Abstract
We recently described a subset of patients with a myeloproliferative variant of hypereosinophilic syndrome (MHES) characterized by elevated serum tryptase levels, increased atypical mast cells in the bone marrow, tissue fibrosis, and the presence of the fusion tyrosine kinase, FIP1L1-PDGFRα, which is a therapeutic target of imatinib mesylate. Seven patients with MHES were treated with imatinib mesylate (300-400 mg daily). Clinical improvement and resolution of eosinophilia was observed in all patients, although cardiac dysfunction, when present, was not altered by therapy. Reversal of bone marrow pathology, including increased cellularity, the presence of spindle-shaped mast cells, and myelofibrosis, was evident in all patients at 4 to 8 weeks following initiation of therapy. This was accompanied by a decrease in activated eosinophils and mast cells in the peripheral blood and bone marrow, respectively. Serum tryptase levels declined rapidly to normal levels in all patients and remained in the normal range throughout therapy. Molecular remission, with disappearance of detectable FIP1L1/PDGFRA (F/P) transcripts, was achieved in 5 of 6 patients tested. The lack of reversal of cardiac abnormalities and persistence of the F/P mutation in some patients suggests that early intervention with higher doses of imatinib mesylate may be desirable in the treatment of patients with MHES.
Introduction
Hypereosinophilic syndrome (HES) is a rare disorder characterized by the presence of more than 1.5 × 109 eosinophils/L in the peripheral blood and evidence of eosinophil-mediated tissue damage. Although increased peripheral blood and bone marrow eosinophilia is the hallmark of HES, the clinical presentations of HES are extremely varied, ranging from asymptomatic eosinophilia to life-threatening organ damage, including endomyocardial fibrosis and restrictive pulmonary disease. It is becoming increasingly clear that HES is not a single disorder, but a group of disorders of varied etiologies that include the clonal proliferation of lymphocytes or myeloid cells. The degree of activation of circulating eosinophils, the cytokines produced, and the cell lineages involved may be determined by the underlying etiology and likely contribute to the wide range of clinical presentations seen in HES.
We recently described a subset of HES patients with a myeloproliferative variant characterized by elevated serum tryptase levels, increased atypical mast cells in the bone marrow, and the presence of tissue fibrosis.1 This clinical subtype appears to correlate with the presence of a recently described fusion tyrosine kinase, FIP1L1-PDGFRα, which is a therapeutic target of imatinib mesylate in HES.1-3 Patients with the myeloproliferative subtype of HES (MHES) demonstrate a rapid and dramatic resolution of peripheral eosinophilia, elevated serum tryptase levels, and clinical symptoms in response to imatinib mesylate therapy. We now report that a well-characterized cohort of patients with MHES demonstrates reversal of bone marrow pathology, which includes myelofibrosis and the presence of spindle-shaped atypical mast cells, in response to imatinib mesylate (400 mg daily) as well as decreased activation of peripheral eosinophils and a reduction in serum cytokines associated with the inflammatory response. Molecular remission, with disappearance of detectable FIP1L1-PDGFRA transcripts, was also rapid and complete in most, but not all, patients.
Patients and methods
Patient population
Patients were eligible for the study if they were 18 years of age or older and met the following diagnostic criteria for the myeloproliferative variant of idiopathic HES1 : (1) eosinophilia more than 1.5 × 109/L on 2 occasions at least 6 months apart, (2) no known etiology for the eosinophilia despite careful clinical evaluation, (3) evidence of end-organ damage (histologic evidence of tissue infiltration by eosinophils or objective evidence of clinical pathology in any organ system that is temporally associated with eosinophilia and not clearly attributable to another cause), and (4) at least 4 of the following laboratory criteria: (a) dysplastic eosinophils on peripheral smear, (b) serum B12 level 1000 pg/mL or higher, (c) serum tryptase level 12 ng/mL or higher, (d) anemia or thrombocytopenia (or both), (e) bone marrow cellularity more than 80% with left shift in maturation, (f) dysplastic (spindle-shaped) mast cells on bone marrow biopsy, (g) evidence of fibrosis on bone marrow biopsy, or (h) dysplastic megakaryocytes on bone marrow biopsy.
