 3 (6) | — | |||
| CR at start | — | — | 5 (25) | |||
| Death | — | — | — | |||
| Courses to CR | ||||||
| 1 | 16 (84) | 33 (70) | 14 (93) | NS | ||
| 2 | 3 (16) | 6 (13) | 1 (7) | |||
| More than 2 | — | 8 (17) | — | |||
| Median days to response [range] | ||||||
| CR | 24 [19-42] | 20 [11-32] | 19 [15-22] | NS | ||
| ANC 109/L or higher | 22 [6-27] | 23 [15-30] | 20 [17-28] | |||
| PLT 100 × 109/L or higher | 24 [20-48] | 25 [18-107] | 21 [17-44] | |||
| Incidence during induction* | ||||||
| FUO | 10 (22) | 31 (41) | 4 (25) | NS | ||
| Infections | 8 (17) | 27 (36) | 5 (31) | |||
| Pneumonia | 4 | 9 | 3 | |||
| Sepsis/bacteremia | 2 | 12 | — | |||
| Fungemia | 1 | 1 | 1 | |||
| Other (minor) | 1 | 5 | 1 | |||
VAD indicates vincristine, doxorubicin, and dexamethasone; PLT, platelet count; —, not applicable; NS, not significant; and FUO, fever of unknown origin.
Only 15 of 20 patients evaluable for induction course toxicity for hyper-CVAD and imatinib mesylate (5 patients in CR at start).
Response
All 15 patients (100%) treated with active disease achieved CR. The CR rates after one cycle of induction therapy were 52% for VAD, 66% for hyper-CVAD with or without rituximab, and 93% for hyper-CVAD and imatinib mesylate (P < .01). Overall CR rates reflected the number of patients who required more than one course to achieve morphologic and hematologic CR (16%, 30%, and 7%, respectively). The median times to CR for the 3 regimens (24, 20, and 19 days); ANC recovery of 1 × 109/L or higher (22, 23, and 20 days); and platelet count of 100 × 109/L or higher (24, 25, and 21 days) were shorter overall for the hyper-CVAD and imatinib mesylate regimen, although this did not reach statistical significance (Table 1).
Bone marrow cytogenetics performed at CR (approximately day 21 of the induction course) showed normalization to diploid karyotype in 13 (87%) of the 15 patients. Levels of marrow quantitative RT-PCR positivity (ratio of bcr-abl/abl × 100) less than 0.05 occurred in 8 patients with the hyper-CVAD and imatinib mesylate regimen alone (Table 2). Complete molecular remission (confirmed by nested PCR) was achieved in 5 patients after hyper-CVAD and imatinib mesylate alone (patient nos. 2, 12, 13, 14, 17), and in 12 (60%) of 20 patients overall. For patients unwilling or unable to undergo allogeneic SCT, serial assessments of quantitative RT-PCR demonstrated stable to decreasing levels of Bcr-Abl transcripts over time, particularly with initiation of higher dose imatinib mesylate during maintenance therapy (Table 2).
Minimal residual disease marrow assessments with hyper-CVAD and imatinib mesylate
Patient no. . | Disease status at entry (breakpoint) . | Age, y/sex . | Response . | Month* . | Cytogenetics (% positivity) . | FISH (% positivity) . | QRT-PCR ratio† . | Status after allo-SCT [imatinib mesylate] . |
|---|---|---|---|---|---|---|---|---|
| 1 | Primary refractory (e1a2) | 28/F | CR | 0 | Ph+ (32%) | Pos (31%) | Pos | Rel ALL mo 12 |
| 1 | Dip | — | Pos | (Refused SCT) | ||||
| 4 | Dip | Neg (1.5%) | Insufficient | |||||
| 9 | Dip | Pos (3%) | 0.0061 | Dead | ||||
| 2 | Primary refractory (b3a2) | 66/M | CR | 0 | Dip | Neg (7%) | Pos | CCR mo 23+ |
| 1 | — | — | Neg | (Refused SCT) | ||||
| 4 | — | — | Neg | |||||
| 6 | Dip | — | 0.0019 | |||||
| 15 | Dip | Neg | Neg | |||||
| 20 | Dip | Neg | 0.330 | |||||
| 3 | De novo (b3a2) | 51/M | CR | 0 | Ph+ (100%) | — | Pos | CCR mo 16+ |
| 1 | Ph+ (5%) | — | — | (SCT mo 6) | ||||
| 2 | Dip | — | — | [Imatinib mesylate 300 mg] | ||||
| 4 | Dip | — | — | skin GVHD | ||||
| 6 | Dip | Neg | Neg | |||||
| 8 | Dip | Neg (donor) | Neg | |||||
| 16 | Dip | Neg (donor) | Neg | |||||
| 23 | Dip | Neg (donor) | Neg | |||||
| 4 | CR (b2a2) | 17/M | NA | 0 | Dip | Neg | — | CCR mo 18+ |
| 2 | Dip | — | Pos | (SCT mo 1) | ||||
| 8 | Dip | — | Neg | [Imatinib mesylate 200 mg] | ||||
| 18 | Dip | Neg | Neg | |||||
| 5 | CR (b3a2) | 53/F | NA | 0 | Ph+ (10%) | Pos (11%) | Pos | CCR mo 22+ |
| 1 | Ph+ (5%) | — | Pos | (No donor) | ||||
| 3 | Dip | — | 0.1000 | |||||
| 5 | — | Pos (5%) | 3.0303 | |||||
| 6 | — | — | 0.4814 | |||||
| 8 | Dip | Neg | 0.5580 | |||||
| 12 | Dip | Neg | 0.0179 | |||||
| 14 | Dip | Neg | 0.0002 | |||||
| 16 | Dip | Neg | 0.1000 | |||||
| 18 | Dip | Neg | 0.0038 | |||||
| 22 | Dip | Neg | 0.0177 | |||||
| 6 | CR (b3a2) | 46/F | NA | 0 | Ph+ (6%) | Pos (12%) | Pos | Rel ALL mo 10 |
| 2 | — | Pos (3%) | Neg | (Off study mo 5) | ||||
| 4 | Ph+ (11%) | — | 0.1168 | MUD SCT at Rel Dead | ||||
| 7 | De novo (e1a2) | 57/M | CR | 0 | Ph+ (100%) | — | Pos | Rel ALL mo 8 |
| 1 | Ph+ (30%) | — | 17.9533 | (SCT mo 3) | ||||
| 2 | Ph+ (30%) | — | — | [No imatinib mesylate] | ||||
| 6 | Dip | Neg | Neg | no GVHD | ||||
| 8 | Dip (donor) | Pos (11%) | — | Dead | ||||
| 8 | De novo (e1a2) | 40/M | CR | 0 | Ph+, −5 | — | — | CCR mo 18+ |
| (56%) | ||||||||
| 1 | Dip | Pos (4.5%) | — | (SCT mo 5) | ||||
| 2 | Dip | Neg | 0.1046 | [No imatinib mesylate] | ||||
| 5 | Dip | — | — | chronic GVHD | ||||
| 6 | Dip | — | — | |||||
| 8 | Dip | — | Neg | |||||
| 10 | — | Neg | Neg | |||||
| 11 | Dip | — | Neg | |||||
| 14 | Dip | — | Neg | |||||
