Abstract
Natural interferon-producing cells (IPCs) specialize in the production of high levels of type 1 interferons (IFNs) in response to encapsulated DNA and RNA viruses. Here we demonstrate that the secretion of type 1 IFN in response to herpes simplex virus type 1 (HSV-1) in vitro is mediated by the toll-like receptor 9 (TLR9)/MyD88 pathway. Moreover, IPCs produce interleukin-12 (IL-12) in response to HSV-1 in vitro, which is also dependent on TLR9/ MyD88 signaling. Remarkably, though TLR9/MyD88-deficiency abrogates IPC responses to HSV-1 in vitro, mice lacking either MyD88 or TLR9 are capable of controlling HSV-1 replication in vivo after local infection, demonstrating that TLR9- and MyD88-independent pathways in cells other than IPCs can effectively compensate for defective IPC responses to HSV-1.
Introduction
Herpes virus simplex type 1 (HSV-1) is a neurotropic α-herpesvirus that causes primary infections of stratified squamous epithelia lining oral mucosa, conjunctiva, cornea, and skin.1 During primary infection, HSV-1 infects the adjacent sensory neuron endings and reaches the sensory ganglia, where it persists in a latent state. Sporadically, HSV-1 is reactivated and travels from the sensory neurons back to the epithelial tissue. Reactivation can cause tissue damage from the cytopathic infection of epithelial cells and inflammation. In immunocompetent hosts, HSV-1 infection triggers the secretion of interferon-α (IFN-α), which has a critical function in host anti–HSV-1 response.2,3 IFN-α inhibits the transcription of immediate-early HSV-1 genes.4 In mice IFN-α is a potent inhibitor of HSV-1 replication in the cornea,5 and it activates host defenses such as natural killer cells, which participate in controlling HSV-1 infection and disease.6 IFN-α has also been shown to limit the progress of infection from peripheral tissues to the nervous system.7 It has been proposed that the IFN-α response to HSV-1 is triggered by recognition of the HSV-1 envelope glycoprotein D (gD).8 Dendritic cells (DCs) may bind HSV-1 glycoproteins through the mannose receptor and possibly other lectins.9 CC-chemokine receptor 3 (CCR3) or CXC-chemokine receptor 4 (CXCR4) may function as coreceptors.8 The intracellular events linking the recognition of HSV-1 glycoproteins with IFN-α secretion are unknown.
As does bacterial DNA, HSV-1 DNA contains abundant deoxycytidylate-phosphate-deoxyguanylate (CpG) motifs.10 These motifs stimulate multiple cellular components of the immune system through the recently characterized toll-like receptor 9 (TLR9).11-14 TLR9 transduces intracellular signals through myeloid differentiation factor 88 (MyD88), which initiates a signaling cascade leading to NF-κB activation and cytokine secretion.15,16 Thus, TLR9 might provide another pathway that links HSV-1 recognition with IFN-α responses. To test this hypothesis we focused on natural interferon-producing cells (IPC). IPCs, also called plasmacytoid dendritic cells (pDCs), respond to HSV-1 and to other DNA and RNA viruses by secreting high levels of IFN-α, -β, and -ω,17-23 releasing IL-1222,24,25 and up-regulating costimulatory molecules.22,24-26 Human and murine IPCs express TLR9 and are activated by single-stranded oligodeoxynucleotides (ODNs) containing CpG motifs.14,27-31 Thus, they represent a valuable biologic system to test the role of TLR9 in immune cell responses to HSV-1. Here we demonstrate that IPC responsiveness to HSV-1 in vitro is indeed mediated by the TLR9/MyD88 pathway. However, mice lacking either MyD88 or TLR9 can control HSV-1 replication after local infection, demonstrating that TLR9- and MyD88-independent mechanisms can effectively compensate for impaired anti–HSV-1 IPC responses in vivo.