Women with childbearing potential were required to have a negative serum pregnancy test prior to starting imatinib mesylate treatment, and all patients at risk were required to use barrier contraceptive measures. All patients were required to have an absolute neutrophil count of 1.0 × 109/L or higher, a platelet count of equal to or more than 10 × 109/L or equal to or more than 50 × 109/L if there was clinical evidence of bleeding, and levels of liver transaminases less than 5 times and bilirubin less than 3 times the upper limit of normal. The study was approved by the Institutional Review Board of the National Institutes of Allergy and Infectious Diseases, and informed consent was obtained from all study participants.
Study design
Study participants received imatinib mesylate in a single daily oral dose of 400 mg. This regimen was selected based on data from phase 2 studies of chronic myelogenous leukemia (CML), which showed a decreased rate of cytogenetic remission at doses less than 300 mg/d despite decreased circulating blasts.4 Imatinib mesylate therapy was interrupted and restarted at a lower dose for severe neutropenia (absolute neutrophil count < 1.0 × 109/L), thrombocytopenia (platelet count < 10 × 109/L or 50 × 109/L with clinical evidence of bleeding), transaminase or bilirubin elevation (> 5 or > 3 times the upper limit of normal, respectively), according to the guidelines proposed for the treatment of CML.5
A comprehensive clinical evaluation, including complete blood count (CBC) with differential, routine chemistries, serum IgE, B12, and tryptase levels, electrocardiogram (ECG), echocardiogram, pulmonary function tests (PFTs), and bone marrow aspirate and biopsy, was performed prior to and at 1 month following the initiation of imatinib mesylate therapy. CBC, liver function tests, and tryptase levels were followed weekly for 1 month after the initiation of therapy, biweekly for 2 additional months and monthly thereafter. Adverse events were graded using the Common Toxicity Criteria of the National Cancer Institute.6
All laboratory testing reported in this study was performed in the Department of Laboratory Medicine at the National Institutes of Health Clinical Center, with the exception of serum total (α and β) tryptase levels, which were measured at the Mayo Medical Labs (Rochester, MN), and serum cytokine levels, which were measured by Pierce Biotechnology (Rockford, IL) using a multiplex sandwich enzyme-linked immunosorbent assay (ELISA), as described previously.7 The normal range for serum tryptase levels (< 11.5 ng/mL) was provided by Mayo Medical Labs. Splenomegaly was assessed by physical examination and confirmed by computed tomography (CT). Pulmonary disease was classified as restrictive or obstructive on the basis of characteristic findings of PFTs, including spirometry, flow volume loops, and assessment of diffusion capacity. Interstitial infiltrates on chest CT were considered supportive evidence of restrictive disease, but were not sufficient for classification in the absence of PFT abnormalities.
Flow cytometric analysis of mast cells
Bone marrow aspirates were obtained from patients who gave their informed consent. The bone marrow aspirate mononuclear cell fraction containing mast cells was then separated using Histopaque (density = 1.077; Sigma, St Louis, MO) gradient centrifugation, and contaminating red cells were lysed by incubation in 0.8% ammonium chloride solution (StemCell Technologies, Vancouver, BC, Canada) for 10 minutes. Mast cells in bone marrow aspirates were identified by flow cytometry as a CD117+ high, side-scatter high population as described.8 Briefly, bone marrow mononuclear cells were incubated in 100-μL aliquots for 30 minutes at 4°C with a phycoerythrin (PE) conjugate of antihuman CD117 (clone 104D2; Becton Dickinson, San Jose, CA) and a fluorescein isothiocyanate (FITC) conjugate of antihuman CD2, CD25, or CD35 (BD Biosciences, San Diego, CA). The cells were then washed, resuspended in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (BSA), and analyzed on a flow cytometer (FACScan; Becton Dickinson).
Flow cytometric analysis of eosinophils
Whole blood was stained with CD25, CD40, HLA-DR, CD9, CD69, IgG1 (BD Biosciences) or CD23 (Beckman Coulter, Brea, CA), all directly conjugated to FITC, in combination with CD16 PE (BD Biosciences) for 2-color flow cytometric analysis. Irrelevant murine IgG1 FITC was used to ascertain background staining. CD9 was used as a positive control. Whole blood (100 μL) was incubated with CD16 PE and the specific surface markers for 30 minutes at 4°C. Red cells were lysed using FACS lysing solution (BD Biosciences). Samples were analyzed on a FACScan (BD Biosciences) using Cellquest software (BD Biosciences). Eosinophils were separated from granulocytes by their characteristically high side-scatter and CD16 dim staining.