| 18 | Dip (donor) | — | Neg | |||||
| 9 | De novo (b3a2) | 30/M | CR | 0 | Ph+, +8 | — | 31.8160 | CCR mo 19+ |
| (100%) | ||||||||
| 1 | IM | — | 17.6260 | (SCT mo 2) | ||||
| 2 | Ph+ (60%) | — | — | [No imatinib mesylate] | ||||
| 3 | Dip | — | — | no GVHD | ||||
| 4 | Dip (donor) | — | — | |||||
| 5 | Dip | — | — | |||||
| 6 | Dip | — | 0.0180 | |||||
| 8 | Dip (donor) | — | Neg | |||||
| 10 | Dip (donor) | — | Neg | |||||
| 13 | Dip (donor) | — | Neg | |||||
| 10 | De novo (e 1a2) | 37/F | CR | 0 | Ph+, oth (95%) | — | 15.8363 | CCR mo 20+ |
| 1 | Dip | — | 0.0646 | (SCT mo 4) | ||||
| 3 | Dip | — | 1.0318 | [No imatinib mesylate] | ||||
| 5 | IM | Neg | Neg | chronic GVHD | ||||
| 6 | Dip | Neg | — | |||||
| 10 | Dip | — | Neg | |||||
| 12 | Dip | — | Neg | |||||
| 16 | Dip | — | Neg | |||||
| 19 | Dip | — | Neg | |||||
| 11 | CR (e 1a2) | 19/F | NA | 0 | Ph+ (5%) | Pos (3%) | — | CCR mo 14+ |
| 1 | Dip | — | — | (SCT mo 2) | ||||
| 2 | Dip (donor) | — | — | [No imatinib mesylate] | ||||
| 3 | Dip | — | Neg | no GVHD | ||||
| 4 | Dip (donor) | — | Neg | |||||
| 5 | Dip | — | Neg | |||||
| 6 | Dip (donor) | — | Neg | |||||
| 12 | Dip (donor) | — | Neg | |||||
| 14 | Dip (donor) | — | Neg | |||||
| 12 | De novo (e 1a2) | 60/M | CR | 0 | Ph+, other (20%) | — | 1.3367 | CCR mo 20+ |
| 1 | Dip | — | 0.0062 | Refused SCT | ||||
| 3 | Dip | — | Neg | |||||
| 8 | Dip | Neg | Neg | |||||
| 10 | Dip | Neg | Neg | |||||
| 14 | Dip | — | Neg | |||||
| 18 | Dip | Neg | Neg | |||||
| 13 | Primary refractory (b3a2) | 75/M | CR | 0 | Ph+, oth (36%) | Pos (81%) | 4.1745 | CCR mo 15 |
| 1 | Dip | Neg | 0.0276 | Ineligible SCT | ||||
| 3 | Dip | Neg | — | Died in CR | ||||
| 9 | Dip | Neg | Neg | |||||
| 15 | — | — | Neg | |||||
| 14 | De novo (e 1a2) | 55/F | CR | 0 | Ph+ (70%) | — | 33.9009 | CCR mo 15+ |
| 1 | Dip | Neg | Neg | Ineligible SCT | ||||
| 2 | Dip | Neg | Neg | Died in CR | ||||
| 4 | Dip | Neg | Neg | |||||
| 6 | Dip | Neg | — | |||||
| 8 | Dip | Neg | Neg | |||||
| 10 | Dip | Neg | Neg | |||||
| 12 | Dip | Neg | — | |||||
| 14 | Dip | Neg | Neg | |||||
| 15 | Dip | — | Neg | |||||
| 15 | De novo (b3a2) | 33/M | CR | 0 | Ph+ (15%) | — | 28.2507 | CCR mo 12+ |
| 1 | Dip | Pos (3%) | 0.3190 | (SCT mo 8) | ||||
| 2 | Dip | Neg | 0.0234 | [Imatinib mesylate 400 mg] | ||||
| 4 | Dip | Neg | 0.3778 | no GVHD | ||||
| 6 | Dip | Neg | 1.0439 | |||||
| 8 | Dip | Neg | 0.0303 | |||||
| 10 | Dip (donor) | Neg | Neg | |||||
| 12 | Dip (donor) | Neg | — | |||||
| 16 | CR (b2a2) | 53/M | NA | 0 | Ph+ (20%) | Pos (13%) | 21.4808 | Rel CG (no ALL) |
| 2 | Dip | Neg | 0.6825 | Off-study mo 5 | ||||
| 4 | Ph+ (21%) | Pos (11%) | 5.2058 | |||||
| 5 | Ph+ (15%) | Pos (12%) | 8.5743 | |||||
| 17 | De novo (e 1a2) | 29/M | CR | 0 | Ph+, oth (93%) | — | 4.5913 | CCR mo 10+ |
| 1 | Dip | Neg | 0.0174 | (SCT mo 3) | ||||
| 3 | Dip | Neg | Neg | [No imatinib mesylate] | ||||
| 4 | Dip | Neg | Neg | |||||
| 5 | Dip (donor) | — | Neg | skin GVHD | ||||
| 6 | Dip | Neg | Neg | |||||
| 9 | Dip (donor) | — | — | |||||
| 18 | Primary refractory (ela2) | 38/M | CR | 0 | Ph+, oth (75%) | — | 7.3448 | CCR mo 6+ |
| 1 | Dip | Neg | 1.5977 | (SCT mo 5) | ||||
| 2 | Dip | Neg | 0.1743 | [No imatinib mesylate] | ||||
| 4 | Ph+ (5%) | Neg (1%) | 0.0830 | skin GVHD | ||||
| 5 | Dip | Neg | 0.5629 | |||||
| 6 | Dip (donor) | Neg | — | |||||
| 19 | De novo (ela2) | 43/M | CR | 0 | Ph+ (75%) | Pos (90%) | 71.6518 | CCR mo 5+ |
| 1 | Dip | Neg | 3.1966 | No donor | ||||
| 2 | Dip | Neg | 0.5743 | |||||
| 4 | Dip | Neg | 0.1990 | |||||
| 20 | De novo (b2a2) | 60/M | CR | 0 | Ph+ (100%) | Pos (95%) | — | CCR mo 3+ |
| 1 | Ph+ (35%) | Pos (40%) | 28.4709 | No donor | ||||
| 2 | IM | Pos (6%) | 5.8824 |
Patient no. . | Disease status at entry (breakpoint) . | Age, y/sex . | Response . | Month* . | Cytogenetics (% positivity) . | FISH (% positivity) . | QRT-PCR ratio† . | Status after allo-SCT [imatinib mesylate] . |
|---|---|---|---|---|---|---|---|---|
| 1 | Primary refractory (e1a2) | 28/F | CR | 0 | Ph+ (32%) | Pos (31%) | Pos | Rel ALL mo 12 |
| 1 | Dip | — | Pos | (Refused SCT) | ||||
| 4 | Dip | Neg (1.5%) | Insufficient | |||||
| 9 | Dip | Pos (3%) | 0.0061 | Dead | ||||
| 2 | Primary refractory (b3a2) | 66/M | CR | 0 | Dip | Neg (7%) | Pos | CCR mo 23+ |
| 1 | — | — | Neg | (Refused SCT) | ||||
| 4 | — | — | Neg | |||||
| 6 | Dip | — | 0.0019 | |||||
| 15 | Dip | Neg | Neg | |||||
| 20 | Dip | Neg | 0.330 | |||||
| 3 | De novo (b3a2) | 51/M | CR | 0 | Ph+ (100%) | — | Pos | CCR mo 16+ |
| 1 | Ph+ (5%) | — | — | (SCT mo 6) | ||||
| 2 | Dip | — | — | [Imatinib mesylate 300 mg] | ||||
| 4 | Dip | — | — | skin GVHD | ||||
| 6 | Dip | Neg | Neg | |||||
| 8 | Dip | Neg (donor) | Neg | |||||
| 16 | Dip | Neg (donor) | Neg | |||||
| 23 | Dip | Neg (donor) | Neg | |||||
| 4 | CR (b2a2) | 17/M | NA | 0 | Dip | Neg | — | CCR mo 18+ |
| 2 | Dip | — | Pos | (SCT mo 1) | ||||
| 8 | Dip | — | Neg | [Imatinib mesylate 200 mg] | ||||
| 18 | Dip | Neg | Neg | |||||
| 5 | CR (b3a2) | 53/F | NA | 0 | Ph+ (10%) | Pos (11%) | Pos | CCR mo 22+ |
| 1 | Ph+ (5%) | — | Pos | (No donor) | ||||
| 3 | Dip | — | 0.1000 | |||||