Materials and methods
Isolation and culture of IPCs and DCs, flow cytometric analyses, and determination of cytokine secretion
MyD88–/– mice32 were backcrossed to C57BL/6, and TLR9–/– mice13 were backcrossed to BALB/c. Mice were between 6 and 12 weeks of age. Spleen DCs were isolated with CD11c+ microbeads (Miltenyi Biotec, Bergisch-Gladbach, Germany). IPCs (CD11c+/Ly-6C+/CD11b– cells), CD11b+ DCs (CD11c+/Ly-6C–/CD11b+ cells, which comprise myeloid DCs), and CD11b– DCs (CD11c+/Ly-6C–/CD11b– cells, which comprise CD8α+ DCs) were sorted from CD11c+ splenocytes using a MoFlo cytometer (Cytomation, Fort Collins, CO; more than 95% purity), as previously described.33 Bone marrow–derived IPCs were generated from bone marrow cells by culture with human recombinant fms-related tyrosine kinase 3 ligand (Flt3L) (10 ng/mL; R&D Systems, Minneapolis, MN) for 7 days and were purified by sorting the CD11c+/CD11blow population as described.33 Cells were cultured with purified KOS HSV-1 (0.01-10 multiplicity of infection [MOI]), CpG ODN 2216 (3 μg/mL; Qiagen, Valencia, CA) or influenza virus PR8 (1 MOI) for 36 hours at the indicated cell density.33 Ultraviolet (UV)–inactivation of diluted HSV-1 stock (5 × 106 and 5 × 105 plaque-forming units (PFU)/mL) was performed using a UV crosslinker (optimal crosslink setting, 1.2 × 105 μJ/cm2).34 IFN-α and IL-12 in culture supernatants were measured using enzyme-linked immunosorbent assay (ELISA; PBL Biomedical Laboratories, New Brunswick, NJ and BD Biosciences, Franklin Lakes, NJ). The IFN-α ELISA detects IFN-αA, α1, α4, α5, α6, and α9 subspecies. Expression of CD86 was determined by flow cytometry33 and is shown as median fluorescence intensity. The median value of the isotype control is indicated in the figures as a dashed line.
Recombinant virus and bioluminescence imaging of HSV-1 infection
To detect HSV-1 replication in living mice, we used a KOS HSV-1 recombinant strain, KOS/Dlux/oriL, which expresses the firefly luciferase (FL) reporter protein.35 KOS/Dlux/oriL was constructed by homologous recombination into a site between UL49 and UL50, with a cassette encoding the UL30 promoter to regulate FL as described previously.36 HSV-1 was injected in the footpads of 6- to 12-week-old (approximate ages) mice (2 × 106 or 107 PFU/footpad as indicated). Viral infection in mouse footpads was detected by bioluminescence imaging of FL using a cooled charge-coupled device camera (IVIS; Xenogen, Cranbury, NJ). Bioluminescence imaging was repeated every 24 hours for 1 week. Signal intensities from regions of interest were expressed as total photon flux (photons per second).
Corneal infection model and HSV-1 plaque assay
Corneal infection, eye swabs, and excision of trigeminal ganglia were performed as described previously.37 Briefly, mice were infected with 2 × 106 PFU per eye after scarification of the corneas. Eye swabs were taken at the indicated time points and diluted in 1 mL culture medium. Trigeminal ganglia were homogenized in 1 mL culture medium. Viral titers were determined by standard plaque assay on Vero cells and are presented as logarithmic mean ± SD of plaque-forming units per milliliter. The limit of detection was 10 PFU/mL.