Detection of FIP1L1-PDGFRA fusion
RNA was isolated from peripheral blood mononuclear cells (PBMCs) using RNA STAT-60 (Tel-Test, Friendswood, TX). First-strand cDNA was synthesized from 2 μg total RNA using Superscript first-strand synthesis system (Invitrogen, Carlsbad, CA) with random hexamer primers. Fusion of FIP1L1 to PDGFRA was detected by nested polymerase chain reaction (PCR) using primers FIP1L1-F1 (5′-acctggtgctgatctttctgat) and PDGFRA-R1 (5′-tgagagcttgtttttcactgga) during the first PCR, and primers FIP1L1-F2 (5′-aaagaggatacgaatgggacttg) and PDGFRA-R2 (5′-gggaccggcttaatccatag) for the second PCR. Control reverse transcription-PCR (RT-PCR) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was performed using the primers GAPDH-F (5′-tggaaatcccatcaccatct) and GAPDH-R (5′-gtcttctgggtggcagtgat).
Statistical analysis
Nonparametric comparisons of paired data were performed using the Wilcoxon signed rank test. Nonparametric comparisons of group means were made using the Mann-Whitney U test. A P value less than .5 was considered statistically significant for all tests.
Results
Baseline characteristics of patients
Between January and April 2003, 8 patients with the myeloproliferative variant of HES were enrolled in a study of imatinib mesylate therapy. All of the patients were men, ranging in age between 29 and 51 years of age. A summary of their clinical manifestations and prior therapies is given in Table 1. Baseline evaluation of all patients prior to the initiation of therapy included RT-PCR analysis for bcr/abl to exclude CML and sequencing of c-kit to exclude systemic mastocytosis.1 Because prior studies suggested that high serum interleukin 5 (IL-5) levels were associated with a lack of response to imatinib mesylate therapy in HES, serum IL-5 levels were measured prior to and during therapy and were either undetectable (4 of 6 patients) or detectable at low levels (1.2 and 1.5 pg/mL) in 2 of the 6 patients tested. Serum IL-5 levels in 5 blood bank donors ranged from less than 0.78 to 12.4 pg/mL in the same assay.
The FIP1L1/PDGFRA mutation was present in RNA from the PBMCs of all 7 patients with myeloproliferative disease and elevated serum tryptase level. Analysis of PBMC RNA from one patient (patient 8; Table 1) who met the inclusion criteria for the imatinib mesylate treatment study but did not have elevated serum tryptase levels did not show evidence of the mutation. This patient was subsequently found to have pre-B-cell acute lymphoblastic leukemia (ALL) and has been excluded from the analysis.
Clinical response to imatinib mesylate
All 7 patients with HES and elevated serum tryptase levels (MHES) experienced a dramatic improvement in clinical symptoms beginning as early as 3 days following the initiation of imatinib mesylate therapy. Mucosal ulcerations, present in 2 patients at the initiation of therapy, began to resolve after 1 week of treatment and had healed completely in both patients within 1 month. Dyspnea and cough, present in 1 patient at the onset of the study, resolved within 3 days of imatinib mesylate therapy. PFTs and chest CT scans performed at the 3-month follow-up confirmed normalization of diffusion capacity and lung volumes, as well as resolution of interstitial infiltrates that had been present at the start of therapy.
Despite a generalized improvement in constitutional symptoms, symptoms and signs of congestive heart failure in the 3 patients with endomyocardial fibrosis remained unaffected by imatinib mesylate therapy. Results of echocardiography and cardiac magnetic resonance imaging performed before treatment were unchanged in the 2 patients for whom posttreatment studies were available. The third patient (patient 6) died 1 month after beginning imatinib mesylate therapy from disseminated cytomegalovirus infection thought to be a result of prolonged high-dose steroid use. At autopsy, extensive endomyocardial fibrosis was evident throughout the ventricular walls and interventricular septum.
Adverse effects of imatinib mesylate therapy (400 mg daily) were limited to transient grade 3 neutropenia leading to interruption of therapy followed by dose reduction to 300 mg daily in one patient, grade 1 pedal and facial edema requiring no therapy in 2 patients, and transient grade 2 myalgias in one patient, which resolved with ibuprofen.
Hematologic and molecular response
In 6 of the 7 patients with MHES and elevated serum tryptase levels, peripheral blood eosinophil counts decreased within a week of beginning imatinib mesylate therapy, despite discontinuation of other therapies. Patient 5, whose eosinophil count had normalized on cyclosporin A and interferon (IFN) therapy, had an initial increase in peripheral eosinophilia at 1 week, followed by a return to normal levels (< 0.75 × 109/L) by 3 weeks after treatment. Anemia (hemoglobin level ≤ 127 g/L [12.7 g/dL]), present in 3 patients at the onset of therapy, resolved within 2 months of the initiation of imatinib mesylate in all patients. Thrombocytopenia (platelets < 154 × 109/L), present in 2 patients prior to therapy, resolved following 3 weeks of therapy in one patient. The other patient (patient 7) remained thrombocytopenic after 1 month of therapy in the setting of right-sided heart failure secondary to severe mitral and tricuspid regurgitation. His platelet count normalized following valve replacement surgery performed 3 months after the initiation of imatinib mesylate therapy.
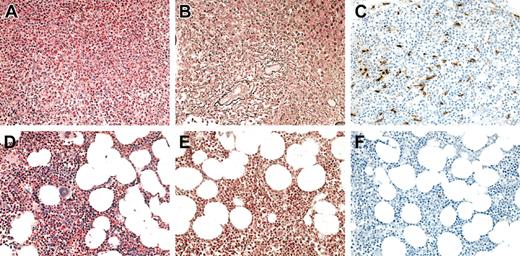
Bone marrow aspirates and biopsies were performed in 6 of 7 patients with MHES at 4 to 8 weeks following the initiation of imatinib mesylate therapy and demonstrated a decrease in cellularity with normalization of the myeloid-erythroid ratio and disappearance of spindle-shaped dysplastic mast cells (Figure 1; Table 2). A decrease in reticulin staining was seen in the bone marrow biopsies from 5 of the 5 patients with increased staining pretreatment, and complete resolution of myelofibrosis was observed in one patient (Figure 1; Table 2).
Bone marrow findings in a representative HES patient before and at 1 month following the initiation of imatinib mesylate therapy. (A-C) Before therapy. (D-F) After therapy. Sections were stained with hematoxylin and eosin (A,D), antireticulin antibody (B,E), and antitryptase antibody (C,F). Not only was there a dramatic decrease in eosinophils and myeloid precursors in response to imatinib mesylate (A,D), but a resolution in the hypercellularity (A,D), reticulin fibrosis (B,E), and atypical spindle-shaped mast cells (C,F) evident prior to therapy.
Bone marrow findings in a representative HES patient before and at 1 month following the initiation of imatinib mesylate therapy. (A-C) Before therapy. (D-F) After therapy. Sections were stained with hematoxylin and eosin (A,D), antireticulin antibody (B,E), and antitryptase antibody (C,F). Not only was there a dramatic decrease in eosinophils and myeloid precursors in response to imatinib mesylate (A,D), but a resolution in the hypercellularity (A,D), reticulin fibrosis (B,E), and atypical spindle-shaped mast cells (C,F) evident prior to therapy.
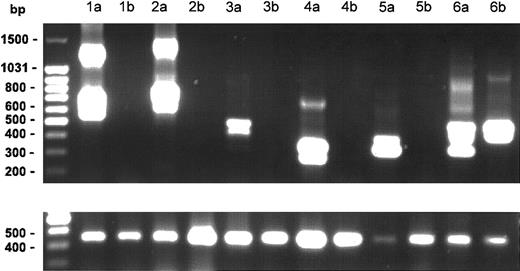
Posttreatment samples of PBMC RNA were available from 6 patients. The FIP1L1/PDGFRA mutation, which was present in all 6 patients at the start of therapy, was undetectable in the samples from 5 of 6 patients treated with imatinib mesylate for 1 to 12 months (Figure 2). The mutation remained detectable in the PBMCs from one patient who had been receiving imatinib mesylate for 18 months despite complete clinical and hematologic remission.
Effect of imatinib mesylate on FIP1L1-PDGFR fusion. Nested RT-PCR results for the FIP1L1/PDGFR fusion gene in PBMC RNA from 6 patients before (lanes a) and after (lanes b) treatment with imatinib mesylate. The lower panel shows the results of RT-PCR for the GAPDH gene as a control for amount of RNA loaded for each patient sample. Various size bands are observed in different cases due to different breakpoints in the FIP1L1 gene as shown previously.1 The different bands in each lane represent splice variants. The FIP1L1-PDGFR fusion became undetectable in 5 of 6 patients after imatinib mesylate therapy.
Effect of imatinib mesylate on FIP1L1-PDGFR fusion. Nested RT-PCR results for the FIP1L1/PDGFR fusion gene in PBMC RNA from 6 patients before (lanes a) and after (lanes b) treatment with imatinib mesylate. The lower panel shows the results of RT-PCR for the GAPDH gene as a control for amount of RNA loaded for each patient sample. Various size bands are observed in different cases due to different breakpoints in the FIP1L1 gene as shown previously.1 The different bands in each lane represent splice variants. The FIP1L1-PDGFR fusion became undetectable in 5 of 6 patients after imatinib mesylate therapy.
Decreased activation of eosinophils and mast cells
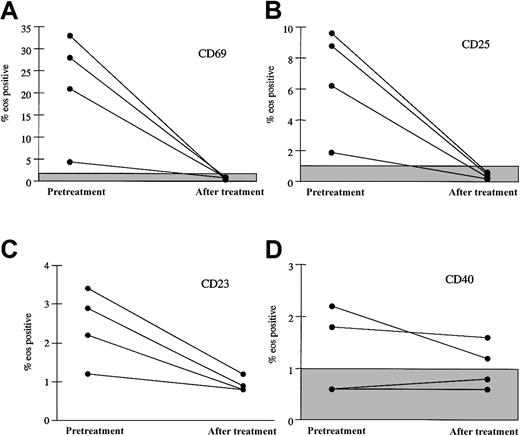
Surface expression of markers associated with eosinophil activation was assessed by flow cytometric analysis of peripheral eosinophils before and at 1 month following the initiation of imatinib mesylate therapy in 4 patients. Increased surface expression of the activation markers CD69 and CD25 was noted on eosinophils from 4 patients prior to imatinib mesylate therapy and normalized following imatinib mesylate therapy (Figure 3). There was no significant change in expression of CD40. Interestingly, oligoclonal T-cell receptor rearrangement patterns, which were seen in 4 of 7 patients prior to therapy and are thought to be reactive, persisted in 2 of 2 patients tested despite the decrease in markers of eosinophil activation (data not shown).
Decrease in surface expression of activation markers on eosinophils 1 month following initiation of imatinib mesylate therapy. The percent of peripheral blood eosinophils expressing CD69 (A), CD25 (B), CD23 (C), and CD40 (D) before treatment and after 1 month of imatinib mesylate therapy is shown for 4 patients with MHES. The shaded boxes indicate the normal ranges for CD69, CD25, and CD40. All of the patient values for percent CD23 are within the normal range (≤ 4.5%). Whereas eosinophil expression of the activation markers CD69, CD25, and CD23 decreased significantly with imatinib mesylate therapy, expression of CD40 was unchanged.
Decrease in surface expression of activation markers on eosinophils 1 month following initiation of imatinib mesylate therapy. The percent of peripheral blood eosinophils expressing CD69 (A), CD25 (B), CD23 (C), and CD40 (D) before treatment and after 1 month of imatinib mesylate therapy is shown for 4 patients with MHES. The shaded boxes indicate the normal ranges for CD69, CD25, and CD40. All of the patient values for percent CD23 are within the normal range (≤ 4.5%). Whereas eosinophil expression of the activation markers CD69, CD25, and CD23 decreased significantly with imatinib mesylate therapy, expression of CD40 was unchanged.
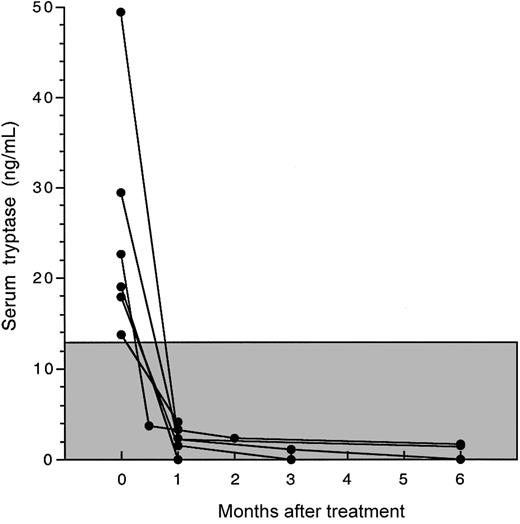
Serum tryptase levels decreased in all patients to within normal limits within 4 weeks of the initiation of therapy (Figure 4). This was accompanied by disappearance of atypical spindle-shaped mast cells (but not normal mast cells) from the bone marrow in all 6 patients for whom posttreatment bone marrow biopsies were available (Table 2; Figure 1). The reduction in atypical mast cells in response to imatinib mesylate treatment was confirmed by flow cytometric analysis of bone marrow aspirates performed before and at 4 to 8 weeks after treatment in 4 patients (Table 2; Figure 5).
Decline in serum tryptase levels in response to imatinib mesylate therapy. Serum tryptase levels before and after imatinib mesylate therapy are shown as a function of time for 6 patients with MHES and elevated serum tryptase levels. The normal range for serum tryptase levels (≤ 11.5 ng/mL) is indicated by the shaded box. Serum tryptase levels for all patients declined rapidly to normal levels and remained in the normal range throughout therapy.
Decline in serum tryptase levels in response to imatinib mesylate therapy. Serum tryptase levels before and after imatinib mesylate therapy are shown as a function of time for 6 patients with MHES and elevated serum tryptase levels. The normal range for serum tryptase levels (≤ 11.5 ng/mL) is indicated by the shaded box. Serum tryptase levels for all patients declined rapidly to normal levels and remained in the normal range throughout therapy.
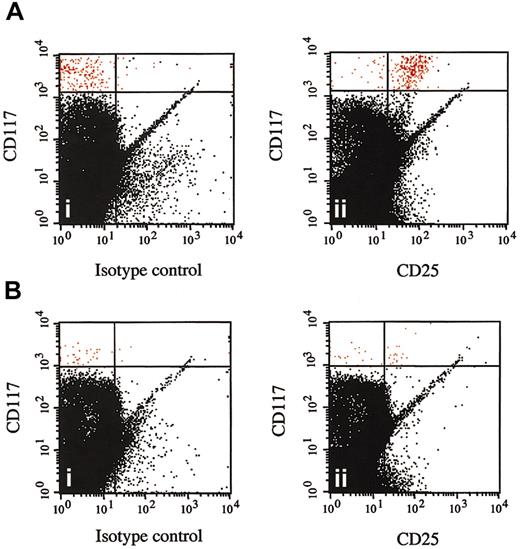
Disappearance of CD25+ bone marrow mast cells following imatinib mesylate therapy. Multicolor flow cytometry results are shown for CD117+ high, side-scatter high bone marrow cells from a representative patient with the myeloproliferative variant of HES before (A) and at 1 month after (B) the initiation of therapy with imatinib mesylate. Cells were stained with FITC-conjugated mouse IgG1 control antibody (isotype control) and antihuman CD25 antibody (CD25). CD117+ high, side-scatter high bone marrow mast cells are shown in red and express CD25 prior to (Aii) but not following (Bii) imatinib mesylate therapy.
Disappearance of CD25+ bone marrow mast cells following imatinib mesylate therapy. Multicolor flow cytometry results are shown for CD117+ high, side-scatter high bone marrow cells from a representative patient with the myeloproliferative variant of HES before (A) and at 1 month after (B) the initiation of therapy with imatinib mesylate. Cells were stained with FITC-conjugated mouse IgG1 control antibody (isotype control) and antihuman CD25 antibody (CD25). CD117+ high, side-scatter high bone marrow mast cells are shown in red and express CD25 prior to (Aii) but not following (Bii) imatinib mesylate therapy.
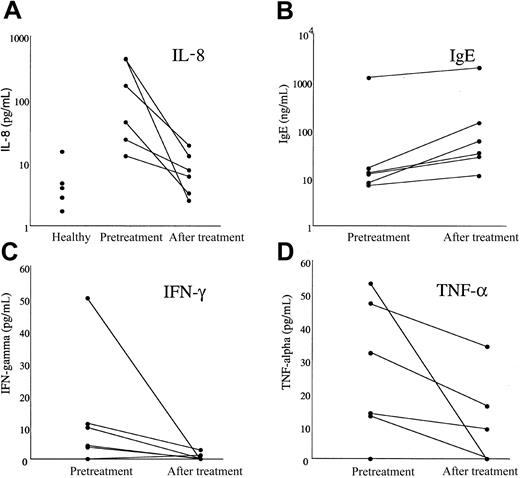
Serum IL-8 levels were significantly increased in patients with MHES (geometric mean [GM] 86.25 pg/mL) prior to imatinib mesylate treatment as compared to healthy blood bank donors (GM 4.22; n = 5) and decreased dramatically following therapy in all MHES patients (GM 6.72 pg/mL; P = .027; Figure 6). Serum levels of IFN-λ and tumor necrosis factor α (TNF-α) also decreased significantly in response to therapy, although pretreatment levels were not different from those in healthy controls (Figure 6). There was no significant change in serum levels of IL-2, IL-4, IL-5, IL-10, IL-12, IL-13, or IL-13 receptor α (data not shown). Interestingly, serum IgE levels increased in all patients following treatment (Figure 6; P = .027), whereas serum IgG, IgM, and IgA levels remained unchanged (data not shown).
Effect of imatinib mesylate therapy on serum cytokine and IgE levels in patients with MHES. Serum levels of IL-8 (A), IgE (B), IFN-λ (C), and TNF-α (D) measured before treatment and after 1 month of imatinib mesylate therapy are shown for 6 patients with MHES. Serum IL-8 values for 5 healthy blood bank donors are also shown to illustrate the increased baseline IL-8 values in patients with MHES. Whereas serum IL-8, IFN-λ, and TNF-α levels decreased in response to imatinib mesylate therapy, IgE levels rose in all patients studied.
Effect of imatinib mesylate therapy on serum cytokine and IgE levels in patients with MHES. Serum levels of IL-8 (A), IgE (B), IFN-λ (C), and TNF-α (D) measured before treatment and after 1 month of imatinib mesylate therapy are shown for 6 patients with MHES. Serum IL-8 values for 5 healthy blood bank donors are also shown to illustrate the increased baseline IL-8 values in patients with MHES. Whereas serum IL-8, IFN-λ, and TNF-α levels decreased in response to imatinib mesylate therapy, IgE levels rose in all patients studied.
Discussion
We have previously demonstrated an association between the presence of the elevated serum tryptase levels in patients with HES and the FIP1L1/PDGFRA (F/P) mutation.1 In view of reports of imatinib mesylate responses in the absence of the F/P mutation in as many as 30% of patients with HES,2 we elected to include patients with clinical and laboratory criteria of HES and myeloproliferative disease in the absence of elevated serum tryptase levels in the present study. Interestingly, the one patient who did not have elevated serum tryptase (patient 8) also did not have the F/P mutation. Although his eosinophilia did respond to imatinib mesylate therapy with a decrease from 151.34 × 109 to 2.41 × 109 eosinophils/L, his eosinophil count did not normalize after 1 month of therapy. He was subsequently found to have pre-B-cell ALL and is currently receiving chemotherapy.
As has been reported for other patients with the F/P mutation,2,9 all the patients with HES and elevated serum tryptase (MHES) in the present study responded to treatment with imatinib mesylate with improvement in clinical symptoms and resolution of laboratory abnormalities and bone marrow pathology. The persistence of cardiac dysfunction in 3 patients despite improvement in other organ systems most likely reflects permanent structural damage to the cardiac tissue rather than ongoing tissue destruction and fibrosis, suggesting that early drug intervention in this subgroup of patients may be desirable. Side effects of imatinib mesylate treatment using standard CML dosing (300-400 mg daily) were minimal, with only a single patient requiring temporary interruption of drug administration due to significant neutropenia at the onset of therapy. To date, none of the 6 patients have shown clinical or laboratory evidence of drug resistance.
The FIP1L1-PDGFRA fusion tyrosine kinase is over 100 times more sensitive to imatinib mesylate than is bcr-abl (inhibitory concentration of 50% [IC50] of 3 nM as compared to 582 nM for bcr/abl),2 explaining the dramatic clinical and hematologic response in HES to imatinib mesylate doses as low as 100 mg weekly.10,11 The demonstration that only 5 of 6 patients with MHES receiving 300 to 400 mg imatinib mesylate daily achieved molecular remission as assessed by RT-PCR of PBMC RNA, however, suggests that higher doses may be necessary to eradicate disease. This hypothesis is supported by recent data demonstrating that despite complete hematologic response to the standard dose of imatinib mesylate (400 mg daily), patients with CML rarely achieve molecular remission unless higher doses are used.12,13
Although the cellular origin of the mutation in F/P-mediated HES remains unknown, the rapid disappearance of dysplastic mast cells and eosinophils in response to imatinib mesylate therapy suggests that both of these cell types arise from the neoplastic clone. Neutrophilia (neutrophils ≥ 7.5 × 109/L), which has been described previously in some patients with HES,14 was observed prior to treatment in only one patient but resolved rapidly with imatinib mesylate therapy, suggesting that other granulocytic lineages may also be affected in this disease. This is consistent with recent data demonstrating the presence of the F/P mutation in enriched eosinophils and neutrophils from one patient.15 In contrast, we observed no change in lymphocyte numbers or surface markers in response to imatinib mesylate, and oligoclonal patterns of T-cell receptor (TCR) expression, when present, were unaffected by therapy (data not shown).
Although the presence of increased numbers of mast cells has been documented in cardiac tissue from patients with HES and endomyocardial fibrosis16 as well as in the bone marrow of patients with HES and myelofibrosis,1 tissue fibrosis in HES has typically been attributed to eosinophil infiltration of the tissues and the subsequent direct stimulation of fibroblast proliferation and extracellular matrix deposition by eosinophil granule proteins and eosinophil-derived fibrogenic cytokines, including transforming growth factor β (TGF-β) and TNF-α.16-18 When mast cells have been implicated in the fibrosis, it has been in the setting of activation felt to be secondary to eosinophil-derived mediators, such as eosinophil major basic protein.19 It is of interest, in this regard, that neither fibrosis nor increased mast cells have been described in the skin of patients with HES and mucosal ulcerations despite marked deposition of eosinophil granule proteins20 (K. Leiferman et al, unpublished data, 2001) or in the bone marrow of patients with HES in the absence of the F/P mutation.1 These findings suggest that mast cells and their interaction with eosinophils and fibroblasts play an essential role in the pathogenesis of fibrosis in MHES.
The cross-talk between activated mast cells and eosinophils is enormously complex and involves numerous cytokines, chemokines, and inflammatory molecules. Nevertheless, recent data suggest that tryptase is one of the key mediators of eosinophil–mast cell–fibroblast interactions.21 Not only does tryptase exert an autocrine action on mast cells and induce fibroblast proliferation, but it is an important inducer of IL-8 release by eosinophils.22 Interestingly, serum levels of IL-8, but not of other cytokines known to be secreted by activated eosinophils, were significantly elevated in patients with MHES prior to imatinib mesylate therapy and declined dramatically in response to therapy. Although the temporal association between the decrease in serum IL-8 levels and the normalization of serum tryptase levels is intriguing, IL-8 can be produced by several different cell types, including neutrophils, fibroblasts, and eosinophils, and is secreted in response to a number of different stimuli, including major basic protein23,24 and secretory phospholipase A2.25,26 Consequently, further studies, including the delineation of the cellular source of IL-8, the lineage specificity of the F/P mutation, and its consequences at the cellular level in patients with MHES, will be necessary to the understanding of the pathogenesis of the fibrotic response in these patients.
In summary, we have demonstrated that imatinib mesylate at doses comparable to those used in CML is effective and well tolerated in patients with MHES. Furthermore, the dramatic clinical and hematologic response to these doses of imatinib mesylate was accompanied by molecular remission in a majority of patients, as well as the disappearance of activated, dysplastic mast cells and eosinophils from the bone marrow and peripheral circulation, a decrease in inflammatory cytokine and serum tryptase levels in the serum, and reversal of myelofibrosis. Limitations of therapy include the lack of reversal of cardiac abnormalities and persistence of the F/P mutation in some patients, suggesting that early intervention with higher doses of imatinib mesylate may be desirable, although further studies are clearly needed to delineate the optimal dose and length of imatinib mesylate therapy in HES and to determine whether or not disease eradication is an achievable goal. The dramatic resolution of myelofibrosis seen in patients with MHES treated with imatinib mesylate in the setting of a defined molecular defect provides a unique opportunity to investigate the pathogenesis of the fibrotic response in this subgroup of patients.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-08-2798.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to acknowledge Barbara Foster for her help with the mast cell flow cytometry, Leigh Bernardino for her help with patient scheduling, and D. Gary Gilliland and Jan Cools for helpful discussions.