| 5 | — | Pos (5%) | 3.0303 | |||||
| 6 | — | — | 0.4814 | |||||
| 8 | Dip | Neg | 0.5580 | |||||
| 12 | Dip | Neg | 0.0179 | |||||
| 14 | Dip | Neg | 0.0002 | |||||
| 16 | Dip | Neg | 0.1000 | |||||
| 18 | Dip | Neg | 0.0038 | |||||
| 22 | Dip | Neg | 0.0177 | |||||
| 6 | CR (b3a2) | 46/F | NA | 0 | Ph+ (6%) | Pos (12%) | Pos | Rel ALL mo 10 |
| 2 | — | Pos (3%) | Neg | (Off study mo 5) | ||||
| 4 | Ph+ (11%) | — | 0.1168 | MUD SCT at Rel Dead | ||||
| 7 | De novo (e1a2) | 57/M | CR | 0 | Ph+ (100%) | — | Pos | Rel ALL mo 8 |
| 1 | Ph+ (30%) | — | 17.9533 | (SCT mo 3) | ||||
| 2 | Ph+ (30%) | — | — | [No imatinib mesylate] | ||||
| 6 | Dip | Neg | Neg | no GVHD | ||||
| 8 | Dip (donor) | Pos (11%) | — | Dead | ||||
| 8 | De novo (e1a2) | 40/M | CR | 0 | Ph+, −5 | — | — | CCR mo 18+ |
| (56%) | ||||||||
| 1 | Dip | Pos (4.5%) | — | (SCT mo 5) | ||||
| 2 | Dip | Neg | 0.1046 | [No imatinib mesylate] | ||||
| 5 | Dip | — | — | chronic GVHD | ||||
| 6 | Dip | — | — | |||||
| 8 | Dip | — | Neg | |||||
| 10 | — | Neg | Neg | |||||
| 11 | Dip | — | Neg | |||||
| 14 | Dip | — | Neg | |||||
| 18 | Dip (donor) | — | Neg | |||||
| 9 | De novo (b3a2) | 30/M | CR | 0 | Ph+, +8 | — | 31.8160 | CCR mo 19+ |
| (100%) | ||||||||
| 1 | IM | — | 17.6260 | (SCT mo 2) | ||||
| 2 | Ph+ (60%) | — | — | [No imatinib mesylate] | ||||
| 3 | Dip | — | — | no GVHD | ||||
| 4 | Dip (donor) | — | — | |||||
| 5 | Dip | — | — | |||||
| 6 | Dip | — | 0.0180 | |||||
| 8 | Dip (donor) | — | Neg | |||||
| 10 | Dip (donor) | — | Neg | |||||
| 13 | Dip (donor) | — | Neg | |||||
| 10 | De novo (e 1a2) | 37/F | CR | 0 | Ph+, oth (95%) | — | 15.8363 | CCR mo 20+ |
| 1 | Dip | — | 0.0646 | (SCT mo 4) | ||||
| 3 | Dip | — | 1.0318 | [No imatinib mesylate] | ||||
| 5 | IM | Neg | Neg | chronic GVHD | ||||
| 6 | Dip | Neg | — | |||||
| 10 | Dip | — | Neg | |||||
| 12 | Dip | — | Neg | |||||
| 16 | Dip | — | Neg | |||||
| 19 | Dip | — | Neg | |||||
| 11 | CR (e 1a2) | 19/F | NA | 0 | Ph+ (5%) | Pos (3%) | — | CCR mo 14+ |
| 1 | Dip | — | — | (SCT mo 2) | ||||
| 2 | Dip (donor) | — | — | [No imatinib mesylate] | ||||
| 3 | Dip | — | Neg | no GVHD | ||||
| 4 | Dip (donor) | — | Neg | |||||
| 5 | Dip | — | Neg | |||||
| 6 | Dip (donor) | — | Neg | |||||
| 12 | Dip (donor) | — | Neg | |||||
| 14 | Dip (donor) | — | Neg | |||||
| 12 | De novo (e 1a2) | 60/M | CR | 0 | Ph+, other (20%) | — | 1.3367 | CCR mo 20+ |
| 1 | Dip | — | 0.0062 | Refused SCT | ||||
| 3 | Dip | — | Neg | |||||
| 8 | Dip | Neg | Neg | |||||
| 10 | Dip | Neg | Neg | |||||
| 14 | Dip | — | Neg | |||||
| 18 | Dip | Neg | Neg | |||||
| 13 | Primary refractory (b3a2) | 75/M | CR | 0 | Ph+, oth (36%) | Pos (81%) | 4.1745 | CCR mo 15 |
| 1 | Dip | Neg | 0.0276 | Ineligible SCT | ||||
| 3 | Dip | Neg | — | Died in CR | ||||
| 9 | Dip | Neg | Neg | |||||
| 15 | — | — | Neg | |||||
| 14 | De novo (e 1a2) | 55/F | CR | 0 | Ph+ (70%) | — | 33.9009 | CCR mo 15+ |
| 1 | Dip | Neg | Neg | Ineligible SCT | ||||
| 2 | Dip | Neg | Neg | Died in CR | ||||
| 4 | Dip | Neg | Neg | |||||
| 6 | Dip | Neg | — | |||||
| 8 | Dip | Neg | Neg | |||||
| 10 | Dip | Neg | Neg | |||||
| 12 | Dip | Neg | — | |||||
| 14 | Dip | Neg | Neg | |||||
| 15 | Dip | — | Neg | |||||
| 15 | De novo (b3a2) | 33/M | CR | 0 | Ph+ (15%) | — | 28.2507 | CCR mo 12+ |
| 1 | Dip | Pos (3%) | 0.3190 | (SCT mo 8) | ||||
| 2 | Dip | Neg | 0.0234 | [Imatinib mesylate 400 mg] | ||||
| 4 | Dip | Neg | 0.3778 | no GVHD | ||||
| 6 | Dip | Neg | 1.0439 | |||||
| 8 | Dip | Neg | 0.0303 | |||||
| 10 | Dip (donor) | Neg | Neg | |||||
| 12 | Dip (donor) | Neg | — | |||||
| 16 | CR (b2a2) | 53/M | NA | 0 | Ph+ (20%) | Pos (13%) | 21.4808 | Rel CG (no ALL) |
| 2 | Dip | Neg | 0.6825 | Off-study mo 5 | ||||
| 4 | Ph+ (21%) | Pos (11%) | 5.2058 | |||||
| 5 | Ph+ (15%) | Pos (12%) | 8.5743 | |||||
| 17 | De novo (e 1a2) | 29/M | CR | 0 | Ph+, oth (93%) | — | 4.5913 | CCR mo 10+ |
| 1 | Dip | Neg | 0.0174 | (SCT mo 3) | ||||
| 3 | Dip | Neg | Neg | [No imatinib mesylate] | ||||
| 4 | Dip | Neg | Neg | |||||
| 5 | Dip (donor) | — | Neg | skin GVHD | ||||
| 6 | Dip | Neg | Neg | |||||
| 9 | Dip (donor) | — | — | |||||
| 18 | Primary refractory (ela2) | 38/M | CR | 0 | Ph+, oth (75%) | — | 7.3448 | CCR mo 6+ |
| 1 | Dip | Neg | 1.5977 | (SCT mo 5) | ||||
| 2 | Dip | Neg | 0.1743 | [No imatinib mesylate] | ||||
| 4 | Ph+ (5%) | Neg (1%) | 0.0830 | skin GVHD | ||||
| 5 | Dip | Neg | 0.5629 | |||||
| 6 | Dip (donor) | Neg | — | |||||
| 19 | De novo (ela2) | 43/M | CR | 0 | Ph+ (75%) | Pos (90%) | 71.6518 | CCR mo 5+ |
| 1 | Dip | Neg | 3.1966 | No donor | ||||
| 2 | Dip | Neg | 0.5743 | |||||
| 4 | Dip | Neg | 0.1990 | |||||
| 20 | De novo (b2a2) | 60/M | CR | 0 | Ph+ (100%) | Pos (95%) | — | CCR mo 3+ |
| 1 | Ph+ (35%) | Pos (40%) | 28.4709 | No donor | ||||
| 2 | IM | Pos (6%) | 5.8824 |
FISH, fluorescent in-situ hybridization; QRT-PCR, quantitative real time polymerase chain reaction; SCT, stem cell transplantation; F, female; CR, complete remission; Pos, positive; Rel, relapse; Dip, diploid; —, not done; Neg, negative; M, male; CCR, continuous CR; GVHD, graft versus host disease; NA, not applicable; CG, cytogenetic; IM, insufficient metaphases; and oth, other.
