Abstract
Human neo-organ formation from stem cells can only be assayed by in vivo xenotransplantation. The human nonobese diabetic–severe combined immunodeficient (HuNOD/scid) CD34+ cell transplantation is a model that allows examination of hematopoietic tissue formation, although human hematopoietic cell maturation is abortive. Conventional humanization of the cytokine microenvironment has depended on generation of human cytokine-transgenic mice in strains appropriate for conventional plasmid microinjection, followed by backcrossing, a costly and time-consuming approach. Lentiviral vector infection of single-cell embryos was recently reported to produce transgenic animals. Using this approach, we have generated direct human granulocyte-macrophage colony-stimulating factor (hGM-CSF) transgenic mice from lentivirus-microinjected NOD/scid embryos, with 68% efficiency and 100% penetrance; this allowed us to obtain NOD/scid transgenic mice with considerable savings of resources. This powerful technique should assist in producing novel mouse models for the study of human blood cell lineage development and other human neo-organs from stem cell xenotransplantation for which a similar “humanization” rationale may be required.
Introduction
Human neo-organ formation from stem cells can only be assayed rigorously by in vivo transplantation experiments in immunodeficient animals.1 Xenotransplantation of human hematopoietic stem cells into nonobese diabetic–severe combined immunodeficient (NOD/scid) mice is the standard method to assay human hematopoietic tissue formation.1,2 Transplanted human hematopoietic scid repopulating cells (SRCs) are able to migrate to murine bone marrow and initiate a differentiation program. Due to the distinct hematopoietic microenvironment in the mouse and the presence of non–species-crossreacting growth factors, this differentiation is abortive, however, and there is no replacement of mouse blood with human blood cells.2 In the mouse, distinct human lineages are represented differentially, depending on the presence or absence of the appropriate crossreacting cytokine. In the macrophage-dendritic lineage, for example, the immature DR++, CD4+, HIV-chemokine receptor–positive cells with dendritic morphology are present, but there is no costaining for mature dendritic cell markers.3
We analyzed the potential of these immature human dendritic cells to support HIV-1 infection and found that productive infection is dependent on the presence of exogenously administered recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF; F.S. et al, manuscript in preparation). In an attempt to provide sufficient hGM-CSF, we studied the use of direct lentivirus-mediated transgenesis of fertilized oocytes in mice on the NOD/scid genetic background. This technique is highly efficient in this strain, eliminating the need for microinjection into the conventional strains used for transgenesis and subsequent backcrossing. This method is a powerful tool for humanizing the lymphohematopoietic microenvironment of NOD/scid mice. It is probable that this approach can be applied to formation of other human neo-organs after tissue-specific human stem cell xenotransplantation.
Study design
Lentiviral vector preparation
The prrl CMV EGFP wpre sin 18 plasmid was obtained from L. Naldini (IRCC, Turin, Italy) and used to generate prrl CMV hGM-CSF. The lentiviral vector was produced in 293T cells and concentrated by ultracentrifugation to 108 IU/mL as described elsewhere.4
Zygote microinjection
Female NOD/scid mice (8-10 weeks old) were used as zygote donors. Fertilized eggs were retrieved from the ampullae (oviducts) in M2 medium5 containing 350 μg/mL hyaluronidase (H-3884; Sigma, St Louis, MO) to remove cumulus cells. After washing in M2 medium at room temperature, zygotes were maintained in mKSOMAA medium6 (37°C, 5% CO2) until use. Zygotes were microinjected in a drop of M2 medium covered with mineral oil (M-8410; Sigma) at 24°C. A 1.0 μm to 1.5 μm inner diameter needle was inserted into the perivitelline (subzonal) space of the zygote, and injection was considered successful when the zona pellucida swelled visibly. Injected zygotes were washed in several drops of mKSOMAA medium and cultured overnight in mKSOMAA medium drops (37°C, 5% CO2). Two cell–stage embryos were transferred into the ampullae (oviduct transfer) of a pseudopregnant recipient CD1 female (+0.5 days after coitus) previously mated with a vasectomized male.