Results
IFN-α and IL-12 responses of IPCs to HSV-1 are impaired in MyD88-deficient mice
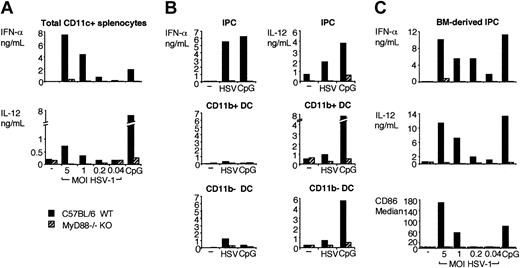
CD11c+ splenocytes were isolated from MyD88-deficient and control mice and were cultured in vitro with HSV-1. After 36 hours, IFN-α and IL-12 in culture supernatants were quantified using ELISA. As shown in Figure 1A, MyD88-deficient CD11c+ splenocytes secreted considerably less IFN-α and IL-12 than did wild-type DCs in response to HSV-1. CD11c+ splenocytes include IPCs, which represent a major source of IFN-α and IL-12.22 In addition, they include CD11b+ DCs and CD11b– DCs, which are also capable of secreting cytokines when exposed to appropriate bacterial and viral stimuli.38,39 Therefore, we asked whether these cell subsets respond to HSV-1 in a MyD88-dependent fashion. IPCs, CD11b+ DCs, and CD11b– DCs were sorted from MyD88–/– and control mice and were stimulated in vitro with HSV-1 to induce the secretion of IFN-α and IL-12. As shown in Figure 1B, wild-type IPCs secreted large amounts of IFN-α and IL-12, whereas cytokine secretion of MyD88–/– IPCs was severely impaired. Wild-type CD11b+ and CD11b– DCs secreted significant levels of IL-12 but very low levels of IFN-α in response to HSV-1. These responses were reduced in MyD88–/– DCs. Finally, we investigated the impact of MyD88 deficiency on the response of IPCs derived from bone marrow precursors to HSV-1. As shown in Figure 1C, the secretion of IFN-α and IL-12 and the up-regulation of CD86 were negligible in MyD88–/– bone marrow–derived IPCs compared to normal IPCs (Figure 1C). We conclude that MyD88 is essential for the IFN-α and IL-12 responses of primary IPCs and DC and of bone marrow–derived IPCs to HSV-1 in vitro.
Cytokine secretion and activation of CD11c+ splenocytes, IPCs, CD11b+ DC, CD11b– DCs, and bone marrow–derived IPCs from wild-type and MyD88–/– mice in response to HSV-1 in vitro. (A) CD11c+ splenocytes were isolated from wild-type (▪) and MyD88–/– (▨) mice and cultured for 36 hours (2 × 106 cells/mL) with KOS HSV-1 at the indicated MOI or 3 μg/mL CpG ODN 2216. (B) IPC (top row), CD11b+ DCs (middle row), and CD11b– DC (bottom row) were sorted from wild-type and MyD88–/– CD11c+ splenocytes and were cultured with 2 MOI HSV-1 or 3 μg/mL CpG ODN 2216 for 36 hours (5 × 104 cells/150 μL/well). (C) Bone marrow–derived wild-type and MyD88–/– IPCs were incubated with HSV-1 (0.04-5 MOI) or CpG ODN 2216 (3 μg/mL) for 36 hours at 106 cells/mL. IFN-α and IL-12 were measured in the supernatants using ELISA. CD86 expression was measured as a marker of activation by flow cytometry and is expressed as median fluorescence intensity. Dashed line indicates the median value of the isotype control sample.
Cytokine secretion and activation of CD11c+ splenocytes, IPCs, CD11b+ DC, CD11b– DCs, and bone marrow–derived IPCs from wild-type and MyD88–/– mice in response to HSV-1 in vitro. (A) CD11c+ splenocytes were isolated from wild-type (▪) and MyD88–/– (▨) mice and cultured for 36 hours (2 × 106 cells/mL) with KOS HSV-1 at the indicated MOI or 3 μg/mL CpG ODN 2216. (B) IPC (top row), CD11b+ DCs (middle row), and CD11b– DC (bottom row) were sorted from wild-type and MyD88–/– CD11c+ splenocytes and were cultured with 2 MOI HSV-1 or 3 μg/mL CpG ODN 2216 for 36 hours (5 × 104 cells/150 μL/well). (C) Bone marrow–derived wild-type and MyD88–/– IPCs were incubated with HSV-1 (0.04-5 MOI) or CpG ODN 2216 (3 μg/mL) for 36 hours at 106 cells/mL. IFN-α and IL-12 were measured in the supernatants using ELISA. CD86 expression was measured as a marker of activation by flow cytometry and is expressed as median fluorescence intensity. Dashed line indicates the median value of the isotype control sample.
IPC responses to HSV-1 are mediated by TLR9 and do not require viral replication
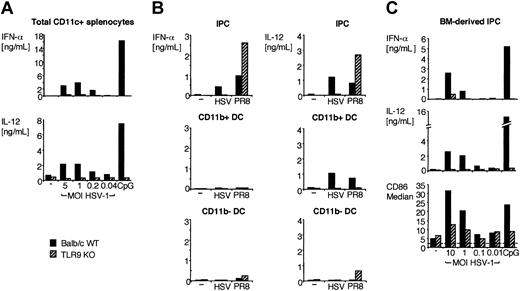
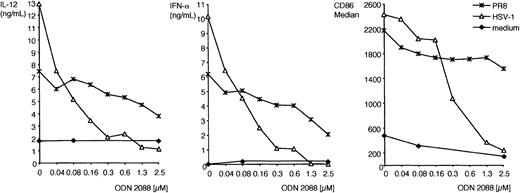
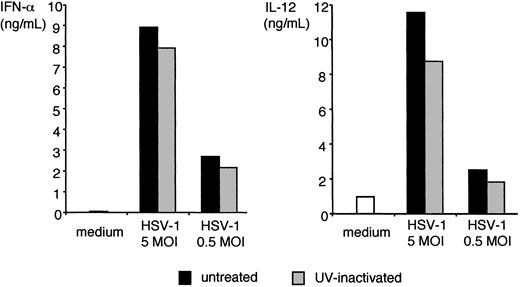
All murine IPCs and DCs express TLR9,30,31 which signals exclusively through MyD88.15,16 Thus, MyD88-dependent responses of IPCs and DCs to HSV-1 may be initiated by TLR9. To test this hypothesis, we prepared total CD11c+ splenocytes, IPCs, CD11b+ DCs, CD11b– DCs, and bone marrow–derived IPCs from TLR9-deficient mice and tested their responses to HSV-1. As shown in Figure 2, TLR9-deficient CD11c+ splenocytes, primary IPCs, and bone marrow–derived IPCs secreted significantly less IFN-α and IL-12 than did their wild-type counterparts. Upregulation of CD86 in response to HSV-1 was also reduced in TLR9-deficient IPCs in comparison with wild-type IPCs, although this marker of activation was less disrupted than cytokine secretion (Figure 2C). Remarkably, IPC responses to influenza virus (strain PR8) were intact in TLR9–/– mice, indicating that IPC recognition of influenza virus is TLR9 independent. Wild-type DCs secreted some IL-12 but no IFN-α, whereas TLR9–/– DCs did not respond to HSV-1 at all. To further corroborate the evidence that TLR9 mediates IPC responses to HSV-1, we cultured wild-type bone marrow–derived IPCs in vitro with HSV-1 either in the presence or the absence of ODN 2088, a competitive inhibitor of TLR9.40 As shown in Figure 3, ODN 2088 inhibited the wild-type IPC secretion of IFN-α and IL-12 and the up-regulation of CD86 in a dose-dependent fashion. ODN 2088 had little effect on IPC responses to influenza virus, demonstrating that ODN 2088 selectively inhibited TLR9-mediated responses, further confirming that recognizing influenza virus is TLR9 independent. We conclude that IPC responses to HSV-1 are critically dependent on TLR9. Finally, we tested whether IPC responses to HSV-1 through TLR9/MyD88 require active viral replication. HSV-1 was treated with UV light to prevent viral replication without denaturing envelope glycoproteins. UV-inactivated HSV-1 was as potent as untreated virus in inducing IFN-α and IL-12 production by bone marrow–derived IPCs (Figure 4) and IPCs sorted from CD11c+ splenocytes (not shown). Thus, IPC responses to HSV-1 in vitro occur in the absence of viral replication.
Cytokine secretion and activation of CD11c+ splenocytes, IPCs, CD11b+ DCs, CD11b– DCs, and bone marrow–derived IPCs from wild-type and TLR9–/– mice in response to HSV-1 in vitro. (A) Wild-type (▪) and TLR9–/– (▨) CD11c+ splenocytes were cultured for 36 hours (2 × 106 cells/mL) with HSV-1 at the indicated MOI or 3 μg/mL CpG ODN 2216. (B) IPCs (top row), CD11b+ DCs (middle row), and CD11b– DCs (bottom row) were sorted from wild-type and TLR9–/– CD11c+ splenocytes and were cultured with 2 MOI HSV-1 and 1 MOI influenza virus PR8 for 36 hours (2 × 104 cells/150 μL/well). (C) Wild-type and TLR9–/– bone marrow–derived IPCs were incubated with HSV-1 (0.01-10 MOI) or CpG 2216 (3 μg/mL). IFN-α and IL-12 were measured in the supernatants using ELISA. CD86 expression was measured by flow cytometry and is expressed as median fluorescence intensity. Dashed line indicates the median value of the isotype control sample.
Cytokine secretion and activation of CD11c+ splenocytes, IPCs, CD11b+ DCs, CD11b– DCs, and bone marrow–derived IPCs from wild-type and TLR9–/– mice in response to HSV-1 in vitro. (A) Wild-type (▪) and TLR9–/– (▨) CD11c+ splenocytes were cultured for 36 hours (2 × 106 cells/mL) with HSV-1 at the indicated MOI or 3 μg/mL CpG ODN 2216. (B) IPCs (top row), CD11b+ DCs (middle row), and CD11b– DCs (bottom row) were sorted from wild-type and TLR9–/– CD11c+ splenocytes and were cultured with 2 MOI HSV-1 and 1 MOI influenza virus PR8 for 36 hours (2 × 104 cells/150 μL/well). (C) Wild-type and TLR9–/– bone marrow–derived IPCs were incubated with HSV-1 (0.01-10 MOI) or CpG 2216 (3 μg/mL). IFN-α and IL-12 were measured in the supernatants using ELISA. CD86 expression was measured by flow cytometry and is expressed as median fluorescence intensity. Dashed line indicates the median value of the isotype control sample.
Inhibition of cytokine production and costimulatory molecule expression by TLR9 antagonist CpG ODN 2088. Bone marrow–derived IPCs (5 × 105 cells/mL, 129/SvJ strain) were preincubated with increasing doses of the competitive TLR9 antagonist CpG ODN 2088 (0.04-2.5 μM) and then were cultured with HSV-1 (5 MOI), influenza virus PR8 (1 MOI), or medium alone for 24 hours. IFN-α and IL-12 were measured in the supernatants using ELISA. CD86 expression was determined by flow cytometry and is expressed as median fluorescence intensity after subtracting the background of the isotype control.
Inhibition of cytokine production and costimulatory molecule expression by TLR9 antagonist CpG ODN 2088. Bone marrow–derived IPCs (5 × 105 cells/mL, 129/SvJ strain) were preincubated with increasing doses of the competitive TLR9 antagonist CpG ODN 2088 (0.04-2.5 μM) and then were cultured with HSV-1 (5 MOI), influenza virus PR8 (1 MOI), or medium alone for 24 hours. IFN-α and IL-12 were measured in the supernatants using ELISA. CD86 expression was determined by flow cytometry and is expressed as median fluorescence intensity after subtracting the background of the isotype control.
IFN-α and IL-12 production by bone marrow–derived IPC in response to UV-inactivated HSV-1. Bone marrow–derived IPCs (5 × 105/mL, 129/SvJ strain) were incubated with untreated and UV-inactivated KOS HSV-1 at 5 and 0.5 MOI for 24 hours. IFN-α (left panel) and IL-12 (right panel) were measured in the supernatants using ELISA. UV-inactivated KOS HSV-1 effectively stimulated IPCs sorted from splenocytes (data not shown).
IFN-α and IL-12 production by bone marrow–derived IPC in response to UV-inactivated HSV-1. Bone marrow–derived IPCs (5 × 105/mL, 129/SvJ strain) were incubated with untreated and UV-inactivated KOS HSV-1 at 5 and 0.5 MOI for 24 hours. IFN-α (left panel) and IL-12 (right panel) were measured in the supernatants using ELISA. UV-inactivated KOS HSV-1 effectively stimulated IPCs sorted from splenocytes (data not shown).
Mice lacking MyD88 or TLR9 can control HSV-1 replication after local infection in vivo
Finally, we investigated the impact of MyD88- and TLR9-mediated responses on HSV-1 replication in vivo. To monitor HSV-1 replication in living animals, we infected mice with KOS/Dlux/oriL HSV-1 strain that expresses the firefly luciferase reporter protein, allowing visualization of HSV-1 replication by bioluminescence imaging.35 After footpad injection, HSV-1 replication was monitored in MyD88–/– mice, TLR9–/– mice, and control mice with a charge-coupled device camera every 24 hours for 6 to 7 days. In all mice, HSV-1 replication was detected exclusively at the site of infection (Figure 5A-D). Replication was sustained up to approximately day 4 or 5 after infection, and then it declined progressively (Figure 5E-F). Measuring photon flux at the site of infection revealed slightly more HSV-1 replication in MyD88–/– mice than in wild-type mice (Figure 5A-B, E). However, no significant difference was detected between TLR9–/– and wild-type mice (Figure 5C-D, F). No systemic spreading of HSV-1, signs of paralysis, or death were detected in MyD88–/– or TLR9–/– mice. It could be argued that bioluminescence imaging is not sensitive enough to detect small differences in HSV-1 replication between wild-type and TLR9/MyD88-deficient mice or that the KOS/Dlux/oriL HSV-1 may be slightly attenuated compared with the unaltered KOS HSV-1. To address this possibility, wild-type and MyD88–/– mice were infected with KOS HSV-1 by corneal scarification, and viral titers were determined in eye swabs and homogenized trigeminal ganglia by standard plaque assay. As shown in Figure 5, panels G and H, HSV-1 replication was not increased in MyD88–/– mice compared with wild-type mice. We conclude that TLR9/MyD88-independent mechanisms can effectively compensate for the absence of TLR9 or MyD88 during an in vivo immune response against localized HSV-1 infection.
Replication of HSV-1 in MyD88–/–, TLR9–/–, and control mice. (A-B, E) MyD88–/– and C57BL/6 control mice (8-week-old males; n = 6) were injected with 107 PFU KOS/Dlux/OriL HSV-1 in each hind footpad. (C-D, F) TLR9–/– and Balb/c controls (6- to 7-week-old males; n = 5) were injected with 2 × 106 PFU KOS/Dlux/OriL HSV-1 in each hind footpad. Viral replication and spatial distribution of replicating virus was visualized by bioluminescence imaging daily up to day 7. Representative images taken 24 hours and 6 days after infection are shown (A-D). Signal intensities from hind feet were measured as photon flux (photons/second) within defined regions of interest (E-F). Mean values ± SEM are shown for each time point. (G-H) C57BL/6J wild-type and MyD88–/– mice were infected with 2 × 106 PFU KOS HSV-1 in each eye after corneal scarification. Viral titers of eye swabs (G) and trigeminal ganglia (H) were measured by plaque assay at different time points. Logarithmic mean values ± SD are shown (n = 3).
Replication of HSV-1 in MyD88–/–, TLR9–/–, and control mice. (A-B, E) MyD88–/– and C57BL/6 control mice (8-week-old males; n = 6) were injected with 107 PFU KOS/Dlux/OriL HSV-1 in each hind footpad. (C-D, F) TLR9–/– and Balb/c controls (6- to 7-week-old males; n = 5) were injected with 2 × 106 PFU KOS/Dlux/OriL HSV-1 in each hind footpad. Viral replication and spatial distribution of replicating virus was visualized by bioluminescence imaging daily up to day 7. Representative images taken 24 hours and 6 days after infection are shown (A-D). Signal intensities from hind feet were measured as photon flux (photons/second) within defined regions of interest (E-F). Mean values ± SEM are shown for each time point. (G-H) C57BL/6J wild-type and MyD88–/– mice were infected with 2 × 106 PFU KOS HSV-1 in each eye after corneal scarification. Viral titers of eye swabs (G) and trigeminal ganglia (H) were measured by plaque assay at different time points. Logarithmic mean values ± SD are shown (n = 3).
Discussion
In this study, we investigated the mechanisms of IPC activation by HSV-1. Our results demonstrate that HSV-1 activates IPC in vitro mainly through the TLR9/MyD88 pathway. In agreement with this conclusion, TLR9- and MyD88-deficient IPCs have severely impaired responses to HSV-1, including dramatically reduced secretion of IFN-α and IL-12 and significantly decreased cell activation. Moreover, blocking TLR9 in wild-type IPCs with an inhibitory CpG ODN had comparable consequences. How does TLR9 recognize HSV-1? UV-inactivated HSV-1 stimulated IPC responses just as untreated HSV-1 did, indicating that HSV-1 replication is not required for the effective stimulation of TLR9/MyD88. Because TLR9 is located in an intracellular endosomal compartment,41 HSV-1 may initially interact with cell surface receptors specific for envelope glycoproteins. After the internalization of encapsulated HSV-1, viral DNA may be released in an endosomal compartment for interaction with TLR9. Thus, IPCs emerge as unique sentinel cells that sense and respond to HSV-1 without becoming productively infected. This conclusion is consistent with the recent demonstration that IPCs recognize HSV-2 through TLR942 and that UV-inactivated HSV-2 and HSV-2 DNAs are sufficient to activate IPCs, whereas a pharmacologic inhibitor of endosomal-lysosomal processing blocks activation.42 Together these studies suggest that other DNAviruses may activate IPCs through the same pathway. Notably, our study also demonstrates that IPCs are major producers of IL-12 in response to HSV-1. IL-12 secretion by IPC and classical DC subsets is also dependent on TLR9/MyD88 signaling, consistent with previous reports that TLR9 is expressed in all murine DC subsets.30,31 It remains unclear why HSV-1 is more effective in stimulating IFN-α responses of IPCs than of DCs. HSV-1 may inhibit DC maturation, as observed in human DCs infected by HSV-1,43 or it may selectively block the TLR9/MyD88 pathway in DCs, as previously reported for the RNaseL and protein kinase regulated by RNA (PKR) pathways, which also mediate host resistance to HSV-1.37,44
Despite the dramatic effect of MyD88 and TLR9 deficiencies on IPC secretion of IFN-α and IL-12 in vitro, we detected no significant increase of HSV-1 replication in MyD88–/– and TLR9–/– mice compared with wild-type mice using the bioluminescent KOS/Dlux/oriL HSV-1 strain. Similarly, the replication of KOS HSV-1 after corneal infection was not greater in MyD88–/– mice than in wild-type mice, as assessed using a standard plaque assay. Moreover, no neurologic symptoms or systemic spreading of HSV-1 was observed in MyD88- or TLR9-deficient mice, even after footpad injection of 5 × 107 PFU of KOS HSV-1 (data not shown). Thus, MyD88 and TLR9 deficiencies had limited or no impact on host ability to control HSV-1 replication in our model of local infection.
In conclusion, our study indicates that TLR9/MyD88-independent responses of cells other than IPCs can mostly compensate for the lack of TLR9/MyD88-dependent responses of IPCs in local HSV-1 infections. Epithelial cells, fibroblasts, macrophages, or other immune cells infiltrating HSV-1–infected skin and mucosa may efficiently recognize and respond to HSV-1 through TLR9/MyD88-independent pathways. These pathways may be initiated by the mannose receptor,9 the chemokine receptors,8 or the herpesvirus entry mediator, which activates nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) transcription factors.45,46 Intracellular replication of HSV-1 may also generate double-stranded (ds) RNA products that activate dsRNA-dependent PKR,47 leading to the activation of IRF3 and the expression of IFN type 1.48 Finally, HSV-1 could activate the recently discovered TRIF pathway downstream of TLR3 or TLR4, inducing IFN-β secretion sufficient to control viral replication.49,50 IPCs and the TLR9/MyD88 pathway may become relevant in IFN-α responses to viruses that access the bloodstream and cause systemic infections in vivo.
Prepublished online as Blood First Edition Paper, October 16, 2003; DOI 10.1182/blood-2003-08-2674.
Supported by the Deutsche Forschungsgemeinschaft (KR 2199/1-1) (A.K.), RO1 EY09083 (D.A.L.), and a Research to Prevent Blindness Lew Wasserman Scholarship (D.A.L.). Supported also by National Institutes of Health grant PA CA94056 awarded to the Molecular Imaging Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Akiko Iwasaki (Yale University) for sharing unpublished results, Arthur M. Krieg (Coley Pharmaceutical Group, Wellesley, MA) for providing TLR9–/– mice, Kathy Frederick and Emil R. Unanue (Washington University) for MyD88–/– mice, William Eades (Siteman Cancer Center flow cytometry facility) for expert cell sorting, and Marina Cella and Susan Gilfillan (Washington University) for advice and helpful discussions.