Abstract
The optimal dose of interferon-alfa (IFN) for chronic myeloid leukemia (CML) is unknown. Retrospective analyses suggest that low doses are as effective as high doses, with less toxicity and fewer patients abandoning the drug. The Dutch Hemato-Oncology Association (HOVON) and British Medical Research Council (MRC) cooperative groups jointly performed randomized trials in newly diagnosed CML patients, comparing high-dose IFN (5 MIU/m2 daily) with low-dose (3 MIU, 5 times a week). Both arms allowed additional hydroxyurea to keep the white blood cell count lower than 5 × 109/L. Quality of life data were collected in a subset of patients. Between 1993 and 2001, 407 patients were randomized. At a median follow-up of 53 months, there were no significant differences in overall survival (odds ratio = 1.09, 95% confidence interval, 0.81-1.46), progression-free survival, and complete hematologic or major cytogenetic responses. Fewer patients in the low-dose group abandoned IFN for reasons other than transplant or progressive disease (P = .002, 58% vs 72% at 5 years). Quality of life data showed comparable results in both arms for most factors. There is no evidence of benefit for high-dose IFN compared with low-dose for the treatment of CML. Therefore, when IFN is combined with other drugs, low-dose IFN is advised, to minimize toxicity and costs. (Blood. 2004;103:4408-4415)
Introduction
For 2 decades, patients with chronic myeloid leukemia (CML) have been treated by interferon-alfa (IFN) in combination with chemotherapy, usually hydroxyurea or low-dose cytarabine (Ara-C).1-12 IFN was the first drug to induce persistent decrease or even disappearance of the Philadelphia chromosome, resulting in an overall 5-year survival benefit of 15% (95% confidence interval [CI], 9%-21%) compared to chemotherapy.13 The minority of patients with a complete cytogenetic response (ie, complete disappearance of the t(9;22) abnormality) have a long-term survival of more than 70% at 10 years.14
It still is not known which dose of IFN is optimal when used either alone or in combination with other compounds. It is evident that dose is an important issue regarding the side effects and costs of IFN.15-17 Most studies have been performed with standard high doses of 5 MIU/m2 per day, either alone or in combination with Ara-C or hydroxyurea. These high doses caused, in varying percentages of patients, serious early and late toxicity such as flu-like symptoms, fatigue, anorexia, weight loss, hair thinning, stomatitis, cardiotoxicity, neurotoxicity, and depression. There is no doubt that therapy with IFN adversely influences the quality of life (QoL),17-19 which, however, also depends on the age of the patient and his/her prognosis, resulting in a better tolerance in those given it for a short period preceding high-dose chemotherapy.20
To establish whether there is a relationship between planned dose of IFN and overall survival in newly diagnosed patients with CML, the Dutch HOVON group and British MRC group jointly performed between 1993 and 2001 randomized trials comparing the standard high IFN dose with a much lower dose. In addition to clinical end points, QoL data were collected prospectively in a subgroup of patients.
With the development of imatinib and the recently reported results of the International Randomized Study of Interferon versus STI571 (IRIS) Trial comparing IFN and imatinib as first-line therapy for newly diagnosed CML,21 IFN is no longer considered standard first-line treatment for CML. However, the drug will remain part of treatment regimens, either alone or combined with other drugs such as imatinib. Therefore, the question of whether low doses of IFN are as effective as high doses remains of great importance for clinicians using this drug in CML.
Patients and methods
Inclusion criteria
Previously untreated patients with newly diagnosed Philadelphia chromosome-positive CML in chronic phase (age 18-60 years in MRC CML IV; age at least 18 years, with no upper age limit in the other trials; very old patients were also allowed) were included. Patients with cytogenetic abnormalities other than loss of chromosome Y or less than 10% of either +8, ider(22)t(9;22)(q34;q11) or a second der(22)t(9;22)(q34;q11) were excluded in the HOVON trial but not from the MRC trial. Patients who did not show the presence of the Philadelphia chromosome on standard cytogenetics could be included if the presence of a BCR/ABL fusion gene was demonstrated by fluorescence in situ hybridization (FISH) or reverse transcriptase-polymerase chain reaction (RT-PCR). A good performance status (World Health Organization [WHO] 0, 1, or 2), adequate hepatic and renal function as defined by bilirubin and creatinine levels below twice the upper limit of normal, and informed consent were required.
Study design
Trials HOVON 20, MRC CML V, and MRC CML IV (subgroup) entered patients. MRC CML IV was a randomized trial of autologous transplantation in which those allocated no transplant were randomized between high- and low-dose IFN. Patients were registered and randomized at central offices in the Netherlands (HOVON 20) and in the United Kingdom (MRC studies). Randomization for MRC CML IV and CML V was done by computer with balancing on age, sex, spleen size, percentage blasts in the blood, and platelet count by minimization; for HOVON, without any stratification factor. All trials had research ethics approval according to each country's regulations at the time. One treatment protocol and one set of case record forms (CRFs) were used. Data entry, data quality control, and generation of queries were performed at both sites. If eligible for the trial, patients received hydroxyurea to reduce the white blood cell (WBC) count to less than 5 × 109/L (HOVON) or to less than 10 × 109/L (MRC) and to obtain a stabilization phase of 3 weeks, during which the WBC count was to be kept below this level. Patients were randomized between high-dose IFN (first week, 3 MIU subcutaneously daily; second week, 5 MIU daily; third week and thereafter, 5 MIU/m2 daily to the maximum tolerated dose but not exceeding a total dose of 35 MIU/m2 per week) and low-dose IFN (a total dose of 3 MIU subcutaneously [not corrected for body surface] 5 days a week [total dose 15 MIU per week]). Both groups could use additional hydroxyurea to maintain the WBC count below 5 × 109/L, preferably between 2 and 4 × 109/L, for as long as possible. If leukopenia or thrombocytopenia occurred, hydroxyurea was initially stopped and, as a second step, IFN. The protocols gave the same dose-adaptation scheme. Two different types of IFN-alfa were used. The HOVON 20 patients used IFN-α2b (Intron A; Schering Plough, Kenilworth, NJ), whereas the MRC CML IV and MRC CML V patients initially used human lymphoblastoid IFN-αn1 (Wellferon; Wellcome Laboratories, Beckenham, United Kingdom). Toward the end of the trials, Wellferon became unavailable, and IFN-α2b was used instead (from March 2000). Patients went off protocol treatment if acceleration or blast crisis occurred,22 if there was failure to control the disease but without fitting the criteria for accelerated or blast phase, if IFN could not be tolerated, if imatinib was started, or if an autologous or allogeneic stem cell transplantation was performed. All patients remained under follow-up for events until death.
Patients were evaluated at least at 3 and 6 months and then at 6-month intervals until death or until a patient went off protocol. Blood counts and IFN/hydroxyurea drug doses were noted on the CRF at each attendance for HOVON 20 and MRC CML V but are unavailable for MRC CML IV. For all patients, dates of going off protocol, stem cell transplantation, acceleration, blast crisis and death, reason for going off protocol, and cause of death were recorded. At 3- to 6-month intervals hematologic responses were registered and verified. In the absence of data on spleen size and/or a leukocyte differential, no complete hematologic response could be scored. Bone marrow (BM) aspirate and cytogenetic analysis were performed at 6-month intervals. In the absence of any cytogenetic response at 24 months, the frequency of BM aspirates could be reduced to once a year.