Southern blot and hGM-CSF ELISA
Tail genomic DNA was digested with BamHI and blotted. A 270–base pair (bp) fragment was used as probe. Mouse serum was analyzed by hGM-CSF–specific enzyme-linked immunosorbent assay (ELISA; R & D Systems, Minneapolis, MN).
Results and discussion
Lentivirus-mediated transgenesis on the NOD/scid background is highly efficient
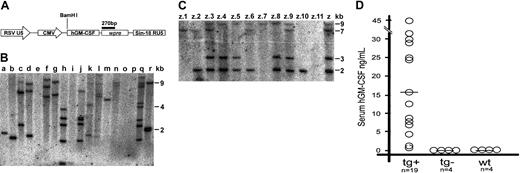
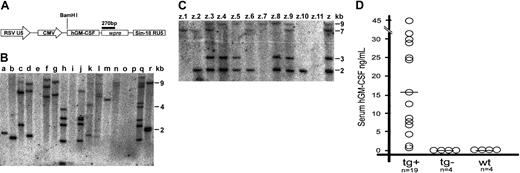
An HIV-1–based lentiviral vector (prrl CMV hGM-CSF) was employed. In this vector, the hGM-CSF gene is expressed under a cytomegalovirus (CMV) promoter (Figure 1A).4 Expression from this construct is directed mainly to the epidermis and musculoskeletal tissue (I.P. et al, manuscript in preparation). Table 1 indicates the number of embryos microinjected. A total of 95 embryos were obtained from hyperovulated NOD/scid donors. Fertilized embryos were microinjected with a lentiviral vector concentrate (108 IU/mL titer) into the perivitelline space, then cultured for 24 hours before embryo transfer. Figure 1B shows Southern blot analysis with a vector probe of genomic DNA from tails of 3-week-old mice. Each band indicates a single integrative event. All 35 pups born had no anomalies; of these, 24 (68.5%) were positive and 11 showed no integration. Transgenic animals had an average of 4 integrations (minimum 1, maximum 8). Control mice did not label with the probe. One male with 4 proviral integrations was crossed with wild-type NOD/scid mice; Figure 1C shows allele segregation and transmission through the germ line.
NOD/scid lentiviral transgenesis and hGM-CSF expression. (A) Schematic diagram of the prrl CMV hGM-CSF lentiviral vector. (B) Proviral transgene insertion in F0 mice (a-r) generated by subzonal hGM-CSF–lentivirus vector microinjection of single-cell embryos. A Southern blot analysis of BamHI-digested tail genomic DNA probed with a 270-bp fragment corresponding to a wpre gene present in the lentiviral vector is shown. Each band represents a proviral insertion. (C) Germ line transmission and allele segregation of the proviral transgene. A Southern blot analysis of genomic DNA of founder (Z) and F1 offspring (z.1 to z.11) resulting from the cross with a wild-type NOD/scid mouse is shown. (D) hGM-CSF expression in serum of hGM-CSF transgenic (tg+), nontransgenic (tg-), and wild-type (wt) NOD/scid mice. Each circle indicates an individual mouse and each horizontal bar shows an arithmetical average.
NOD/scid lentiviral transgenesis and hGM-CSF expression. (A) Schematic diagram of the prrl CMV hGM-CSF lentiviral vector. (B) Proviral transgene insertion in F0 mice (a-r) generated by subzonal hGM-CSF–lentivirus vector microinjection of single-cell embryos. A Southern blot analysis of BamHI-digested tail genomic DNA probed with a 270-bp fragment corresponding to a wpre gene present in the lentiviral vector is shown. Each band represents a proviral insertion. (C) Germ line transmission and allele segregation of the proviral transgene. A Southern blot analysis of genomic DNA of founder (Z) and F1 offspring (z.1 to z.11) resulting from the cross with a wild-type NOD/scid mouse is shown. (D) hGM-CSF expression in serum of hGM-CSF transgenic (tg+), nontransgenic (tg-), and wild-type (wt) NOD/scid mice. Each circle indicates an individual mouse and each horizontal bar shows an arithmetical average.
Expression of the hGM-CSF transgene
hGM-CSF expression was measured in mouse serum in a human-specific ELISA (Figure 1D). All transgenic animals expressed hGM-CSF (range: 200 pg/mL-44 ng/mL; mean: 15 ng/mL). There was no correlation between expression level and number of integrations. Nontransgenic littermates had undetectable hGM-CSF levels, similar to uninjected wild-type NOD/scid mice. F1 mice also expressed variable hGM-CSF levels (not shown).
There is considerable interest in obtaining human organs and tissues from transplanted human stem cells. This human neo-organ formation can only be experimentally assayed in vivo using immunodeficient mouse strains in which the human stem cells find a niche in which they can proliferate and differentiate into organ tissue–specific mature cells.1 Human hematopoietic grafts in the NOD/scid mouse strain have been studied thoroughly. Due to the different hematopoietic microenvironment between man and the mouse, however, human blood lineage formation is abortive. Initial attempts to humanize the cytokine microenvironment of NOD/scid animals used conventional plasmid microinjections into non-NOD strains, followed by backcrossing onto the NOD/scid background.7-9 This approach is time consuming, and insertion of multiple human cytokine genes may require many backcrosses.
A highly efficient lentiviral vector infection method was recently reported for generating transgenic mice and other animals species in which conventional transgenesis often fails.10,11 Single-cell embryos are exposed to lentiviral vector–containing medium with no damage to embryo membranes. After several attempts to produce transgenic NOD/scid mice by conventional techniques,12 we tested lentiviral vector microinjection into the perivitelline space. Previous tests of this method on conventional genetic backgrounds resulted in efficient generation of transgenic mice and Mendelian transmission of the lentiviral provirus in successive generations with no significant silencing of retroviral expression (not shown).
The hGM-CSF lentiviral vector microinjection resulted in generation of transgenic NOD/scid mice in 68.5% of mice born, a higher percentage than that obtained by conventional transgenesis in appropriate strains.12 Most animals had multiple integrations, concurring with observations by other investigators.10 All transgenic mice expressed hGM-CSF in serum, as measured by human-specific ELISA. We found no correlation between integration number and expression levels, indicative that positional effects play an important role in expression. As predicted, transgenic mice transmitted the provirus to their progeny with independent allele segregation. This suggests that contrary to conventional transgenesis, in which most multiple integrations are in tandem, the integrated proviruses represent independent integrative events in separate areas of the genome.12
Direct lentiviral vector transgenesis is thus a highly efficient method for obtaining NOD/scid transgenic mice. A single round of viral concentrate microinjection into the perivitelline space is sufficient to obtain founder mice with an array of transgene expression levels. This procedure should be useful for humanizing the cytokine microenvironment of mice, which will assist in human stem cell research.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-07-2298.
Supported in part by Plan Nacional de Salud y Farmacia, CICYT (SAF2001-2262), and a specific grant (3026/99) from the FIPSE Foundation to A.B. F.S. received support from the FIPSE Foundation. The Department of Immunology and Oncology was founded and is supported by the Spanish Council for Scientific Research (CSIC) and by Pfizer.
I.P. and L.M.C. contributed equally to this work.
F.S. and A.B. are senior authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the workers of the Centro Nacional Biotecnología (CNB) animal facility for their assistance, C. Mark for editorial work, and the organizers and teachers of the European Molecular Biology Organization (EMBO) course “Lentiviral Vectors” held at the Institute for Cancer Research and Treatment (IRCC), Turin, Italy.