Abstract
Two helper-dependent (HD) adenoviral vectors encoding a canine factor VIII B-domain–deleted transgene (cFVIII) were constructed and evaluated in 4 hemophilia A dogs. One vector was regulated by the cytomegalovirus (CMV) promoter (HD-CMV-cFVIII), while the other vector contained a tissue-restricted promoter comprised of the human FVIII proximal promoter with an upstream concatemer of 5 hepatocyte nuclear factor 1 binding sites (HD-HNF-cFVIII). We detected no toxicity at low dose (5 × 1011 vp/kg), but at higher vector doses (> 1 × 1012 vp/kg) transient hepatotoxicity and thrombocytopenia were observed. Low-level increases in FVIII activity were detected in all 3 HD-HNF-cFVIII–treated dogs, which corresponded with decreased whole blood clotting times. None of the animals receiving the HD-HNF-cFVIII vector developed FVIII inhibitors, and in 1 of the 3 animals, FVIII activity was sustained for over 6 months after treatment. One animal, which received the HD-CMV-cFVIII vector, achieved peak levels of FVIII above 19 000 mU/mL, but FVIII activity disappeared within 1 week, coincident with the development of a potent anti–canine FVIII antibody response. This study supports previous demonstrations of improved safety using HD gene transfer and suggests that these vectors can provide transient FVIII expression with minimal, acute toxicity in the absence of inhibitor formation.
Introduction
Over the past 50 years, critical advances in the treatment of hemophilia A have been made, which have helped to vastly extend and improve the life of hemophiliacs. However, each of these innovations has been fraught with problems such as infectious agent transmission (including HIV and hepatitis C), catheter infections, inhibitor formation, high costs, and factor shortages.1 It is, therefore, crucial that the next generation of treatment approaches address these potential concerns.
The most promising new avenue of treatment may be provided by gene therapy.2 With the capability of sustained factor VIII (FVIII) production, many of the complications associated with hemophilia, such as recurrent joint bleeding and the related chronic arthropathy, would be minimized. In addition, endogenous FVIII production would obviate reliance on inconvenient and costly recombinant products, which are often in short supply. Unfortunately, the development of neutralizing FVIII inhibitors is one potential complication that remains a serious threat even with the gene transfer approach.3 Thus, it is essential that whatever strategy of gene therapy is employed does not increase the risk of inhibitor formation.
Adenoviral (Ad) vectors are a promising candidate for gene transfer. They have a high insert capacity (up to 32 kb), are efficient at transducing a wide range of tissues, can be produced at high titer, and are not associated with a risk of insertional mutagenesis or germline transmission.4,5 Conversely, Ad vectors do carry the possibility of transient hepato- and hematologic toxicities and are known to initiate a host immune response.
Preliminary studies in mice with early generation Ad vectors provided evidence of sustained FVIII expression in the absence of inhibitor formation.6-8 However, when these vectors were subsequently tested in large animal models of hemophilia A, FVIII expression was only transient (< 2weeks), and neutralizing FVIII inhibitors were observed, sometimes for the lifetime of the animal.9 In addition, elevated alanine aminotransferase (ALT) levels indicated severe liver damage, contraindicating the potential use of these vectors for hemophiliacs suffering from chronic hepatitis C.
Detailed analysis of studies with early Ad vectors revealed that, although replication-incompetent, low-level expression of viral peptides were responsible, at least in part, for the observed toxicity and immunogenicity.10-12 Subsequently, improvements in Ad design have been carried out to eliminate additional viral coding sequences and thereby reduce vector-related toxicity.4
Most notably, the helper-dependent Ad (HD) system was developed in which all viral open reading frames are deleted from the vector, leaving only the inverted terminal repeats (ITRs) necessary for replication and the packaging signal sequence required for vector packaging.13 Viral capsids are produced in trans by E1-E3–deleted Ad (ΔE1ΔE3-Ad) helper viruses containing the necessary coding sequences, but devoid of the packaging signal sequence required for their own incorporation into vector capsids. HD-Ad vectors produced using this system retain the high transduction efficiency of Ads while eliminating the expression of viral antigens.
Initial studies of HD vectors in normal baboons demonstrated reduced vector toxicities from previous Ad vectors and the ability to mediate sustained transgene expression of the human alpha1 antitrypsin gene.14 Furthermore, in murine models of hemophilia, we and others have shown that HD vectors are capable of providing long-term FVIII expression in the absence of inhibitor formation.15 However, as noted, murine studies do not necessarily predict treatment outcomes in higher order mammals. Thus, it is important that HD vectors be tested in a large animal model of hemophilia A before future clinical trials are carried out.