Hematologic responses
Complete hematologic remission was defined as WBC count less than 10 × 109/L with a normal differential and less than 5% circulating immature cells (myelocytes or metamyelocytes), platelet count of less than 450 × 109/L, and the disappearance of all signs and symptoms related to CML activity, including palpable splenomegaly.23 Partial hematologic remission was defined as WBC count less than 20 × 109/L or normal WBC count but with more than 5% circulating immature cells (blasts, promyelocytes, myelocytes, or metamyelocytes), or platelet count of more than 450 × 109/L or palpable splenomegaly, or the presence of other signs of disease.23 No hematologic response (failure) was defined as WBC count higher than 20 × 109/L.
Prognostic factors
For all patients, prognostic scores were recorded according to Sokal24 (age, spleen size in centimeters below the left costal margin, platelet count, and percentage of circulating blasts) and to the European risk score25 (age, spleen size in centimeters below the left costal margin, platelet count, percentage of circulating blasts, eosinophils, and basophils). They were then ranked in 3 groups: Sokal low (score less than 0.8), intermediate (more than 0.8 but less than 1.2), and high (more than 1.2), and European risk score low (less than 780), intermediate (780-1480), and high (more than 1480).
Cytogenetic analysis
Cytogenetic analysis was centrally performed in the HOVON 20 study and in the respective regional cytogenetics laboratories in the MRC studies.11 At least 30 metaphases obtained from BM aspirates had to be analyzed. Analysis of peripheral blood was acceptable only for diagnostic purposes. If 10 or fewer metaphases were analyzed, the data were not used unless they fitted into a pattern obtained from the previous and subsequent tests. If 5 or fewer metaphases were analyzed, the data were excluded. If only one normal metaphase among the remaining Ph-positive metaphases was observed, this was not interpreted as a cytogenetic response. In Ph-negative/BCR-ABL-positive patients and in patients in whom cytogenetic analysis failed on BM obtained during follow-up, the cytogenetic response was assessed with help of FISH on metaphase or interphase cells. A complete cytogenetic response was defined as Ph chromosome present in none of the metaphases; a partial cytogenetic response as Ph chromosome present in 1% to 35% of the metaphases; minimal cytogenetic response as Ph present in 36% to 95% of the metaphases; and no cytogenetic response as Ph present in more than 95% of the metaphases or more than 90% if 10 or fewer metaphases were analyzed.
Quality of life assessment
QoL was prospectively assessed in MRC CML V trial patients. The EORTC QLQ-C30 questionnaire was used.26 In addition, an in-house questionnaire developed in the MRC CML III trial was used.18 Data were collected at randomization and subsequently every 6 months while the patients remained in chronic phase, whether or not the patient remained on treatment.
Statistics
End points for the study were overall survival time (any cause of death) and progression-free survival (= duration of chronic phase, censoring at death from causes other than CML in chronic phase) from randomization, hematologic response at 6 months, and best cytogenetic responses at any time after randomization. Patients who went off protocol for any reason were followed up and assessed according to the intention-to-treat principle. The survival analyses were done first including all events and second with patients who started imatinib or who underwent stem cell transplantation in chronic phase censored at the date of starting imatinib/transplantation. The sample size was based on the following assumptions: to detect an absolute difference of 15% in survival (from 50% at 5 years to 65%) between patients randomized to low- and high-dose IFN, with 80% power and using a significance level of 2P = .05 would require 360 randomized patients. Allowing some loss to follow-up, the trial's first target was 400. To detect a 10% difference in survival would require 760 patients. This second target was not fulfilled due to the fall in recruitment caused by the introduction of imatinib into clinical practice, which resulted in the trials closing to entry in May 2001. Kaplan-Meier curves and the log-rank test, stratified for study with a trend analysis if appropriate, were used to compare the survival and duration of chronic phase in different subgroups, with surviving MRC patients censored on October 31, 2002, when follow-up was complete for most patients. HOVON patients (and the small number of MRC patients lost to follow-up) were censored at the date at which they were last known to be alive. The observed minus expected (O-E) number of events in the high-dose arm and its variance (V) were calculated from the log-rank survival analysis and used to calculate the odds ratio = exp[(O-E)/V]. An odds ratio (OR) greater than unity indicates more events in the high-dose arm. Percentages were compared between groups using chi-square tests, stratified for study if applicable. QoL data were compared by treatment arm using the Mann-Whitney test at each time point. Only P values less than .01 are quoted, as the large number of significance tests performed increases the probability of obtaining a conventionally significant result (P < .05) by chance alone.
Results
Patient characteristics
Patients from the study came from 111 different hospitals from the United Kingdom, 42 from The Netherlands, 1 from Romania, and 1 from the Czech Republic (see “Appendix”). A total of 407 patients (247 MRC CML V, 130 HOVON 20, and 30 MRC CML IV) were registered and randomized between October 1993 and May 2001, when the studies closed because of falling recruitment rates. At that time, the sample size for the first target (detection of a 15% difference in survival) was fulfilled. Median duration of follow-up in survivors was 53 months (range, 0-101 months). The patients were well balanced between study arms, apart from a difference in sex (in the HOVON trial 59% of those allocated high-dose IFN but only 30% allocated low-dose IFN were female, P = .001). Both arms showed a high median age (60 years) and a high percentage of patients in the unfavorable subgroups according to the Sokal score (38% and 41%), which was less pronounced when the European risk score was applied (13% and 21%) (Table 1). Eight patients in the MRC trials had additional abnormalities recorded at registration (t(7;18); t(11;17); t(12;17); der(22); +18p; +8 (2 cases); +2q, and -7).
