Abstract
Polymorphonuclear neutrophils (PMNs) produce an abundance of bactericidal and cytotoxic molecules consistent with their role as first-line defense against bacterial infection. PMNs, however, also cause efficient cellular cytotoxicity when targeted through Fc receptors to appropriate antibody-coated target cells. Although this so-called antibody-dependent cellular cytotoxicity (ADCC) was described many years ago, the mechanism of killing is still elusive. We now have found that PMNs contain perforin and granzyme B, the 2 molecules known as the cytotoxic entity of natural killer cells and of cytotoxic T lymphocytes as well. Lysates of PMNs were lytic for chicken erythrocytes in a time-, temperature-, and Ca2+-dependent manner. Moreover, apoptosis of Jurkat cells was induced, consistent with the observation that the PMN lysates contain enzymatically active granzyme B. Taken together, our data provide evidence for the presence of perforin and granzyme B within the cytotoxic arsenal of PMNs. (Blood. 2004;103:1099-1104)
Introduction
Polymorphonuclear neutrophils (PMNs) are mainly recognized for their bactericidal activity and their role as “first-line defense.” However, ample evidence indicates that PMNs are also potent effector cells of antibody-dependent cellular cytotoxicity (ADCC). Since the first description of ADCC by PMNs,1 different target cells for PMN-mediated ADCC have been identified, notably erythrocytes, tumor cells, or virus-infected cells.2-5 Although the participation of the different immunoglobulin receptors has been studied in great detail, as has the effect of cytokines on the efficacy of cytotoxicity,6-13 the mechanism of killing is still elusive.
A whole array of toxic substances is stored in PMNs in addition to radicals generated during phagocytosis and host defense. Their contribution to ADCC, however, has not been unequivocally demonstrated. A participation of reactive oxygen species had been described in lysis of antibody-coated erythrocytes,14,15 but not for tumor cells lysis.16-18 Moreover, inhibition of RNA or protein synthesis apparently did not inhibit the ADCC,10 implying preformed mediators.
Early ultrastructural studies with virus-infected cells as targets pointed to the formation of transmembrane channels generated by direct contact of the PMNs with their target cell.19 The latter finding would be compatible with the release of perforin, as it has been described for other cytotoxic effector cells such as natural killer (NK) cells or cytotoxic T cells. Perforin inserts into target membranes, where it assembles to form transmembrane pores (for reviews, see Podack20 and Tschopp and Nabholz21 ).
Perforin is lytic for some target cells only; together with granzymes, however, a large number of target cells are killed by induction of apoptosis. Although the exact sequence of events leading to cell death is not yet completely understood, there is agreement that both granzymes and perforin, acting synergistically, are the cytotoxic entity of NK cells and of cytotoxic T cells as well (for reviews, see Tschopp and Nabholz,21 Froelich et al,22 and Shresta et al23 ).
On the basis of these findings, we tested whether perforin and granzymes were also expressed by PMNs.
Materials and methods
Media for cell culture
AIM V and RPMI were obtained from Gibco (Eggenstein, Germany); hydroxyethyl starch (HES) was from Fresenius (Gräfelfing, Germany). Propidium iodide (PI) was obtained from Sigma (München, Germany) and interferon γ (IFN-γ) from Boehringer Mannheim (Mannheim, Germany). The following antibodies were used: antihuman perforin (clone δG9; PharMingen, obtained through Becton and Dickinson, Heidelberg, Germany), anti-granzyme B (clone 2C5/F5l; Silenius, Boronia, Australia), anti-CD66b (Coulter Immunotech, Marseille, France), mouse IgG as isotype control (Coulter Immunotech), and as secondary antibodies fluorescein isothiocyanate (FITC)-labeled antimouse IgG (from goat) and cyanine 3 (Cy3)-conjugated antimouse antibody (from goat), both obtained from Jackson ImmunoResearch (West Grove, PA).
The chromogenic substrate Boc-Ala-Ala-Asp-pNA was obtained from Bachem (Weil am Rhein, Germany).
Isolation and characterization of PMNs and T lymphocytes
Blood was taken from healthy donors by venous puncture using 7.5-mL heparin-coated tubes (Sarstedt, Nümbrecht, Germany). PMNs were separated from heparinized blood by PolymorphPrep (PMP; Nycomed, Oslo, Norway). PMNs were positively selected by anti-CD15 beads and T cells by anti-CD3 beads using Automacs (Miltenyi Biotec, Bergisch-Gladbach, Germany). Cell purity was higher than 99% as judged by cytofluorometry.