Month from start of study.
Ratio bcr-abl/abl ×100 by competitive real-time PCR; Negs confirmed by nested PCR.
Remission duration and survival
The median follow-up of the study group is 20 months (range, 4+ to 24+ months). All but 5 of the 20 patients remained alive in CR with hyper-CVAD and imatinib mesylate-based therapy (9/10 after hyper-CVAD and imatinib mesylate followed by allogeneic SCT, 6/10 after hyper-CVAD and imatinib mesylate alone) (Figure 1). Of the deaths with hyper-CVAD and imatinib mesylate with or without SCT, 2 were related to ALL relapse. The first death was a 28-year-old female (patient no. 1) with primary refractory disease (failure to induction chemotherapy with the “Linker regimen” and active CNS disease at presentation) who had simultaneous marrow and CNS relapse 12 months from study entry despite continuance on maintenance therapy (refused allogeneic SCT). The patient subsequently failed multiple salvage regimens and died of Aspergillus pneumonia prior to planned matched unrelated donor (MUD) SCT. The second death was a 57-year-old male (patient no. 7) with de novo disease who underwent matched related allogeneic SCT after 3 courses of hyper-CVAD and imatinib mesylate, and relapsed on day 160 of SCT (9 months from start of hyper-CVAD and imatinib mesylate) with marrow and CNS disease (had completed CNS prophylaxis for low-risk disease). The patient succumbed to complications of probable fungal pneumonia on day 26 of reinduction therapy with hyper-CVAD and imatinib mesylate.
Disease-free survival of patients treated with hyper-CVAD and imatinib mesylate compared with those treated with non-imatinib mesylate-containing hyper-CVAD and VAD regimens. Note: a long-term survivor at 15 years in the VAD group is not shown.
Disease-free survival of patients treated with hyper-CVAD and imatinib mesylate compared with those treated with non-imatinib mesylate-containing hyper-CVAD and VAD regimens. Note: a long-term survivor at 15 years in the VAD group is not shown.
Of the deaths, 2 occurred in patients who had maintained continuous CR. The first was a 75-year-old male (patient no. 13) who died in CR after 15 months related to sequelae of osteomyelitis from vertebroplasty for trauma-related vertebral fracture (with underlying osteoporosis). The second death was in a 55-year-old female (patient no. 14) with underlying diabetes mellitus who died in CR after 16 months related to sequelae of sinus infection with mucormycosis unresponsive to antifungal therapy.
There were 2 patients who discontinued protocol therapy for persistence or recurrence of Ph-positive disease by routine karyo-typing without evidence of overt ALL. One 46-year-old female (patient no. 6) subsequently relapsed 5 months later (11 months from diagnosis) despite treatment with higher dose imatinib mesylate concurrently with an asparaginase-containing regimen. Salvage therapy regimen was given without success, with demise resulting from complications of matched unrelated donor allogeneic SCT and recurrent disease (Figure 2). The other patient (no. 16) remained on single-agent high-dose imatinib mesylate for 5+ months (12 months from diagnosis) with persistence of Ph-positive metaphases in the marrow without recurrence of ALL at the time of this report.
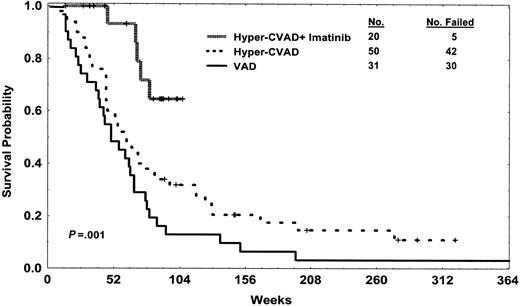
Overall survival of patients treated with hyper-CVAD and imatinib mesylate compared with those treated with non-imatinib mesylate-containing hyper-CVAD and VAD regimens. Curves represent intention to treat, without censoring for allogeneic SCT. Differences remain significant with exclusion of the 5 patients in CR at start of hyper-CVAD and imatinib mesylate (not shown). Note: a long-term survivor at 15 years in the VAD group is not shown.
Overall survival of patients treated with hyper-CVAD and imatinib mesylate compared with those treated with non-imatinib mesylate-containing hyper-CVAD and VAD regimens. Curves represent intention to treat, without censoring for allogeneic SCT. Differences remain significant with exclusion of the 5 patients in CR at start of hyper-CVAD and imatinib mesylate (not shown). Note: a long-term survivor at 15 years in the VAD group is not shown.
There were 2 patients who had completed all therapy per current protocol, including both intensifications during maintenance therapy, without proceeding to allogeneic SCT. Both remained in continuous CR but were placed on alternate therapy after study completion. The first was a 66-year-old male (patient no. 2) who achieved RT-PCR negativity and was initially observed, declining nonmyeloablative allogeneic SCT. Surveillance marrow assessment performed 3 months after discontinuing therapy showed continued CR with detectable RT-PCR transcripts. He resumed maintenance therapy with imatinib mesylate, vincristine, and prednisone. The second is a 53-year-old female (patient no. 5) without an identifiable stem cell transplant donor who remained RT-PCR positive and was continued on maintenance therapy with high-dose imatinib mesylate and prednisone (without vincristine because of peripheral neuropathy).