To this end, we have constructed HD vectors encoding a canine B domain–deleted FVIII transgene (cFVIII) under the control of either a ubiquitously expressed viral promoter or a tissue-restricted mammalian promoter and have treated 4 adult hemophilia A dogs. Each animal was monitored for vector-induced toxicities, as well as changes in FVIII levels and inhibitor development.
Materials and methods
Construction, propagation, and purification of recombinant adenovirus
Two different adenoviral vectors were constructed as follows: (1) HD-CMV-cFVIII. An NsiI-SwaI-MluI linker was added to plasmid pDC111 (Microbix Biosystems, Toronto, ON, Canada) at the HindIII site to create plasmid pDC111S1. A pBK vector containing a canine FVIII B-domain–deleted transgene under the control of a cytomegalovirus (CMV) promoter, previously described,16 was digested with MluI and NsiI. The insert, containing the CMV-cFVIII cassette, was isolated and ligated into pDC111S1, creating pDC111S1-CMV-FVIII. Subsequently, a PacI linker was added to pDC111S1-CMV-cFVIII at the NsiI site to create pDC111S1-CMV-cFVIII-PacI, which was then cut with MluI and PacI for ligation into the packaging vector, pC4-HSU-PacI.17 This produced pHD-CMV-cFVIII which, in addition to the expression cassette, contained the adenoviral packaging sequence (ψ) and the inverted terminal repeats (ITRs), the cis acting elements that are necessary and sufficient for adenovirus production. The stuffer sequence of pC4-HSU was previously described by Sandig et al.17 (2) HD-HNF-cFVIII. A PacI linker was added to pBK-HNF-cFVIII, previously described,16 at the SpeI site to create pBK-HNF-cFVIII-PacI. The plasmid was cut with MluI and PacI, the expression cassette was gel purified and finally ligated into pC4-HSU-PacI to create pHD-HNF-cFVIII.
Analysis of helper-dependent adenoviral vectors
Adenovirus DNA from virions purified by CsCl density gradient was isolated by mixing virus with an equal amount of sodium dodecyl sulfate (SDS)–pronase solution (0.05% pronase in 10 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH7.5, 10 mM EDTA [ethylenediaminetetraacetic acid], and 0.5% SDS) then incubating for 2 hours at 37°C, followed by phenol extraction and ethanol precipitation. Viral particle concentration was estimated based on fluorimeter measurement of viral DNA as described by Shi et al.19 The concentration of virus particles (virus particles/L) was determined by the following formula: [virus DNA concentration (g/L)] × [6.02 × 1023]/[virus DNA molecular weight].
The genomic structure of the virus was assessed by HindIII digestion followed by agarose gel electrophoresis. Gels were stained with ethidium bromide and viewed under ultraviolet light. The level of helper-virus contamination in all preparations of HDAd was determined by real-time polymerase chain reaction (PCR). Real-time PCR was carried out using the Roche DNA Master SYBR Green I kit (Roche, Mississauga, ON, Canada), with the sense and antisense primers, 5′-TCTGAGTTGGCACCCCTATTC-3′ and 5′-GTTGCTGTGGTCGTTCTGGTA-3′, respectively. Real-time fluorescent detection was assessed on a Cepheid Smartcycler (Cepheid, Sunnyvale, CA) under these conditions: 5 minutes at 94°C, followed by 40 cycles of successive incubations at 94°C for 20 seconds, 66°C for 20 seconds, and an extension at 72°C for 20 seconds. Quantification of helper-virus concentration was performed using a standard curve consisting of dilutions from 6.74 × 102 pg/mL to 6.74 × 10–3 pg/mL Ad2 DNA. The percent of helper virus contamination is reported as the quotient of [sample Ad2 DNA]/[total sample viral DNA].
Animal procedures
The experimental animals used in this study were mixed-breed dogs from the hemophilia A colony housed at Queen's University.20 These animals all have the same FVIII RNA processing mutation that has similarities to the common human FVIII inversion mutation.21
HD vectors were diluted in Hanks buffered saline solution (Life Technologies, Gaithersburg, MD) and administered through an in-dwelling cephalic vein catheter at a rate of 2 mL/minute. Within 5 minutes of vector infusion, dogs B, C, and D were administered 2 mg/kg diphenhydramine and 100 mg of hydrocortisone by intravenous injection.
Sampling for FVIII and inhibitor analysis was carried out by cephalic vein phlebotomy into 10% buffered citrate (0.1 M citric acid, 0.1 M sodium citrate, 0.1 M EACA [[epsilon]-aminocaproic acid]) anticoagulant. Whole blood was centrifuged for 2 minutes at 10 000g, and plasma was collected and snap-frozen on dry ice. Samples were stored at –70°C.
All animals were housed in facilities accredited by the Canadian Council for Animal Care, and experimental procedures were approved by the Queen's University Animal Care Committee.
Toxicity assays
To assay liver toxicity, serum was collected before and at the indicated times following vector administration, centrifuged, aliquoted, and frozen. Each serum sample was analyzed for the presence of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AP), creatine kinase (CK), urea, creatinine, and total bilirubin levels.
Coagulation and inhibitor assays
Biologically active FVIII was measured in dog plasma using the Coamatic chromogenic bioassay (DiPharma, West Chester, OH). Pooled human plasma (George King Bio-Medical, Overland Park, KS) was used to generate a standard curve in which 1 U (1000 mU) was defined as the amount of FVIII activity in 1 mL of pooled human plasma, or 100 to 200 ng/mL. When compared to human plasma, normal canine plasma FVIII levels are approximately 6-fold higher. The Coatest assay displayed a limit of sensitivity of 10 mU/mL in normal canine pooled plasma.
Anti–canine FVIII inhibitory antibodies were measured using the Bethesda assay. Various dilutions of test plasmas were mixed 1:1 with a normal canine plasma pool and incubated at 37°C for 2 hours. Residual FVIII coagulant activity was determined with a conventional one-stage FVIII:C assay. The dilution with residual activity closest to 50% was used to calculate the inhibitor titer, in which 50% residual FVIII activity equals 1 Bethesda unit (BU) per milliliter.
Whole blood clot times (WBCTs) were measured by monitoring the time to complete clot formation of 1.5 mL whole blood in a 37°C water bath. A minimum of 2 whole blood aliquots were taken and averaged for each WBCT measurement.
RT-PCR analysis
Tissue was collected from dog A and B on day 420 and 210, respectively, by survival surgery liver biopsy. DNA and RNA were isolated by Tripure Isolation Reagent (Roche Molecular Biochemicals, Laval Quebec) as directed by the manufacturer. Reverse transcription was carried out using an RNA template-specific PCR (RS-PCR) reaction.22 This technique is used to reduce the possibility of amplifying transgene DNA during PCR amplification.
Briefly, 10 μg of sample RNA was DNase I–treated (Ambion, Austin, TX) for 1 hour at 37°C and reverse transcribed (RT) with SuperScript II (Invitrogen, Burlington, ON, Canada) using a 43mer antisense primer with 18 nucleotides specific for the canine FVIII transcript (italics) and 25 unique nucleotides (underlined); 5′-GCCGTCATACCGTGTAGGCTCAGGACTTGCTTTGCCCACATAG-3′. Subsequent to RT, the 43mer was removed by QiaQuick column purification (Qiagen, Burlington, Canada), and cFVIII RS-cDNA was amplified using sense and antisense primers, 5′-GATCTCCGAGAGGCCACAGATAGAG-3′ and 5′-GCCGTCATACCGTGTAGGCTCAGGA-3′, which bind to the cFVIII B-domain and the unique 25mer sequence, respectively.
The presence of adenoviral vector DNA in tissues was assessed by PCR using sense and antisense primers, 5′-ATAATCAGCCATACCACATTTG-3′ and 5′-ACTCCAGCACATTTAAATAC-3′, respectively, which are specific for regions in the stuffer sequence of both HD vectors.
PCR reactions for the detection of sequences in the HD vector genome were performed on a Techne Thermocycler (Fischer Scientific, Nepean, Canada) using these conditions: 94°C for 4 minutes, followed by 40 cycles of 94°C/1 minute, 55°C/1 minute, and 72°C/1 minute. For canine FVIII-specific PCR, a 2-step reaction was carried out for 40 cycles at 94°C/1 minute and 72°C/1 minute 20 seconds. Reactions were analyzed on a 2% agarose gel by ethidium bromide staining and ultraviolet light visualization.
For quantitative PCR analysis of helper and HD vectors, a real-time PCR reaction was carried out using the primers described above. Amplicon quantitation was measured by Sybr green incorporation using an Applied Biosystems 7000 Sequence Detection System (Foster City, CA).
Results
FVIII expression and phenotypic correction of bleeding abnormalities in canine hemophilia A
Two adenoviral vectors were constructed, each encoding the canine FVIII (cFVIII) B-domain–deleted transgene. The 2 vectors were amplified with help from Ad2LC8cCARP and were thus packaged in serotype 2–derived virion capsids.18 One vector, HD-CMV-cFVIII, expressed cFVIII under the control of the cytomegalovirus immediate early promoter, and the other, HD-HNF-cFVIII, was regulated by a tissue-restricted promoter, constructed in our laboratory and comprising the proximal human FVIII promoter with an upstream concatemer of 5 hepatocyte nuclear factor 1 binding sites.16
Amplified vector preparations were further purified by CsCl buoyant density centrifugation23 and tested for helper contamination by quantitative PCR directed against sequences specific to the helper virus. All HD viruses showed levels of helper-virus contamination under 1% of total viral yield.
All 4 animals used in this study are deficient in FVIII due to a spontaneous mutation that results in the aberrant processing of FVIII mRNA.21 These animals do not display any functional FVIII activity as determined by FVIII chromogenic bioassay (< 10 mU/mL), and measurements of whole blood clotting times (WBCTs) revealed prolonged coagulation (14-16 minutes) in all 4 dogs (normal canine WBCT: 2-6 minutes).
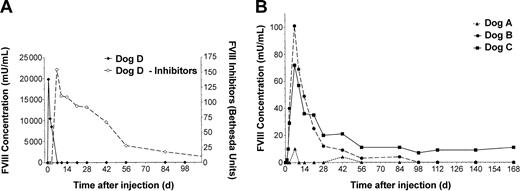
HD vectors were administered by cephalic vein infusion over 10 minutes (Table 1). Plasma samples were taken at the times indicated and assayed for FVIII activity by chromogenic assay (Figure 1) and WBCT (Figure 2). Dog D was administered 2 × 1012 vp/kg HD-CMV-cFVIII and monitored for changes in FVIII levels. Within 5 hours of treatment, FVIII activity rose to 247 mU/mL and by 24 hours, FVIII concentrations reached 19 830 mU/mL. FVIII levels quickly declined below the level of detection however, and by day 7 high titer anti–canine FVIII inhibitors (> 100 BU) were observed.
FVIII activity and inhibitor analysis. Plasma was collected from each animal prior to and following vector administration at each time point indicated. Chromogenic bioassay analysis was carried out on the samples to determine FVIII activity. The results were compared against pooled human plasma and reported in units of human FVIII activity. A Bethesda assay was employed to establish inhibitor titers for dog D. (A) FVIII activity (solid line, left) and inhibitor analysis (dashed line, right) of HD-CMV-cFVIII–treated dog. The anti–FVIII antibody response showed cross-reactivity with both canine and human plasma. The titer of anti–canine FVIII inhibitors are reported here. (B) FVIII activity of dogs treated with HD-HNF-cFVIII.
FVIII activity and inhibitor analysis. Plasma was collected from each animal prior to and following vector administration at each time point indicated. Chromogenic bioassay analysis was carried out on the samples to determine FVIII activity. The results were compared against pooled human plasma and reported in units of human FVIII activity. A Bethesda assay was employed to establish inhibitor titers for dog D. (A) FVIII activity (solid line, left) and inhibitor analysis (dashed line, right) of HD-CMV-cFVIII–treated dog. The anti–FVIII antibody response showed cross-reactivity with both canine and human plasma. The titer of anti–canine FVIII inhibitors are reported here. (B) FVIII activity of dogs treated with HD-HNF-cFVIII.
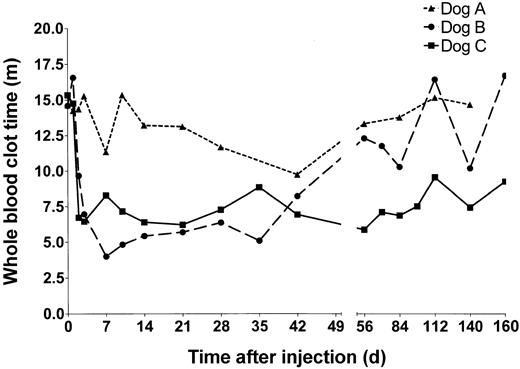
Whole blood clotting times of HD-HNF-cFVIII–treated hemophilic dogs. Blood was collected at the time points indicated from dogs A (▴),B(•), and C (▪), and whole blood clot time was measured.
Whole blood clotting times of HD-HNF-cFVIII–treated hemophilic dogs. Blood was collected at the time points indicated from dogs A (▴),B(•), and C (▪), and whole blood clot time was measured.
Dog A received 5 × 1011 vp/kg of HD-HNF-cFVIII, the lowest vector dose of any of the animals. WBCTs in dog A showed only a minimal decline at 7 and 42 days after infusion. These declines may not have been significant but were, however, associated with a minor increase in FVIII activity to 10 mU/mL at both times. No inhibitors were detected in dog A at any time tested (> 1 year).
Dogs B and C both received 1.25 × 1012 vp/kg HD-HNF-cFVIII. In dog B, the WBCT began to decline within 5 hours of vector administration. By 7 days, dog B's WBCT was 4 minutes, similar to a normal dog, and FVIII levels were as high as 100 mU/mL, or 10% of normal human FVIII activity. FVIII expression subsequently declined, and by day 56 the animal's WBCT had returned to pretreatment values. In dog C, WBCT shortened to 6 minutes within 2 days of vector administration, and FVIII levels increased to 10 mU/mL. FVIII concentrations peaked by day 7 at 72 mU/mL and were sustained at around 10 mU/mL for the duration of the study. These levels of plasma FVIII correlated with WBCTs under 8 minutes, half of the animal's pretreatment values. FVIII inhibitors were not detected in either dog B or C.
All of the hemophilic dogs in this colony experience about 5 spontaneous bleeding episodes per year. When this occurs they are treated with canine cryoprecipitate. No clinical bleeding events were observed in any of the dogs, including dog D, subsequent to treatment with the HD-Ad vector.
HD vector–associated transient hepatotoxicity
Early generation adenoviral vectors previously have been shown to be severely toxic in higher order mammals and have even been implicated in the death of one human subject.9,24,25 HD vectors do show an improved safety margin over previous Ad vectors when administered to rodents,26 but their affect in large animal models is less well described.
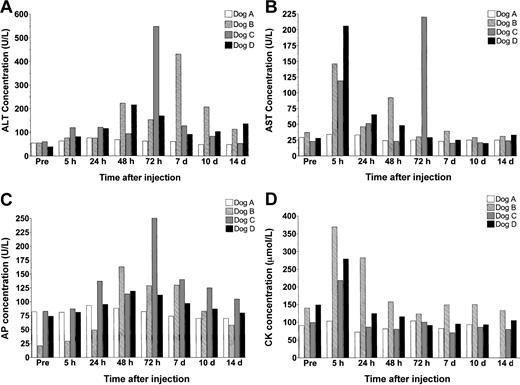
In these studies, we monitored serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, creatine kinase (CK), bilirubin, urea, and creatinine to assess vector-induced hepato-, renal, and muscle toxicity (Figure 3). In dog A, the animal receiving the lowest vector dose, levels of serum enzymes did not change significantly in response to treatment. In contrast, all 3 animals receiving vector doses over 1012 vp/kg showed signs of acute transient hepatotoxicity.
Serum enzyme analysis. (A) ALT (normal range, 15-84 U/L), (B) AST (8.2-57.3 U/L), (C) AP (10.6-100.7 U/L), and (D) CK (13.7-119 μmol/L).
Serum enzyme analysis. (A) ALT (normal range, 15-84 U/L), (B) AST (8.2-57.3 U/L), (C) AP (10.6-100.7 U/L), and (D) CK (13.7-119 μmol/L).
Immediately after injection, dogs B, C, and D all showed a marked change in demeanor, which included sluggishness and in dog C even gagging. Diphenhydramine and hydrocortisone were intravenously administered within 5 minutes of vector infusion to successfully return the animals to their normal behavior. By 5 hours after treatment, AST levels rose to over 6 times their normal concentration but returned to baseline by 24 hours. In one animal, dog C, a spike in AST levels was observed again at 72 hours.
Surprisingly, although ALT levels did increase in each of the high-dose animals, the response was heterogeneous and did not display a dose-dependent effect. Dog D, which received the highest vector dose, showed only minor elevations in serum ALT, which did not exceed 200 U/L. Dogs B and C both showed higher peak elevations in ALT concentration than dog D, but these changes occurred at different times in the 2 animals. By day 14 after treatment, transaminase levels had returned to normal in dog C but remained at approximately 2-fold baseline values in dogs B and D until day 28.
At no time was any variation observed in bilirubin, urea, or creatinine levels (data not shown).
High HD vector doses are associated with thrombocytopenia but not an acute phase response
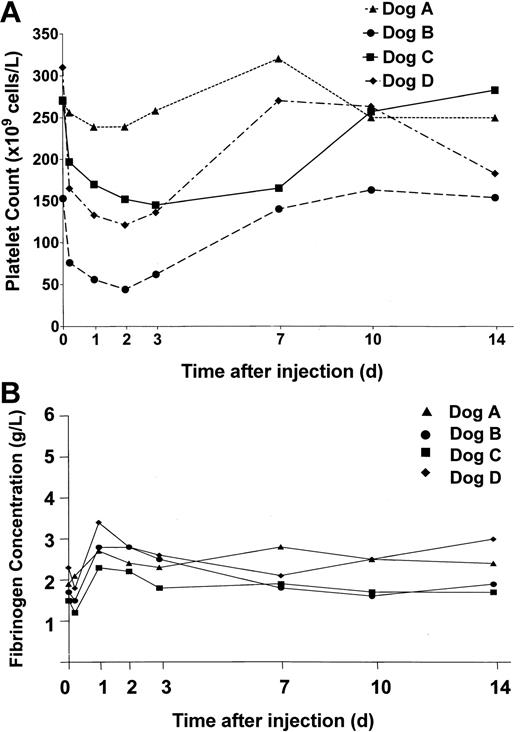
In view of previous reports of adenovirus-mediated thrombocytopenia,27 platelet levels in the dogs were monitored and are shown in Figure 4A. In the low-dose animal, dog A, we did not observe a significant change in circulating platelets, but in the 3 dogs receiving vector doses over 1012 vp/kg, platelet counts dropped between 40% and 70% within 48 hours of vector administration. Concomitant with the drop in platelet count was an increase in fibrin degradation products (FDPs). In addition, gross qualitative assessment of whole blood clots between 5 hours and 3 days after treatment revealed that clots were unstable and could be disrupted by mild agitation. In dog B, which had platelet counts less than 100 × 109/L, the site of venipuncture routinely bled for the first 48 hours after vector administration. Platelet counts and FDPs returned to baseline in all 3 high-dose animals within 2 weeks of treatment.
Platelet counts and fibrinogen levels in treated dogs. Blood was collected from the dogs at the times indicated prior to and following treatment. Platelet and fibrinogen levels were measured. (A) Platelet counts in dogs A (▴), B (•), C (▪), and D (♦) treated with HD-Ad vectors. (B) Fibrinogen levels in dogs treated with HD-Ad vectors (normal canine fibrinogen levels, 2-4g/L).
Platelet counts and fibrinogen levels in treated dogs. Blood was collected from the dogs at the times indicated prior to and following treatment. Platelet and fibrinogen levels were measured. (A) Platelet counts in dogs A (▴), B (•), C (▪), and D (♦) treated with HD-Ad vectors. (B) Fibrinogen levels in dogs treated with HD-Ad vectors (normal canine fibrinogen levels, 2-4g/L).
Unlike previous studies in large animal models employing early Ad vectors,9,24 fibrinogen levels were not significantly altered in response to treatment with these HD vectors. Thus, indirect evidence of an acute phase response to this therapy was not observed (Figure 4B).
No changes were observed in red blood cell count or hemoglobin levels in any of the animals, and only a minor elevation in white blood cells was recorded in dog D at 5 hours (data not shown).
Liver histology and hepatic DNA and RNA analysis
Dogs A and B underwent liver biopsy procedures on days 420 and 210 after therapy, respectively. Routine histologic examination of hematoxylin and eosin–stained liver sections showed evidence of a very mild, nonspecific hepatitic process in dog B (rare, small clusters of lymphocytes and Kupffer cells) but no abnormalities in dog A.
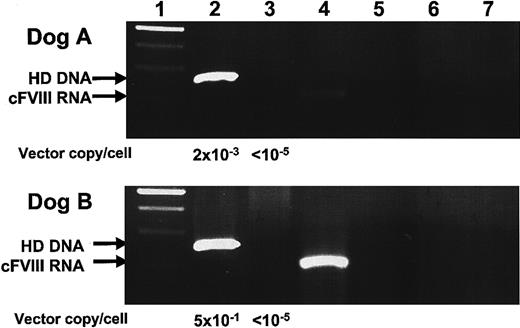
Nucleic acids were extracted from liver tissue by standard isolation methods and analyzed for the presence of HD vector genomes, helper virus, and cFVIII RNA. Our results, shown in Figure 5, indicate that HD vector DNA was present in both dog A and B, but that helper virus was not detected.28 cFVIII RNA expression was analyzed by RS-PCR, a technique designed to discriminate between transgene DNA and RNA.22 Our results indicate that cFVIII mRNA expression was present in both dogs A and B, although FVIII activity in plasma was undetectable by chromogenic bioassay at these same times. Unfortunately, RS-PCR limits quantitative analysis, and we are currently unable to ascertain transcript copy number.
Hepatic DNA and RNA analysis of dogs A and B. Liver biopsies were taken from dogs A and B on days 420 and 210, respectively. DNA and RNA were isolated. One microgram of DNA was subject to PCR amplification for detection of HD and helper-virus genomes. RNA was reverse transcribed using a cFVIII-specific primer with a unique 25 nucleotide tag. Subsequent RS-PCR amplification enabled discriminate amplification of tagged cFVIII cDNA molecules. (1) 100-bp ladder, (2) HD PCR, (3) helper-virus PCR, (4) cFVIII RS-PCR, (5) HD PCR–negative control, (6) helper-virus PCR-negative control, and (7) cFVIII-PCR–negative control. Vector copies/cell was determined by Sybr green incorporation quantitative PCR. One-tenth microgram of liver-derived DNA was amplified, and the value 1 μg DNA = 105 liver cells28 was used to calculate the value for vector copies/cell shown here.
Hepatic DNA and RNA analysis of dogs A and B. Liver biopsies were taken from dogs A and B on days 420 and 210, respectively. DNA and RNA were isolated. One microgram of DNA was subject to PCR amplification for detection of HD and helper-virus genomes. RNA was reverse transcribed using a cFVIII-specific primer with a unique 25 nucleotide tag. Subsequent RS-PCR amplification enabled discriminate amplification of tagged cFVIII cDNA molecules. (1) 100-bp ladder, (2) HD PCR, (3) helper-virus PCR, (4) cFVIII RS-PCR, (5) HD PCR–negative control, (6) helper-virus PCR-negative control, and (7) cFVIII-PCR–negative control. Vector copies/cell was determined by Sybr green incorporation quantitative PCR. One-tenth microgram of liver-derived DNA was amplified, and the value 1 μg DNA = 105 liver cells28 was used to calculate the value for vector copies/cell shown here.
Discussion
We report here the first comprehensive study employing the recently developed HD-Ad gene transfer system for the treatment of hemophilia A in a large animal model. We treated 4 hemophilia A dogs with HD vectors expressing a canine FVIII B domain-deleted transgene and assessed both the safety and efficacy of these vectors. Animals treated with HD-HNF-cFVIII showed increased FVIII activity, ranging from 10-100 mU/mL, which correlated with shortened WBCTs. None of the animals treated with the tissue-restricted vector developed neutralizing FVIII inhibitors, and in one of the dogs, FVIII activity was observed for over 6 months after treatment. At the lowest dose, the vector was shown to be nontoxic, however, at higher doses (> 1012 vp/kg) transient hepatotoxicity and thrombocytopenia, lasting no longer than 14 days, was observed.
In previous studies, comparable doses of early generation Ad vectors in hemophiliac dogs resulted in a biphasic pattern of hepatotoxicity with peak elevations in ALT concentrations occurring at 2 days (250-500 U/L) and 10 days (1500-2000 U/L) after treatment.9 In contrast, hepatotoxicity in this study was much less severe (peak ALT 550 U/L) and did not present in the biphasic manner observed with early generation Ad vectors. Thus, HD vector design, while not eliminating initial cellular toxicity, reduces delayed tissue destruction and provides an increased margin of safety over previous Ad systems.29 Nevertheless, these toxicities developed with just a 2.5-fold increase in vector dose, and there was a significant and unpredictable between-animal variation in the manifestation of these toxicities.
Stable levels of plasma fibrinogen, measured in this study, suggest that an acute phase response did not occur in any of the animals. This is unexpected, however, as fibrinogen levels are responsive to increases in the inflammatory cytokine interleukin-6 (IL-6),30 and adenovirus infection, through either direct cell toxicity or stimulation of the innate immune system, is known to elicit an IL-6 response.31,32 Indeed, Rhesus Macaques treated with early generation Ad presented a biphasic acute phase response, as measured by changes in fibrinogen levels, which directly correlated with increases in serum IL-6 concentration.24 Administration of hydrocortisone is known to reduce IL-6 production33,34 and may have served to diminish the initial acute phase response in this study, while the absence of viral gene expression in the HD system prevented a secondary stress response from occurring. Unfortunately, assays are not currently available to measure canine IL-6, so our analysis is limited to the detection of perturbations in fibrinogen levels. However, studies in humans suggest that the dose of hydrocortisone administered here would be sufficient to attenuate an IL-6 and fibrinogen response.35
In the dog that received the HD-CMV-cFVIII vector, we observed supraphysiological levels of FVIII (> 19 U/mL) followed by the rapid development of cFVIII inhibitors within 1 week of vector administration. This occurred even though the animal had been previously exposed to canine cryoprecipitate, and thus highlights the potential immunogenicity of this therapy. Similar observations, with respect to inhibitor formation, were reported in rodent studies employing CMV-regulated vectors and have been attributed, at least in part, to FVIII expression in professional antigen-presenting cells (APCs).36 Inhibitor development also may have been a response to the supraphysiological levels of FVIII expression as shown in a murine study with HD-Ad.37
In the dogs treated with the HD-HNF-cFVIII vector, inhibitor development was circumvented, probably in part by the restriction of transgene expression to the liver. This is somewhat surprising, however, as studies employing tissue-specific promoters with early generation Ad vectors did not always evade immunity.9 Improved vector design and the coadministration of hydrocortisone may have contributed to the lack of inhibitors seen in our studies by reducing the inflammatory milieu. Activation of innate immunity is required to trigger an adaptive immune response, and in the absence of an inflammatory response, under conditions of minimal toxicity, sufficient maturation of professional APCs would not be expected.38 Thus, both the tissue-restricted transgene expression and improved toxicity profile of the HD-Ad system are important factors for eluding subsequent humoral immunity to the transgene product.
Although FVIII expression was achieved in the dogs treated with HD-HNF-cFVIII, expression peaked at 1 week and declined over time. Diminished FVIII expression did not correlate with the development of an inhibitor, and nucleic acid analysis demonstrated that HD vector DNA and cFVIII RNA were present in the liver more than 12 weeks after treatment. This suggests that a significant cytotoxic T-lymphocyte (CTL) response did not target HD-Ad–infected cells and supports the finding that improved vector design can reduce the host cellular immune response.14 Conversely, helper-virus genomes were not detected in either animal, implying that clearance of the ancillary virus did occur. CTL deletion of Ad2LC8cCARP-infected cells may have inadvertently resulted in the loss of HD-Ad vectors coharbored in the same cells and resulted in decreased FVIII expression over time.
Studies of Ad biodistribution, upon systemic administration, indicate that the vector preferentially transduces Kupffer cells, liver-resident macrophages.39 Initial expression and subsequent clearance in Kupffer cells also may contribute to the diminishing FVIII activity profile observed in our studies and has been reported elsewhere.26 Further reducing helper-virus contamination and the use of macrophage-depleting agents such as clodronate could serve to reduce treatment dose and minimize vector clearance in future protocols.37,40
This study demonstrates, for the first time, that correction of canine hemophilia A can be achieved by systemic administration of an HD-Ad vector encoding the canine FVIII cDNA, under tissue-restricted control, without the induction of anti-FVIII inhibitors. This is a noteworthy achievement, and although acute hepatotoxicity and thrombocytopenia were observed, they were relatively mild and transient in nature, and occurred only at doses over 1012 vp/kg. It should be noted that the peak levels of FVIII induced by the CMV promoter were 200 times greater than the maximum level achieved using a vector containing FVIII expressed from the tissue-specific promoter. Thus, it may be possible to administer HD vectors with more efficient promoters at lower doses to achieve reduced toxicity but increased FVIII activity.
We do not believe that current HD technology is ready for the treatment of human monogenic disease, especially in light of the relatively small therapeutic index and the significant interindividual variability of toxicity seen in this project. Nevertheless, improved HD vectors may ultimately provide an effective alternative to integrating vector systems, and their continued study as gene therapy vehicles is clearly warranted.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-05-1426.
Supported by funds from the Canadian Institutes of Health Research (grant MOP-10912 and MT6762) and the Bayer/Canadian Blood Services Partnership Fund. B.D.B. was the recipient of an Ontario Graduate Scholarship in Science and Technology. D.L. is a Career Investigator of the Heart and Stroke Foundation of Ontario and holds a Canada Research Chair in Molecular Hemostasis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.