The Jurkat cell line was cultivated in RPMI, supplemented with fetal calf serum (5%) and antibiotics.
Cultivation of PMNs
Purified PMNs (1 × 106/mL) were cultivated in AIM V with 2.5% autologous serum (heat inactivated at 56°C for 30 minutes) for 24 to 48 hours with or without IFN-γ (100 U/mL). Under those conditions up to 80% of PMNs survived.24 Lysates were prepared by sonicating 107 PMNs on ice 5 times for 20 seconds each (22 W). To prevent proteolysis, a mixture of 5 mM EDTA (ethylenediaminetetraacetic acid), 100 μM phenylmethylsulfonyl fluoride (PMSF), and 10 μL/mL proteinase inhibitor cocktail (Sigma P-8340) was used. When preparing lysates for functional testing, the EDTA and the protease inhibitor cocktail were omitted.
Cytofluorometry
For detection of intracellular antigens, whole blood was mixed with HES (2:1 vol/vol); following incubation for 30 minutes at room temperature, the cell-rich plasma was recovered. To 100 μL, 500 μL permeabilizing solution (Becton Dickinson) was given, and after 20 minutes of incubation at room temperature, the cells were washed in fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline [PBS], containing 1% bovine serum albumin, 0.1% sodium azide). To the cell pellet, the respective antibodies or mouse IgG were given (30 minutes, room temperature), and following washing with FACS buffer, a phycoerythrin (PE)-labeled antibody to mouse IgG. After 30 minutes of incubation in the dark, the cells were washed twice in FACS buffer and resuspended on 1% paraformaldehyde. For double staining a FITC-labeled antibody to CD66b was used, which was added incubated with the washed PMNs for 20 minutes prior to adding the paraformaldehyde. Fluorescence was measured by FACScalibur using CellQuest software (Becton Dickinson). PMNs were identified by CD66b, and the gate was set accordingly.
Confocal laser microscopy
Purified PMNs (3 × 105) were resuspended in 500 μL FACS buffer, centrifuged onto PolyPrep slides (Sigma) by cytospin (700 rpm/5 min), and fixed with ice-cold ethanol for 30 seconds. To reduce nonspecific binding, cells were incubated with 100 μL PBS, containing 0.5% normal mouse serum and 0.1% sodium azide for 30 minutes at room temperature. The following antibodies were used: antihuman perforin, anti-granzyme B, anti-CD66b, and mouse IgG as isotype control (all diluted 1:50) and as secondary antibody a Cy3-conjugated antimouse IgG. Slides were incubated for 1 hour at room temperature with the respective antibodies. After repeated washing, cells were fixed with Dako mounting medium (Dako, Carpinteria, CA). The slides were examined by confocal laser microscopy (Leica microscope DM RBE, Leica laser system TCS NT; Leica, Bensheim, Germany) and Windows NT as software.
Immunoadsorption
Antibodies to perforin, granzyme B, or mouse IgG (1 μg each) were coupled to 500 μL protein A Sepharose (Pharmacia, Uppsala, Sweden) at 4°C overnight. Free-binding sites were blocked with 0.2 M glycine. The material was then washed twice (0.1 M sodium acetate/0.5 M NaCl), suspended in 0.1 M NaHCO3/0.5 M NaCl, and was then ready for use. Freshly isolated PMNs (5 × 107) or PMNs cultivated for 24 hours in the presence of IFN-γ (100 U/mL) were washed twice in PBS, pelleted, and resuspended in 500 μL lysing buffer (1 mg/mL CHAPS [3[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid] in PBS, pH 7.4, containing 0.01 M EDTA, 1 mM PMSF, and 0.1% sodium azide). After 10 minutes, the sample was centrifuged (10 minutes at 100 000g) and the supernatant collected and incubated with the immobilized antibodies for 3 hours at room temperature. Then the supernatants were harvested for functional testing. To release perforin, the Sepharose was washed extensively with PBS and then eluted with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer.
Western blotting
Lysates of PMNs (1-5 × 106) were applied to 9% SDS-PAGE and then blotted onto nitrocellulose. Binding of the antibody to granzyme B was determined using the enhanced chemiluminescence (ECL) Western blotting analysis system (Amersham Pharmacia Biotech, Freiburg, Germany).
RT-PCR for perforin and granzyme B
PMNs were cultivated in AIM V/2.5% heat-inactivated serum with or without IFN-γ for 0, 2, 4, 6, or 10 hours. Then the total cellular RNA was isolated using the High Pure RNA Isolation Kit from Roche (Roche Diagnostics, Mannheim, Germany). For reverse transcription (RT) the SuperScript Preamplification System (Life Technologies, Gibco BRL, Gettysburg, PA) was used. Polymerase chain reaction (PCR) for granzyme B and perforin was carried out in a Hybaid thermal cycler as follows: to 5 μL RNA 10 pmol dNTP was added, 25 pmol of the respective primers and 1 U Taq DNA polymerase in 2 mM MgCl2. After preheating to 94°C for 5 minutes, 10 cycles were performed (94°C 30 seconds; 66°C 30 seconds; 73°C 30 seconds) followed by 25 cycles (94°C 1 minute; 63°C 1 minute; 73°C 1 minute) followed by a final step at 73°C for 5 minutes. The following primers were used: granzyme B sense: 5′ TGC AGG AAG ATC GAA AGT GCG 3′; antisense: 5′ GAG GCA TGC CAT TGT TTC GTC 3′ amplifying a 824-bp fragment25 ; perforin-specific primers sense: 5′ CAG TAC AGC TTC AGC ACT GAC 3′; antisense: 5′ ATG AAG TGG GTG CCG TAG TTG 3′ were used as described,26 yielding a 176-base pair (bp) fragment. The primers were synthesized by ARK Scientific Biosystems (Darmstadt, Germany). The PCR products were analyzed by gel electrophoreses (1.5% agarose) and stained with Sybr green (Molecular Probes, Leiden, The Netherlands). As size markers, DNA molecular weight markers III and VI were used (Boehringer Mannheim). The bands were quantified using a FLA 2000 scanner (Fuji, Tokyo, Japan) and Image Gauge version 3.0 as software.
Quantitative detection of granzyme B
A commercially available trapping assay (Hölzel Diagnostika, Köln, Germany) was used. The antibody-coated beads provided with the assay were also used for depletion of granzyme B from the PMN lysates in selected experiments as indicated.
Granzyme B enzymatic activity was tested in lysates of 107 highly purified PMNs, using a previously published protocol.27 The granzyme B inhibitor 1 (Z-AAd-CMK) and Z-VAD were purchased from Calbiochem (Marburg, Germany).
Hemolytic test
Chicken erythrocytes (106/100 μL) were incubated with PMN lysates (100 μL) in various concentrations. Release into the supernatant of hemoglobin was measured after various times at 37°C.
Killing test
Jurkat cells were propagated in RPMI containing 10% fetal calf serum and 1% penicillin. Cells were washed in RPMI and resuspended to a final concentration of 1 × 107/mL. One to 2 mL cells/well were placed into a 24-well culture plate (Greiner, Nürtingen, Germany). After various times, aliquots were taken and viability was determined. For PI staining cells were centrifuged and PI solution (1 mg/mL PBS) was added to the pellet. After incubation for 10 minutes, cells were analyzed by FACScalibur and CellQuest software. Cell death was measured different times after adding the PMN lysates or an antibody to CD95 (anti-Fas) used as “positive” control.
Killing was also determined by trypan blue exclusion. For this procedure, at least 100 cells were counted, and cell death expressed as percentage of blue, that is, dead cells.
Results
Detection of granzyme B and perforin in PMNs
By confocal laser microscopy ethanol-fixed PMNs were visualized by CD66b expression. In these cells also perforin and granzyme B were seen. Both perforin and granzyme B displayed a diffuse staining pattern with exclusion of the nucleus (Figure 1).
Visualization of perforin and granzyme B by confocal microscopy. Isolated, ethanol-fixed PMNs were incubated with either mouse IgG or monoclonal antibodies to CD66b, granzyme B, or perforin. Then, Cy3-labeled antibody to mouse IgG was added. The bottom row shows an overview (original magnification × 400); the top row is a zoom (original magnification × 2.4) on selected cells to show details.
Visualization of perforin and granzyme B by confocal microscopy. Isolated, ethanol-fixed PMNs were incubated with either mouse IgG or monoclonal antibodies to CD66b, granzyme B, or perforin. Then, Cy3-labeled antibody to mouse IgG was added. The bottom row shows an overview (original magnification × 400); the top row is a zoom (original magnification × 2.4) on selected cells to show details.
In highly purified, permeabilized PMNs, granzyme B and perforin could be detected by cytofluorometry (Figure 2A-B). When PMNs of different donors were compared, a considerable variation of the mean fluorescence was seen, indicating donor-inherent differences. In all donors, however, perforin and granzyme B were detectable (data of 7 donors are summarized in Figure 2C).
Presence of granzyme B and perforin in highly purified permeabilized PMNs as detected by cytofluorometry. (A) Saponin-permeabilized PMNs were incubated with an antibody to perforin or granzyme B. Next, a PE-conjugated antimouse IgG was added and after extensive washing an FITC-labelled antibody to CD666. On the top, the isotype controls are shown. (B) In another set of experiments, highly purified, saponin-permeabilized PMNs were incubated with antibodies to CD66b, perforin, or granzyme B; then, antimouse IgG-FITC was added (thick lines). The thin lines represent the isotype controls. (C) Expression of perforin and granzyme B was measured in 7 individuals; the data are summarized as box blots with the boxes containing 50% of the values. Horizontal bars represent the median; squares, the mean.
Presence of granzyme B and perforin in highly purified permeabilized PMNs as detected by cytofluorometry. (A) Saponin-permeabilized PMNs were incubated with an antibody to perforin or granzyme B. Next, a PE-conjugated antimouse IgG was added and after extensive washing an FITC-labelled antibody to CD666. On the top, the isotype controls are shown. (B) In another set of experiments, highly purified, saponin-permeabilized PMNs were incubated with antibodies to CD66b, perforin, or granzyme B; then, antimouse IgG-FITC was added (thick lines). The thin lines represent the isotype controls. (C) Expression of perforin and granzyme B was measured in 7 individuals; the data are summarized as box blots with the boxes containing 50% of the values. Horizontal bars represent the median; squares, the mean.
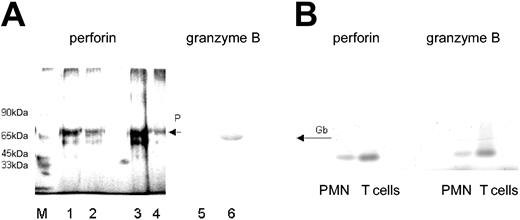
Because the antibody to perforin was not suitable for Western blotting, the presence of perforin in PMNs was confirmed by immunoadsorption. A protein with an apparent molecular weight of 70 kDa was obtained, similar to that derived from T cells, which were used as “positive control” (Figure 3A). Moreover, by using primer pairs designed for the NK cell-derived perforin, from PMN-RNA an amplification product could be generated by RT-PCR, which was similar in size to that obtained with T cell-derived RNA (Figure 3B).
Presence of perforin and granzyme B in lysates of PMNs. (A) By immunoadsorption, perforin was found in lysates of freshly isolated T lymphocytes (2 and 1 × 106; lanes 1 and 2, respectively) and freshly isolated PMNs (5 × 106; lane 3), and to a lesser degree in PMNs cultivated for 48 hours in IFN-γ (100 U/mL lane 4; detected by silver staining). The presence of granzyme B in PMN lysates was shown by Western blotting (lane 6). Lane 5 shows the respective negative control (mouse IgG in place of antibody to granzyme B). M represents the molecular size marker. (B) Detection of perforin- and granzyme B-specific message by RT-PCR. RNA was extracted from highly purified PMNs (106) or T cells (106); then, RT-PCR was performed with primers specific for either perforin or granzyme B. The RT-PCR products obtained from PMN RNA were of similar size as those derived from T cells.
Presence of perforin and granzyme B in lysates of PMNs. (A) By immunoadsorption, perforin was found in lysates of freshly isolated T lymphocytes (2 and 1 × 106; lanes 1 and 2, respectively) and freshly isolated PMNs (5 × 106; lane 3), and to a lesser degree in PMNs cultivated for 48 hours in IFN-γ (100 U/mL lane 4; detected by silver staining). The presence of granzyme B in PMN lysates was shown by Western blotting (lane 6). Lane 5 shows the respective negative control (mouse IgG in place of antibody to granzyme B). M represents the molecular size marker. (B) Detection of perforin- and granzyme B-specific message by RT-PCR. RNA was extracted from highly purified PMNs (106) or T cells (106); then, RT-PCR was performed with primers specific for either perforin or granzyme B. The RT-PCR products obtained from PMN RNA were of similar size as those derived from T cells.
The presence of granzyme B in PMN lysates was verified by Western blotting (Figure 3A). Moreover, an RT-PCR product could be obtained with primers designed for NK cells, in size similar to the T-cell product (Figure 3B). By a trapping assay, granzyme B was determined quantitatively. The lysates of 5 × 107 PMNs contained 175 ± 27 pg granzyme B. Compared to T cells, the abundance of granzyme B was about 5 times less: 107 T cells contained 250 ± 35 pg granzyme B (data are mean values derived from experiments using cells of 3 different donors; the large SD reflects most probably the interindividual differences that were seen also by cytofluorometry).
Effects of IFN-γ on perforin and granzyme B
IFN-γ is known to activate PMNs, particularly the protein synthesis and the escape from constitutive apoptosis. Therefore, the effect of IFN-γ on perforin and granzyme B in highly purified PMNs was examined. In doses known to induce interleukin 8 (IL-8)-specific mRNA and synthesis (100 U/mL), IFN-γ did not enhance the abundance of the perforin-specific message. In the same cells, however, a 2- to 2.5-fold increase of the granzyme B RT-PCR product was seen 2 to 4 hours after stimulation (1 of 3 experiments is shown in Figure 4).
Effect of IFN-γ on granzyme B and perforin mRNA. (A) Highly purified PMNs (106) were incubated with IFN-γ (100 U/mL) for the times indicated; then, mRNA was extracted and RT-PCR was performed with primers specific for either perforin or granzyme B. β-actin was used as a housekeeping gene. (B) Semiquantitative analysis using the data derived from scanning corrected for the β-actin data (nonstimulated PMNs, □; IFN-stimulated PMN, ▪).
Effect of IFN-γ on granzyme B and perforin mRNA. (A) Highly purified PMNs (106) were incubated with IFN-γ (100 U/mL) for the times indicated; then, mRNA was extracted and RT-PCR was performed with primers specific for either perforin or granzyme B. β-actin was used as a housekeeping gene. (B) Semiquantitative analysis using the data derived from scanning corrected for the β-actin data (nonstimulated PMNs, □; IFN-stimulated PMN, ▪).
There was, however, no evidence for an increase in protein synthesis. After 24 hours rather a reduction of the granzyme B protein was seen. By the quantitative trapping assay, 120 ± 8 pg/mL was found after 24 hours of culture with IFN-γ compared to 178 ± 16 pg/mL in the freshly isolated PMNs (values represent mean of triplicates).
Enzymatic activity of the PMN lysates
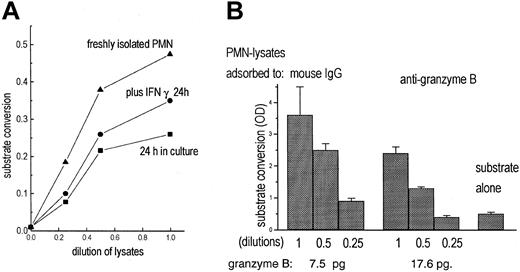
To test for functional activity of granzyme B, a chromogenic substrate (Boc-Ala-Ala-Asp-pNA), specific for enzymes, cleaving off terminal aspartic acid, was used. With lysates of freshly isolated, highly purified PMNs substrate conversion was seen (Figure 5A). Lysates of cultivated PMNs (IFN-γ for 24 hours) were also enzymatically active, but to a lesser degree.
Detection of granzyme B activity in PMNs. (A) Lysates of freshly isolated PMNs (5 × 10; (▴) contained enzyme activity measured by cleavage of the chromogenic substrate Boc-Ala-Ala-Asp-pNA. Lysates of unstimulated PMNs (•) or of PMNs cultivated for 24 hours with IFN-γ (100 U/mL; ▪) contained less enzymatic activity. (B) PMN lysates were adsorbed to mouse IgG or antigranzyme B; then substrate conversion was measured as increase of optical density (OD) in 3 dilutions. In parallel, granzyme B adsorbed to the antibody-coated beads was determined by quantitative cytofluorometry (data represent mean of triplicates).
Detection of granzyme B activity in PMNs. (A) Lysates of freshly isolated PMNs (5 × 10; (▴) contained enzyme activity measured by cleavage of the chromogenic substrate Boc-Ala-Ala-Asp-pNA. Lysates of unstimulated PMNs (•) or of PMNs cultivated for 24 hours with IFN-γ (100 U/mL; ▪) contained less enzymatic activity. (B) PMN lysates were adsorbed to mouse IgG or antigranzyme B; then substrate conversion was measured as increase of optical density (OD) in 3 dilutions. In parallel, granzyme B adsorbed to the antibody-coated beads was determined by quantitative cytofluorometry (data represent mean of triplicates).
To assess whether the enzymatic activity was due to granzyme B, effects of antibodies to granzyme B were tested. The soluble antibodies did not inhibit the substrate conversion (that the antibody is not inhibiting function is also stated in the data sheet supplied by the manufacturer). Following incubation of the lysates with the immobilized antibodies, provided with the trapping assay, the enzymatic activity was considerably reduced, whereas granzyme B was detected on the beads (Figure 5B). That inhibition was not complete indicates that PMNs contain substrate-converting activities other than granzyme B.
Hemolysis induced by PMN lysates
To measure lytic activity of PMN lysates, chicken erythrocytes were used as target cells. Lysates from freshly isolated PMNs, cultivated PMNs, or of supernatants derived from PMNs were added to the erythrocytes and after various times, release into the supernatant of hemoglobin was measured. Considerable hemolysis was seen only with lysates of freshly isolated PMNs. After 20 minutes of incubation at 37°C up to 56% lysis was seen (41 ± 12.0; data derived from 5 independent experiments) and increased for up to 2 hours (Figure 6).
Hemolysis induced by PMN lysates. Chicken erythrocytes (1 × 106/100 μL Hanks balanced salt solution [HBSS]) were incubated with lysates obtained from 106 PMNs in 100 μL HBSS (undiluted) or in the dilutions indicated (A). Hemolysis was measured after 20 minutes of incubation. (B) Hemolysis induced by the undiluted lysate was measured after various times (all values represent the mean ± SD of 3 independent experiments).
Hemolysis induced by PMN lysates. Chicken erythrocytes (1 × 106/100 μL Hanks balanced salt solution [HBSS]) were incubated with lysates obtained from 106 PMNs in 100 μL HBSS (undiluted) or in the dilutions indicated (A). Hemolysis was measured after 20 minutes of incubation. (B) Hemolysis induced by the undiluted lysate was measured after various times (all values represent the mean ± SD of 3 independent experiments).
The presence of EDTA (0.01 M) prevented hemolysis, as did temperatures below 25°C. Depleting perforin by the immobilized antibody used for immunoadsorption resulted in a loss of hemolytic activity.
Induction of target cells killing
Jurkat cells were used as targets and induction of cell death was measured by PI incorporation. For comparison, an antibody to CD95 (anti-Fas), a bona fide inducer of apoptosis, was used. Lysates of freshly isolated PMNs induced cell death, progressing with time (an example is shown in Figure 7; data from different experiments are summarized in Table 1). Killing of Jurkat cells was also assessed by determining trypan blue exclusion. Here cell death became apparent after 2 to 4 hours (Table 1).
Induction of cell death by PMN lysates. PI incorporation was measured in untreated Jurkat cells and in cells treated with anti-Fas, culture medium, or lysates of PMNs for 1 or 4 hours.
Induction of cell death by PMN lysates. PI incorporation was measured in untreated Jurkat cells and in cells treated with anti-Fas, culture medium, or lysates of PMNs for 1 or 4 hours.
As expected, soluble antibodies to either granzyme B or perforin did not inhibit cell killing; however, depletion of granzyme B or of perforin by Sepharose-coated antibodies reduced the cytotoxicity (Table 1). The granzyme B inhibitor 1 (Z-AAD-CMK) inhibited killing by 50%, when 1 μM was used; with Z-VAD all cells survived.
Discussion
PMNs mediate ADCC through their Fc receptors, particularly after appropriate stimulation.1,6-13 This long-recognized function has gained increasing interest, particularly with regard to a role of PMNs in tumor defense2,3,5,12,13 or elimination of virus-infected cells.4 We now provide evidence that PMNs contain perforin and granzyme B, 2 proteins described to act synergistically in the induction of cytotoxicity either by T cells of by NK cells.20-23
By various techniques preformed perforin and granzyme B were detected in PMNs of healthy donors. The reactivity with antibodies raised against T cell- or NK cell-derived proteins, respectively, and the amplification products obtained by RT-PCR using primers designed for the NK cell-derived proteins, indicate that the PMN-derived proteins are similar to the proteins derived from T lymphocytes or NK cells.
That both proteins are preformed is consistent with previous data by others showing that PMNs do not require protein synthesis for efficient ADCC to occur, but that preformed mediators play a major role in that process.10,28,29
Although the data clearly indicate that PMNs contain perforin and granzyme B, their participation in ADCC awaits further study. Consistent with the pore-forming capacity of perforin, lysates of PMNs caused hemolysis in a time-, temperature- and Ca2+-dependent manner30 ; consistent with granzyme B activity, lysates of PMNs induced killing of Jurkat cells, presumably by apoptosis.31,32 Depleting the lysates of perforin or granzyme B greatly reduced the respective functional activity. Even though functional activities of other cytotoxic proteins cannot be excluded, our data provide evidence for functional activity of both perforin and granzyme B.
Thus, so far nothing argues against the proposition that PMNs use exactly the same mechanisms for ADCC as NK cells: a vectorial exocytosis of perforin and granzyme(s), which synergistically kill the target cells by puncturing their membrane and by inducing apoptosis. One discrepancy appears to be the effect of IFN-γ. Although ample evidence suggests that IFN-γ enhanced ADCC by PMNs,6,8,10,11,33 it apparently does not enhance availability of either perforin or granzyme B. On the other hand, IFN-γ up-regulates expression of Fc receptors, particularly of CD64, and also expression of CD11b/CD18. Because both receptors are critically involved in ADCC, IFN-γ might rather promote the contact between the PMNs and the target cell and hence the cytotoxic synapse formation than increase the availability of either perforin or granzyme B.12,13,34-36 This interpretation again fits with previous data that de novo synthesis of proteins is not required for ADCC.10
Participation in ADCC might not be the only function of granzyme B. Presence of a peptide sequence with bactericidal activity was described in granzyme B, as well as killing of intracellular pathogens in T lymphocytes.37,38 Although a role of granzyme B in bacterial defense has not been established as yet, it would be within the scope of the PMN function.
Taken together, our data provide relevant information regarding the cytotoxic potential of PMNs, which, in light of the increasing interest in PMNs also as effector cells for tumor cell killing, add to the understanding of their physiologic role as cytotoxic cells.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-04-1069.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




![Figure 6. Hemolysis induced by PMN lysates. Chicken erythrocytes (1 × 106/100 μL Hanks balanced salt solution [HBSS]) were incubated with lysates obtained from 106 PMNs in 100 μL HBSS (undiluted) or in the dilutions indicated (A). Hemolysis was measured after 20 minutes of incubation. (B) Hemolysis induced by the undiluted lysate was measured after various times (all values represent the mean ± SD of 3 independent experiments).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/3/10.1182_blood-2003-04-1069/6/m_zh80030455840006.jpeg?Expires=1766215496&Signature=itTH2pQjRGlSZF1R6q4LAHsCmOvL-S05BJZ-atMbRRB4zfug3rPVUtXFzV5D10ous3GdFbOnlyErmXQeK0HQg2Uk72XyAAtnDorbsuLX9Bws0fZa4vIvHzpUTgkoPgQ-W4TfguyHWkNdJMpzCaTQmAv75Ebzjx7SK6lHejmISUM~jGMZH0ux3US9cWfcbOjvZwJc-UorQ4BTSXnGUWfG5ftj9ZX0W2sldRKwxA5E1w-ZKTz9rgncNifu3yXRMevs0pL5SlKil4T2MqsBzUXflL732rG2aM9r0iHry5D~pOgTu0j3Ku7NqLmOA7nY-Mge-oYT~hAfrWxOdCKxMPjE-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