Figures 1 and 2 illustrate the respective DFS and survival of patients treated with hyper-CVAD and imatinib mesylate compared with those treated with non-imatinib mesylate-containing hyper-CVAD or VAD regimens. DFS and survival of the study group with current follow-up was not different whether patients were censored or uncensored at the time of allogeneic SCT (data not shown). Figures 3 and 4 show the DFS and survival by disease status prior to start of therapy (eg, de novo, CR at start, or primary refractory).
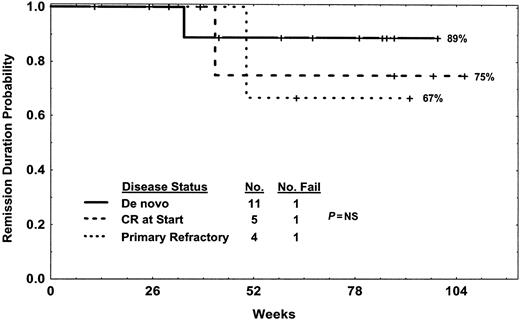
Disease-free survival of Ph-positive ALL by status of disease at study entry for those treated with hyper-CVAD and imatinib mesylate. One de novo relapsed after allogeneic SCT (patient no. 7), one CR at start relapsed after change in therapy (patient no. 6), and one primary refractory (patient no. 1) relapsed on therapy without SCT. Deaths in complete remission (one de novo, one primary refractory) are censored.
Disease-free survival of Ph-positive ALL by status of disease at study entry for those treated with hyper-CVAD and imatinib mesylate. One de novo relapsed after allogeneic SCT (patient no. 7), one CR at start relapsed after change in therapy (patient no. 6), and one primary refractory (patient no. 1) relapsed on therapy without SCT. Deaths in complete remission (one de novo, one primary refractory) are censored.
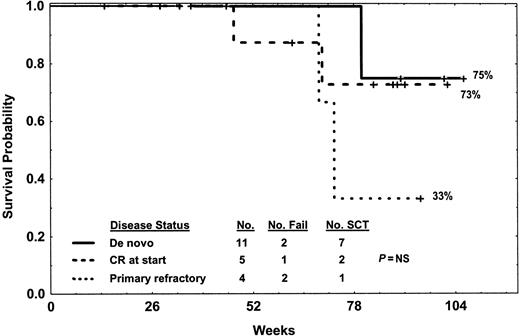
Survival by disease status at study entry. Deaths were related to relapse in patient nos. 7, 6, and 1 (one de novo after allogeneic SCT, one CR at start after change in therapy, one primary refractory while on therapy) or other causes while in CR for one de novo and one primary refractory (patient nos. 14, 13).
Survival by disease status at study entry. Deaths were related to relapse in patient nos. 7, 6, and 1 (one de novo after allogeneic SCT, one CR at start after change in therapy, one primary refractory while on therapy) or other causes while in CR for one de novo and one primary refractory (patient nos. 14, 13).
Allogeneic stem cell transplantation
There were 10 patients (50%) who underwent allogeneic SCT in first CR at a median time of 3.5 months (range, 1-8 months) from start of therapy. Of the 10 patients who did not undergo SCT, reasons were lack of donor or older age (n = 5), persistence/recurrence of Ph-chromosome without leukemia (n = 2), and/or refusal of procedure (n = 3). Allogeneic SCT in first CR was performed at M. D. Anderson Cancer Center in all cases. Conditioning regimens included CTX, rituximab, and total body irradiation (TBI) (n = 4); CTX and TBI (n = 3); CTX, alemtuzumab, and TBI (n = 2); or fludarabine and TBI (n = 1). Donor sources were matched related sibling (n = 8), matched unrelated donor (n = 1), and cord blood (n = 1). Of the 10 patients (90%) who underwent allogeneic SCT, 9 remained alive in CR with a median time from allogeneic SCT of 12 months (range, 1-17 months). Imatinib mesylate (daily doses ranging from 100 to 400 mg) was resumed after allogeneic SCT as maintenance therapy in 4 patients (usually commenced after full hematologic recovery from SCT).
Mild to moderate graft-versus-host disease (GVHD) was observed in 5 patients. One de novo patient (no. 7) undergoing matched related allogeneic SCT after 3 courses of chemotherapy with hyper-CVAD and imatinib mesylate relapsed on day 160 of SCT with bone marrow and CNS disease (received CNS prophylaxis for low-risk disease). Conversion to RT-PCR negativity (confirmed by nested PCR) was observed in 8 of 9 patients who were RT-PCR positive prior to allogeneic SCT (patient no. 18 after allogeneic SCT quantitative RT-PCR pending, patient no. 17 negative for Bcr-Abl by nested PCR prior to SCT).
Results of SCT in first CR with Ph-positive ALL patients treated prior to hyper-CVAD and imatinib mesylate included 5 (16%) of 31 patients previously treated with VAD chemotherapy from 1981 to 1992. Median time to SCT was 3 months (range, 2 to 7 months). Conditioning regimen for all 5 patients was etoposide, CTX, and TBI. Of the patients, 2 were consolidated with autologous SCT and 3, with matched related sibling SCT; 2 patients died from infectious complications of allogeneic SCT, and 2 patients died after relapse at 4 and 8 months after SCT. One patient remained alive without evidence of disease after the autologous intensification at 15 years (not shown in Figures 1, 2).
Of 50 patients treated with hyper-CVAD without imatinib mesylate, 12 (24%) underwent SCT in first CR, within a median time of 4 months (range, 1-7 months). No autologous SCTs were performed in this group. Conditioning regimens included CTX and TBI (n = 5); etoposide, CTX, and TBI (n = 3); thiotepa, CTX, and TBI (n = 3); or CTX, rituximab, and TBI (n = 1). Matched related sibling donors were used in 7, matched unrelated donors in 4, and 1-antigen mismatch related donor in 1 patient. Of the patients, 4 died in CR related to infectious complications or GVHD; 6 relapsed a median time of 6 months from SCT (range, 2 to 38 months); and 2 remained alive without evidence of disease at 45 and 60 months from SCT. Overall, 7 patients (58%) had mild acute or chronic GVHD.
Toxicity
The median time to hematologic recovery after each cycle of chemotherapy with the hyper-CVAD and imatinib mesylate regimen was not significantly different from the prior non-imatinib mesylate regimens. Median time to complete remission was 19 days (range, 15-22 days; Table 1). The median time to recovery varied by cycle with each of the subsequent courses of consolidation therapy. The median time to neutrophil recovery to ANC of 109/L or higher for the odd cycles (hyper-CVAD) ranged from 12 to 28 days, and 12 to 24 days for the even cycles (high-dose methotrexate and ara-C). The median time to recovery of the platelet count to 50 × 109/L or higher ranged from 14 to 21 days for the odd cycles, and 13 to 37 days for the even cycles. The incidence of febrile episodes and documented infections was similar among the 3 programs. No induction mortality was observed. Toxicities observed with the hyper-CVAD and imatinib mesylate combination were not significantly different from the prior regimens, with the majority of expected events related to each of the chemotherapy components of the regimen. Toxicities observed with the hyper-CVAD and imatinib mesylate regimen are summarized in Table 3.
Toxicities of hyper-CVAD and imatinib mesylate in 91 postinduction courses
. | No. (%)* . | . | |
|---|---|---|---|
| Parameter . | Grades 1-2 . | Grades 3-4 . | |
| Infections (overall)† | — | 23 (25) | |
| FUO | — | 7 (8) | |
| Pneumonia | — | 6 (7) | |
| Bacterial | — | 3 (3) | |
| Atypical | — | 2 (2) | |
| Fungal/presumed fungal | — | 1 (1) | |
| Sepsis | — | 9 (10) | |
| GNR bacteremia | — | 5 (5) | |
| Catheter-related bacteremia | — | 4 (4) | |
| Other | — | 8 (9) | |
| Sinusitis | — | 3 (3) | |
| Osteomyelitis | — | 2 (2) | |
| Herpes zoster | — | 2 (2) | |
| Upper respiratory infections (RSV) | — | 1 (1) | |
| Cardiovascular | |||
| Fluid retention | 5 (25) | 1 (5) | |
| Arrythmia (supraventricular) | 2 (10) | 2 (10) | |
| Deep venous thrombosis | — | 2 (10) | |
| Syncope | — | 2 (10) | |
| Reduction in ejection fraction | 2 (10) | — | |
| Hepatic | |||
| Increase in bilirubin | 5 (25) | — | |
| Increase in transaminases | 5 (25) | — | |
| Neuromuscular | |||
| Fatigue | 6 (30) | 2 (10) | |
| Peripheral neuropathy | 6 (30) | 1 (5) | |
| Headaches (postlumbar puncture) | 2 (10) | 2 (10) | |
| Bone pain | 3 (15) | — | |
| Myalgias | 2 (10) | — | |
| Fracture (femur/vertebral) | — | 2 (10) | |
| Gastrointestinal | |||
| Stomatitis | 5 (25) | — | |
| Constipation | 4 (20) | 1 (5) | |
| Diarrhea | 2 (10) | 2 (10) | |
| Nausea | 2 (10) | 1 (5) | |
| Reflux | 1 (5) | 2 (10) | |
| Ileus | — | 1 (5) | |
| Coagulopathy | |||
| Hypofibrinogenemia | 2 (10) | 1 (5) | |
| Hemorrhage | |||
| Gastrointestinal | — | 1 (5) | |
| Skin | |||
| Rash | 2 (10) | — | |
| Renal | |||
| Increase in creatinine | 5 (25) | — | |
| Hyponatremia | — | 1 (5) | |
. | No. (%)* . | . | |
|---|---|---|---|
| Parameter . | Grades 1-2 . | Grades 3-4 . | |
| Infections (overall)† | — | 23 (25) | |
| FUO | — | 7 (8) | |
| Pneumonia | — | 6 (7) | |
| Bacterial | — | 3 (3) | |
| Atypical | — | 2 (2) | |
| Fungal/presumed fungal | — | 1 (1) | |
| Sepsis | — | 9 (10) | |
| GNR bacteremia | — | 5 (5) | |
| Catheter-related bacteremia | — | 4 (4) | |
| Other | — | 8 (9) | |
| Sinusitis | — | 3 (3) | |
| Osteomyelitis | — | 2 (2) | |
| Herpes zoster | — | 2 (2) | |
| Upper respiratory infections (RSV) | — | 1 (1) | |
| Cardiovascular | |||
| Fluid retention | 5 (25) | 1 (5) | |
| Arrythmia (supraventricular) | 2 (10) | 2 (10) | |
| Deep venous thrombosis | — | 2 (10) | |
| Syncope | — | 2 (10) | |
| Reduction in ejection fraction | 2 (10) | — | |
| Hepatic | |||
| Increase in bilirubin | 5 (25) | — | |
| Increase in transaminases | 5 (25) | — | |
| Neuromuscular | |||
| Fatigue | 6 (30) | 2 (10) | |
| Peripheral neuropathy | 6 (30) | 1 (5) | |
| Headaches (postlumbar puncture) | 2 (10) | 2 (10) | |
| Bone pain | 3 (15) | — | |
| Myalgias | 2 (10) | — | |
| Fracture (femur/vertebral) | — | 2 (10) | |
| Gastrointestinal | |||
| Stomatitis | 5 (25) | — | |
| Constipation | 4 (20) | 1 (5) | |
| Diarrhea | 2 (10) | 2 (10) | |
| Nausea | 2 (10) | 1 (5) | |
| Reflux | 1 (5) | 2 (10) | |
| Ileus | — | 1 (5) | |
| Coagulopathy | |||
| Hypofibrinogenemia | 2 (10) | 1 (5) | |
| Hemorrhage | |||
| Gastrointestinal | — | 1 (5) | |
| Skin | |||
| Rash | 2 (10) | — | |
| Renal | |||
| Increase in creatinine | 5 (25) | — | |
| Hyponatremia | — | 1 (5) | |
GNR indicates gram-negative rod;—, not applicable.
Incidence of infections episodes/courses; all other toxicities according to occurrence by patient since usually intermittent in nature (no. at risk = 20).
Refer to Table 2 for infections specific for induction (course 1).
Discussion
Outcome with conventional chemotherapy programs for adults with Ph-positive ALL has been poor (Table 4).7,22,54-66 Remission rates have ranged from 46% to 90%, with median remission durations of 6 to 11 months. Overall 3-year survival rates without allogeneic SCT are less than 20%, and reach only 30% to 40% with SCT in selected patients. The hyper-CVAD regimen, although effective in producing high CR rates similar to the non-Ph-positive counterparts, has not improved long-term DFS or overall survival in Ph-positive ALL.
Outcome with conventional chemotherapy for adults with Ph-positive ALL
First author/reference no. . | Year . | No. . | % CR . | Median CRD, mo . | Median survival, mo . | % DFS (y) . | % OS (y) . |
|---|---|---|---|---|---|---|---|
| Bloomfield54 | 1989 | 29 | 46 | 7 | 11 | — | — |
| Secker-Walker23 | 1991 | 23 | 64 | — | 8 | — | — |
| Gotz55 | 1992 | 25 | 76 | 9 | 13 | — | 6 (3) |
| Westbrook56 | 1992 | 12 | 71 | 10 | 11 | — | — |
| Larson57 | 1995 | 25 | 70 | 7 | — | 11 (3) | 16 (3) |
| GFCH58 | 1996 | 127 | 59 | — | 5 | — | 5 (3) |
| Chim59 | 1997 | 6 | 83 | — | — | — | — |
| Secker-Walker60 | 1997 | 40 | 83 | 11 | — | 13 (3) | — |
| Thomas61 | 1998 | 43 | 64 | 6 | 9 | — | — |
| Wetzler62 | 1999 | 67 | 82 | 11 | 16 | 8 (5) | 11 (5) |
| Faderl7 | 2000 | 67 | 90 | 10 | 16 | — | — |
| Ko63 | 2001 | 26 | 64 | — | — | 0 (5) | 7 (5) |
| Thomas64 | 2001 | 51 | — | — | — | 13 (5) | 10 (5) |
| Annino65 | 2002 | 47 | 83 | 9 | — | — | — |
| Dombret66 | 2002 | 154 | 67 | — | — | — | 19 (3) |
| Thomas (current study) | 2003 | 20 | 100 | 15+ | 18+ | 84 (2) | 64 (2) |
First author/reference no. . | Year . | No. . | % CR . | Median CRD, mo . | Median survival, mo . | % DFS (y) . | % OS (y) . |
|---|---|---|---|---|---|---|---|
| Bloomfield54 | 1989 | 29 | 46 | 7 | 11 | — | — |
| Secker-Walker23 | 1991 | 23 | 64 | — | 8 | — | — |
| Gotz55 | 1992 | 25 | 76 | 9 | 13 | — | 6 (3) |
| Westbrook56 | 1992 | 12 | 71 | 10 | 11 | — | — |
| Larson57 | 1995 | 25 | 70 | 7 | — | 11 (3) | 16 (3) |
| GFCH58 | 1996 | 127 | 59 | — | 5 | — | 5 (3) |
| Chim59 | 1997 | 6 | 83 | — | — | — | — |
| Secker-Walker60 | 1997 | 40 | 83 | 11 | — | 13 (3) | — |
| Thomas61 | 1998 | 43 | 64 | 6 | 9 | — | — |
| Wetzler62 | 1999 | 67 | 82 | 11 | 16 | 8 (5) | 11 (5) |
| Faderl7 | 2000 | 67 | 90 | 10 | 16 | — | — |
| Ko63 | 2001 | 26 | 64 | — | — | 0 (5) | 7 (5) |
| Thomas64 | 2001 | 51 | — | — | — | 13 (5) | 10 (5) |
| Annino65 | 2002 | 47 | 83 | 9 | — | — | — |
| Dombret66 | 2002 | 154 | 67 | — | — | — | 19 (3) |
| Thomas (current study) | 2003 | 20 | 100 | 15+ | 18+ | 84 (2) | 64 (2) |
CR indicates complete remission; CRD, CR duration; DFS, disease-free survival; OS, overall survival; —, not reported; and GFCH, Group Francais de Cytogenetique Hematologique.
In our study, we treated 20 patients with de novo Ph-positive ALL with a combination of hyper-CVAD and imatinib mesylate. All 15 patients with active disease at the start of therapy achieved CR. With a median follow-up of 20 months, 15 patients (75%) were alive without evidence of ALL. Of the 20 patients, 10 (50%) were able to undergo allogeneic SCT. With a median follow-up of 12 months after allogeneic SCT, all but 1 of these 10 patients remained alive without disease. Outcome in the 10 patients who had not undergone allogeneic SCT (for reasons of lack of donor, older age, prohibitive comorbid conditions, or refusal) included 6 patients who remained alive without disease after a median follow-up of 20 months (1 primary refractory patient relapsed at 12 months, 1 patient relapsed after change from hyper-CVAD and imatinib mesylate to alternate therapy for persistent Ph-positive metaphases at 10 months, and 2 patients died in CR at 15 and 16 months related to comorbid conditions).
Outcome with the hyper-CVAD and imatinib mesylate regimen is significantly better than the historical control group treated with VAD50 (n = 31) or hyper-CVAD without imatinib mesylate46,51 (n = 50) with respect to CR rates (P < .01), DFS (P < .001), and overall survival rates (P = .001) (Table 1; Figures 1, 2). With current follow-up of the study group, neither median remission duration nor median survival has been reached. There was no significant difference in survival or DFS with censoring at the time of allogeneic SCT. The regimen was tolerable, with toxicity profile of the hyper-CVAD and imatinib mesylate combination similar to that of hyper-CVAD alone (Tables 1, 3). The historical rate of proceeding to allogeneic SCT in first CR for Ph-positive ALL at our institution was 20% with prior ALL programs, compared with 50% in this study. Several factors could account for the increased success of performing allogeneic SCT in first CR, including increased availability of unrelated donors, timeliness of referral for consideration of transplantation, and longer duration of remission allowing time for potential search for donors and eventual SCT.
Allogeneic SCT in Ph-positive ALL in first CR remains the standard intervention until optimal uses and long-term results of imatinib mesylate in combination with chemotherapy are known. Published series of allogeneic SCT in adults have shown DFS rates ranging from 31% to 65% and survival rates of 37% to 72% (Table 5).11,14-16,18,66-70 In a large prospective randomized trial of 167 patients with Ph-ALL treated between 1993 and 2000 on UKALL12/ECOG 2993 protocols, 88 patients (53%) received chemotherapy alone, 49 (29%) underwent MRS SCT in first CR, 23 (14%) had an MUD SCT, and 7 (4%) received autologous intensification.18 The actuarial 5-year relapse risk was lower with allogeneic SCT (related or unrelated donor) compared with chemotherapy or autologous SCT (32% versus 81%, P < .0001). Allogeneic SCT was also associated with a better 5-year DFS rate (36% versus 17%, P = .02) and survival rate (42% versus 19%, P = .02) compared with chemotherapy. Dombret et al66 reported similar results in a prospective study of LALA-94 chemotherapy with planned allogeneic SCT in 154 patients with Ph-positive ALL. All patients with a matched related or unrelated donor were assigned to allogeneic SCT; those without a donor underwent autologous SCT. The availability of a donor and absence of minimal residual disease (MRD) prior to SCT were both associated with longer DFS (P < .001 and P = .01, respectively) and survival (P = .02 and P = .01, respectively). The 3-year survival rates were 37% for the donor group versus 12% for the no-donor group (P = .02). The role of SCT after hyper-CVAD and imatinib mesylate remains to be determined, as durable remissions were observed with hyper-CVAD and imatinib mesylate alone.