Entry characteristics of both groups of patients
. | High dose; n = 201 . | Low dose; n = 206 . |
|---|---|---|
| HOVON-20, n (%) | 63 (31) | 67 (33) |
| MRC CML IV, n (%) | 15 (7) | 15 (7) |
| MRC CML V, n (%) | 123 (61) | 124 (60) |
| Age, y (range) | 60.3 (20-78) | 60.5 (20-81) |
| Female, % | 52 | 42 |
| Spleen palpable, n (%) | 114 (57) | 130 (63) |
| If yes, median in cm below Icm (range) | 7 (1-30) | 7 (1-25) |
| Hemoglobin level, g/L (range) | 116 (63-179) | 115 (66-174) |
| WBC count, × 109/L (range) | 146 (12-530) | 150 (11-650) |
| Basophils, % (range) | 4 (0-18) | 3 (0-29) |
| Eosinophils, % (range) | 2 (0-20) | 2 (0-16) |
| Blasts, % (range) | 1.2 (0-12) | 1.4 (0-16) |
| Platelet count, × 109/L (range) | 436 (78-2225) | 399 (63-1752) |
| Additional cytogenetic abnormalities, n | 3 | 5 |
| Sokal score lower than 0.8, % | 19 | 22 |
| Sokal score 0.8-1.2, % | 43 | 36 |
| Sokal score higher than 1.2, % | 38 | 41 |
| European risk score lower than 780, %* | 23 | 21 |
| European risk score 780-1480, % | 53 | 50 |
| European risk score higher than 1480, % | 13 | 21 |
. | High dose; n = 201 . | Low dose; n = 206 . |
|---|---|---|
| HOVON-20, n (%) | 63 (31) | 67 (33) |
| MRC CML IV, n (%) | 15 (7) | 15 (7) |
| MRC CML V, n (%) | 123 (61) | 124 (60) |
| Age, y (range) | 60.3 (20-78) | 60.5 (20-81) |
| Female, % | 52 | 42 |
| Spleen palpable, n (%) | 114 (57) | 130 (63) |
| If yes, median in cm below Icm (range) | 7 (1-30) | 7 (1-25) |
| Hemoglobin level, g/L (range) | 116 (63-179) | 115 (66-174) |
| WBC count, × 109/L (range) | 146 (12-530) | 150 (11-650) |
| Basophils, % (range) | 4 (0-18) | 3 (0-29) |
| Eosinophils, % (range) | 2 (0-20) | 2 (0-16) |
| Blasts, % (range) | 1.2 (0-12) | 1.4 (0-16) |
| Platelet count, × 109/L (range) | 436 (78-2225) | 399 (63-1752) |
| Additional cytogenetic abnormalities, n | 3 | 5 |
| Sokal score lower than 0.8, % | 19 | 22 |
| Sokal score 0.8-1.2, % | 43 | 36 |
| Sokal score higher than 1.2, % | 38 | 41 |
| European risk score lower than 780, %* | 23 | 21 |
| European risk score 780-1480, % | 53 | 50 |
| European risk score higher than 1480, % | 13 | 21 |
Data presented are median values for each category. Icm indicates left costal margin.
Percentages for European risk score do not sum up to 100% due to missing data.
Overall survival and duration of chronic phase
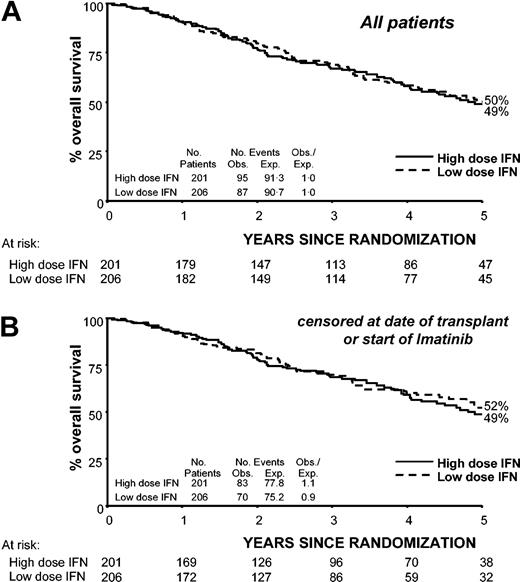
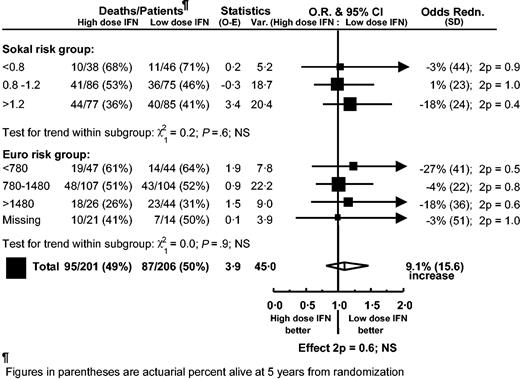
Overall survival from randomization for all patients, including those who went off protocol for reasons not related to disease progression, was not significantly different between the low- and high-dose arms (OR = 1.09, 95% CI, 0.81-1.46, P = .6). At 5 years from randomization, overall survival was 50% in the low-dose arm and 49% in the high-dose arm (Figure 1). Repeating the analysis, but censoring at the start of imatinib or stem cell transplantation in chronic phase, gave similar results (OR = 1.18, 95% CI, 0.86-1.63, P = .3) with 5-year survival from randomization being 52% in the low-dose group and 49% in the high-dose group (Figure 1). Similarly, no significant difference in duration of chronic phase was found between the low- and high-dose arms whether the results were censored on the date of starting imatinib or stem cell transplantation (OR = 1.14, 95% CI, 0.82-1.57, P = .4 and OR = 1.24, 95% CI, 0.89-1.74, P = .2, respectively), with 59% in the low-dose group versus 55% in the high-dose group (59% vs 52% censored at imatinib and stem cell transplantation) still in chronic phase at 5 years from randomization. Stratifying by sex did not materially alter these results. Analysis by risk group (European or Sokal) showed that no single subgroup benefited from IFN dose more than any other (Figure 2). The Sokal and the European risk scores discriminated between high- and low-risk patients in both treatment arms.
Overall survival by randomized dose calculated for all patients and censored at date of stem cell transplantation or start of imatinib in chronic phase. Solid line indicates high-dose IFN-alfa (5 MIU/m2 daily); dotted line, low-dose IFN-alfa (3 MIU 5 times a week). (A) All patients. (B) Patients censored at date of stem cell transplantation or the start of imatinib.
Overall survival by randomized dose calculated for all patients and censored at date of stem cell transplantation or start of imatinib in chronic phase. Solid line indicates high-dose IFN-alfa (5 MIU/m2 daily); dotted line, low-dose IFN-alfa (3 MIU 5 times a week). (A) All patients. (B) Patients censored at date of stem cell transplantation or the start of imatinib.
Overall survival by randomized dose subdivided by risk group (Sokal risk and European score risk). Each subgroup result is represented by a square, with a horizontal line indicating the 99% CI. Large squares indicate larger subgroups that provide more information. The overall result for each comparison is represented by a diamond, the width of which shows the 95% CI.
Overall survival by randomized dose subdivided by risk group (Sokal risk and European score risk). Each subgroup result is represented by a square, with a horizontal line indicating the 99% CI. Large squares indicate larger subgroups that provide more information. The overall result for each comparison is represented by a diamond, the width of which shows the 95% CI.
Responses
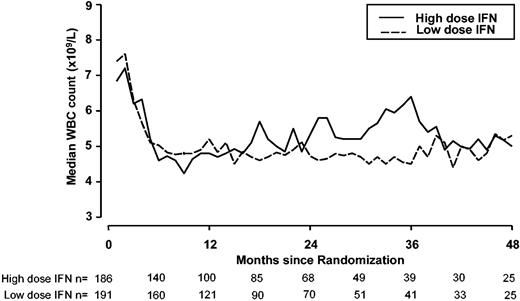
Table 2 gives the hematologic and cytogenetic responses by randomized allocation. Of the 340 patients either with hematologic response data at 6 months or dying from disease before 6 months, the complete hematologic response rate was similar in the 2 arms, at 71% and 75% for high and low dose, respectively (P = .4). Partial and complete cytogenetic response rates (including all patients who went off protocol within the first 6 months because of IFN-related side effects or progressive disease) were 13% and 7% for the high-dose arm compared with 15% and 9% for the low-dose arm (P = .4). The incidence of major cytogenetic response (partial or complete) in the European risk groups for each dose level was similar: 35% versus 45% for the low-risk, 18% versus 25% in the intermediate-risk, and 0% versus 3% in the high-risk group. The time to major cytogenetic response in those who achieved a major response was not significantly different for the 2 randomized dose levels (median, 12.6 months in both arms, Mann Whitney P = .9). WBC count control over time by randomized dose showed that from 6 months after randomization onwards, median WBC counts were around 5 × 109/L, without differences between both arms. The high-dose IFN arm did not perform better (Figure 3).
Responses
. | High-dose IFN; n = 201 . | Low-dose IFN; n = 206 . |
|---|---|---|
| Hematologic response at 6 mo, n (%) | ||
| Evaluable patients | 170 | 170 |
| Complete response | 121 (71) | 128 (75) |
| Partial response | 35 (21) | 28 (16) |
| Failure | 12 (7) | 12 (7) |
| Dead from disease at 6 mo | 2 (1) | 2 (1) |
| Nonevaluable patients | 31 | 36 |
| Dead from cause unrelated to disease at 6 mo | 3 | 3 |
| Missing data | 28 | 33 |
| Best cytogenetic response, n (%) | ||
| Evaluable patients | 173 | 181 |
| Complete response (0% Ph+) | 12 (7) | 17 (9) |
| Partial response (1%-35% Ph+) | 23 (13) | 27 (15) |
| Minimal response (36%-95% Ph+) | 49 (28) | 53 (29) |
| No response | 70 (40) | 73 (40) |
| Early progression/death | 13 (8) | 8 (4) |
| Early side effects | 6 (3) | 3 (2) |
| Nonevaluable patients | 28 | 25 |
| Not tested | 25* | 23 |
| Early transplantation | 1 | 2 |
| IFN abandoned early or never started | 2 | 0 |
. | High-dose IFN; n = 201 . | Low-dose IFN; n = 206 . |
|---|---|---|
| Hematologic response at 6 mo, n (%) | ||
| Evaluable patients | 170 | 170 |
| Complete response | 121 (71) | 128 (75) |
| Partial response | 35 (21) | 28 (16) |
| Failure | 12 (7) | 12 (7) |
| Dead from disease at 6 mo | 2 (1) | 2 (1) |
| Nonevaluable patients | 31 | 36 |
| Dead from cause unrelated to disease at 6 mo | 3 | 3 |
| Missing data | 28 | 33 |
| Best cytogenetic response, n (%) | ||
| Evaluable patients | 173 | 181 |
| Complete response (0% Ph+) | 12 (7) | 17 (9) |
| Partial response (1%-35% Ph+) | 23 (13) | 27 (15) |
| Minimal response (36%-95% Ph+) | 49 (28) | 53 (29) |
| No response | 70 (40) | 73 (40) |
| Early progression/death | 13 (8) | 8 (4) |
| Early side effects | 6 (3) | 3 (2) |
| Nonevaluable patients | 28 | 25 |
| Not tested | 25* | 23 |
| Early transplantation | 1 | 2 |
| IFN abandoned early or never started | 2 | 0 |
Includes 1 patient with a dry tap.
Control of WBC counts during follow-up by randomized dose (data unavailable for MRC CML IV patients). The aim was to keep the WBC count between 2 and 4 × 109/L, using additional hydroxyurea if necessary. Solid line indicates high-dose IFN-alfa (5 MIU/m2 daily); dotted line, low-dose IFN-alfa (3 MIU 5 times a week).
Control of WBC counts during follow-up by randomized dose (data unavailable for MRC CML IV patients). The aim was to keep the WBC count between 2 and 4 × 109/L, using additional hydroxyurea if necessary. Solid line indicates high-dose IFN-alfa (5 MIU/m2 daily); dotted line, low-dose IFN-alfa (3 MIU 5 times a week).
IFN dose adherence
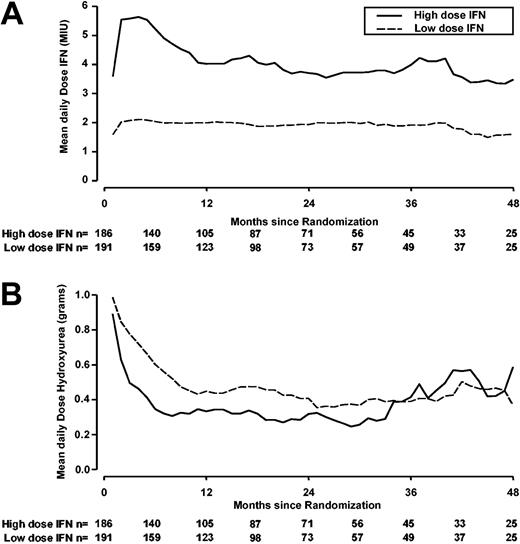
Figure 4 shows the actual doses of IFN and hydroxyurea used over time by randomized therapy. Dose reduction was seen predominantly in the high-dose arm compared with the low-dose arm. At 3, 6, 9, and 12 months from randomization, the percentages of patients who received at least 75% of the target dose were 35%, 28%, 22%, and 17% in the high-dose arm and 91%, 89%, 86%, and 85% in the low-dose arm. The difference in daily IFN dose used between the arms was still at least 2-fold in the first 4 years of follow-up.
Mean daily received doses of interferon-alfa and hydroxyurea by randomized dose (data unavailable for MRC CML IV patients). Solid line indicates high-dose IFN-alfa (5 MIU/m2 daily); dotted line, low-dose IFN-alfa (3 MIU 5 times a week). Note: some patients started IFN several weeks after randomization. (A) Doses of interferon-alfa. (B) Doses of hydroxyurea.
Mean daily received doses of interferon-alfa and hydroxyurea by randomized dose (data unavailable for MRC CML IV patients). Solid line indicates high-dose IFN-alfa (5 MIU/m2 daily); dotted line, low-dose IFN-alfa (3 MIU 5 times a week). Note: some patients started IFN several weeks after randomization. (A) Doses of interferon-alfa. (B) Doses of hydroxyurea.
Reasons for going off protocol
At a median interval of 17 months (range, 0.2-81 months) in the high-dose arm and 16 months (range, 0-84 months) in the low-dose arm, 325 patients (171 of 201 high dose and 154 of 206 low dose) discontinued the protocol treatment (Table 3) for reasons including progressive disease, adverse reactions, intercurrent disease, stem cell transplantation in chronic phase, indication for imatinib, and patient/doctor preference. The actuarial risk of abandoning IFN for any reason was not significantly different in the 2 arms (OR = 1.24 95% CI, 0.99-1.54, log-rank P = .06) and by 5 years from randomization was 78% in the low-dose group compared with 85% in the high-dose group. In 121 cases, 76 (38%) in the high-dose arm and 45 (22%) in the low-dose arm, the reason given for leaving the study was recorded as being due to IFN-related side effects. About half of this group had already left the study at 1 year. These numbers were 43 (21%) and 20 (10%), respectively. However, this is likely to be an underestimate, as those who refused treatment (patient and/or physician decision) and those who started imatinib may well have done so due to side effects of IFN. The actuarial risk of abandoning IFN for any reason other than transplantation and disease was significantly lower in the low-dose arm than in the high-dose arm (OR = 1.58 95% CI, 1.19-2.10, log-rank P = .002) and by 5 years from randomization was 58% in the low-dose group compared with 72% in the high-dose group. An analysis per year did not show any trend or heterogeneity in effect by time period between treatment arms.
Reasons for ending protocol treatment
. | High-dose IFN . | Low-dose IFN . |
|---|---|---|
| Overall, n (%) | 171 of 201 (85) | 154 of 206 (75) |
| IFN-related adverse reactions | 76 (44) | 45 (29) |
| CML-related (acceleration or blast crisis) | 40 (23) | 48 (31) |
| Stem cell transplantation | 12 (7) | 20 (13) |
| Indication for imatinib | 23 (13) | 16 (10) |
| Intercurrent other diseases | 7 (4) | 6 (4) |
| Refusal, violation, other | 6 (4) | 12 (8) |
| Unknown | 7 (4) | 7 (5) |
| Over the first year, n (%) | 73 | 59 |
| IFN-related adverse reactions | 43 (59) | 20 (34) |
| CML-related | 15 (21) | 21 (36) |
| Stem cell transplantation | 7 (10) | 8 (14) |
| Indication for imatinib | 2 (3) | 2 (3) |
| Intercurrent other diseases | 3 (4) | 3 (5) |
| Refusal, violation, other | 0 (0) | 3 (5) |
| Unknown | 3 (4) | 2 (3) |
. | High-dose IFN . | Low-dose IFN . |
|---|---|---|
| Overall, n (%) | 171 of 201 (85) | 154 of 206 (75) |
| IFN-related adverse reactions | 76 (44) | 45 (29) |
| CML-related (acceleration or blast crisis) | 40 (23) | 48 (31) |
| Stem cell transplantation | 12 (7) | 20 (13) |
| Indication for imatinib | 23 (13) | 16 (10) |
| Intercurrent other diseases | 7 (4) | 6 (4) |
| Refusal, violation, other | 6 (4) | 12 (8) |
| Unknown | 7 (4) | 7 (5) |
| Over the first year, n (%) | 73 | 59 |
| IFN-related adverse reactions | 43 (59) | 20 (34) |
| CML-related | 15 (21) | 21 (36) |
| Stem cell transplantation | 7 (10) | 8 (14) |
| Indication for imatinib | 2 (3) | 2 (3) |
| Intercurrent other diseases | 3 (4) | 3 (5) |
| Refusal, violation, other | 0 (0) | 3 (5) |
| Unknown | 3 (4) | 2 (3) |
Causes of death
Causes of death did not differ between arms, most having died because of disease progression or disease-related complications (Table 4), although in the elderly CML group, a considerable number of patients died due to non-CML-related diseases. Four patients died because of IFN-related adverse events, one from depression-related suicide (low dose), one from sepsis and leukocytopenia (low dose), one from renal insufficiency caused by hemolytic uremic syndrome (high dose), and one from a central nervous system (CNS) bleed when the platelet count was 55 on IFN (high dose). In addition, one patient died after going off protocol therapy due to splenic rupture on imatinib in accelerated phase (high dose).
Causes of death
. | High-dose IFN; n = 95 . | Low-dose IFN; n = 87 . |
|---|---|---|
| Therapy related | 2 | 2 |
| CML related | 58 | 51 |
| Stem cell transplantation | 14 | 9 |
| Intercurrent other diseases | 20 | 22 |
| Not known | 1 | 3 |
. | High-dose IFN; n = 95 . | Low-dose IFN; n = 87 . |
|---|---|---|
| Therapy related | 2 | 2 |
| CML related | 58 | 51 |
| Stem cell transplantation | 14 | 9 |
| Intercurrent other diseases | 20 | 22 |
| Not known | 1 | 3 |
QoL study
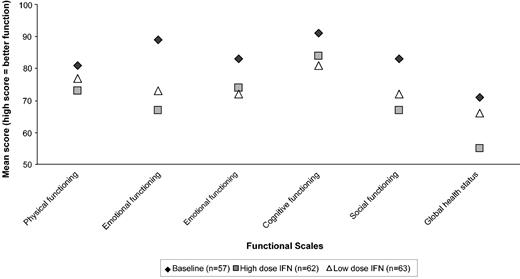
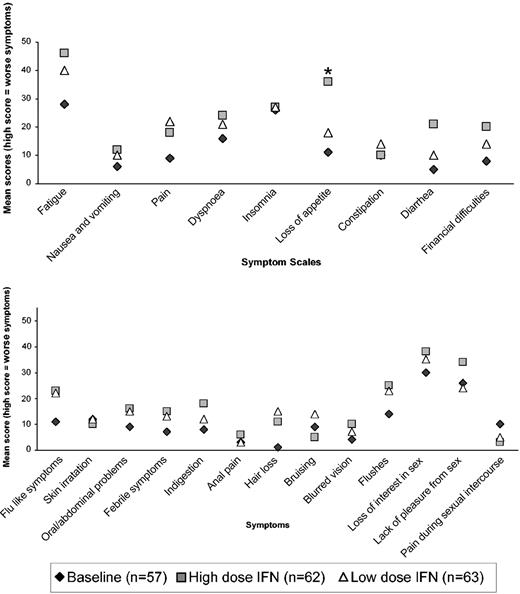
In total, 974 eligible forms were received from 223 patients. Unfortunately, a baseline QoL form was received only in 57 of 247 randomized MRC CML V patients. The reason was that IFN therapy had frequently been started by the time the patient completed the first QoL questionnaire. Figure 5 shows the results of the EORTC functional scales questionnaire within 6 months of IFN therapy. Both low-dose and high-dose IFN showed reduction in the various functional levels and in global health status compared to baseline, but there were no significant differences between the 2 arms (at P < .01). No significant differences by allocated dose were noted at other time periods up to 24 months, but all the functional scales remained lower than baseline over the full 24 months of therapy analyzed. Figure 6 shows the EORTC and in-house symptom scales over the first 6 months of therapy. Again, both high- and low-dose IFN scored higher for symptoms compared with baseline, but there was little difference between the 2 arms except for significant lack of appetite (P = .0007) in the high-dose arm. Sexual activity and pleasure were significantly reduced in the high-dose arm compared with the low-dose arm but only when assessed within 6 to 12 months of therapy (P ≤ .01). There were few significant differences between male and female patients except for more females reporting problems with hair loss (P < .0001), flushing (P = .003), diarrhea (P = .01), and pain during sexual intercourse (P = .008) at 6 to 12 months. Younger patients (age ≤ 65 years) had significantly more financial concerns over the 24 months than older patients (P < .01 in each time period). Older patients reported significantly more problems with physical functioning and global health status over the first 6 months of therapy (P = .01) and constipation over the second 6 months (P = .002). Modeling showed that the effect of randomized dose on QoL was similar in male compared to female patients and in younger compared to older patients for most areas of quality of life.
QoL as assessed by EORTC questionnaire at 6 months for different functional scales and global QoL for patients on low- or high-dose IFN-alfa. The mean result at baseline prior to starting IFN-alfa is also given for comparison.
QoL as assessed by EORTC questionnaire at 6 months for different functional scales and global QoL for patients on low- or high-dose IFN-alfa. The mean result at baseline prior to starting IFN-alfa is also given for comparison.
QoL assessed by EORTC and in-house symptom scales at 6 months for patients on low- or high-dose IFN-alfa. The mean result at baseline prior to starting IFN-alfa is also given for comparison. The asterisk reflects a significant difference for the loss-of-appetite item (P < .01). (A) EORTC symptom scales. (B) In-house module.
QoL assessed by EORTC and in-house symptom scales at 6 months for patients on low- or high-dose IFN-alfa. The mean result at baseline prior to starting IFN-alfa is also given for comparison. The asterisk reflects a significant difference for the loss-of-appetite item (P < .01). (A) EORTC symptom scales. (B) In-house module.
Discussion
We have shown in 3 joint multicenter randomized studies performed mainly in 2 different countries that low doses of IFN may be as effective as high doses for newly diagnosed patients with CML. No significant differences in overall survival and duration of chronic phase were seen. The target recruitment of 400 (or 800) patients, giving 80% power to detect a 15% (or 10%) difference in survival, was not reached for any individual trial. However, with 407 patients in total and very similar survival in the 2 treatment arms, the 95% CI for the difference at 5 years was from 10% worse to 12% better with high-dose IFN. Unfortunately, with this number of patients, it is not possible to rule out smaller differences. In addition, intermediate end points such as hematologic and cytogenetic responses were similar. The number of dropouts due to adverse effects of IFN was significantly higher in the high-dose arm compared with the low-dose arm. This means that a considerable number of patients may thereby have lost the chance of obtaining a cytogenetic response.
In both arms, more prominently in the high-dose than in the low-dose arm, the dose of IFN was reduced over time, but at least a 2-fold difference in dose was maintained for as long as 4 years. All previous studies incorporating IFN showed marked dose reductions, often more than 50% after 5 years with also a large percentage of discontinuations.3,4,6,27 Although this may weaken the difference seen, it demonstrates the difference between the policies of giving 2 different doses of IFN rather than between the actual doses used.
One of the reasons for performing these randomized trials was to try to clarify the role of IFN dose in relation to cytogenetic response and outcome. Although case reports suggest a dose response effect between the percentage of cytogenetic responses and daily IFN dose,28 other studies have shown equally good responses at much lower IFN doses, down to 15 MIU per week.6,11,15,29 Moreover, in a large data set of patients who obtained a complete cytogenetic response, no relationship could be found between IFN-alfa type, IFN-alfa dose (which ranged from 9 to 88 MIU actually administered per week preceding the response), and time to first complete cytogenetic response, duration of response, or survival.14
In reviewing the evidence from multicenter randomized trials of IFN, those randomized trials that used high doses (5 MIU/m2) of IFN versus chemotherapy alone3-5,30 had cytogenetic responses (8%-19%) and overall survival at 5 years (54%-59%) similar to those that used lower doses (11%-16%; 5-year overall survival, 54%-55%).6 It is important to realize that those studies reporting much higher percentages of major cytogenetic responses (38%-43%) and overall survival (63%-68%)31,32 all included patients with a much more favorable (40% to even 50% in lowest risk group) Sokal risk profile, whereas this percentage in our study was 21%. Moreover, these (nonrandomized) trials were single institute-based, thereby likely reflecting a different referral pattern compared with patients entered in multicenter trials. Of note, the French9 and Italian12 multicenter randomized trials, which also included high percentages of low-risk patients and compared high doses of IFN to IFN plus Ara-C, showed lower major cytogenetic response rates (24% and 18%, respectively) than the single-center studies. The results of our trial need to be set in the context of an older patient population with a high proportion of unfavorable patients and a substantial proportion dying of unrelated causes probably due to the significantly higher median age in the study.
Applying the rule of thumb that the major cytogenetic response rate in a study is approximately equal to half the proportion of patients with low Sokal risk score, the rate in our study should have reached about 10% responses. However, we observed more than twice this amount. Possibly the required strict control of the WBC count with additional hydroxyurea might have contributed to the better outcome.4,11 The median WBC counts were indeed lower in our study than in most other studies.
The QoL analysis showed that both low- and high-dose IFN were associated with lower functional scores than baseline, but little measured difference was seen when comparing the randomized doses. Thus, both low- and high-dose IFN are associated with reduced QoL. Interpretation of quality of life scores may be influenced by the relatively high number of patients abandoning IFN due to adverse side effects, especially in the first 12 months of therapy (21% and 10%, respectively). These rates are not significantly different from previously reported studies, particularly given the older age of patients in this study,19 and reflect the difficulty in maintaining therapy, particularly with higher doses of IFN. Those patients who continue on IFN are likely to be those who attain hematologic and cytogenetic responses and have a motivation to continue the drug. In addition, they—over a period of time—have not had severe intolerance to the drug.
With the advent of imatinib, IFN is no longer considered the preferred drug for newly diagnosed patients with CML. Given the fact that imatinib on its own probably will not cure patients and that resistance may develop in the future, combinations with other drugs are foreseen. IFN will be especially important in this regard, given the differences in the character of remissions achieved with either drug.33 Therefore, the issue of dose remains important, and our findings can be applied to schemes where IFN is combined with other drugs. We would advise the use of low doses of IFN, thus sparing costs and enabling more patients to continue to receive this important drug.
Appendix
The CML Working Group is part of the National Cancer Research Institute Adult Leukemia Working Party. The following doctors contributed to the studies. Doctors are grouped by number of patients randomized by each doctor.
MRC CML IV and V
Twelve patients: Dr T. J. Littlewood, Oxford Radcliffe Hospitals, Oxford. Seven patients: Dr D. Bareford, City Hospital NHS Trust, Birmingham. Five patients: Dr C. S. Chapman, Leicester Royal Infirmary, Leicester; Prof A. R. Green, Addenbrooke's NHS Trust, Cambridge; and Dr A. E. Milne, The North Hampshire Hospital, Hants. Four patients: Dr P. Ganly, Western General Hospital, Edinburgh; Dr M. S. Hamilton, Good Hope Hospital NHS Trust, W. Midlands; Dr M. Sivakumuran, Peterborough District Hospital, Peterborough; Dr J. Tucker, Good Hope Hospital NHS Trust, W. Midlands; and Dr D. Wright, Pontefract General Infirmary, West Yorkshire. Three patients: Dr V. E. Andrews, Dartford and Gravesham NHS Trust, Dartford, Kent; Dr R. E. Clark, Royal Liverpool University Hospital, Liverpool; Dr D. J. Culligan, Aberdeen Royal Infirmary, Aberdeen; Dr A. M. Deane, Norfolk and Norwich University Hospital N, Norfolk; Dr A. E. Hunter, Leicester Royal Infirmary, Leicester; Dr P. D. Micallef Eynaud, Crosshouse Hospital, Ayrshire; Dr D. C. Mitchell, Derbyshire Royal Infirmary, Derby; Dr J. Ropner, Gloucestershire Royal Hospital, Gloucester; Dr R. R. Slade, Southmead Hospital, Bristol; Dr J. Van DePette, Frimley Park Hospital, Surrey; and Dr M. Vasilica, Fundeni Hospital, Romania. Two patients: Dr Z. Abboudi, Central Middlesex Hospital, London; Dr H. F. Barker, Rotherham District General, S Yorks; Dr S. Basu, Warwick Hospital, Warwick; Dr A. D. J. Birch, Falkirk District Royal Infirmary, Falkirk; Dr A. Brownell, Oldchurch Hospital, Essex; Dr P. Cachia, Dundee Teaching Hospitals NHS Trust, Dundee; Prof J. C. Cawley, Royal Liverpool University Hospital, Liverpool; Dr P. Cervi, Basildon Hospital, Essex; Dr D. Chan-Lam, Barnsley District General Hospital NHS Trust, S Yorks; Dr R. C. Chasty, North Staffs Hospital Centre, Stoke-On-Trent; Dr P. Chu, Royal Liverpool University Hospital, Liverpool; Dr R. Collin, Royal Chesterfield Hospital, Chesterfield; Dr J. A. Copplestone, Derriford Hospital, Devon; Dr H. Davis, Queen Elizabeth II Hospital, Herts; Dr C. De Alwis, Royal Glamorgan Hospital, M Glamorgan; Dr C. DeSilva, Whipps Cross Hospital, London; Dr Z. R. Desai, Belfast City Hospital, Belfast; Dr N. J. Dodd, Ipswich Hospital, Suffolk; Dr A. Duncombe, Southampton University Hospital Trust, Southampton; Dr C. Fegan, Birmingham Heartlands Hospital, Birmingham; Prof J. M. Goldman, Hammersmith Hospital, London; Dr H. W. Habboush, Nevill Hall Hospital, Gwent; Dr J. Hanley, Ninewells Hospital and Medical School, Dundee; Dr P. Harrison, Russells Hall Hospital, W Midlands; Dr P. Hillmen, Pinderfields General Hospital, W Yorks; Dr J. B. Houghton, Salford Royal Hospitals NHS Trust, Salford; Dr M. T. Jeha, James Paget Hospital, Norfolk; Dr S. Kelly, Wycombe General Hospital, Bucks; Dr M. Krahulova, Masaryk University Hospital, Czech Republic; Prof S. R. McCann, St James Hospital, Eire; Dr D. W. Milligan, Birmingham Heartlands Hospital, Birmingham; Dr J. P. Ng, Barnsley District General Hospital NHS Trust, S Yorks; Dr D. V. O'Brien, Ormskirk District General, Lancs; Dr L. A. Parapia, Bradford Royal Infirmary, W Yorks; Dr A. C. Parker, Western General Hospital, Edinburgh; Dr A. Prentice, Derriford Hospital, Devon; Dr C. D. L. Reid, Northwick Park Hospital, Middlesex; Dr E. Rhodes, Countess of Chester Hospital, Chester; Dr S. G. N. Richardson, Russells Hall Hospital, W Midlands; Dr S. Sadullah, Borders General Hospital, Roxburghshire; Dr P. Shepherd, Western General Hospital, Edinburgh; Dr G. P. Summerfield, James Cook University Hospital, Middlesborogh; Dr P. C. Taylor, Rotherham District General, S Yorks; Dr G. E. Turner, Norfolk and Norwich University Hospital NHS Trust, Norfolk; and Dr J. M. Voke, Queen Elizabeth II Hospital, Herts. One patient: Dr A. H. M. Abdul-Cader, Hospital of St Cross, Warks; Dr M. A. Adelman, Lincoln County Hospital, Lincoln; Dr R. Aitchison, Wycombe General Hospital, Bucks; Dr N. Akhtar, King George Hospital, Essex; Dr S. Allard, Northwick Park Hospital, Middlesex; Dr J. Apperley, Hammersmith Hospital, London; Dr B. J. Bain, St Mary's Hospital, London; Dr D. L. Barnard, St James's University Hospital, Leeds; Dr J. Behrens, St Helier Hospital, Surrey; Dr D. P. Bentley, Llandough Hospital NHS Trust, S Glamorgan; Dr D. H. Bevan, St George's Hospital, London; Dr N. Bienz, Wexham Park Hospital, Berks; Dr N. E. Blesing, The Great Western Hospital, Wiltshire; Dr L. R. Bond, York District Hospital, York; Dr F. Booth, Torbay Hospital, Devon; Dr P. Burnside, Causeway Hospital, Coleraine; Prof J. A. Child, Leeds General Infirmary, Leeds; Dr R. Chopra, Glasgow Royal Infirmary, Glasgow; Dr P. Clarke, Vale of Leven District General Hospital, Dunbartonshire; Dr J. V. Clough, Countess of Chester Hospital, Chester; Dr G. Cook, Monklands District General, Lanarkshire; Dr M. K. Cook, St Johns Hospital at Howden, West Lothian; Dr T. Cranfield, Queen Alexandra Hospital, Hants; Dr R. G. Dalton, Cheltenham General Hospital, Gloucestershire; Dr M. El Agnaf, Ards Hospital, N Ireland; Dr R. Evely, United Bristol Healthcare Trust, Bristol; Dr R. S. Evely, Southmead Hospital, Bristol; Dr M. J. Galloway, Bishop Auckland General Hospital, County Durham; Dr G. P. Galvin, Manor Hospital, W Midlands; Dr M. Ganczakowski, Queen Alexandra Hospital, Hants; Dr D. S. Gillett, Pembury Hospital, Kent; Dr R. J. Grace, Eastbourne District General, East Sussex; Dr A. G. Gray, The Great Western Hospital, Wiltshire; Dr H. Grech, Royal Berkshire Hospital, Berks; Dr M. D. Hamon, Derriford Hospital, Devon; Dr D. Harvey, Barnet General Hospital, Herts; Dr A. P. Haynes, Nottingham City Hospital, Nottingham; Dr A. M. Hendrick, South Tyneside Hospital, Tyne and Wear; Dr R. G. Hughes, West Middlesex Hospital, Middlesex; Dr R. M. Hutchinson, Leicester Royal Infirmary, Leicester; Dr A. Iofciulescu, Fundeni Hospital, Romania; Dr S. Jalihal, Scunthorpe General Hospital, Scunthorpe; Dr P. R. E. Johnson, Western General Hospital, Edinburgh; Dr R. J. Johnson, Birmingham Heartlands Hospital, Birmingham; Dr L. Jones, Epsom General Hospital, Surrey; Dr P. J. Kingston, Gloucestershire Royal Hospital, Gloucester; Dr H. E. T. Korn, Ysbyty Gwynedd, Gwynedd; Dr A. Lennard, Royal Victoria Infirmary, Newcastle; Dr J. Leslie, Norfolk and Norwich University Hospital NHS Trust, Norfolk; Dr M. L. Lewis, Kidderminster Hospital, Worcester; Dr M. J. Mackie, Western General Hospital, Edinburgh; Dr C. Mattock, Jersey General Hospital, Jersey; Dr P. McKay, Royal Alexandra Hospital, Renfrewshire; Dr M. F. McMullin, Belfast City Hospital, Belfast; Dr M. F. McMullin, Mater Infirmorum Hospital, Belfast; Dr G. McQuaker, Glasgow Royal Infirmary, Glasgow; Dr B. A. McVerry, St James's University Hospital, Leeds; Dr A. B. Mehta, Royal Free Hospital, London; Dr J. Mercieca, St Helier Hospital, Surrey; Dr A. Moiceanu, Fundeni Hospital, Romania; Dr J. A. Murphy, Monklands District General, Lanarkshire; Dr J. A. Murray, Selly Oak Hospital, Birmingham; Dr H. Myint, Royal Bournemouth Hospital, Bournemouth; Dr J. Neilson, Russells Hall Hospital, W Midlands; Dr A. M. O'Hea, Stoke Mandeville Hospital, Bucks; Dr A. C. Parker, Royal Infirmary of Edinburgh, Edinburgh; Dr E. J. Parker Williams, St George's Hospital, London; Prof M. J. Pippard, Ninewells Hospital and Medical School, Dundee; Dr A. Poenaru, Fundeni Hospital, Romania; Dr C. Poynton, University Hospital of Wales, Cardiff; Dr D. R. Prangnell, Lincoln County Hospital, Lincoln; Prof H. G. Prentice, Royal Free Hospital, London; Dr C. G. L. Raper, Hull Royal Infirmary, Hull; Dr S. Rassam, Queen Mary's Sidcup NHS Trust, Kent; Dr J. T. Reilly, Royal Hallamshire Hospital, Sheffield; Dr S. Y. Rogers, Victoria Hospital, Fife; Dr J. R. Y. Ross, Northampton General Hospital, Northampton; Prof N. H. Russell, Nottingham City Hospital, Nottingham; Dr M. F. Ryan, Altnagelvin Area Hospital, Londonderry; Dr W. Sadik, University Hospital Aintree, Liverpool; Dr G. L. Scott, Bristol Royal Infirmary, Bristol; Dr M. Sekhar, West Middlesex Hospital, Middlesex; Dr M. J. Semple, Epsom General Hospital, Surrey; Dr S. Shahriari, Hairmyres Hospital, Glasgow; Dr A. G. Smith, Southampton University Hospital Trust, Southampton; Dr S. R. Smith, Torbay Hospital, Devon; Dr R. Soutar, Monklands District General, Lanarkshire; Dr P. J. Stableforth, Sandwell General Hospital, W Midlands; Dr A. Stark, Dumfries and Galloway R. Infirmary, Dumfries; Dr P. A. Stevenson, Walton Hospital, Liverpool; Dr P. Stross, St Richard's Hospital, West Sussex; Dr J. Tighe, Aberdeen Royal Infirmary, Aberdeen; Dr F. Toolis, Dumfries and Galloway R. Infirmary, Dumfries; Dr J. Tucker, Borders General Hospital, Roxburghshire; Dr W. Watson, Monklands District General, Lanarkshire; Dr J. Z. Wimperis, Norfolk and Norwich University Hospital NHS Trust, Norfolk; Dr J. K. Wood, Leicester Royal Infirmary, Leicester; Dr M. Wood, Colchester General Hospital, Essex; and Dr B. E. Woodcock, Southport District General, Southport.
HOVON trials group (HOVON 20)
Fifteen patients: Prof Dr J. H. F. Falkenburg, University Medical Center Leiden. Nine patients: Dr P. Muus, University Medical Center, Nijmegen. Eight patients: Dr H. van der Lelie, University Medical Center, Amsterdam; and Dr K. J. Roozendaal, OLVG Amsterdam. Five patients: Dr L. Siegenbeek van Heukelom, Medical Center Alkmaar; Dr J. Baars and Dr J. F. M. Pruijt, Netherlands Cancer Institute, Amsterdam; Dr J. J. Mol, Rijnstate Hospital, Arnhem; Dr S. M. G. J. Daenen, University Hospital, Groningen; Dr P. Joosten, Medical Center, Leeuwarden; and Dr A. W. Dekker, University Medical Center, Utrecht. Four patients: Dr H. A. M. Sinnige, Bosch Medicenter, Den Bosch; and Dr Ph. H. B. Sybesma, Merwede Hospital, Dordrecht. Three patients: Dr M. H. H. Kramer, Eemland Hospital Amersfoort; Dr E. Maartense, R. de Graaf Hospital Delft; Dr A. Vlasveld, Diakonessen Hospital, Eindhoven; Dr R. E. H. Smeets, St Anna Hospital, Geldrop; and Dr G. Woolthuis, St Antonius Hospital, Sneek. Two patients: Dr A. C. J. M. Holdrinet, Ignatius Hospital Breda; Dr W. Gerrits, Leyenburg Hospital, Den Haag; Dr K. J. Heering, St Joseph Hospital, Gouda; Dr M. Fickers, De Wever Hospital Heerlen; Dr F. Cluitmans, Rijnland Hospital, Leiderdorp; Dr J. J. Keuning, St Joseph Hospital Veldhoven; Dr M. J. B. P. Abegg-Werter, St Elisabeth Hospital, Venray; Dr J. B. Ruit, Holy Hospital, Vlaardingen; and Dr J. G. S. Breed, St Jans Hospital, Weert. One patient: Dr J. Albada, Medical Center, Molendael Baarn; Dr H. Muller, Hospital Gooi-Noord Blaricum; Dr G. Van Deijk, Red Cross Hospital, Den Haag; Dr R. Vriesendorp, Westeinde Hospital, Den Haag; Dr H. van Kamp, Nij Smellinge Hospital, Drachten; Dr D. De Laan, Scheeper Hospital, Emmen; Dr G. de Klerk, Kennemer Hospital, Haarlem; Dr B. Runhaar, Regional Hospital, Hardenberg; Dr D. v.d. Stadt, Spaarne Hospital, Heemstede; Dr K. T. Njo, Bethesda Hospital, Hoogeveen; Dr M. Herben, St Antonius Hospital, Leidschendam; Dr M. Soesan, Slotervaart Hospital, Amsterdam; Dr H. R. Oosten, Canisius-Wilhelmina Hospital, Nijmegen; Dr J. A. M. J. Wils, St Laurentius Hospital, Roermond; Dr J. J. Braun, Slingeland Hospital, Schiedam; Dr C. v.d. Heul, Elisabeth Hospital, Tilburg; and Dr R. L. Stuijver, Hospital Velp.
Prepublished online as Blood First Edition Paper, March 9, 2004; DOI 10.1182/blood-2003-10-3605.
Supported by the Medical Research Council (United Kingdom trials) and Schering Plough and Glaxo-Wellcome research grants.
Lists of members of the UK CML Working Group of NCRI and the HOVON trials group appear in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prof Dr A. Hagemeijer and Dr R. Slater performed most of the cytogenetic analyses from the HOVON 20 patients during the early years of the study. We thank staff at CTSU (Clinical Trials Support Unit) for the randomization and data management of the MRC trials; Mr J. F. van der Burgh for his help with the HOVON 20 database; and Mr J. de Roo and Mrs P. Bakels for their help with the data management of HOVON 20.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal