Abstract
Dendritic cells (DCs) play a key role in the induction and control of immunity. Genetic engineering of DCs is a promising approach for the development of a broad range of immunomodulatory strategies, for purposes ranging from genetic immunization to tolerance induction. The development of DC-based immunotherapies is limited by the inability to efficiently transfect DCs using naked DNA. Here we demonstrate that after plasmid DNA delivery, the transgene expression level controlled by the human immediate-early cytomegalovirus promoter (hIE-CMVp) is higher in mature DCs than in immature DCs and is further increased after terminal differentiation of DCs by agonist anti-CD40 monoclonal antibody (mAb) or after DC interaction with CD4+ T cells. CD40 signaling of DCs resulted in nuclear translocation of the transcription factors nuclear factor-κB (NF-κB), activator of protein-1 (AP-1), and cyclic adenosine monophosphate (cAMP)–responsive element, necessary for the activation of hIE-CMVp. Transgene expression by DCs diminished after the inhibition of these transcription factors or the blockade of adhesion molecules involved in the DC–T-cell synapse. Importantly, CD40 signaling of DCs results in the highly efficient expression and presentation of transgenic antigens and the induction of “in vivo” cytotoxic T-cell (CTL) responses specific for transgenic antigen peptides, demonstrating the functional potential of genetically engineered DCs.
Introduction
Dendritic cells (DCs) have a key role in initiating and controlling immune responses. In addition to being the most potent antigen-presenting cells (APCs), DCs determine the nature and magnitude of immune responses and provide a link between innate and acquired immunity.1,2 Genetic engineering of DCs offers potential for the development of immune-regulatory strategies for purposes ranging from immunization to tolerance induction. The capacity of DCs engineered to express transgenic antigens, cytokines, or T-cell costimulatory molecules to induce or bias T helpers 1 and 2 (TH1/TH2)–skewed immune responses, or to promote tolerance, is the subject of current investigation.3-5
DNA-based immunization has potential advantages over protein-based vaccines.5-8 These include the simultaneous delivery of transgenic antigens and immunoregulatory genes to DCs. Despite advances in the understanding of DC biology, the development of genetic immunization strategies using DCs transfected with plasmid DNA has been limited by low transfection efficiencies.9 Currently, the most efficient method for DC transduction is infection by recombinant adenovirus (rAd) at a relatively high multiplicity of infection (MOI). However, the applicability of viral vectors is limited by their potential to interfere with DC function and by the coadministration of viral antigens that elicit strong B- and T-cell responses in the host that may limit the readministration of viral vectors.10-12
Indirect evidence suggests that the signaling pathways involved in maturation/terminal differentiation of DCs may enhance the level of transgene expression driven by the human immediate-early cytomegalovirus promoter (hIE-CMVp). We and others have shown that rAd induces the maturation of mouse bone marrow (BM)–derived DCs (BMDCs) mainly by nuclear translocation of nuclear factor-κ B (NF-κB).11 NF-κB is one of the factors necessary to initiate mRNA transcription by hIE-CMVp, and nuclear translocation of NF-κB occurs immediately after CMV infection.13,14 Furthermore, low doses of rAds, engineered to be internalized by cell surface CD40, resulted in enhanced hIE-CMVp–driven transgene expression by DCs.15 CD40 ligand (L) (CD154) is expressed transiently on the surfaces of activated T cells and mediates the terminal differentiation of DCs. This phenomenon takes place during formation of the immunologic synapse between DCs and CD4+ T cells.16-20 Therefore, we hypothesized that signaling DCs through CD40 may increase DC transgene expression controlled by the hIE-CMVp.
In this study we have compared the transfection efficiency of different methods to deliver naked DNA to murine DCs and have analyzed the mechanism(s) involved in transgene expression. We found that gene gun delivery of transgenes controlled by the hIE-CMVp was higher in mature DCs than in immature DCs and further increased after terminal differentiation of mature DCs by CD40 stimulation or interaction with CD4+ T cells. This enhanced transgene expression in mature DCs depended on nuclear translocation of the transcription factors NF-κB, activator of protein-1 (AP-1), and cyclic adenosine monophosphate (cAMP)–responsive element. Our results show that transfection with naked DNA of mature DCs signaled by CD40 or those adhesion molecules involved in DC–T-cell interactions resulted in the highly efficient expression of transgenic proteins to levels induced by rAds. Importantly, CD40-induced terminal differentiation of mature DCs resulted in efficient expression, processing, and major histocompatibility complex (MHC) class I–restricted presentation of transgenic antigens to T cells. Moreover, DCs transfected with the gene gun and signaled through CD40 induced a strong cytotoxic T-cell (CTL) response against DNA-encoded transgenic antigens in vivo, demonstrating the potential for genetically engineering DCs with naked DNA.
Materials and methods
Mice
Eight- to 12-week-old C57BL/6 mice or OT-1 mice (Jackson Laboratories, Bar Harbor, ME) were housed in the pathogen-free animal facility of the University of Pittsburgh and used according to institutional guidelines.
Genetic vectors
Plasmids encoding the reporter protein firefly luciferase, enhanced green fluorescence protein (EGFP), or β-galactosidase (pCMV-LacZ) were generated by subcloning the gene of interest into the pcDNA3.1+ plasmid vector (Invitrogen, Carlsbad, CA) at the XbaI-HindIII restriction site of the multiple cloning site. The plasmid pCMV-OVA-489 encodes a truncated form of ovalbumin (OVA) sequence 138 to 386 that remains intracytoplasmic. The plasmid pCMV-OVA–transmembrane (TR) encodes a membrane-bound form of the OVA sequence 138 to 386 linked to the sequence encoding the first 118 amino acids of the transferrin receptor subcloned into XbaI-HindIII restriction sites of pcDNA3.1+. All transgenes were under the control of the hIE-CMVp. Plasmids were amplified in Escherichia coli DH5α (Gibco BRL, Life Technologies, Gaithersburg, MD) and purified using Endofree Qiagen Maxi kits (Qiagen, Chatsworth, CA). Generation and purification of the E1-E3 deleted rAds encoding luciferase (rAd-Luc) OVA-489 (rAd-OVA489) or β-galactosidase (rAd-LacZ) under the control of hIE-CMVp was performed as decribed.11 All rAds were used to infect DCs in vitro at an MOI of 100.
Generation, purification, and terminal differentiation and apoptosis analysis of DCs
DCs were generated after culturing BM precursors11,17 and used on day 6 after culture. Mature DCs were separated from immature DCs by 14.5% (wt/vol) metrizamide gradient centrifugation (Sigma, St. Louis, MO) (purity of mature DCs [CD11c+ CD86+] was 83% or higher). Terminal differentiation of DCs was performed with anti-CD40 monoclonal antibody (mAb) (BD PharMingen, San Diego, CA) (10 μg/mL) as previously described.17 As control, DCs were incubated with low endotoxin/nonazide (LE/NA) irrelevant hamster immunoglobulin M (IgM; 10 μg/mL). For flow cytometric analysis, DCs were incubated with phycoerythrin (PE) anti-CD11c mAb in combination with fluorescein isothiocyanate (FITC) anti-CD86, anti-H-2Kb, anti-IAb, anti-CD80, or anti-CD40 mAbs (BD PharMingen) followed by analysis with FACScalibur (Becton Dickinson, Palo Alto, CA). Species- and isotype-matched immunoglobulins were included as negative controls. Percentage of apoptotic cells was assessed by quantification of hypoploid DNA in nuclei, as described.21
Transfection of DCs
DCs (106) were transfected using gene gun, lipofection, Ca2PO4 precipitation, electroporation, or rAd infection. For gene gun, naked DNA was precipitated into 1-μm gold particles (Bio-Rad Laboratories, Hercules, CA) as described.22 Cells were resuspended in 200 μL complete medium, and gene gun transfection was performed by delivering one shot using either a Helios (Bio-Rad Laboratories) or an Accel Gene Gun (PowderJect Vaccines, WI) device at a helium pressure of 250 psi. For lipofection, DCs were transfected using Lipofectamine-Plus reagent (Gibco) according to the manufacturer's protocols. Electroporation and Ca2PO4 precipitation were performed as described.23 For rAd infection, pCMV-rAd was used at an MOI of 100, as described.11 After transfection, cells were cultured in complete medium without cytokines, and transgene expression was assessed at indicated time points. Luciferase expression was analyzed as described previously.22
Transmission electron microscopy
DCs were gene gun–transfected with pCMV-OVA-TR, cultured for 24 hours, and labeled with anti-OVA mAb (Sigma) followed by bead-conjugated antimouse immunoglobulin G (IgG; Miltenyi Biotec, Auburn, CA) and immunobead-sorted by their surface expression of transgenic OVA (OVA+ DC purity 90% or greater). OVA+ or OVA– DCs were fixed in 2.5% glutaraldehyde and processed for transmission electron microscopy (TEM). Ultrathin sections were analyzed using a JEOL 1210 electron microscope (JEOL, Chicago, IL).
Quantification of transgenic proteins/peptides in transduced-DCs
Total DCs or mature DCs were transfected with plasmid DNA or rAd encoding pCMV-OVA 489, pCMV-OVA-TR, or pCMV-EGFP and were cultured in the presence or absence of agonist hamster (IgM) anti-CD40 mAb or irrelevant hamster IgM. Transgene expression was analyzed by flow cytometry or by immunofluorescence microscopy in cytospins 24 hours later. For cell membrane staining, DCs were collected, rinsed in cold phosphate-buffered saline (PBS), and incubated with anti-OVA mAb (Sigma) for 1 hour at 4°C, followed by FITC F(ab')2 antimouse IgG (Sigma). For intracytoplasmic detection of OVA, DCs were fixed in 2% paraformaldehyde and permeabilized in buffer containing 0.1% saponin; this was followed by 1-hour incubation with FITC-conjugated rabbit anti-OVA polyclonal antibody (Sigma) at 4°C.
We analyzed the loading of transgenic OVA peptide in H-2Kb molecules by transfected mature DCs by labeling with the mAb 25-D1.16 specific for the OVA257-264 (SIINFEKL) peptide-H2Kb complex, followed by FITC F(ab')2 antimouse IgG (Sigma).24 As negative controls, we included DCs transduced with a plasmid encoding pCMV-LacZ as an irrelevant transgene, followed by labeling with anti-OVA antibody or 25-D1.16 mAb, as described. After immunostaining, DCs were fixed in 1% paraformaldehyde, and OVA expression was analyzed by flow cytometry.
OT-1 CD8+ CTL proliferation assays
Naive CD8+ T cells were obtained from transgenic OT-1 mice.25 Spleen and lymph node single-cell suspensions were depleted of red blood cells, and naive CD8+ T cells were purified using nylon wool columns followed by complement-mediated lysis of macrophages, CD4+ T cells, DCs, B cells, and memory T cells expressing LyC6 (purity of naive CD8+ OT-1 T cells 85% or greater by flow cytometry). Monoclonal antibodies included anti-F4/80 (macrophages), anti-IAb (M5/114.15.2) (DCs), anti-B220 (B lymphocytes), anti-CD4 (GK1.5) (ATCC, Rockville, MD), and anti-LyC6 (memory T cells) (BD PharMingen). Mature or immature control or pCMV-OVA-TR–transfected DCs were cultured with or without agonist anti-CD40 mAb. After 24 hours, DCs (stimulators) were γ-irradiated (2000 Gy) and cocultured with naive CD8+ OT-1 T cells at different stimulator/responder ratios in round-bottom, 96-well plates for 3 days. For the final 18 hours, wells were pulsed with 1 μCi (0.037 MBq) [3H] thymidine. The amount of radioisotope incorporated was determined using a β scintillation counter. Assays were performed in triplicate, and results are expressed as mean counts per minute ± 1 SD.
Quantitative analysis of NF-κB, AP-1, and CREB translocation
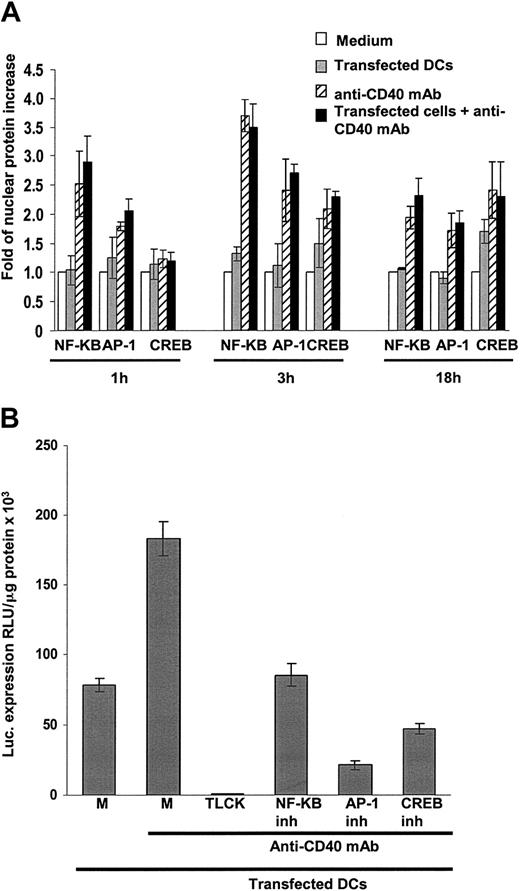
Nuclear translocation of transcription factors was quantified in DC nuclear extracts using TransAM enzyme-linked immunosorbent assay (ELISA)–based kits specific for human-, mouse-, and rat-activated NF-κB, phosphorylated AP-1, or cAMP-responsive element binding protein (CREB) (Active Motif, Carlsbad, CA) according to the manufacturer's protocols and was analyzed after 1, 3, or 18 hours. For NF-κB, nuclear extracts were plated on 96-well plates coated with the immobilized oligonucleotide containing the activated NF-κB consensus site (5′-GGGACTTTCC-3′).26 For AP-1, nuclear extracts were placed in wells containing the immobilized DNA 12-O-tetradecanoylphorbol-13-acetate (TPA)–responsive element oligonucleotide (5′-TGAGTCA-3′), which is bound specifically by AP-1 dimers,27 followed by incubation with an antibody recognizing phosphorylated c-Jun. For CREB, nuclear extracts were placed in wells coated with immobilized oligonucleotide containing the cAMP-responsive element site (5′-TGACGTCA-3′), followed by incubation with an antibody recognizing the Ser133-phosphorylated CREB.28-30 Results are expressed as nuclear -fold increase of the 3 different factors and were calculated according to the formula [nuclear content in treated DCs/nuclear content in control DCs] at different time points.
Blocking assays of nuclear factor translocation and adhesion molecules
Nuclear translocation of NF-κB, AP-1, or cAMP-responsive element was blocked by preincubating DCs with one of the following inhibitory agents: (1) NF-κB SN50 cell-permeable inhibitor peptide (18 μM; Calbiochem-Novabiochem, San Diego, CA)31 ; (2) SP600125, a cell-permeable inhibitor of the c-Jun N terminal kinase (10 μM; Calbiochem)32,33 ; or (3) cAMP-dependent protein kinase peptide inhibitor (10 μM; Promega, Madison, WI).30 After transfection with pCMV-Luc, DCs were cultured in medium containing the specific inhibitors or their solvents (negative controls) and agonist anti-CD40 mAb. Adhesion molecules were blocked with 25 μg/mL of the following LE/NA blocking mAbs: anti-CD54 (3E2), anti-CD102 (3C4), anti-CD11a (17/4), anti-CD11b (M1/70), or anti-CD18 (GAME-46) (BD PharMingen), or with 100 μg/mL mannan to block dendritic cell–specific ICAM-grabbing nonintegrin molecule (DC-SIGN).34 Controls included incubation of transfected-DCs with species- and isotype-matched irrelevant mAbs.
DC CD4+ T-cell cocultures
Naive CD4+ T lymphocytes were purified from naive C57BL/6 mice. Memory CD4+ T cells were isolated from C57BL/6 mice gene gun–immunized with pCMV-Luc on the abdominal skin (1 priming dose + 2 boosts 7 days apart). On day 21, naive or pCMV-Luc–immunized mice were killed, and naive or memory CD4+ T lymphocytes were isolated from lymph nodes and spleens using mouse naive or memory CD4+ T-cell purification column kits (R&D Systems) (purity 95% or greater by flow cytometry). Mature or immature DCs (106) were transfected with pCMV-Luc and cocultured with naive or memory CD4+ T cells at a 1:6 ratio. After 24 hours, luciferase expression was assessed on CD11c+ DCs purified by immunomagnetic bead sorting (Miltenyi Biotec). As controls, gene gun–transfected DCs cultured in medium with or without agonist anti-CD40 mAb were included. Controls included pCMV-Luc–transfected DCs cultured alone, with agonist anti-CD40 mAb, or in the presence of CD4+ T cells with or without species- and isotype-matched mAbs.
In vivo generation of OVA-specific CTL response
For OVA-specific CTL generation in vivo, C57BL/6 mice were immunized by intradermal injection of 3 × 106 mature DCs transfected with the different naked DNA delivery methods (gene gun, lipofection, Ca2PO4, electroporation) or rAd encoding OVA489 followed by signaling through the CD40 molecule. Mature DCs were transfected in vitro and were cultured in the presence of agonist CD40 mAb for 24 hours. After culture, DCs were extensively washed in sterile PBS and were resuspended at a concentration of 3 × 106 cells/200 μL sterile PBS. C57BL/6 mice were immunized intradermally in the lower flanks (100 μL/dose). Mice were given a priming dose of transfected mature DCs on day 1 and boosted on days 7, 21, and 28. Thirty-five days after priming, the mice were humanely killed. Lymph nodes draining immunized skin and spleens were dissected to obtain single-cell suspensions, 3 × 107 cells were restimulated in culture in the presence of OVA protein (1 mg/mL) and SIINFEKL peptide (20 ng/mL), and CTL assays were performed as described.6 Positive controls included mice immunized with mature DCs pulsed with SIINFEKL peptide and signaled with agonist anti-CD40 mAb. Negative controls included groups of mice injected with mature DCs signaled with agonist anti-CD40 mAb and transfected with pCMV-LacZ or rAd LacZ and naive mice injected with PBS.
Statistical analysis
Comparisons of 3 or more means (±1 SD) were performed using analysis of variance (ANOVA) and then the Student Newman-Keuls test.35 Comparisons of 2 means (±1 SD) were performed using the Student t test. A P value of less than .05 was considered significant.
Results
Efficiency of transgene expression by BMDCs transfected with different methods
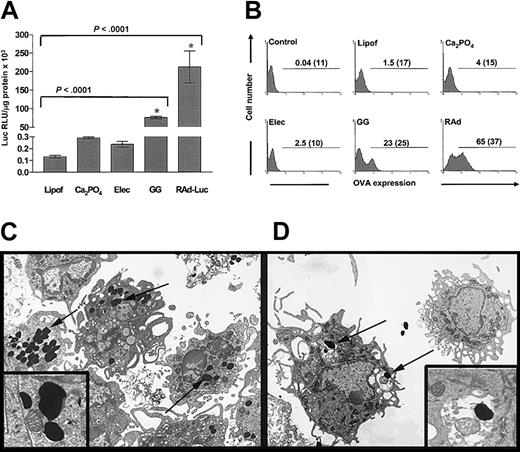
The expression of transgenic luciferase by murine BMDCs (day 6) DNA-transfected by lipofection, calcium phosphate precipitation (Ca2PO4), electroporation, and gene gun or infected with rAd was quantified by luminometry (Figure 1A). DCs expressed the highest levels of luciferase after rAd infection [211 676 ± 43 000 relative light unit (RLU)/μg protein]. Gene gun induced higher levels of transgene expression (74 718 ± 3400 RLU/μg protein) than lipofection, Ca2PO4, or electroporation (131 ± 12, 290 ± 9, and 235 ± 25 RLU/μg protein, respectively) (Figure 1A). To determine the number of DCs that express the transgene, DCs were transduced with plasmid DNA or rAd encoding the intracytoplasmic form of chicken OVA (pCMV-OVA-489), and the percentage of DCs expressing the transgene was assessed by flow cytometry. Expression of OVA was detected in 62.4% ± 4% of DCs infected with rAds and in 21.3% ± 3% of DCs transfected with the gene gun. Transfection efficiencies induced by lipofection, Ca2PO4, or electroporation remained low (1.3% ± 0.3%, 3.2% ± 0.2%, and 2.0% ± 0.5% of OVA+ DCs, respectively) (Figure 1B). The enhanced transgene expression observed after gene gun transfection compared with other methods may be ascribed to the direct delivery of DNA-loaded gold particles to the cytosol of DCs or, alternatively, to the uptake of free gold particles from culture medium by DCs. To address this question, the correlation between level of transgene expression and intracellular location of gold particles was analyzed ultrastructurally in DCs transfected with a plasmid encoding the transmembrane form of OVA (pCMV-OVA-TR). Twenty-four hours after transfection, DCs expressing OVA on their surfaces were separated from OVA-TR– DCs by immunomagnetic bead sorting, and both DC populations were analyzed by TEM. OVA-TR+ DCs showed a high number of gold particles free in the cytosol (probably delivered directly by the gene gun shot), whereas OVA-TR– DCs contained few gold particles inside membrane vesicles, suggesting bead internalization by endocytosis (Figure 1C-D).
Gene gun transfection induces high transgene expression by DCs compared with other naked DNA transfection methods. (A) Luciferase expression by DCs after transfection with naked DNA using lipofection (Lipof), Ca2PO4, electroporation (Elec), or gene gun (GG), or after infection with rAd-Luciferase (rAd-Luc). Although rAd-Luc induces the highest luciferase expression, highly significant expression is found with the gene gun compared with other nonviral methods used. Means ± 1 SD from 3 independent experiments are shown. *indicates P < .01. (B) Expression of cytoplasmic OVA by DCs transfected with different naked DNA methods or infected with rAd. Numbers in graphs are percentages of OVA-positive DCs and mean fluorescence intensity (MFI) (in parentheses). (C-D, insets) High transgene expression (OVA-TR+ DCs) correlated with the presence of gold particles (arrows) free in the cytosol of DCs (shot directly) (C). Transgene-negative DCs (OVA-TR– DCs) exhibited fewer gold particles inside membrane vesicles (likely acquired from the medium by endocytosis) (D). Panels C and D, TEM (original magnification × 3000; insets, original magnification × 14 000).
Gene gun transfection induces high transgene expression by DCs compared with other naked DNA transfection methods. (A) Luciferase expression by DCs after transfection with naked DNA using lipofection (Lipof), Ca2PO4, electroporation (Elec), or gene gun (GG), or after infection with rAd-Luciferase (rAd-Luc). Although rAd-Luc induces the highest luciferase expression, highly significant expression is found with the gene gun compared with other nonviral methods used. Means ± 1 SD from 3 independent experiments are shown. *indicates P < .01. (B) Expression of cytoplasmic OVA by DCs transfected with different naked DNA methods or infected with rAd. Numbers in graphs are percentages of OVA-positive DCs and mean fluorescence intensity (MFI) (in parentheses). (C-D, insets) High transgene expression (OVA-TR+ DCs) correlated with the presence of gold particles (arrows) free in the cytosol of DCs (shot directly) (C). Transgene-negative DCs (OVA-TR– DCs) exhibited fewer gold particles inside membrane vesicles (likely acquired from the medium by endocytosis) (D). Panels C and D, TEM (original magnification × 3000; insets, original magnification × 14 000).
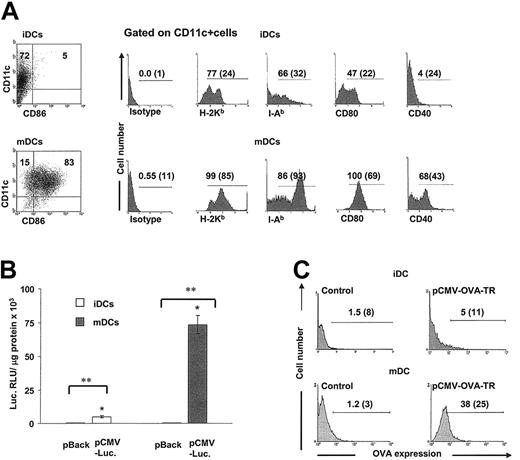
Because rAd induces efficient transgene expression and activation/maturation in BMDCs, we investigated in BMDC cultures whether transgenes delivered by the gene gun were expressed preferentially by mature DCs. Our BMDC cultures were composed of immature DCs (CD11c+, MHC 1lo, MHC 2lo, CD80lo, CD86–, CD40–) and mature DCs (CD11c+, MHC 1hi, MHC 2hi, CD80hi, CD40+ CD86lo/hi) (Figure 2A). After transfection with pCMV-Luc, mature DCs expressed significantly higher levels of luciferase than immature DCs (73 435 ± 6780 vs 5032 ± 670 RLU/μg protein, respectively; P < .001) (Figure 2B). To assess the percentage of DCs expressing the transgene according to the stage of DC maturation, immature DC and mature DC cultures were gene gun–transfected with pCMV-OVA-TR was analyzed by flow cytometry. Transgenic OVA expression was detected in 34% ± 4% of mature DCs and 4% ± 1% of immature DCs (Figure 2C).
High transgene expression is mainly observed in the population of mature DCs. (A) Day-6 DCs were composed of CD11c+ CD86– (immature DCs) and CD11c+ CD86+ cells (mature DCs). The phenotype of purified immature DCs and mature DCs gated on CD11c+ cells is displayed. Numbers represent percentages of positive cells and MFI (between parentheses). Results from 1 of 10 representative experiments are shown. (B-C) Transgene expression in DCs is related to their maturation stage. (B) Luciferase expression in purified immature DCs or mature DCs shows that most of the transgenic luciferase was expressed in the population of mature DCs (P < .001). Negative controls are DCs transfected with plasmid backbone. Means ± 1 SD from 3 independent experiments are shown. * indicates P < .001; **, P < .0001. (C) Expression of OVA-TR is preferentially observed on the cell membranes of mature DCs. Numbers in graphs are percentages of OVA-positive DCs and MFI (between parentheses).
High transgene expression is mainly observed in the population of mature DCs. (A) Day-6 DCs were composed of CD11c+ CD86– (immature DCs) and CD11c+ CD86+ cells (mature DCs). The phenotype of purified immature DCs and mature DCs gated on CD11c+ cells is displayed. Numbers represent percentages of positive cells and MFI (between parentheses). Results from 1 of 10 representative experiments are shown. (B-C) Transgene expression in DCs is related to their maturation stage. (B) Luciferase expression in purified immature DCs or mature DCs shows that most of the transgenic luciferase was expressed in the population of mature DCs (P < .001). Negative controls are DCs transfected with plasmid backbone. Means ± 1 SD from 3 independent experiments are shown. * indicates P < .001; **, P < .0001. (C) Expression of OVA-TR is preferentially observed on the cell membranes of mature DCs. Numbers in graphs are percentages of OVA-positive DCs and MFI (between parentheses).
CD40 signaling increases transgene expression by BMDCs
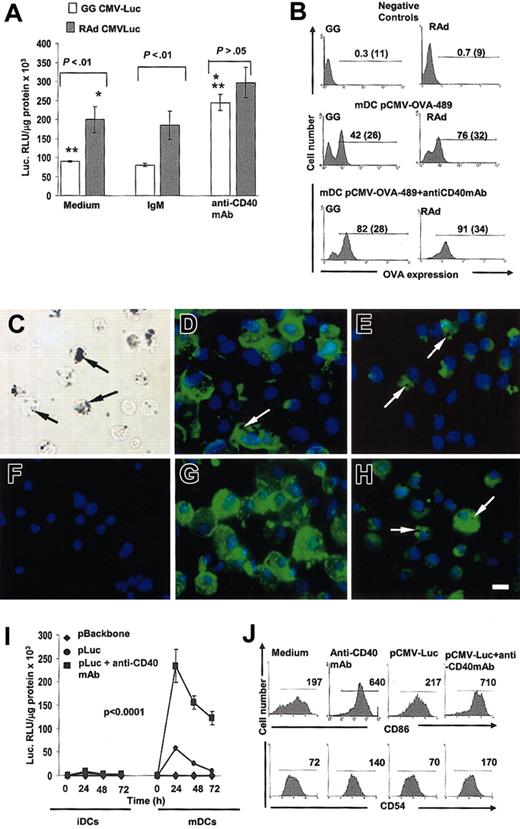
Based on the facts that mature DCs expressed higher levels of hIE-CMVp–driven transgenes than immature DCs and that signaling by CD40 triggers terminal differentiation (maturation) of DCs, we tested whether CD40 signaling of mature DCs further enhanced transgene expression up to levels observed with rAds. For this purpose mature DCs were transfected with the gene gun or infected with rAds encoding luciferase and cultured with agonist (IgM) anti-CD40 mAb or with control IgM. Adding anti-CD40 mAb increased luciferase expression 3-fold in mature DCs transfected with the gene gun and 1.5-fold in mature DCs infected with rAd (Figure 3A). We then assessed the enhancing effect of CD40 stimulation on transgene expression regarding the percentage of DCs expressing intracytoplasmic OVA. Mature DCs were gene gun–transduced with pCMV-OVA-489 or infected with rAd-OVA-489, cultured with or without anti-CD40 mAb and analyzed by flow cytometry. After CD40 ligation the number of OVA+ mature DCs increased from 40% ± 2% to 79% ± 3% in DCs transduced with the gene gun and from 73% ± 3% to 88% ± 3% in DCs infected with rAd (Figure 3B). The results obtained with the gene gun were further confirmed in cytospins of mature DCs gene gun–transfected with pCMV-OVA-TR or pCMV-EGFP (Figure 3C-H).
Signaling DCs through CD40 increases transgene expression to protein levels similar to those induced by rAd-Luc. (A) Transgene expression in mature DCs, gene gun–transfected with pCMV-Luc or infected with rAd-CMV-Luc and cultured in the presence or in the absence of the agonist anti-CD40 mAb. Mature DCs infected with rAd expressed significantly higher levels of luciferase compared with mature DCs transfected with gene gun (P < .01). The enhancing effect of CD40 signaling was observed in mature DCs transfected with gene gun or rAd by the induction of similar levels of luciferase expression (P > .05). Controls included mature DC transfected and incubated in the presence of hamster antimouse IgM. (B) The efficiency of transfection after CD40 ligation was determined by the expression of cytoplasmic OVA in mature DC gene gun–transfected with pCMV-OVA-489 or infected with rAd-OVA-489. Numbers in graphs are percentages and MFI of OVA+ cells (parentheses). Negative controls included mature DCs transfected with pCMV-LacZ or infected with rAd LacZ and cultured with agonist anti-CD40 mAb and immunostained as DCs transfected with OVA-489. (C) Mature DCs transfected with gene gun showed numerous gold particles in the cytoplasm (arrows). (D-E) Expression of transmembrane OVA (D) or cytoplasmic EGFP (E) by mature DCs 24 hours after gene gun transfection. (F) As negative controls, mature DCs were transfected with pCMV-LacZ and labeled with anti-OVA mAb. (G-H) Enhanced expression of transmembrane OVA (G) or cytoplasmic EGFP (H) by mature DCs 24 hours after gene gun transfection plus agonist anti-CD40 mAb. Transgene expression is observed in DCs with gold particles in the cytoplasm (arrows). (C) Phase-contrast microscopy. (D-H) Fluorescence microscopy. Original magnification × 400. Bar = 10 μm. (I-J) Effect of CD40 signaling of mature DCs on gene expression is maintained for 72 hours and is attributed to the terminal differentiation of mature DCs. (I) Time-point curve showing that the high transgene expression observed in mature DCs transfected with gene gun and signaled through CD40 was sustained up to 72 hours. (J) DC terminal differentiation by CD40 signaling was assessed by the increase in the level of expression of the activation markers CD86 and CD54 in mature DCs, indicated by the significant increase of the MFI (numbers in graphs).
Signaling DCs through CD40 increases transgene expression to protein levels similar to those induced by rAd-Luc. (A) Transgene expression in mature DCs, gene gun–transfected with pCMV-Luc or infected with rAd-CMV-Luc and cultured in the presence or in the absence of the agonist anti-CD40 mAb. Mature DCs infected with rAd expressed significantly higher levels of luciferase compared with mature DCs transfected with gene gun (P < .01). The enhancing effect of CD40 signaling was observed in mature DCs transfected with gene gun or rAd by the induction of similar levels of luciferase expression (P > .05). Controls included mature DC transfected and incubated in the presence of hamster antimouse IgM. (B) The efficiency of transfection after CD40 ligation was determined by the expression of cytoplasmic OVA in mature DC gene gun–transfected with pCMV-OVA-489 or infected with rAd-OVA-489. Numbers in graphs are percentages and MFI of OVA+ cells (parentheses). Negative controls included mature DCs transfected with pCMV-LacZ or infected with rAd LacZ and cultured with agonist anti-CD40 mAb and immunostained as DCs transfected with OVA-489. (C) Mature DCs transfected with gene gun showed numerous gold particles in the cytoplasm (arrows). (D-E) Expression of transmembrane OVA (D) or cytoplasmic EGFP (E) by mature DCs 24 hours after gene gun transfection. (F) As negative controls, mature DCs were transfected with pCMV-LacZ and labeled with anti-OVA mAb. (G-H) Enhanced expression of transmembrane OVA (G) or cytoplasmic EGFP (H) by mature DCs 24 hours after gene gun transfection plus agonist anti-CD40 mAb. Transgene expression is observed in DCs with gold particles in the cytoplasm (arrows). (C) Phase-contrast microscopy. (D-H) Fluorescence microscopy. Original magnification × 400. Bar = 10 μm. (I-J) Effect of CD40 signaling of mature DCs on gene expression is maintained for 72 hours and is attributed to the terminal differentiation of mature DCs. (I) Time-point curve showing that the high transgene expression observed in mature DCs transfected with gene gun and signaled through CD40 was sustained up to 72 hours. (J) DC terminal differentiation by CD40 signaling was assessed by the increase in the level of expression of the activation markers CD86 and CD54 in mature DCs, indicated by the significant increase of the MFI (numbers in graphs).
Signaling by CD40 sustained transgene expression in DCs transfected with the gene gun for up to 72 hours (Figure 3I). The enhanced transgene expression detected in mature DCs after CD40 stimulation correlated with the terminal differentiation of mature DCs (assessed by further up-regulation of CD86 and CD54) (Figure 3J). Gene gun transfection by itself did not induce the terminal differentiation of mature DCs, but it did not abrogate CD40-mediated DC terminal differentiation (Figure 3J). The effect of CD40 stimulation on transgene expression was not due to higher viability of DCs because apoptosis of mature DCs 24, 48, and 72 hours after gene gun transfection was not significantly different (5%-10% of apoptotic DCs) between experimental groups (data not shown).
CD40 signaling increases transgenic antigen presentation by BMDCs to T cells
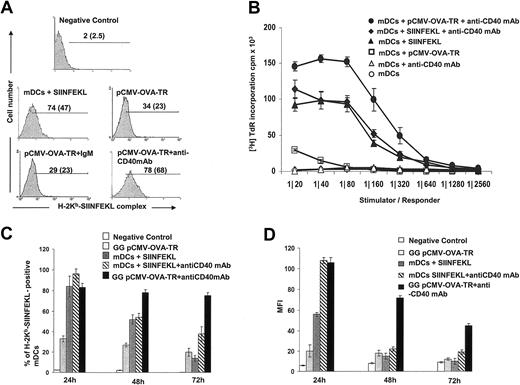
To determine the effect of CD40 stimulation on the ability of BMDCs to process and present transgenic antigenic peptides in MHC 1 molecules, mature DCs were transfected with pCMV-OVA-TR by gene gun, and the level of expression of OVA257-264 (SIINFEKL) peptide-H-2Kb was assessed on the cell surface by flow cytometry. Percentages of mature DCs expressing SIINFEKL-H-2Kb and the density of peptide-MHC 1 complexes (assessed by mean fluorescence intensity [MFI]) increased substantially after treatment with anti-CD40 mAb (34%-78% in DCs expressing SIINFEKL-H-2Kb and 23%-68% in MFI) (Figure 4A).
Transfected mature DCs signaled through CD40 increase H-2Kb loading of transgenic peptides and antigen presentation to specific CD8+ T cells. (A) H-2Kb-SIINFEKL complex expression by: (1) mature DCs after transfection with pCMV-LacZ (negative control); (2) nontransfected mature DCs incubated with a saturating dose of synthetic peptide SIINFEKL (positive control); or (3) mature DCs after transfection with pCMV-OVA-TR and cultured in medium alone (control), with irrelevant IgM (control), or with agonist anti-CD40 (IgM) mAb. Numbers represent percentages of positive mature DCs and MFI (in parentheses). Results from 1 of 3 representative experiments are shown. (B) Induction of OT-1 CD8+ T-cell proliferation after stimulation with syngeneic mature DCs at different cell ratios. • Mature DCs transfected with pCMV-OVA-TR and cultured for 24 hours with anti-CD40 mAb induced significantly higher proliferation of OT-1 CD8 + T cells than □ mature DCs transfected with pCMV-OVA-TR and cultured in medium alone (P < .0001). Negative controls were ▵ nontransfected mature DCs cultured with anti-CD40 mAb and ▵ nontransfected mature DCs cultured in medium alone. Positive controls were mature DCs pulsed with a saturating dose of SIINFEKL and cultured for 24 hours with ♦ or without ▴ anti-CD40 mAb. (C-D) High OT-1 proliferation induced by mature DCs transfected with the gene gun and CD40 signaled corresponded with high and sustained expression of H-2Kb-SIINFEKL complex in the surfaces of mature DCs. (C) Percentage of mature DCs expressing H-2Kb-SIINFEKL complex in the cell membrane. (D) MFI after gene gun transfection with pCMV-OVA-TR or after incubation with OVASIINFEKL peptide in the presence or in the absence of the agonist CD40 mAb. Means ± SD of 3 independent experiments are displayed.
Transfected mature DCs signaled through CD40 increase H-2Kb loading of transgenic peptides and antigen presentation to specific CD8+ T cells. (A) H-2Kb-SIINFEKL complex expression by: (1) mature DCs after transfection with pCMV-LacZ (negative control); (2) nontransfected mature DCs incubated with a saturating dose of synthetic peptide SIINFEKL (positive control); or (3) mature DCs after transfection with pCMV-OVA-TR and cultured in medium alone (control), with irrelevant IgM (control), or with agonist anti-CD40 (IgM) mAb. Numbers represent percentages of positive mature DCs and MFI (in parentheses). Results from 1 of 3 representative experiments are shown. (B) Induction of OT-1 CD8+ T-cell proliferation after stimulation with syngeneic mature DCs at different cell ratios. • Mature DCs transfected with pCMV-OVA-TR and cultured for 24 hours with anti-CD40 mAb induced significantly higher proliferation of OT-1 CD8 + T cells than □ mature DCs transfected with pCMV-OVA-TR and cultured in medium alone (P < .0001). Negative controls were ▵ nontransfected mature DCs cultured with anti-CD40 mAb and ▵ nontransfected mature DCs cultured in medium alone. Positive controls were mature DCs pulsed with a saturating dose of SIINFEKL and cultured for 24 hours with ♦ or without ▴ anti-CD40 mAb. (C-D) High OT-1 proliferation induced by mature DCs transfected with the gene gun and CD40 signaled corresponded with high and sustained expression of H-2Kb-SIINFEKL complex in the surfaces of mature DCs. (C) Percentage of mature DCs expressing H-2Kb-SIINFEKL complex in the cell membrane. (D) MFI after gene gun transfection with pCMV-OVA-TR or after incubation with OVASIINFEKL peptide in the presence or in the absence of the agonist CD40 mAb. Means ± SD of 3 independent experiments are displayed.
We then evaluated the ability of mature DCs transfected with pCMV-OVA-TR with the gene gun to present the SIINFEKL-H-2Kb complex to naive OT-1 CD8+ T cells, which bear transgenic T-cell receptors for SIINFEKL-H-2Kb.25 Mature DCs were transfected with pCMV-OVA-TR, cultured with or without anti-CD40 mAb for 24 hours, washed and cocultured with naive OT-1 CD8+ T cells (depleted from LyC6-positive T cells).36 Mature DCs transfected with pCMV-OVA-TR induced low proliferation of OT-1 CD8+ T cells (Figure 4B). By contrast, mature DCs transfected with pCMV-OVA-TR and activated by CD40 induced the highest proliferation of naive OT-1 CD8+ T cells, even higher than mature DCs pulsed with saturating doses of synthetic SIINFEKL and activated by CD40 (Figure 4B). This difference could have resulted from a sustained and higher expression of SIINFEKL-H-2Kb on the surfaces of mature DCs transfected with the gene gun plus anti-CD40 mAb compared with mature DCs pulsed with SIINFEKL plus anti-CD40 mAb. To address this point, we analyzed the expression of SIINFEKL-H-2Kb in DCs at different intervals for 72 hours (minimum time required to detect proliferation of OT-1 CD8+ T cells). Mature DCs were gene gun–transfected with pCMV OVA-TR or pulsed with SIINFEKL and cultured with or without anti-CD40 mAb. The number of DCs expressing SIINFEKL-H-2Kb and the density of peptide–MHC complexes on the DC surface (assessed by MFI) were analyzed by flow cytometry. The number of DCs transfected with the gene gun (+ anti-CD40 mAb) expressing SIINFEKL-H-2Kb remained high throughout the 72-hour interval but decreased significantly in DCs cultured with the peptide (+anti-CD40 mAb; P < .001) (Figure 4C). The density of SIINFEKL-H-2Kb decreased more rapidly in DCs pulsed with SIINFEKL than in gene gun–transfected DCs (both with anti-CD40 mAb; P < .001) (Figure 4D).
Mechanism(s) of CD40-dependent enhancement of hIE-CMVp-driven transgenes in DCs
Because all transgenes tested were driven by hIE-CMVp, we ascertained whether the increase in transgene expression by DCs after CD40 signaling was caused by nuclear translocation of the transcription factors NFκB, AP-1, or CREB, which are involved in initiation of hIE-CMVp–controlled transcription.13,14 Translocation of NFκBp65 and AP-1 was observed 1 hour after CD40 signaling, whereas translocation of CREB was detectable 3 hours later. The maximum level of translocation of the 3 factors occurred 3 hours later, with amounts still detected after 18 hours. The highest nuclear increase was observed for NFκB (3.69 ± 0.3-fold increase at 3 hours; P < .0001) and to a lower extent for AP-1 (2.7 ± 0.16; P = .0005) and CREB (2.29 ± 0.1; P = .0014) (Figure 5A). Gene gun treatment by itself did not induce significant nuclear translocation of transcription factors compared with nontreated cells (P > .05), and there was no difference between translocation induced by anti-CD40 mAb alone or in combination with the gene gun (P > .05) (not shown). Accordingly, the specific inhibition of nuclear translocation of NFκB, AP-1, and CREB significantly diminished hIE-CMVp–dependent luciferase expression in mature DCs (P < .0001) (Figure 5B).
Signaling mature DCs through CD40 induces nuclear translocation of NF-κB, AP-1, and CREB. (A) One hour after incubation with agonist anti-CD40 mAb, mature DCs translocated NF-κB(P = .0004), and to a lower extent AP-1 (P < .0008), into their nuclei. The highest amounts of nuclear translocation factors occurred 3 hours after incubation with the agonist anti-CD40 mAb (P < .0001, P < .0005, and P < .001 for NF-κB, AP-1, and CREB, respectively). Transfection with gene gun did not induce translocation compared with untreated mature DCs, (P > .05) and signaling through CD40 induced nuclear translocation regardless of gene gun transfection (P > .05). Translocation of transcription factors was quantified by ELISA and expressed as -fold of protein increase versus untreated control cells. A value of 1 was set for untreated cells in each situation. Means ± 1 SD from 3 independent experiments are shown. (B) Specific inhibition of NF-κB, AP-1, and CREB resulted in significantly decreased transgene expression by CD40-signaled mature DCs (P < .0001). Controls included transfected mature DCs cultured in medium alone (M) or with anti-CD40 mAb or transfected mature DCs incubated with anti-CD40 mAb in the presence of the serine proteases blocking agent TLCK. Means ± 1 SD from 4 (A) or from 10 (B) independent experiments are shown. P < .0001.
Signaling mature DCs through CD40 induces nuclear translocation of NF-κB, AP-1, and CREB. (A) One hour after incubation with agonist anti-CD40 mAb, mature DCs translocated NF-κB(P = .0004), and to a lower extent AP-1 (P < .0008), into their nuclei. The highest amounts of nuclear translocation factors occurred 3 hours after incubation with the agonist anti-CD40 mAb (P < .0001, P < .0005, and P < .001 for NF-κB, AP-1, and CREB, respectively). Transfection with gene gun did not induce translocation compared with untreated mature DCs, (P > .05) and signaling through CD40 induced nuclear translocation regardless of gene gun transfection (P > .05). Translocation of transcription factors was quantified by ELISA and expressed as -fold of protein increase versus untreated control cells. A value of 1 was set for untreated cells in each situation. Means ± 1 SD from 3 independent experiments are shown. (B) Specific inhibition of NF-κB, AP-1, and CREB resulted in significantly decreased transgene expression by CD40-signaled mature DCs (P < .0001). Controls included transfected mature DCs cultured in medium alone (M) or with anti-CD40 mAb or transfected mature DCs incubated with anti-CD40 mAb in the presence of the serine proteases blocking agent TLCK. Means ± 1 SD from 4 (A) or from 10 (B) independent experiments are shown. P < .0001.
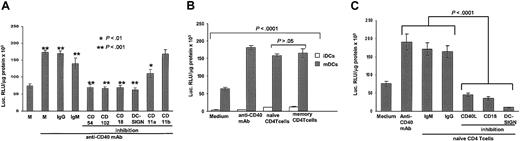
Stimulation by CD40 induces DC clustering because of the up-regulation and increased affinity of adhesion molecules (ie, intracellular adhesion molecules [ICAMs], β2 integrins, and DC-SIGN).37-41 Thus, we investigated whether the effect of CD40 on transgene expression by mature DCs was a combined effect of direct stimulation by CD40 and interaction of these adhesion molecules. Specific blockade of CD18, CD54, CD102, DC-SIGN (P < .0001), and, to a lesser extent, CD11a (P < .001) resulted in significant inhibition of transgene expression and DC aggregation (Figure 6A).
Effect of CD40 ligation and role of adhesion molecules in transgenic expression by mature DCs. (A) Blockade of CD11a, CD18, CD54, CD102, or DC-SIGN significantly inhibited transgene expression by mature DCs. No inhibition was detected after blocking CD11b (P > .05). Transgene expression by mature DCs transfected and cultured with irrelevant IgGs or IgMs were included as controls. Means ± 1 SD from 10 independent experiments are shown. (B-C) Coculture of naive or memory CD4+ T cells with transfected mature DCs increases transgene expression. (B) Coculture of naive or memory CD4+ T cells with mature DCs increased transgene expression by mature DCs up to 4-fold compared with transfected mature DCs in medium alone (P > .0001). (C) Specific blockade of CD18, DC-SIGN, and CD40 ligand (CD40L) significantly inhibited the expression of transgenic luciferase (P < .0001). Means ± 1 SD from 5 independent experiments are shown.
Effect of CD40 ligation and role of adhesion molecules in transgenic expression by mature DCs. (A) Blockade of CD11a, CD18, CD54, CD102, or DC-SIGN significantly inhibited transgene expression by mature DCs. No inhibition was detected after blocking CD11b (P > .05). Transgene expression by mature DCs transfected and cultured with irrelevant IgGs or IgMs were included as controls. Means ± 1 SD from 10 independent experiments are shown. (B-C) Coculture of naive or memory CD4+ T cells with transfected mature DCs increases transgene expression. (B) Coculture of naive or memory CD4+ T cells with mature DCs increased transgene expression by mature DCs up to 4-fold compared with transfected mature DCs in medium alone (P > .0001). (C) Specific blockade of CD18, DC-SIGN, and CD40 ligand (CD40L) significantly inhibited the expression of transgenic luciferase (P < .0001). Means ± 1 SD from 5 independent experiments are shown.
The adhesion molecules that mediate DC-DC interaction are also involved in the organization of the immunologic synapse between DCs and T cells. Thus, we tested whether transgene expression by mature DCs increased during the formation of the synapse. For this, mature DCs or immature DCs were transfected with pCMV-Luc with the gene gun and cocultured with syngeneic C57BL/6 naive or memory CD4+ T cells. Interaction with either CD4+ T-cell population increased transgene expression similarly by mature DCs, with lower effect by immature DCs (Figure 6B). The increase in transgene expression was similar to that induced by CD40 signaling of mature DCs, and was blocked by anti-CD40L mAb, anti-CD18 mAb, or the DC-SIGN inhibitor mannan (P < .0001) (Figure 6C).
In vivo induction of OVA-specific CTLs
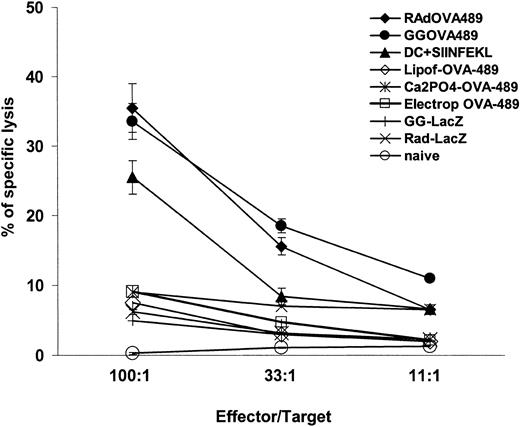
Generation of antigen-specific CTLs plays a key role in the development of immunity to tumors and infectious diseases. To evaluate the ability of mature DCs transfected and signaled by CD40 to induce immune responses, we investigated the in vivo induction of a CTL response. Mature DCs were transfected with pCMV-OVA-489 by lipofection, Ca2PO4, electroporation, the gene gun, or rAd-OVA-489, cultured with anti-CD40 mAb for 24 hours, and injected intradermally into C57BL-6 mice. Mature DCs infected with rAd or transfected with the gene gun induced the highest CTL response when compared with the other methods of transfection with naked DNA (Figure 7). There was no significant difference between CTL responses induced by mature DCs infected with rAd-OVA-489 compared with mature DCs transfected with the gene gun (P > .05). A higher CTL response was observed after immunization with mature DCs infected with rAd or transfected with gene gun compared with immunizations with mature DCs pulsed with OVA257-264 peptide (SIINFEKL) (P < .05) (Figure 7).
Immunization of mice with gene gun–transfected mature DCs with pCMV-OVA 489 or infected with rAd-CMV-OVA-489 induces OVA-specific CTL response. Induction of OVA-specific CTL response in mice immunized with mature DCs transfected with pCMV-OVA-489 or infected with rAd CMV-OVA-489 and signaled through CD40. Comparisons of CTL response induced by mature DCs transfected with lipofectamine, electroporation, Ca2PO4 precipitation, and gene gun or infected with rAd are displayed. Gene gun and rAd induced the highest CTL response compared with other naked DNA transfection methods (P < .001). There were no significant differences between CTL responses induced by mature DCs transfected with gene gun or infected with rAd (P > .05). Immunizations with mature DCs transfected with gene gun or infected with rAd induced a higher CTL response than immunizations performed with mature DCs pulsed with SIINFEKL peptide (P < .05). Mature DCs transfected with other DNA methods induced CTL levels similar to negative controls. Negative controls included mature DCs signaled with CD40 and gene gun transfected with pCMV-LacZ or rAd CMV-LacZ, and naive mice injected with PBS. Means ± 1 SE from triplicates are shown. Results from 1 of 3 representative experiments are displayed.
Immunization of mice with gene gun–transfected mature DCs with pCMV-OVA 489 or infected with rAd-CMV-OVA-489 induces OVA-specific CTL response. Induction of OVA-specific CTL response in mice immunized with mature DCs transfected with pCMV-OVA-489 or infected with rAd CMV-OVA-489 and signaled through CD40. Comparisons of CTL response induced by mature DCs transfected with lipofectamine, electroporation, Ca2PO4 precipitation, and gene gun or infected with rAd are displayed. Gene gun and rAd induced the highest CTL response compared with other naked DNA transfection methods (P < .001). There were no significant differences between CTL responses induced by mature DCs transfected with gene gun or infected with rAd (P > .05). Immunizations with mature DCs transfected with gene gun or infected with rAd induced a higher CTL response than immunizations performed with mature DCs pulsed with SIINFEKL peptide (P < .05). Mature DCs transfected with other DNA methods induced CTL levels similar to negative controls. Negative controls included mature DCs signaled with CD40 and gene gun transfected with pCMV-LacZ or rAd CMV-LacZ, and naive mice injected with PBS. Means ± 1 SE from triplicates are shown. Results from 1 of 3 representative experiments are displayed.
Discussion
For the purpose of immune therapy using genetically engineered DCs, transfection with naked DNA may be desirable over recombinant viral vectors. So far, DCs have been shown to be exceedingly difficult to transfect with naked DNA, and the highest transfection efficiencies have been achieved using recombinant viral vectors.11,15,23 Previous reports have shown effective naked DNA transfection of primary cells such as CD34+ bone marrow stem cells and glial cells using the gene gun.42,43 Here we analyzed the transfection efficiencies induced by different naked DNA delivery methods in DCs and showed that gene gun induced significantly higher transgene expression. This effect was a consequence of shooting DNA-loaded gold particles directly into the cytosol of DCs rather than of the uptake of gold particles from the culture medium by macropinocytosis. By using naked DNA, we demonstrated that the efficient transfection of DCs was limited to the population of mature DCs and that transgene expression increased dramatically after DC terminal differentiation induced by signaling of DCs by CD40, which was responsible for the nuclear translocation of transcription factors required by hIE-CMVp. Importantly, when combined with CD40 ligation of mature DCs, gene gun and rAd induced similar levels of transgene expression and similar numbers of DCs expressing transgenic antigenic proteins. Our mechanistic study suggests that increased transgene expression was caused by the nuclear translocation of NF-κB, AP-1, and CRE. These transcription factors bind to sequences in the enhancer region of hIE-CMVp, contributing to the strength of the promoter.13,14
We have previously described that the gene gun induces rapid activation and migration of skin DCs.5,21 Interestingly, under our experimental conditions, the gene gun did not induce further maturation or terminal differentiation of mature DCs, as demonstrated by unchanged levels of expression of the DC activation markers CD54 and CD86 and by the inability to translocate NF-κB and AP-1 to the DC nucleus. These opposite results can be explained by the fact that the gene gun induces the activation and migration of skin DCs transfected in vivo or in ex vivo models of skin explants. Under such experimental conditions, the activation of skin DCs can be ascribed to the damage caused to epidermal keratinocytes that secrete proinflammatory cytokines necessary to trigger DC maturation and migration.5,21
Signaling DCs through CD40 in vitro induces the formation of homotypic DC clusters. This effect is caused by an increase in the levels of ICAM-1 (CD54), ICAM-2 (CD102), and ICAM-3 (CD50) and to an enhanced affinity for their ligands, the β2 integrins, or DC-SIGN.37-41 Our results demonstrate that the blockade of CD54, CD11a, CD18, or DC-SIGN consistently diminishes transgene expression in terminal differentiated DCs. These observations strongly suggest that transgene expression controlled by the hIE-CMVp in DCs that are signaled by CD40 is regulated by a complex mechanism that also combines signaling through adhesion molecules. Importantly, the adhesion molecules involved in the formation of DC clusters are also implicated in the initial steps of the organization of the immunologic synapse between DC and CD4+ T cells. As a consequence of this interaction, DCs receive the necessary CD4+ T-cell “help” (by CD40-CD40L interaction) to initiate the CD8+ T-cell response.44-46 Therefore, we hypothesized that the DC-CD4+ T-cell interaction might affect the level of transgene expression by DCs in a similar fashion to CD40 signaling and homotypic DC aggregation. Our results demonstrated that coculture of transfected mature DCs with naive or antigen-specific memory CD4+ T cells induces transgene expression equivalent to that observed after CD40 signaling. This observation suggests that the interaction of maturing migratory DCs, transfected in peripheral tissues, with T cells in secondary lymphoid organs results in a further increase in transgene expression. This in turn facilitates the delivery of transgenic antigens and immunoregulatory factors by transfected DCs where antigen presentation occurs.
The enhanced transgene expression by DCs is functionally significant, as demonstrated by the efficient processing and presentation of a transgenic antigen-derived peptide by DCs. The success of preventive or therapeutic immunization against tumors or intracellular pathogens depends on the generation of antigen-specific CTL responses.3 Effective CTL induction requires efficient antigen and adjuvant delivery to induce effective antigen presentation and T-cell costimulation by mature DCs. We have shown that CD40 signaling of transfected DCs induces efficient loading of transgenic antigen peptides into MHC class 1 and potent antigen presentation to naive CD8+ specific T cells. The increased expression of SIINFEKL–H-2Kb complexes on the surfaces of DCs correlated with enhanced T-cell stimulatory capacity. Importantly, DCs that were transfected and terminally differentiated by CD40 ligation induced significantly higher antigen-specific T-cell proliferation than DCs loaded with saturating doses of synthetic peptide, even when the latter population was identically matured by CD40 ligation. This observation could be explained by a sustained synthesis of transgenic proteins, followed by sustained exportation of MHC class 1 peptide complexes to the DC surface in gene gun–transfected and CD40-activated mature DCs.47 Indeed the turnover of MHC class 1 molecules, together with a decreased concentration of OVA peptide in the culture wells over a period of 72 hours, may account for the different OT-1 proliferation activity observed in OT-1 assays stimulated with CD40-signaled gene gun–transfected mature DCs compared with OVA (SIINFEKL)–pulsed CD40-signaled mature DCs.
Importantly, the effect we observed in ex vivo OT-1 proliferation assays was further confirmed in “in vivo” CTL assays. As expected, gene gun transfection and rAd infection of mature DCs signaled with agonist CD40 mAb were similar, and both induced significantly higher CTL response than mature DCs transfected with other naked DNA methods. Furthermore the CTL response induced by gene gun–transfected or rAd-infected mature DCs was slightly higher than the CTL response induced by OVA257-264 (SIINFEKL)–peptide-pulsed mature DCs.
Taken together, our results demonstrate that high-level transgene expression can be achieved in DCs by combining an efficient DNA delivery method with an appropriate signaling strategy able to translocate to the DC nucleus those transcription factors required for functioning of the transpromoter used. The data presented here further elucidate the mechanisms regulating efficient transgene expression by DCs and contribute to the development of DC engineering strategies for the purpose of immunoregulation.
Prepublished online as Blood First Edition Paper, October 9, 2003; DOI 10.1182/blood-2003-02-0524.
Supported by grants from the Dermatology Foundation (A.T.L) and by National Institutes of Health grants RO1 AI43916, PO1 AI0550794, and PO1 CA73743 (L.D.F.), RO1 DK49745 and RO1 AI41011 (A.W.T.), and R21 HL69725 and R21 AI55027 (A.E.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr P. Robbins and Mrs J. Duke for providing the rAd CMV-OVA-489.