Outcome with allogeneic stem cell transplantation in Philadelphia-positive acute lymphocytic leukemia
First author/reference no. . | No. . | Age, y (range) . | Status (no.) . | Donor (no.) . | % RR (status) . | % TRM (status or donor) . | % DFS (y) . | % OS (y) . |
|---|---|---|---|---|---|---|---|---|
| Barrett14 | 67 | 5-49 | CR1 (33) | MRS | 34 (CR1) | 42 (CR1) | 31 (2) | NR |
| 32 (> CR1) | 40 (> CR1) | |||||||
| Chao15 | 38 | NR | CR1 (17) | MRS | NR | NR | 46 (2) | NR |
| Stockschlader67 | 10 | NR | CR1 (10) | MRS (9) | 10 | 30 | NR | NR |
| MUD (1) | ||||||||
| Sierra68 | 18 | 25 (2-51) | CR1 (7) | MUD | 14 (CR1) | NR | 49 (2)* | NR |
| Snyder16 | 23 | 30 (6-44) | CR1 (23) | MRS | 9 | 30 | 65 (3) | NR |
| Arico11 | 38 | 8 (0-20) | CR1 (38) | MRS | 24 | 8 | 65 (5) | 72 (5) |
| Goldstone69 | 72 | NR | CR1 (72) | MRS (49) | 32 | 37 (MRD) | NR | 42 (5) |
| MUD (23) | 43 (MUD) | |||||||
| Cornelissen70 | 62 | 31 (16-54) | CR1 (48) | MUD | NR | NR | NR | 40 (2) |
| Dombret66 | 56 | NR | CR1 (51) | MRS (44) | 37 (CR1) | 25 (CR1) | NR | 37 (3) |
| MUD (12) | ||||||||
| Lee71 | 23 | 36 (15-44) | CR1 (14) | MRS | 35 (CR1) | NR | 43 (2)* | 28 (2)* |
First author/reference no. . | No. . | Age, y (range) . | Status (no.) . | Donor (no.) . | % RR (status) . | % TRM (status or donor) . | % DFS (y) . | % OS (y) . |
|---|---|---|---|---|---|---|---|---|
| Barrett14 | 67 | 5-49 | CR1 (33) | MRS | 34 (CR1) | 42 (CR1) | 31 (2) | NR |
| 32 (> CR1) | 40 (> CR1) | |||||||
| Chao15 | 38 | NR | CR1 (17) | MRS | NR | NR | 46 (2) | NR |
| Stockschlader67 | 10 | NR | CR1 (10) | MRS (9) | 10 | 30 | NR | NR |
| MUD (1) | ||||||||
| Sierra68 | 18 | 25 (2-51) | CR1 (7) | MUD | 14 (CR1) | NR | 49 (2)* | NR |
| Snyder16 | 23 | 30 (6-44) | CR1 (23) | MRS | 9 | 30 | 65 (3) | NR |
| Arico11 | 38 | 8 (0-20) | CR1 (38) | MRS | 24 | 8 | 65 (5) | 72 (5) |
| Goldstone69 | 72 | NR | CR1 (72) | MRS (49) | 32 | 37 (MRD) | NR | 42 (5) |
| MUD (23) | 43 (MUD) | |||||||
| Cornelissen70 | 62 | 31 (16-54) | CR1 (48) | MUD | NR | NR | NR | 40 (2) |
| Dombret66 | 56 | NR | CR1 (51) | MRS (44) | 37 (CR1) | 25 (CR1) | NR | 37 (3) |
| MUD (12) | ||||||||
| Lee71 | 23 | 36 (15-44) | CR1 (14) | MRS | 35 (CR1) | NR | 43 (2)* | 28 (2)* |
RR indicates relapse rate; TRM, treatment related mortality; DFS, disease-free survival; OS, overall survival; CR1, first complete remission; MRS, matched related donor; and NR, not reported or not available for the group; and MUD, matched unrelated donor.
Includes both CR1 and > CR1.
Sensitive quantitative RT-PCR techniques enable detection of a single leukemia cell in 104 to 106 normal cells. In Ph-positive ALL, the level of residual leukemia has predicted the probability of relapse after allogeneic SCT.10,23,71-74 Radich et al24 reported a significantly increased relapse risk in patients with a positive PCR for Bcr-Abl after transplantation (relapse rate 43%) compared with patients with negative PCR. Return to negative RT-PCR status has been reported after the development of chronic GVHD, suggesting a graft-versus-leukemia (GVL) effect, with decreased incidence of relapse compared with patients who did not develop chronic GVHD (15% versus 100%, P = .01).71 Serial measurements of quantitative PCR for Bcr-Abl in patients with relapsed or refractory Ph-positive ALL treated with single-agent imatinib mesylate showed median reductions in Bcr-Abl/glyceraldehydes-3-phosphate dehydrogenase (GAPDH) ratios of 2 to 3 logs, with lower Bcr-Abl levels (< 10-2) correlating with longer time to progression.75 In another study, a higher log-reduction of Bcr-Abl levels over time was noted in patients treated with single-agent imatinib mesylate compared with those treated with chemotherapy, attributed to the high specificity of this agent.76
In our study, serial monitoring of MRD by quantitative RT-PCR demonstrated detectable transcripts in some patients treated with hyper-CVAD and imatinib mesylate (Table 2). Overall, 12 (60%) of 20 patients remained RT-PCR negative and 10 (66%) of 15 patients remained negative for Bcr-Abl by FISH, without relapses observed to date after achieving RT-PCR-negative status except in one patient after SCT (who became FISH positive prior to relapse). The proportion of patients with the e1a2 (p190bcr-abl) or b3a2 (p210bcr-abl) was approximately equal, and no differences with respect to response or outcome were observed. Despite achievement of molecular remission by RT-PCR after hyper-CVAD and imatinib mesylate followed by unmaintained allogeneic SCT, relapse was observed. There were 3 other failures: 1 patient with p190bcr-abl ALL with overt relapse on maintenance therapy and 2 patients with p210bcr-abl ALL with persistence or recurrence of Ph-positive metaphases during intensive chemotherapy.
Potential reasons for the hyper-CVAD and imatinib mesylate failures in our study include development of resistance by mechanisms reported previously (eg, mutations in the adenosine triphosphate [ATP]-binding domain of Abl, amplification of the Bcr-Abl gene, and/or up-regulation of Bcr-Abl transcription).42,43,77 Alternatives could be pharmacologic interactions that decreased drug levels of imatinib mesylate (metabolized via cytochrome P450).44,45 The minimum level of imatinib mesylate required for 50% inhibition of cellular phosphorylation of Bcr-Abl is 0.25 μM. In vivo animal studies have shown that imatinib mesylate penetrated the blood-brain barrier poorly.78
Analysis of CSF levels of imatinib mesylate in patients treated with single-agent imatinib mesylate for Ph-positive ALL or CML in lymphoid blast crisis have shown average CSF imatinib mesylate levels in the subtherapeutic range (0.88 μM).79 Thus, concurrent intrathecal CNS prophylaxis should be administered to all patients with de novo Ph-positive ALL, as was done in our study. No isolated CNS relapse had been observed at the time of this report.
The success of our study, in part, has been to increase the proportion of patients able to undergo allogeneic SCT compared with historical rates. Although 10 patients were unable to undergo allogeneic SCT, it is possible that long-term remission status can be achieved in the absence of SCT (2 patients treated with hyper-CVAD and imatinib mesylate alone remain in complete cytogenetic remission for more than 2 years with continuation of imatinib mesylate-based maintenance therapy). Longer follow-up of these and other cohorts unable to undergo allogeneic SCT will be required to determine the optimal therapeutic strategy in Ph-positive ALL.
Future directions include consideration of pharmacokinetic assays during therapy to determine the effects of various components of the chemotherapy on imatinib mesylate levels to ensure optimal dose delivery. Given the tolerance of the regimen to date, and dose-response relationship observed in Ph-positive CML, increased duration of exposure and dosing of imatinib mesylate with chemotherapy is under investigation. The addition of rituximab to the hyper-CVAD and imatinib mesylate regimen in patients with CD20 expression will be studied, given the preliminary favorable results observed with the hyper-CVAD and rituximab regimens in de novo ALL and Burkitt leukemia/lymphoma.51 Use of imatinib mesylate with or without rituximab as maintenance therapy after allogeneic SCT should be studied prospectively. The significance of MRD in relation to relapse and prognosis will need to be determined to guide therapeutic modifications. Enrollment of patients into clinical trials will be crucial to furthering our knowledge regarding the role of imatinib mesylate in the treatment of de novo Ph-positive ALL.
Prepublished online as Blood First Edition Paper, October 9, 2003; DOI 10.1182/blood-2003-08-2958.
Supported in part by the National Institutes of Health (NIH) grant 5K12 CA88084-2.
Presented in part at the 43rd and 44th Annual Meeting of the American Society of Hematology, Orlando, FL and Philadelphia, PA, respectively, and the 39th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL.80-82
Two of the authors (L.L., A.S.) are employed by Novartis Pharmaceuticals Corporation, whose product was studied in the present work.
An Inside Blood analysis of this article appears in the front of this issue.
Imatinib mesylate was supplied by Novartis Pharmaceuticals Corporation to the participants of this study free of charge.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal